Following encouraging preclinical animal data, stem cell therapy has been tested in a variety of clinical trials in patients with both acute and chronic ischemic myocardial injury with the goal of repairing damaged myocardium and/or inducing the growth of new blood vessels so as to improve cardiac function and symptoms. Many cell types have been studied, but most clinical trials to date have utilized autologous bone marrow mononuclear cells (or cellular fractions thereof) with good safety data but heterogeneous results in terms of efficacy.1 Two new studies using this cell type were recently completed by the Cardiac Cell Therapy Research Network established by the National Heart, Lung, and Blood Institute to accelerate the development of cell-based therapies in the USA. Both failed to demonstrate any beneficial effects on cardiac function.2,3 By contrast, two recently published trials using stem cells derived from the heart itself have reported positive results.4,5 In this Commentary, we describe these trials in detail and provide possible explanations for their differing results.
Acute myocardial infarctions (AMIs), or heart attacks, are caused by interruption of blood supply to a part of the heart, leading to death of heart tissue, most often due to rupture of an atherosclerotic plaque and blockage of a coronary artery. In most heart attack patients, the blocked coronary artery can be reopened using either thrombolytic drugs or percutaneous coronary intervention (PCI) employing balloon angioplasty and stents. However, depending mainly on the time delay between the attack and therapeutic intervention, many patients fail to achieve complete recovery of the damaged heart tissue and sustain substantial injury, leading to impaired cardiac function and heart failure. Several evidence-based drug therapies can improve the symptoms and cardiac dysfunction associated with the resulting ischemic cardiomyopathy, but there remains a need for new therapies to treat patients whose clinical condition fails to improve or continues to deteriorate. It is now recognized that both extracardiac (e.g., bone marrow) and intracardiac progenitor cells are mobilized and home to the site of the myocardial injury to participate in the compensatory healing response. There is also growing evidence that such cells participate in the maturation and induction of collateral vascular growth and neovasculogenesis and may acquire phenotypic properties of neighboring cardiac myocytes.6 However, these natural self-renewing processes, while sufficient to sustain normal homeostasis, are insufficient to salvage heart muscle following massive injury. This situation has prompted efforts to isolate, propagate, and reintroduce into the body various types of progenitor cells associated with the healing response.
LateTIME
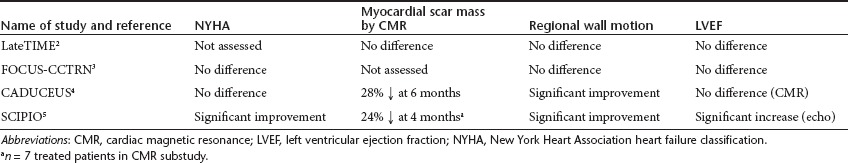
The LateTIME study is a randomized, double-blind, placebo-controlled trial of 150 million autologous nucleated bone marrow mononuclear cells administered by intracoronary injection to patients with AMI and a left ventricular ejection fraction (LVEF) below 45% (Table 1). Patients were recruited 2–3 weeks after their initial event, a time window chosen to determine whether patients who present to centers that lack expertise in cell therapy or who are initially too unstable to be treated could still benefit from the therapy. The same investigators are conducting a parallel study with a recruitment window of 3–7 days after the initial AMI (the TIME study). Patients randomized to placebo received 5% (v/v) human serum albumin–saline solution to which 100 ml of autologous blood was added to ensure that the color and consistency of the solution matched those of the experimental therapy. After 6 months' follow-up, the cardiac magnetic resonance imaging (MRI) data showed no effect of the treatment on either overall or regional cardiac function or infarct size2 (Table 2). These data contrast with those from the previously published double-blinded, placebo-controlled Repair-AMI trial, which reported a significant benefit on ejection fraction following intracoronary administration of bone marrow mononuclear cells at a median of 4 days after the clinical event.7 Taken together, these two studies suggest that bone marrow mononuclear cell therapy may be efficacious only if administered early after AMI.
Table 1. Summary of design and methodology.
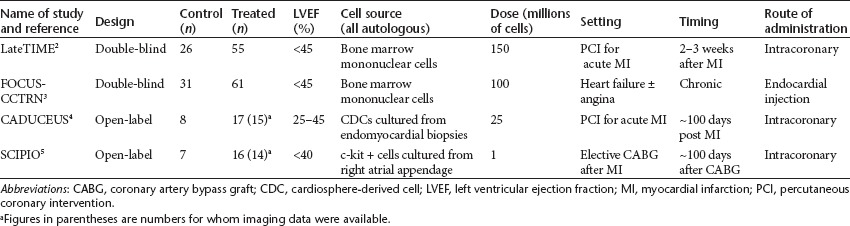
Table 2. Summary of effects of cell therapy.
FOCUS-CCTRN
In the FOCUS-CCTRN trial, patients with chronic ischemic left ventricular dysfunction with heart failure and/or angina were entered into a randomized, double-blinded, controlled trial comparing 100 million autologous nucleated bone marrow cells or placebo administered by catheter-mediated intramyocardial injection using an existing electromechanical mapping system to identify appropriate sites for injection (Table 1). This trial had three co-primary end points: the change in left ventricular end-systolic volume assessed by echocardiography, maximal oxygen consumption, and reversal of myocardial perfusion abnormalities identified using single-photon emission tomography. After 6 months' follow-up, there were no statistically significant differences between the groups for any of the primary end points and no differences in patient symptoms3 (Table 2). Regression analysis suggested a positive relationship between the CD34+ and CD133+ cell count in the cell population and absolute change in ejection fraction. In this regard, it should be noted that injection of purified CD34 cells previously yielded therapeutic benefit in a population of patients with chronic myocardial ischemia.8 In addition, it has been recognized that the functional properties of stem cells are impaired in older patients and in those with advanced ischemic heart disease and heart failure. In FOCUS-CCTRN, a prespecified subgroup analysis showed a statistically significant treatment effect on LVEF of patients younger than 62 years (the median age of the study population), with no benefit in those older than 62 years, suggesting that age is an important consideration for inclusion in such trials.
Cardiac stem cells (CSCs) were first identified in the murine heart in 1999 and isolated and characterized in human myocardium by Messina et al. in 2004 (refs. 9, 10). The cardiac source of these cells may more readily ensure a therapeutic benefit as compared with naive bone marrow mononuclear cells. In animal models, CSCs injected into the heart have been shown to differentiate into all three major cardiac cell types—cardiomyocytes, endothelial cells, and vascular smooth muscle cells—and this led to improved cardiac function, suggesting that the delivered cells directly contributed to generation of new myocardial tissue. The results of two small prospective randomized first-in-human studies of stem cells derived from cardiac tissue and delivered back to the heart were recently reported. These studies employed two distinct CSC populations, but both purport to show, for the first time, evidence of myocardial regeneration within previously infarcted areas.
CADUCEUS
In the CADUCEUS (Cardiosphere-Derived Autologous Stem Cells to Reverse Ventricular Dysfunction) study, 31 patients with AMI who had undergone successful PCI with stent implantation but were left with reduced cardiac function were randomized in a ratio of 2:1 to receive usual care or 12.5–25 million autologous cardiosphere-derived cells (CDCs) cultured from percutaneous endomyocardial biopsy tissues obtained approximately 2–4 weeks after the AMI4 (Table 1). Cell preparation required an average of 36 days, and most patients were treated 6–12 weeks after the MI (average 65 days). The cells were administered by intracoronary injection into the infarct-affected artery using a balloon catheter inflated at the site of the previously implanted stent. As might be expected in such a small trial, there were differences in the patient characteristics between the two groups, including racial origin, history of prior MI, and location of the damage within the heart. Although cardiac function was impaired in both groups, there was no description of the standard drug therapies used in such patients. Only 25 patients completed the study because of various modes of failure of the cell culture4, presence of an occluded artery at the time of planned cell therapy preventing delivery of the cell therapy1, or withdrawal of consent1.
Importantly, the investigators report that the US Food and Drug Administration would not allow the use of a sham procedure, so this trial was not blinded. However, the MRI scans used to evaluate left ventricular function, volumes, and infarct size were analyzed in a blinded fashion by an independent “core” lab. The primary end point of the trial was a combination of safety outcomes, and in this respect the trial was positive with no reported adverse events at the time of cell infusion and no incidence of ventricular arrhythmias, sudden death, or the development of cardiac tumors during 6 months of follow-up. There was one new MI between 6 and 12 months in the cell-treated group but no other major adverse cardiac events. Serious adverse events were three times more common in the cell therapy group (36% vs. 13%), but this difference was not significant, clearly because of the small numbers involved. The efficacy outcomes are summarized in Table 2. No improvements in symptoms or quality of life were reported by the patients. The excitement generated by this study relates to the cardiac MRI results, which showed a marked reduction in the volume of myocardial scar assessed using the well-validated technique of late gadolinium enhancement. At 6 months, between-group differences in function in the infarct zones also favored the CDC-treated patients, although the within-subject changes in these parameters were not reported and overall left ventricular volumes and function were similar.
SCIPIO
In the SCIPIO (Stem Cell Infusion in Patients With Ischemic Cardiomyopathy) trial, the published work represents an interim report of 23 randomized patients from a study described as “ongoing”5 (Table 1). These patients all had remote MI an average of 3.7 years before recruitment and were scheduled to undergo elective coronary artery bypass graft surgery (CABG). During this surgery, the right atrial appendage is routinely removed and can be used to initiate cell culture. In each patient studied, it was possible to generate approximately 2 million c-Kit-positive CSCs over a period of several weeks. Patients randomized to cell therapy received 0.5–1 million cells by intracoronary injection via a native coronary artery or bypass graft approximately 4 months after surgery. Clearly, blinding would have been feasible in SCIPIO because the cardiac tissue was harvested under general anesthesia during cardiac surgery rather than percutaneously under local anesthesia as a separate procedure. However, the investigators indicated that they did not wish to undertake any additional invasive procedures in the control group, and so this trial was also not blinded. Cardiac function was assessed using three-dimensional echocardiography with only a subgroup undergoing cardiac MRI. Hence, there are only limited data on infarct size. Cell culture was successful in 80 of 81 patients screened for entry into the trial, although many subjects were excluded in the period between CABG and planned cell administration, mainly because of withdrawal of consent or an improvement in LVEF post CABG to >40%.
Again, the primary end point was a combination of safety outcomes, and, like CADUCEUS, the trial was positive in this respect, with no reported incidence of ventricular arrhythmias, sudden death, or the development of cardiac tumors during up to 12 months of follow-up. There was one periprocedural adverse event due to catheter-related damage to a bypass graft that was being used as the conduit artery for cell administration to the heart. As a result, this bypass graft required treatment by additional coronary intervention. Other serious adverse events occurred with similar frequency in the two groups. The efficacy outcomes are summarized in Table 2. In contrast to CADUCEUS, cell-treated patients in SCIPIO demonstrated significant improvements in symptoms, quality of life, and LVEF at 4 months' follow-up. The seven treated patients who participated in the cardiac magnetic resonance (CMR) substudy showed a statistically significant reduction in myocardial scar mass from baseline to 4 months, which was further reduced in the six patients who underwent a second cardiac MRI at 12 months.
Therefore, CADUCEUS and SCIPIO achieved their goal of demonstrating the feasibility and medium-term safety of culturing therapeutic doses of autologous CSCs from myocardial biopsy tissues and subsequently administering these cells by intracoronary injection to patients with prior MI. Although not powered for efficacy, both studies demonstrated a reduction in the volume/mass of scar on cardiac MRI, and this has been interpreted as showing replacement of the scar tissue with new viable heart muscle. If functionally important myocardial regeneration had taken place, an improvement in cardiac function and symptoms would have been expected. Despite similar reductions in scar volume (see Table 2), only the patients in SCIPIO showed improvements in quality of life and overall cardiac function. These findings may reflect differential efficacy of the two cell types.11 In addition, the patients in CADUCEUS were treated 2–3 months after their original MIs as compared with 3–4 years in SCIPIO.
Conclusions
In terms of translation and wider applicability, the intracoronary route of administration is obviously most attractive because it is easier and safer than direct injection of therapeutics into the heart muscle. In autologous CSC–derived therapy, the time taken for cell culture could mean that the window for optimal benefit may be missed in patients with recent AMI. If the efficacy of CSCs is confirmed in larger phase II studies, this would represent a paradigm shift in the management of patients with cardiac dysfunction due to prior MI. However, the results of LateTIME and FOCUS-CCTRN highlight the critical importance of study design. No matter how logistically difficult, future trials with CSCs should have a double-blind design with matched study procedures because proof of clinically meaningful efficacy will be required before wider adoption can be recommended.
REFERENCES
- Malliaras K., and, Marban E. Cardiac cell therapy: where we've been, where we are, and where we should be headed. Br Med Bull. 2011;98:161–185. doi: 10.1093/bmb/ldr018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traverse JH, Henry TD, Ellis SG, Pepine CJ, Willerson JT, Zhao DXM.et al. (2011Effect of intracoronary delivery of autologous bone marrow mononuclear cells 2 to 3 weeks following acute myocardial infarction on left ventricular function. The LateTIME Randomized Trial JAMA 3062110–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perin EC, Willerson JT, Pepine CJ, Henry TD, Ellis SG, Zhao DXM.et al. (2012Effect of transendocardial delivery of autologous bone marrow mononuclear cells on functional capacity, left ventricular function and perfusion in chronic heart failure. The FOCUS-CCTRN trial JAMAe-pub ahead of print 24 March 2012. [DOI] [PMC free article] [PubMed]
- Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LEJ, Berman D.et al. (2012Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial Lancet 379895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolli R, Chugh AR, D'Amario D, Loughran JH, Stoddard MF, Ikram S.et al. (2011Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial Lancet 3781847–1857. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bartunek J, Vanderheyden M, Hill J., and, Terzic A. Cells as biologics for cardiac repair in ischemic heart failure. Heart. 2010;96:792–780. doi: 10.1136/hrt.2007.139394. [DOI] [PubMed] [Google Scholar]
- Schächinger V, Erbs S, Elsässer A, Haberbosch W, Hambrecht R, Hölschermann H.et al. (2006Intra-coronary bone marrow-derived progenitor cells in acute myocardial infarction N Engl J Med 3551210–1221. [DOI] [PubMed] [Google Scholar]
- Losordo DW, Henry TD, Davidson C, Lee JS, Costa MAA, Bass T.et al. (2011Intramyocardial autologous CD34+ cell therapy for refractory angina Circ Res 109428–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisher TA. Cardiac-derived stem cells. IDrugs. 2000;3:649–653. [PubMed] [Google Scholar]
- Messina E, De Angelis L, Frati G, Morrone S, Chimenti S, Fiordaliso F.et al. (2004Isolation and expansion of adult cardiac stem cells from human and murine heart Circ Res 95911–921. [DOI] [PubMed] [Google Scholar]
- Li T-S, Cheng K, Malliaras K, Smith RR, Zhang Y, Sun B.et al. (2012Direct comparison of different stem cell types and subpopulations reveals superior paracrine potency and myocardial repair efficacy with cardiosphere-derived cells J Am Coll Cardiol 59942–953. [DOI] [PMC free article] [PubMed] [Google Scholar]


