Abstract
INTRODUCTION
A stem cell model of prostate cancer tumourigenesis explains progression to castration resistant prostate cancer (CRPC) and offers novel perspectives in targeting this cancer in its more advanced forms. Androgen receptor (AR) regulated pathways are central mechanisms in progression to CRPC. However, AR was thought to be lacking in prostate stem cell enriched fractions. Potential low levels of AR expression in stem cell enriched cells were investigated and potential direct effects of androgen were examined.
METHODS
Human prostate stem cell enriched populations, based on high α2β1 integrin expression (α2β1hi), were selected from primary human prostate tissue in men undergoing transurethral prostatectomy or cystoprostatectomy. Effects on differentiation were assayed with flow cytometry using differentiation-specific markers.
RESULTS
Low levels of AR were demonstrable in α2β1hi cells following inhibition of the proteasome using MG132. Furthermore, a direct effect of androgen was shown in stabilising/inducing AR expression. Androgen treatment of α2β1hi cells was associated with the induction of differentiation using a number of differentiation-specific markers (prostatic acid phosphatase, cytokeratin 18 and AR) with increases ranging from 49% to 67% (p<0.05). These effects were blocked with the AR-specific inhibitor bicalutamide (p<0.05). These data support a role of direct androgen activity on stem cell enriched cells in the prostate and the implications of these findings are discussed.
Keywords: Prostate cancer, Stem cells, Androgen receptor
Prostate cancer is the most common male cancer with an incidence of over 37,000 new cases and over 10,000 cancer deaths in the UK in 2008.1 The Nobel-Prize-winning work of Huggins and Hodges in the 1940s first described the dependence on androgen for these tumours and to this day the management of advanced prostate cancer centres on androgen deprivation. Although most patients respond to androgen deprivation therapy, the majority will eventually develop castration-resistant prostate cancer (CRPC). At this point the median time to death is 18–24 months, despite contemporary chemotherapies.
Recently, new models based on stem cell biology to describe mechanisms for hormone independent disease have emerged and offer a fresh approach to targeting CRPC. The stem cell is a specialised self-renewing cell that is maintained for the life of the tissue and sustains lineage-specific cellular compartments. These cells differentiate into a highly proliferative cell type, the transiently amplifying population (TAP), that expand cell numbers before committing to terminal differentiation to replace cells that undergo natural wastage. Stem cells are appealing candidates as the cell of origin for cancer because of their long-lived capacity for self renewal, which provides a greater opportunity to accumulate the multiple mutations required for tumourigenesis.2,3
Within the prostate epithelium, the luminal population comprises terminally differentiated specialised secretory cells that are maintained by androgens. Castration causes the adult prostate gland to undergo involution as the androgen-dependent luminal epithelial cells undergo apoptosis.4 The remaining basal layer is able to regenerate the luminal prostate epithelium following the readministration of androgen and therefore houses the stem cell. Prostate epithelial stem cells can be enriched by selecting basal cells expressing high levels of the α2β1 integrin (α2β1hi).5 These progenitor cells can be further defined into stem (α2β1hi CD133+) and TAP cells (α2β1hi CD133-) (Fig 1).6
Figure 1.
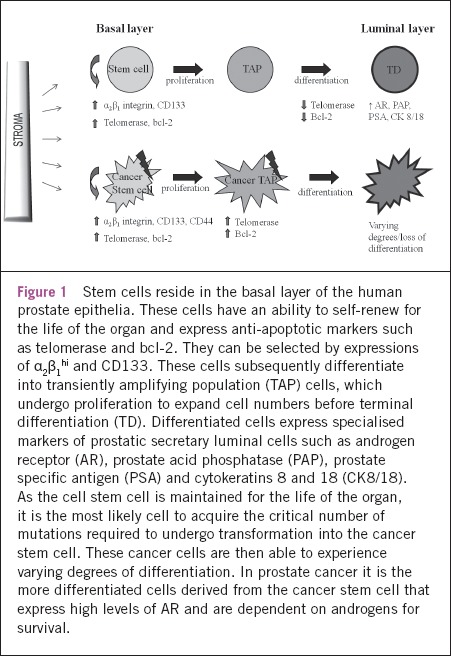
Stem cells reside in the basal layer of the human prostate epithelia. These cells have an ability to self-renew for the life of the organ and express anti-apoptotic markers such as telomerase and bcl-2. They can be selected by expressions of α2β1hi and CD133. These cells subsequently differentiate into transiently amplifying population (TAP) cells, which undergo proliferation to expand cell numbers before terminal differentiation (TD). Differentiated cells express specialised markers of prostatic secretary luminal cells such as androgen receptor (AR), prostate acid phosphatase (PAP), prostate specific antigen (PSA) and cytokeratins 8 and 18 (CK8/18). As the cell stem cell is maintained for the life of the organ, it is the most likely cell to acquire the critical number of mutations required to undergo transformation into the cancer stem cell. These cancer cells are then able to experience varying degrees of differentiation. In prostate cancer it is the more differentiated cells derived from the cancer stem cell that express high levels of AR and are dependent on androgens for survival.
Recent identification of α2β1hi CD133+ CD44- prostate cancer stem cells highlights the relevance of the stem cell model to prostate transformation.7 Cancer stem cells, a minute fraction of tumour cells that drive the propagation of the tumour, share many molecular and functional characteristics of normal progenitor cells, suggesting a common origin.7–10 Androgens play an integral role in the regulation of prostate epithelial differentiation and aberrant control of androgen receptor (AR) pathways are considered to be a key mechanism behind the formation of prostate cancer.11,12 During androgen deprivation treatment, similar to the androgen deprivation-related involution described in the normal prostate, the differentiated tumour cells undergo apoptosis. However, the cancer stem cells, which lack AR expression, are thought to persist as unlike the rest of the tumour they are not dependent on androgen for survival. These cells can subsequently expand and lead to the emergence of CRPC. Current prostate cancer treatment strategies may be failing in that we are only treating the differentiated cells and not the cancer stem cells at the ‘root’.11
Interestingly, when prostate cancers progress despite castration to an androgen independent form of the disease, AR activity persists.13 This phenomenon is clinically evident in castrate resistant disease where we commonly see hallmark surges of prostate specific antigen (PSA) expression, which is a reporter of AR activity. In CRPC a number of different mechanisms have been extensively documented, acting alone or in combination, to allow constitutive activation of the AR.14 The cancer stem cell model provides additional insights into the potential mechanism for CRPC development.15–17 However, the absence of AR expression in prostate stem cells appears to be at odds with the more established mechanisms of CRPC in which AR activity remains a central mechanism.13 According to the stem cell model of prostate cancer, if the tumour initiating cells (AR-) undergo selective growth in CRPC,12,14 how do we see AR amplification, mutation and constitutive activation? Androgens are believed to exert an indirect effect on the prostate epithelium differentiation, which offers a potential explanation to this issue in that AR expression could be acquired through differentiation.4 The indirect effects are mediated through paracrine growth factor signalling via adjacent androgen sensitive stroma.18,19 However, in the context of a cancer stem cell model, the direct effects of androgen in a stem cell model of differentiation were ill defined. The study of AR expression in the human prostate epithelial stem cell model formed the basis of the work described in this Hunterian lecture.
Methods
Human prostatic tissue was obtained from 60 patients (age range: 56–85 years) undergoing either transurethral transaction of the prostate for benign prostatic hyperplasia or a cystoprostatectomy for bladder cancer. All tissue was obtained with patient consent and ethical approval. Benign histology was confirmed by a clinical pathologist. Epithelial structures were separated from the stromal fraction by differential gravity centrifugation (epithelial purity >98%)20 and further purified with selection of human epithelial antigen (CD326; immunomagnetic isolation kit, Miltenyi Biotec Ltd, Bisley, UK). To enrich the stem cell population, α2β1hi basal cells were selected by rapid adherence to type I collagen (5 minutes) (BD Biosciences, Oxford, UK).5
To examine for low levels of AR expression in prostatic epithelial cells an inhibitor of proteasomal pathways (MG132, 10mM; Calbiochem®, Merck Chemicals Ltd, Nottingham, UK), was used. The effect of human androgen analogue (R1881, 10nM; AstraZeneca, Macclesfield, UK) on α2β1hi cell differentiation was explored. The specificity of this reaction was tested using the anti-androgen bicalutamide (10mM; AstraZeneca). Epithelial differentiation of prostatic cells was validated using antibodies specific for prostate acid phosphatase (PAP; rabbit A0627, Dako Ltd, Ely, UK), PSA (goat sc-26023, Santa Cruz Biotechnology Inc, Santa Cruz, CA, US), cytokeratin 18 (CK18; mouse F7212, Dako Ltd) and AR (rabbit sc-815, Santa Cruz Biotechnology Inc). Furthermore, the effect on the expression of the α2β1 integrin was examined (anti-α2(β1), PIE6, Dako Ltd).
Expression of these differentiation markers were assessed by fluorescence activated cell sorting (FACS) analysis. The following controls were used for these experiments: no primary antibody and isotype IgG-specific controls. For the detection of epithelial differentiation, the cells were permeabilised with 0.01% Triton™ X-100 (Dow Chemical Company, Midland, MI, US) to allow the detection of intracellular antigens. The permeabilised cells were incubated with primary anti-human antibodies for one hour at 48°C.
After washing in Isoton (Beckman Coulter Ltd, High Wycombe, UK), samples were pre-incubated with unconjugated primary antibody and subsequently incubated with a fluorochrome conjugated antibody for 30 minutes at 48°C. Immediately prior to FACS analysis, propidium iodide at a final concentration of 0.025mg/ml was added to gate out debris. The samples were studied using an LSR II FACS machine and data analysis was performed using Lysis™ II software (Becton Dickinson, Oxford, UK). Increased expression of differentiation markers were taken over the 97th percentile of isotype control on quadrant analysis.
Results
AR protein expression was demonstrated within the prostatic epithelial α2β1hi basal cells
AR messenger ribonucleic acid (mRNA) expression was confirmed in stem cell enriched α2β1hi basal cells and low levels of AR protein were suspected below the threshold of detection using standard techniques such as Western blotting.21 The regulation of AR protein expression by translational synthesis and proteasomal degradation is recognised.22 In the absence of androgen ligand (as with the cells in this study), it is established that AR expression is subject to proteasomal breakdown.22,23 Hence, the proteasomal inhibitor (MG132) was added to prostatic epithelial α2β1hi cells for one hour and AR protein expression measured by flow cytometry (Fig 2). Treatment with MG132 demonstrates induction of AR protein expression compared with untreated α2β1hi cells (in an ethanol vehicle control). By inhibiting the proteasome, AR expression was ‘unmasked’, allowing an increase in the overall AR to levels adequate for protein detection in approximately 60% of the cells.
Figure 2.
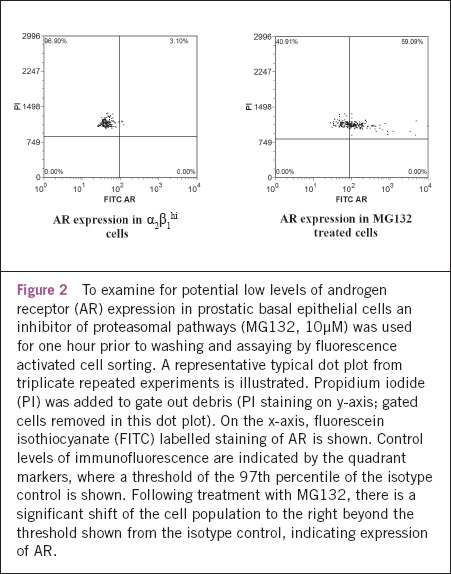
To examine for potential low levels of androgen receptor (AR) expression in prostatic basal epithelial cells an inhibitor of proteasomal pathways (MG132, 10μM) was used for one hour prior to washing and assaying by fluorescence activated cell sorting. A representative typical dot plot from triplicate repeated experiments is illustrated. Propidium iodide (PI) was added to gate out debris (PI staining on y-axis; gated cells removed in this dot plot). On the x-axis, fluorescein isothiocyanate (FITC) labelled staining of AR is shown. Control levels of immunofluorescence are indicated by the quadrant markers, where a threshold of the 97th percentile of the isotype control is shown. Following treatment with MG132, there is a significant shift of the cell population to the right beyond the threshold shown from the isotype control, indicating expression of AR.
Androgen stabilised AR expression in prostatic epithelial α2β1hi basal cells and stimulated differentiation
Having confirmed low levels of measurable AR expression, the potential direct effects of R1881 on α2β1hi progenitor basal cells were explored. The addition of androgen to the prostate basal cells was shown to stabilise and/or induce AR expression by 63% (p<0.001; two sided paired t-test) (Fig 3).
Figure 3.
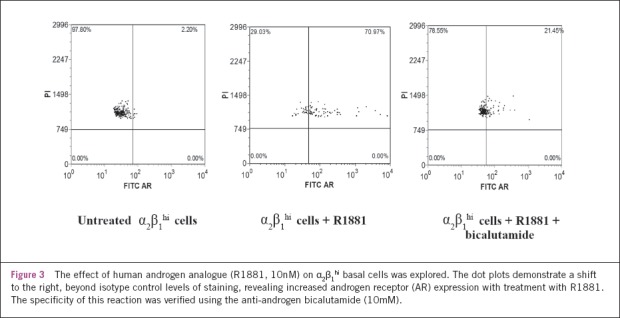
The effect of human androgen analogue (R1881, 10nM) on α2β1hi basal cells was explored. The dot plots demonstrate a shift to the right, beyond isotype control levels of staining, revealing increased androgen receptor (AR) expression with treatment with R1881. The specificity of this reaction was verified using the anti-androgen bicalutamide (10mM).
Having demonstrated AR expression in basal cells, the potential direct effects of androgen on differentiation were examined. Using FACS analysis, prostatic epithelial α2β1hi cells treated with R1881 demonstrated induction of prostate-specific markers of differentiation, namely PAP, cytokeratin 18 (CK18) and AR expression, with mean increases of 67% (p=0.004, two sided paired t-test), 49% (p=0.011) and 63% (p<0.001) respectively (Fig 4). The expression of the α2β1 integrin was down-regulated by 60% (p=0.042), which is associated with differentiation.19 These observed effects of androgens on the selected prostatic epithelial basal cells were inhibited by its specific inhibitor, bicalutamide (p<0.05).
Figure 4.
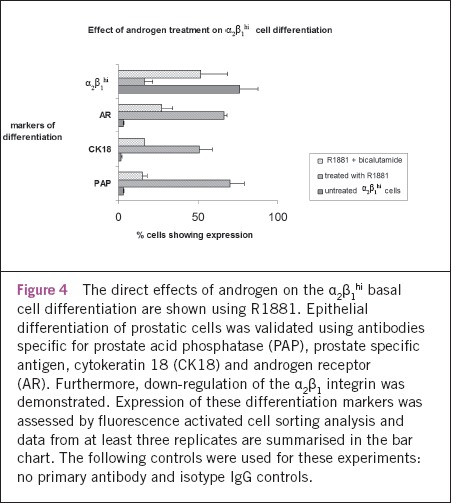
The direct effects of androgen on the α2β1hi basal cell differentiation are shown using R1881. Epithelial differentiation of prostatic cells was validated using antibodies specific for prostate acid phosphatase (PAP), prostate specific antigen, cytokeratin 18 (CK18) and androgen receptor (AR). Furthermore, down-regulation of the α2β1 integrin was demonstrated. Expression of these differentiation markers was assessed by fluorescence activated cell sorting analysis and data from at least three replicates are summarised in the bar chart. The following controls were used for these experiments: no primary antibody and isotype IgG controls.
Discussion
Androgens are thought to have both direct and indirect effects on prostate epithelial differentiation.18,24 It has long been established that androgens are required to maintain the terminally differentiated luminal cells in vivo. The indirect effects via androgen regulated growth factors are becoming increasingly better characterised in the human prostate.19 However, the direct effects on regulating adult α2β1hi basal epithelial differentiation were not unknown. In the in vitro model described in this work, additional effects of androgen are demonstrated as a direct independent regulator of prostate stem cell enriched α2β1hi basal cell differentiation.
The direct effects of androgen on prostate cell differentiation are significant as α2β1hi cells were considered to lack AR and PSA expression.5,19 However, AR transcript expression had been confirmed and observations of direct androgen responsiveness allowed speculation that AR protein expression may exist albeit at low levels, beneath the threshold of detection by conventional protein-based assays such as Western blotting and flow cytometry. AR protein is rapidly degraded in the absence of ligand23 and, on androgen binding, leads to stabilisation of AR.25 This mechanism can be similarly used to explain findings by others where cultured prostate epithelium in the absence of androgen has been associated with the undetectable AR protein expression even though mRNA can be detected.26 Inhibiting proteasomal activity revealed detection of AR protein using FACS, confirming that AR protein is present and regulated by a tight balance between production and turnover in androgen depleted conditions in stem cell enriched basal cells. These data are supported by occasional reports of basal AR expression using immunohistochemistry.10
Furthermore, the results presented show that androgen itself is able to directly induce de novo AR protein expression and/or stabilise AR protein in the α2β1hi cells. These data have been confirmed with immunofluorescence studies showing the induction of AR by R1881,21 where a pattern of co-expression of PSA expression and nuclear translocation of AR verified the presence of a functional AR. Of particular relevance is the α2β1 integrin managed extracellular matrix (ECM) interaction, which has been shown to maintain the basal cell phenotype and negatively to regulate differentiation.19 Similar to previous findings with keratinocyte growth factor, in this study androgens induced differentiation through the down-regulation of the α2β1 integrin, outlining a mechanism of escape from ECM regulated maintenance of the immature basal phenotype.
In summary, a role for androgens directly to regulate differentiation of stem cell enriched basal cells and to modulate AR protein expression is described. The demonstrable expression and regulation of AR within this population represents a significant finding as it allows for a potential description of a cancer stem cell model incorporating AR dependent pathways that are relevant in CRPC. Greater degrees of stem cell enrichments within the basal cells are described by selecting CD133+ expression and current evidence supports a lack of AR expression in these cells.6 These cells are thought to be the target for malignant transformation and CD133+ cancer stems are similarly thought not to express AR. As mutations and amplifications of AR are recognised as major genetic events in CRPC and if the CD133+ cancer cells are the tumour initiating cells in this hormone depleted environment, then these cells would be expected to have AR expression.
Future work should build on the data presented here and revisit the expression of AR in α2β1hi CD133+ and α2β1hi CD133- epithelial fractions using the most sensitive molecular biological techniques. These investigations should ideally be supported with in situ investigations within the stem cell niche as ex vivo experiments are associated with altered phenotype.27 In particular, potential AR expression in α2β1hi CD133+ stem cells would have significant clinical relevance to prostate cancer as it remains the most likely cell of origin for tumourigenesis.
References
- 1.Prostate cancer statistics. Cancer Research UK. http://info.cancerresearchuk.org/cancerstats/types/prostate/> (cited June 2011)
- 2.Isaacs JT. Prostate stem cells and benign prostatic hyperplasia. Prostate. 2008;68:1,025–1,034. doi: 10.1002/pros.20763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lawson DA, Witte ON. Stem cells in prostate cancer initiation and progression. J Clin Invest. 2007;117:2,044–2,050. doi: 10.1172/JCI32810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sugimura Y, Cunha GR, Donjacour AA. Morphological and histological study of castration-induced degeneration and androgen-induced regeneration in the mouse prostate. Biol Reprod. 1986;34:973–983. doi: 10.1095/biolreprod34.5.973. [DOI] [PubMed] [Google Scholar]
- 5.Collins AT, Habib FK, Maitland NJ, Neal DE. Identification and isolation of human prostate epithelial stem cells based on alpha(2)beta(1)-integrin expression. J Cell Sci. 2001;114(Pt 21):3,865–3,872. doi: 10.1242/jcs.114.21.3865. [DOI] [PubMed] [Google Scholar]
- 6.Richardson GD, Robson CN, Lang SH, et al. CD133, a novel marker for human prostatic epithelial stem cells. J Cell Sci. 2004;117(Pt 16):3,539–3,545. doi: 10.1242/jcs.01222. [DOI] [PubMed] [Google Scholar]
- 7.Collins AT, Berry PA, Hyde C, et al. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:1,0946–10,951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 8.Miki J, Furusato B, Li H, et al. Identification of putative stem cell markers, CD133 and CXCR4, in hTERT-immortalized primary nonmalignant and malignant tumor-derived human prostate epithelial cell lines and in prostate cancer specimens. Cancer Res. 2007;67:3,153–3,161. doi: 10.1158/0008-5472.CAN-06-4429. [DOI] [PubMed] [Google Scholar]
- 9.Patrawala L, Calhoun-Davis T, Schneider-Broussard R, Tang DG. Hierarchical organization of prostate cancer cells in xenograft tumors: the CD44+alpha2beta1+ cell population is enriched in tumor-initiating cells. Cancer Res. 2007;67:6,796–6,805. doi: 10.1158/0008-5472.CAN-07-0490. [DOI] [PubMed] [Google Scholar]
- 10.Bonkhoff H. Role of the basal cells in premalignant changes of the human prostate: a stem cell concept for the development of prostate cancer. Eur Urol. 1996;30:201–205. doi: 10.1159/000474170. [DOI] [PubMed] [Google Scholar]
- 11.Maitland NJ, Collins AT. Prostate cancer stem cells: a new target for therapy. J Clin Oncol. 2008;26:2,862–2,870. doi: 10.1200/JCO.2007.15.1472. [DOI] [PubMed] [Google Scholar]
- 12.Chen CD, Welsbie DS, Tran C, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–39. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 13.Attard G, Cooper CS, de Bono JS. Steroid hormone receptors in prostate cancer: a hard habit to break? Cancer Cell. 2009;16:458–462. doi: 10.1016/j.ccr.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1:34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- 15.Isaacs JT. The biology of hormone refractory prostate cancer. Why does it develop? Urol Clin North Am. 1999;26:263–273. doi: 10.1016/s0094-0143(05)70066-5. [DOI] [PubMed] [Google Scholar]
- 16.Hudson DL, O'Hare M, Watt FM, Masters JR. Proliferative heterogeneity in the human prostate: evidence for epithelial stem cells. Lab Invest. 2000;80:1,243–1,250. doi: 10.1038/labinvest.3780132. [DOI] [PubMed] [Google Scholar]
- 17.English HF, Santen RJ, Isaacs JT. Response of glandular versus basal rat ventral prostatic epithelial cells to androgen withdrawal and replacement. Prostate. 1987;11:229–242. doi: 10.1002/pros.2990110304. [DOI] [PubMed] [Google Scholar]
- 18.Sugimura Y, Foster BA, Hom YK, et al. Keratinocyte growth factor (KGF) can replace testosterone in the ductal branching morphogenesis of the rat ventral prostate. Int J Dev Biol. 1996;40:941–951. [PubMed] [Google Scholar]
- 19.Heer R, Collins AT, Robson CN, et al. KGF suppresses alpha2beta1 integrin function and promotes differentiation of the transient amplifying population in human prostatic epithelium. J Cell Sci. 2006;119(Pt 7):1,416–1,424. doi: 10.1242/jcs.02802. [DOI] [PubMed] [Google Scholar]
- 20.Robinson EJ, Neal DE, Collins AT. Basal cells are progenitors of luminal cells in primary cultures of differentiating human prostatic epithelium. Prostate. 1998;37:149–160. doi: 10.1002/(sici)1097-0045(19981101)37:3<149::aid-pros4>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 21.Heer R, Robson CN, Shenton BK, Leung HY. The role of androgen in determining differentiation and regulation of androgen receptor expression in the human prostatic epithelium transient amplifying population. J Cell Physiol. 2007;212:572–578. doi: 10.1002/jcp.21154. [DOI] [PubMed] [Google Scholar]
- 22.Lin HK, Wang L, Hu YC, et al. Phosphorylation-dependent ubiquitylation and degradation of androgen receptor by Akt require Mdm2 E3 ligase. EMBO J. 2002;21:4,037–4,048. doi: 10.1093/emboj/cdf406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kemppainen JA, Lane MV, Sar M, Wilson EM. Androgen receptor phosphorylation, turnover, nuclear transport, and transcriptional activation. Specificity for steroids and antihormones. J Biol Chem. 1992;267:968–974. [PubMed] [Google Scholar]
- 24.Cunha GR, Young P. Inability of Tfm (testicular feminization) epithelial cells to express androgen-dependent seminal vesicle secretory proteins in chimeric tissue recombinants. Endocrinology. 1991;128:3,293–3,298. doi: 10.1210/endo-128-6-3293. [DOI] [PubMed] [Google Scholar]
- 25.Krongrad A, Wilson CM, Wilson JD, et al. Androgen increases androgen receptor protein while decreasing receptor mRNA in LNCaP cells. Mol Cell Endocrinol. 1991;76:79–88. doi: 10.1016/0303-7207(91)90262-q. [DOI] [PubMed] [Google Scholar]
- 26.Uzgare AR, Xu Y, Isaacs JT. In vitro culturing and characteristics of transit amplifying epithelial cells from human prostate tissue. J Cell Biochem. 2004;91:196–205. doi: 10.1002/jcb.10764. [DOI] [PubMed] [Google Scholar]
- 27.Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441:1,075–1,079. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]


