Abstract
INTRODUCTION
The aim of this study was to determine if cardiopulmonary exercise testing (CPET) predicts 30-day and midterm outcomes when assessing suitability for abdominal aortic aneurysm (AAA) repair.
METHODS
Since July 2006 consecutive patients from a single centre identified with a large (≥5.5cm) AAA were sent for CPET. Follow-up was completed on 1 August 2009. Univariate logistical regression was used to compare CPET parameters with the Detsky score, the Acute Physiology and Chronic Health Evaluation (APACHE) II score and the Vascular Physiological and Operative Severity Score for the enUmeration of Mortality and morbidity (VPOSSUM) in predicting predefined early and late outcome measures.
RESULTS
Full data were available for 102 patients (93% male, median age: 75 years, interquartile range (IQR): 70–80 years, median follow up: 28 months, IQR: 18–33 months). Ventilatory equivalents for oxygen and APACHE II predicted postoperative inotrope requirement (p=0.018 and p=0.019 respectively). The Detsky score predicted the length of stay in the intensive care unit (p=0.008). Midterm (30-month) survival was predicted by the anaerobic threshold (p=0.02).
CONCLUSIONS
CPET provided the only means in this study of predicting both 30-day outcome and 30-month mortality. CPET could therefore become an increasingly important tool in determining the optimum management for AAA patients.
Keywords: Aortic aneurysm, abdominal; Preoperative care; Exercise testing
Abdominal aortic aneurysm (AAA) is a life-threatening condition commonly affecting men over 65 years. A benefit for the repair of small AAAs (<5.5cm) has not been demonstrated.1,2 UK mortality rates from open repair of large (≥5.5cm) AAAs are reported to be on average as high as 7%.3 Randomised controlled trials have confirmed reduced 30-day AAA-related mortality with endovascular repair but not for those considered unfit for open repair.4,5 For this reason patient selection for these high-risk procedures will continue to be of paramount importance in management of AAA.
Numerous techniques designed to assess cardiopulmonary capacity are currently employed in the selection of patients suitable for AAA repair, demonstrating the lack of consensus for a gold standard test. In 2007 Carlisle et al reported on cardiopulmonary exercise testing (CPET) in 130 patients undergoing open AAA repair. CPET was better able to predict 30-day and mid-term mortality than all other physiological scoring tools.6 CPET has been used to predict long-term outcomes in patients with heart failure and to select patients for surgery with high cardiorespiratory risk.7,8 The advantage of CPET is the ability to predict cardiopulmonary capacity without requiring the patient to reach a state of maximum cardiovascular stress (often impossible due to co-morbidity).
The ability to predict long-term mortality has become increasingly important in the assessment of AAA patients. The long-term outcome of the EVAR 1 trial on endovascular aneurysm repair (EVAR) demonstrates that the AAA-related survival advantage of EVAR is lost after four years and suggests economic and re-intervention advantages for open repair beyond six years.9
The message from the EVAR 2 trial is disputed and endovascular repair is frequently used to treat AAA patients previously thought unfit for open AAA repair.4 Without an accurate tool for predicting the survival of patients after repair, many of these patients are likely to die early from other causes, rendering the AAA repair for some of these patients an expensive folly. In the current economic climate vascular specialists have a responsibility to ensure patients offered endovascular repair have a reasonable life expectancy. It would seem appropriate to put this at >3 years (∼50% survival for EVAR 2 trial patients).4
This study aimed to assess the usefulness of CPET and the Detsky score to predict mid-term mortality in AAA patients assessed for elective open repair. A secondary aim was to compare the ability of CPET, the Detsky score, the Acute Physiology and Chronic Health Evaluation (APACHE) II score and the Vascular Physiological and Operative Severity Score for the enUmeration of Mortality and morbidity (VPOSSUM) to predict 30-day perioperative morbidity.
Methods
Between July 2006 and June 2009 consecutive patients presenting to a single vascular unit with an asymptomatic AAA of ≥5.5cm and considered for an open AAA repair were included in our study. Potential risk factors for postoperative survival were recorded (age, sex, smoking history, lung disease, hypercholesterolaemia, hypertension, diabetes, ischaemic heart disease, cerebrovascular accident/transient ischaemic attack, heart failure and creatinine levels). All patients were referred for CPET and underwent physiological scoring by the Detsky index method.10 CPET was carried out by exercising patients on a stationary bicycle. CPET data were collected using BreezeSuite™ 6.4.1 (Medical Graphics, St Paul, MN, US) measuring metabolic variables. The electrocardiography was measured using CardioPerfect™ (Welch Allyn, Skaneateles Falls, NY, US). The ventilator minute volume, O2 consumption and CO2 excretion of a subject was measured with Ultima™ (Medical Graphics, St Paul, MN, US) linked to a cycle ergometer.
Four variables were derived from the CPET graphs: the anaerobic threshold (AT), peak oxygen consumption (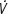 peak), and ventilatory equivalents for oxygen (
peak), and ventilatory equivalents for oxygen (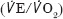 ) and carbon dioxide (
) and carbon dioxide ( ).11 Fitness for open AAA repair was decided taking into account all CPET measurements together with co-morbidities and size of the AAA. As a guide an AT >11 was considered the ideal. Those who underwent open AAA repair on the basis of CPET were further assessed by APACHE II and VPOSSUM.12,13
).11 Fitness for open AAA repair was decided taking into account all CPET measurements together with co-morbidities and size of the AAA. As a guide an AT >11 was considered the ideal. Those who underwent open AAA repair on the basis of CPET were further assessed by APACHE II and VPOSSUM.12,13
Follow-up morbidity and mortality data were collected retrospectively from hospital records, including the electronic intensive care unit (ICU) patient database (Eclipsys® software, Eclipsys, Atlanta, GA, US), and cross-referenced with primary care records. A cardiac event was defined as newly diagnosed arrhythmia or acute coronary syndrome. A respiratory event was defined by an admission to hospital for a respiratory-related pathology or a newly diagnosed respiratory condition during a hospital stay. Data on cause of death were not reliably collected and AAA-related deaths remained unknown in both groups.
Statistical analysis
Data were analysed using binary logistic regression analyses to assess the association of cardiopulmonary exercise, Detsky score and selected co-morbidities on postoperative adverse events and death. Binary logistic analyses were also used to assess whether CPET, the Detsky score, APACHE II score or the VPOSSUM are able to predict the need for perioperative inotropes. Receiver operating characteristic (ROC) analysis was conducted to confirm the associations of any factors identified as being statistically significant on logistic regression. A linear regression analysis was performed to assess the effect of cardiopulmonary exercise, Detsky score, APACHE II score and VPOSSUM on the duration of intensive care required postoperatively. Survival analysis was conducted using the Kaplan–Meier method with differences between groups of interest quantified using the logrank chisquare methodology. Statistical significance was assumed at the 5% level. All statistical analysis was performed using SPSS® v15 (SPSS Inc, Chicago, IL, US).
Results
A total of 107 patients identified with a large AAA (≥5.5cm) were considered for open repair. Five patients were excluded as they never had CPET (one ruptured prior to testing, four had AAA considered too big to wait for CPET assessment). Of the 102 patients tested, 36 were deemed unfit for open repair on the basis of full interpretation of the CPET data. The median AAA anteroposterior ultrasound diameter was 59mm (interquartile range (IQR): 56–67mm). Of the 66 patients offered open surgery, 3 requested endovascular repair and were referred to another unit (as at that time EVAR was not undertaken here) and the remaining 63 patients underwent open repair within our centre (Fig 1). One patient from the unfit group underwent an endovascular repair elsewhere.
Figure 1.
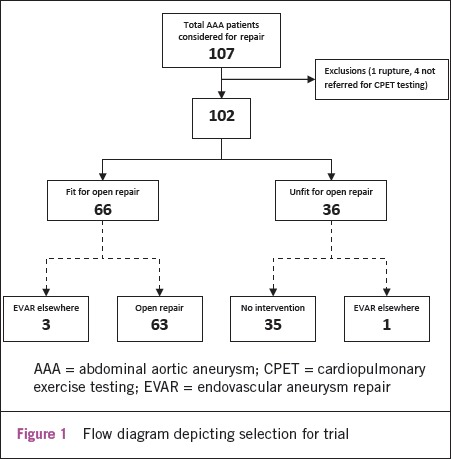
Flow diagram depicting selection for trial
The median follow-up period for all patients was 28 months (IQR: 18–33 months). There were eight deaths in the unfit group and six deaths in the group offered open repair. The unfit group was significantly older (p=0.019). Otherwise there were no significant differences in baseline characteristics between the groups (Table 1). 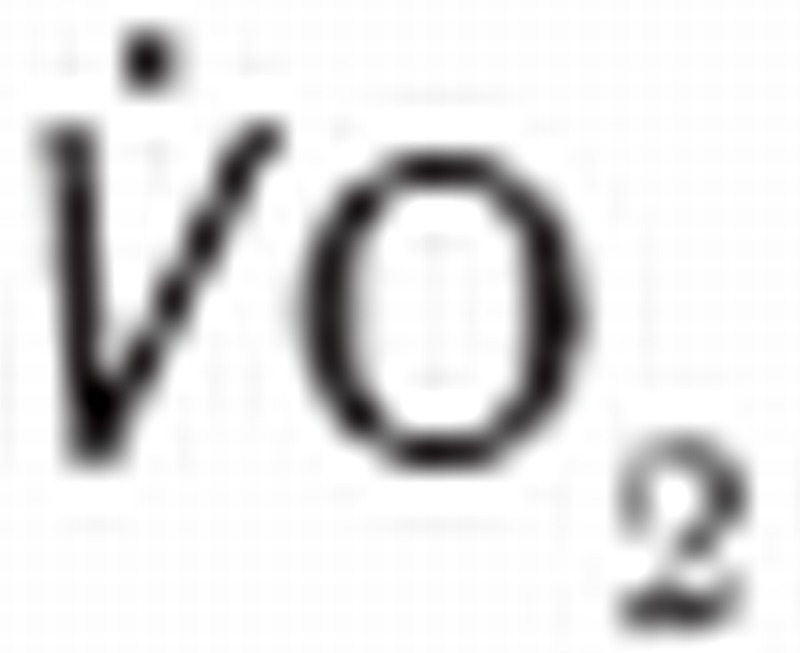 peak, AT and
peak, AT and 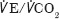 were significantly different between the groups (p<0.001, p<0.001 and p=0.005) (Table 2). Survival after CPET was significantly longer in the repair group (p=0.009) but there were no significant differences in other adverse events (Table 3). There was one 30-day death in the open repair group. Preoperative assessment concluded the patient was at increased risk but there remained a benefit from repair given a 90mm AAA with 68mm and 40mm common iliac aneurysms. (The values reported by CPET were:
were significantly different between the groups (p<0.001, p<0.001 and p=0.005) (Table 2). Survival after CPET was significantly longer in the repair group (p=0.009) but there were no significant differences in other adverse events (Table 3). There was one 30-day death in the open repair group. Preoperative assessment concluded the patient was at increased risk but there remained a benefit from repair given a 90mm AAA with 68mm and 40mm common iliac aneurysms. (The values reported by CPET were: 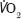 peak 12.9, AT 10.5,
peak 12.9, AT 10.5, 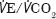 28 and
28 and 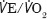 28.)
28.)
Table 1.
Patient characteristics
| Fit for AAA repair (n=66) | Not fit for AAA repair (n=36) | p-value | |
|---|---|---|---|
| Median age at CPET (range) | 74 (49–88) | 77 (49–87) | 0.019† |
| Sex | 0.365* | ||
| Male | 62 (93.9%) | 32 (88.9%) | |
| Female | 4 (6.1%) | 4 (11.1%) | |
| Smoking status | 0.300* | ||
| Non-smoker | 12 (18.2%) | 5 (13.9%) | |
| Ex-smoker | 25 (37.9%) | 15 (41.7%) | |
| Smoker | 23 (34.8%) | 9 (25.0%) | |
| Unknown | 76 (9.1%) | 7 (19.4%) | |
| Co-morbidities | |||
| Median number per patient (range) | 3 (0–5) | 2 (0–5) | 0.321† |
| Respiratory disease | 20 (30.3%) | 10 (32.3%) | 0.846* |
| Hypercholesterolaemia | 54 (81.8%) | 27 (87.1%) | 0.514* |
| Hypertension | 58 (87.9%) | 27 (84.4%) | 0.632* |
| Diabetes | 8 (12.1%) | 1 (3.2%) | 0.264‡ |
| Ischaemic heart disease | 32 (48.5%) | 12 (38.7%) | 0.367* |
| CVA/TIA | 5 (7.6%) | 0 (0.0%) | 0.174‡ |
| Heart failure | 4 (6.1%) | 3 (9.7%) | 0.677‡ |
| Serum creatinine (range) | 99.5μmol/l (49–275μmol/l) | 104μmol/l (55–748μmol/l) | 0.563† |
AAA = abdominal aortic aneurysm; CPET = cardiopulmonary exercise testing; CVA = cerebrovascular accident; TIA = transient ischaemic attack
Pearson's chi-square test;
Mann–Whitney U test;
Fisher's exact test
(The data on co-morbidities for five patients ‘not fit for repair' were incomplete. Percentages and p-values are based on a reduced sample of n=31.)
Table 2.
Mortality prediction data for cardiopulmonary exercise testing; values are given as a median (25th to 75th centile)
| Fit for AAA repair (n=63) | Not fit for AAA repair (n=30) | p-value* | |
|---|---|---|---|
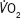 peak peak |
15.1 (13.3–17.1) | 13.1 (10.8–14.3) | <0.001 |
| Anaerobic threshold | 12.0 (10.8–13.6) | 10.7 (9.0–11.4) | <0.001 |
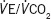 |
35.0 (32.0–39.0) | 37.0 (35.0–41.0) | 0.005 |
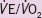 |
31.0 (29.0–36.5) | 34.0 (29.0–39.0) | 0.176 |
| Detsky score | 10.0 (5.0–16.3) | ||
| APACHE II score | 16.0 (14.0–22.3) | ||
| VPOSSUM physiology score | 27.0 (22.8–31.3) | ||
| VPOSSUM operative score | 12.0 (10.0–16.0) |
AAA = abdominal aortic aneurysm; 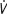 E = ventilatory equivalent; APACHE = Acute Physiology and Chronic Health Evaluation; VPOSSUM = Vascular Physiological and Operative Severity Score for the enUmeration of Mortality and morbidity
E = ventilatory equivalent; APACHE = Acute Physiology and Chronic Health Evaluation; VPOSSUM = Vascular Physiological and Operative Severity Score for the enUmeration of Mortality and morbidity
Mann–Whitney U test
Table 3.
Patient outcomes
| Fit for AAA repair (n=66) | Not fit for AAA repair (n=36) | p-value | |
|---|---|---|---|
| Mean ICU stay (range) | 1 day (0–19 days) | – | – |
| Inotropes required | 24 (36.4%) | – | – |
| Adverse events | |||
| 30-day mortality | 1 (1.5%) | – | – |
| All mortality | 6 (9.1%) | 8 (22.2%) | 0.066* |
| 30-month survival | 58 (87.9%) | 22 (61.1%) | 0.009† |
| Cardiac event | 12 (18.2%) | 4 (11.1%) | 0.390* |
| Cerebrovascular accident | 0 (0%) | 1 (2.8%) | 0.343‡ |
| Respiratory event | 10 (15.2%) | 4 (11.1%) | 0.766‡ |
AAA = abdominal aortic aneurysm; ICU = intensive care unit
Pearson's chi-square test;
Mantel–Cox logrank test;
Fisher's exact test
Five patients from the group unfit for open repair and three from the fit group did not reach the AT due to an inability to pedal the bicycle effectively. In patients achieving the AT (n=94) and given Detsky scores, AT was the only marker able to predict death and major events successfully (p=0.02, p=0.037) (Table 4).
Table 4.
Logistic regression analysis to assess power of cardiopulmonary exercise testing and Detsky score in predicting cardiorespiratory event or death for all patients (operated and not operated)
| Odds ratio | 95% confidence interval | p-value | |
|---|---|---|---|
| Cardiac event | |||
| Anaerobic threshold | 0.882 | 0.682–1.140 | 0.337 |
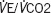 |
0.921 | 0.828–1.026 | 0.135 |
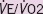 |
0.936 | 0.847–1.034 | 0.191 |
| Detsky score | 1.052 | 0.995–1.113 | 0.075 |
| Respiratory event | |||
| Anaerobic threshold | 0.824 | 0.621–1.094 | 0.181 |
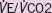 |
1.068 | 0.986–1.157 | 0.107 |
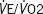 |
1.058 | 0.980–1.142 | 0.152 |
| Detsky score | 0.990 | 0.926–1.059 | 0.774 |
| Cerebrovascular event | |||
| Anaerobic threshold | 0.473 | 0.174–1.284 | 0.142 |
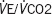 |
0.942 | 0.647–1.373 | 0.757 |
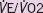 |
0.874 | 0.560–1.362 | 0.551 |
| Detsky score | 0.970 | 0.750–1.253 | 0.813 |
| Any major event | |||
| Anaerobic threshold | 0.789 | 0.631–0.985 | 0.037 |
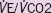 |
0.997 | 0.933–1.066 | 0.932 |
 |
0.996 | 0.933–1.063 | 0.902 |
| Detsky score | 1.030 | 0.982–1.080 | 0.230 |
| Death | |||
| Anaerobic threshold | 0.675 | 0.484–0.940 | 0.020 |
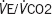 |
1.047 | 0.965–1.136 | 0.272 |
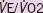 |
1.059 | 0.981–1.144 | 0.141 |
| Detsky score | 1.056 | 0.995–1.121 | 0.071 |
| Death or any major event | |||
| Anaerobic threshold | 0.789 | 0.631–0.985 | 0.037 |
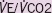 |
1.028 | 0.964–1.097 | 0.398 |
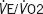 |
1.025 | 0.963–1.091 | 0.434 |
| Detsky score | 1.031 | 0.983–1.080 | 0.209 |
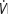 E = ventilatory equivalent
E = ventilatory equivalent
ROC analysis was performed for those outcomes achieving statistical significance. These demonstrated that for the AT to predict any event excluding death, any event or death alone, the area under the curve was 0.618 (p=0.076), 0.618 (p=0.076) and 0.694 (p=0.046) respectively. A history of diabetes was predictive of cardiac events (p=0.024) and a history of respiratory disease was predictive of future respiratory events (p=0.002) (Table 5). A comparison of 30-day outcomes in the open repair group demonstrated the ability of  and APACHE II scores to predict inotropic requirements (p=0.018, p=0.019) and of Detsky scores to predict the length of ICU stay (p=0.008) (Tables 6 and 7).
and APACHE II scores to predict inotropic requirements (p=0.018, p=0.019) and of Detsky scores to predict the length of ICU stay (p=0.008) (Tables 6 and 7).
Table 5.
Logistic regression to assess ability of co-morbidities on predicting adverse event or death following abdominal aortic aneurysm repair
| Odds ratio | 95% confidence interval | p-value | |
|---|---|---|---|
| Cardiac event | |||
| Respiratory disease | 1.156 | 0.304–4.397 | 0.831 |
| Hypercholesterolaemia | 1.023 | 0.191–5.482 | 0.979 |
| Hypertension | – | – | – |
| Diabetes | 6.125 | 1.268–29.576 | 0.024 |
| Ischaemic heart disease | 1.568 | 0.441–5.572 | 0.487 |
| CVA/TIA | – | – | – |
| Heart failure | 5.100 | 0.641–40.573 | 0.124 |
| Smoking history | 1.026 | 0.188–5.604 | 0.977 |
| Respiratory event | |||
| Respiratory disease | 14.333 | 2.681–76.631 | 0.002 |
| Hypercholesterolaemia | 2.000 | 0.227–17.633 | 0.533 |
| Hypertension | 1.102 | 0.118–10.281 | 0.932 |
| Diabetes | 0.762 | 0.083–6.966 | 0.810 |
| Ischaemic heart disease | 0.384 | 0.090–1.642 | 0.197 |
| CVA/TIA | 1.417 | 0.142–14.173 | 0.767 |
| Heart failure | – | – | – |
| Smoking history | – | – | – |
| Adverse event or death | |||
| Respiratory disease | 5.250 | 1.693–16.377 | 0.004 |
| Hypercholesterolaemia | 1.448 | 0.343–6.108 | 0.615 |
| Hypertension | 3.405 | 0.384–30.236 | 0.271 |
| Diabetes | 3.922 | 0.841–18.288 | 0.082 |
| Ischaemic heart disease | 0.795 | 0.284–2.229 | 0.795 |
| CVA/TIA | 0.464 | 0.049–4.425 | 0.505 |
| Heart failure | 2.050 | 0.269–15.633 | 0.489 |
| Smoking history | 3.103 | 0.609–15.810 | 0.173 |
CVA = cerebrovascular accident; TIA = transient ischaemic attack
Table 6.
Logistic regression analysis to assess power of cardiopulmonary exercise testing, the Detsky score, the Acute Physiology and Chronic Health Evaluation (APACHE) II score and the Vascular Physiological and Operative Severity Score for the enUmeration of Mortality and morbidity (VPOSSUM) in predicting the need for inotropes following abdominal aortic aneurysm repair
| Odds ratio | 95% confidence interval | p-value | |
|---|---|---|---|
| Inotropic requirement | |||
| Anaerobic threshold | 0.749 | 0.539–1.041 | 0.085 |
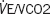
|
1.118 | 0.992–1.260 | 0.068 |
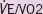
|
1.151 | 1.024–1.293 | 0.018 |
| Detsky score | 1.020 | 0.954–1.089 | 0.564 |
| APACHE II score | 1.143 | 1.022–1.278 | 0.019 |
| VPOSSUM physiology score | 1.064 | 0.974–1.163 | 0.171 |
| VPOSSUM operative score | 1.142 | 0.978–1.334 | 0.094 |
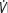 E = ventilatory equivalent
E = ventilatory equivalent
Table 7.
Logistic regression analysis to assess power of cardiopulmonary exercise testing, the Detsky score, the APACHE II score and VPOSSUM in predicting the length of intensive care unit (ICU) stay following abdominal aortic aneurysm repair; β represents the likelihood for each unit increase in the test variable (days in ICU)
| β | 95% confidence interval | p-value | |
|---|---|---|---|
| Length of ICU stay | |||
| Anaerobic threshold | -0.033 | -0.574 to 0.508 | 0.902 |
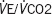 |
0.003 | -0.028 to 0.034 | 0.837 |
| APACHE II score | 0.106 | -0.046 to 0.257 | 0.169 |
| Detsky score | 0.139 | 0.038 to 0.240 | 0.008 |
| VPOSSUM physiology score | 0.065 | -0.065 to 0.196 | 0.319 |
| VPOSSUM operative score | 0.030 | -0.202 to 0.262 | 0.797 |
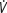 E = ventilatory equivalent
E = ventilatory equivalent
None of the scoring tools were able to predict 30-day major morbidity or mortality as defined by perioperative complications (p>0.05). Figure 2 is a survival plot for patients deemed fit and not fit for AAA repair. Figure 3 is a Kaplan–Meier plot illustrating the effect of the AT on time to death.
Figure 2.
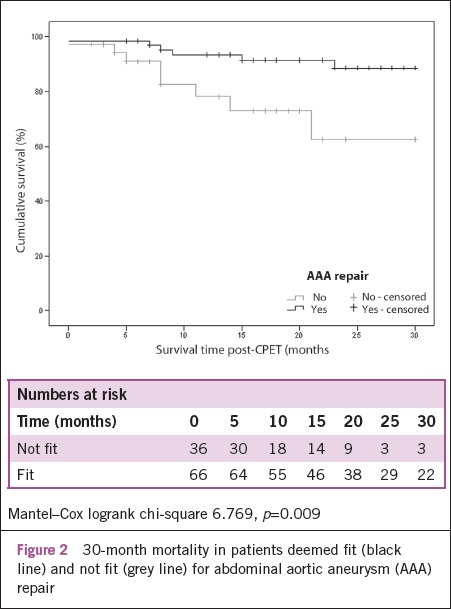
30-month mortality in patients deemed fit (black line) and not fit (grey line) for abdominal aortic aneurysm (AAA) repair
Figure 3.
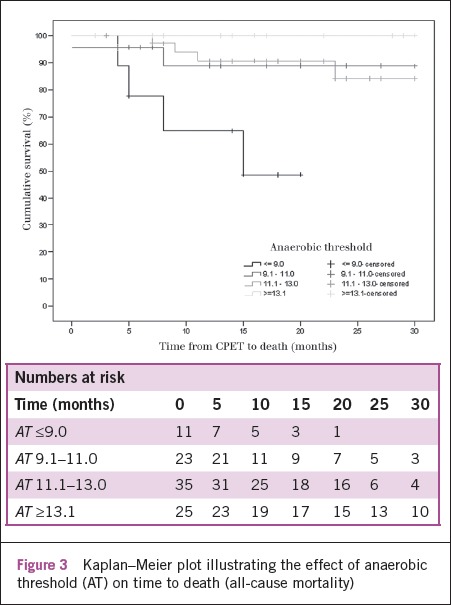
Kaplan–Meier plot illustrating the effect of anaerobic threshold (AT) on time to death (all-cause mortality)
Discussion
This study supports the use of CPET as a tool to predict the 30-month outcome of all AAA patients being considered for repair. CPET-derived AT was associated with 30-month survival of all patients considered for AAA repair (p=0.02), consistent with the reported literature.6 The mortality for the unfit group over a median 22-month period was 22.2% (8/36) as compared to 9.1% (6/66) in the fit group (Fig 2). This mortality is comparable with that reported by the EVAR trial participants4,5 and suggests that the preoperative selection criteria used by this unit (guided by CPET) has provided a similar division to that of the EVAR 1 and EVAR 2 cohorts.
A criticism of this study is the lack of detail available on the cause of death. It would have been beneficial to know how many deaths were attributable to AAA-related pathology, particularly between the fit and unfit groups. However, this study did not aim to demonstrate a superior method of treatment selection for AAA patients and therefore the information would not detract from the main finding of this study, namely that CPET is a good predictor of overall mortality in all patients being considered for repair.
A history of respiratory disease was able to predict morbidity and mortality into the mid term (similar to AT in this study). However, measures of respiratory function (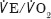 ,
, 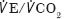 ) were unable to confirm this as a functional association. Previously,
) were unable to confirm this as a functional association. Previously, 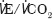 has been reported as the most reliable predictor of mid-term survival mortality following open AAA repair.6 The absence of association with
has been reported as the most reliable predictor of mid-term survival mortality following open AAA repair.6 The absence of association with 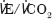 in this study may represent a type 2 error due to the small study size. Prior to this, our unit has reported on the association of Detsky scores and long-term outcome following AAA repair.14
in this study may represent a type 2 error due to the small study size. Prior to this, our unit has reported on the association of Detsky scores and long-term outcome following AAA repair.14
The most important feature of this study is the inclusion of AAA patients deemed unfit for open repair. In many centres these patients would have been considered for endovascular repair. At the time of this study, endovascular AAA repair was not funded through the primary care trust. Previous studies have demonstrated strong associations between stress testing and morbidity following intervention, usually open surgery. This study shows that CPET stress testing is able to predict mortality, into the mid term, for patients being considered for AAA repair.
The importance of accurately predicting mid-term survival in the management of AAA patients who are either fit or not fit for open repair has been brought back under the spotlight by the reporting of the long-term outcomes of the EVAR 1 and EVAR 2 trials.9,15 The EVAR 2 trial reported improved AAA survival in the intervention group after eight years of follow up. This advantage was only seen in 20% of participants in our study who survived the follow-up period and it will be argued that these patients should have been selected into the cohort of those not fit for open repair.
Equally, the long-term outcome of the EVAR 1 trial demonstrates that there is no advantage for endovascular repair in patients living more than six years and that the very fit AAA patient should be offered the choice of open repair. It appears that to provide AAA patients with an informed choice they need to be informed of not only their perioperative risk but also their mid-term predicted survival. In clinical practice this calculation is made all the time, guided by experience. Accurately predicting survival as well as perioperative morbidity could help standardise these decisions with the benefits of impartiality and audit.
It has been known for a long time that exercise capacity is the single best predictor of survival.16 In this study, 25% of the unfit cohort was recorded as having an AT >11.4. (An AT of ≥11 is used as the unit guideline for recommending open surgery.) In these cases other co-morbidities resulted in them being selected for conservative management despite their predicted survival being favourable (Fig 3). It is likely that some of these cases would survive for long enough to benefit from endovascular repair although their perioperative risk for open surgery is high.
In the sample of 63 open AAA repairs, days spent in ICU and inotropic requirement were used as surrogate markers for perioperative morbidity. CPET-derived 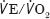 was equivalent to APACHE II scores in predicting inotropic requirement and, by extrapolation, the risk of perioperative morbidity. Detsky scores were also able to predict the length of ICU stay. These findings support the use of CPET to predict 30-day mortality, in keeping with the literature.6 This study was not powered to demonstrate any direct association with 30-day morbidity or mortality.
was equivalent to APACHE II scores in predicting inotropic requirement and, by extrapolation, the risk of perioperative morbidity. Detsky scores were also able to predict the length of ICU stay. These findings support the use of CPET to predict 30-day mortality, in keeping with the literature.6 This study was not powered to demonstrate any direct association with 30-day morbidity or mortality.
Conclusions
Our study reiterates the usefulness of CPET in predicting perioperative morbidity in AAA patients undergoing open repair. In addition, it demonstrates the potential of CPET to predict all-cause mortality for all patients being considered for AAA repair. CPET may serve as a tool to standardise the allocation of treatment modalities (best medical therapy, endovascular and open repair).
Acknowledgments
The authors would like to thank Timothy Catton, who helped with data collection for this study.
The material in this article was presented at the 44th annual scientific meeting of the Vascular Society of Great Britain and Ireland held in Liverpool, UK, 18–20 November 2009. It was also published as an abstract (Peters N, Thompson A, Lovegrove R et al. Cardio-pulmonary exercise testing provides a discriminating predictive tool for early and late outcomes in patients with an AAA. Br J Surg 2010; 97(S1): 15).
References
- 1.Mortality results for randomised controlled trial of early elective surgery or ultrasonographic surveillance for small abdominal aortic aneurysms. The UK Small Aneurysm Trial Participants. Lancet. 1998;352:1,649–1,655. [PubMed] [Google Scholar]
- 2.Lederle FA, Johnson GR, Wilson SE, et al. The aneurysm detection and management study screening program: validation cohort and final results. Aneurysm Detection and Management Veterans Affairs Cooperative Study Investigators. Arch Intern Med. 2000;160:1,425–1,430. doi: 10.1001/archinte.160.10.1425. [DOI] [PubMed] [Google Scholar]
- 3.Holt PJ, Poloniecki JD, Loftus IM, et al. Epidemiological study of the relationship between volume and outcome after abdominal aortic aneurysm surgery in the UK from 2000 to 2005. Br J Surg. 2007;94:441–448. doi: 10.1002/bjs.5725. [DOI] [PubMed] [Google Scholar]
- 4.Endovascular aneurysm repair and outcome in patients unfit for open repair of abdominal aortic aneurysm (EVAR trial 2): randomised controlled trial. Lancet. 2005;365:2,187–2,192. doi: 10.1016/S0140-6736(05)66628-7. [DOI] [PubMed] [Google Scholar]
- 5.Endovascular aneurysm repair versus open repair in patients with abdominal aortic aneurysm (EVAR trial 1): randomised controlled trial. Lancet. 2005;365:2,179–2,186. doi: 10.1016/S0140-6736(05)66627-5. [DOI] [PubMed] [Google Scholar]
- 6.Carlisle J, Swart M. Mid-term survival after abdominal aortic aneurysm surgery predicted by cardiopulmonary exercise testing. Br J Surg. 2007;94:966–969. doi: 10.1002/bjs.5734. [DOI] [PubMed] [Google Scholar]
- 7.Older P, Hall A, Hader R. Cardiopulmonary exercise testing as a screening test for perioperative management of major surgery in the elderly. Chest. 1999;116:355–362. doi: 10.1378/chest.116.2.355. [DOI] [PubMed] [Google Scholar]
- 8.Gitt AK, Wasserman K, Kilkowski C, et al. Exercise anaerobic threshold and ventilatory efficiency identify heart failure patients for high risk of early death. Circulation. 2002;106:3,079–3,084. doi: 10.1161/01.cir.0000041428.99427.06. [DOI] [PubMed] [Google Scholar]
- 9.Greenhalgh RM, Brown LC, Powell JT, et al. Endovascular versus open repair of abdominal aortic aneurysm. N Engl J Med. 2010;362:1,863–1,871. doi: 10.1056/NEJMoa0909305. [DOI] [PubMed] [Google Scholar]
- 10.Detsky AS, Abrams HB, Forbath N, et al. Cardiac assessment for patients undergoing noncardiac surgery. A multifactorial clinical risk index. Arch Intern Med. 1986;146:2,131–2,134. [PubMed] [Google Scholar]
- 11.Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol. 1986;60:2,020–2,027. doi: 10.1152/jappl.1986.60.6.2020. [DOI] [PubMed] [Google Scholar]
- 12.Copeland GP, Jones D, Walters M. POSSUM: a scoring system for surgical audit. Br J Surg. 1991;78:355–360. doi: 10.1002/bjs.1800780327. [DOI] [PubMed] [Google Scholar]
- 13.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 14.Payne SP, Galland RB. The use of a simple clinical cardiac risk index predictive of long-term outcome after infrarenal aortic reconstruction. Eur J Vasc Endovasc Surg. 1995;9:138–142. doi: 10.1016/s1078-5884(05)80082-6. [DOI] [PubMed] [Google Scholar]
- 15.Brown LC, Greenhalgh RM, Thompson SG, Powell JT. Does EVAR alter the rate of cardiovascular events in patients with abdominal aortic aneurysm considered unfit for open repair? Results from the randomised EVAR trial 2. Eur J Vasc Endovasc Surg. 2010;39:396–402. doi: 10.1016/j.ejvs.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Myers J, Prakash M, Froelicher V, et al. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346:793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]


