Abstract
Systemic lupus erythematosus (SLE) is a chronic inflammatory multi-system disease characterised by varied clinical manifestation and immunological abnormalities. The clinical presentation of the disorder has wide spectra, from an asymptomatic presentation to a severe life-threatening disease affecting several organs. The sole manifestation of lupus erythematosus could be neurological syndrome, where diagnosis of SLE is difficult to establish. The authors intended to report a young female, who initially developed left-sided hemiparesis due to tumefactive demyelination, later on diagnosed as case of SLE. The association of tumefactive demyelination and SLE has not been previously reported in the literature.
Background
Central nervous system lupus is potentially lethal treatable disorder and sometimes presents difficult diagnostic challenges for physicians. Systemic lupus erythematosus (SLE) is multi-system, auto-immune disorder presenting as combination of clinical manifestations depending upon system involvement.1 In full florid disease the diagnosis is straight forward but sometimes initial unusual presentation can pose great difficulty in diagnosis of this treatable disorder.
Parkinsonism, cognitive dysfunction, neuromyelitis optica, limbic encephalitis and posterior leucoencephalopathy syndrome are some of the examples of initial presentation of SLE mentioned in the literature. We report a young female who presented with left-sided hemiparesis due to tumefactive demyelination as the initial manifestation of SLE not ever emphasised in the literature.
Case presentation
A 13-year-old female presented with progressive left-sided face, arm, leg weakness and difficulty in walking for the duration of 7 days. During the course, the patient also reported having headache and occasional vomiting. There was no history of seizures, fever before the development of focal neurological deficit, altered sensorium and vision loss. The patient did not have joint pain, chest pain and urinary problems.
On examination, pulse rate was 96/min, regular, synchronous with no radio femoral delay. Cardiovascular system and respiratory examination did not reveal any abnormality.
Neurological examination disclosed left-sided hemiparesis. Plantar response was extensor on left side. The patient was well conscious and higher mental functions were intact. Other parts of the neurological evaluation were normal. At this juncture, routine hematological parameters and MRI of cranium with contrast was advised. Hb%, serum creatinine, erythrocyte sedimentation rate, C reactive protein, blood sugar, antinuclear antibody (ANA) titre and x-ray chest posteroanterior view were with in normal limits. MRI of cranium with contrast revealed white matter oedema with adjacent gray matter involvement with sulcal effacement in right parietal region showing patchy mild post contrast enhancement (figures 1 and 2). The lesion is causing mass effect over body of the right lateral ventricle (figure 3). Magnetic spectroscopy findings showed mild increase in choline with decrease in NAA with no other significant findings. Based on this profile, possibility of inflammatory demyelinating pseudotumour (tumefactive demyelination) was suspected. MRI of spine did not reveal any abnormality. The patient belongs to endemic area of tuberculosis and due to positive mantoux test and positive ELISA for tuberculosis IgM, IgG antibody, antituberculous chemotherapy was started. She also received tablet prednisolone (1 mg/kg body weight) for 4 weeks and tapered in further 2 weeks. The patient responded very well to treatment and her motor deficit improved with in 2 months. During the course of treatment she started developing fever which was low to moderate grade in nature.
Figure 1.
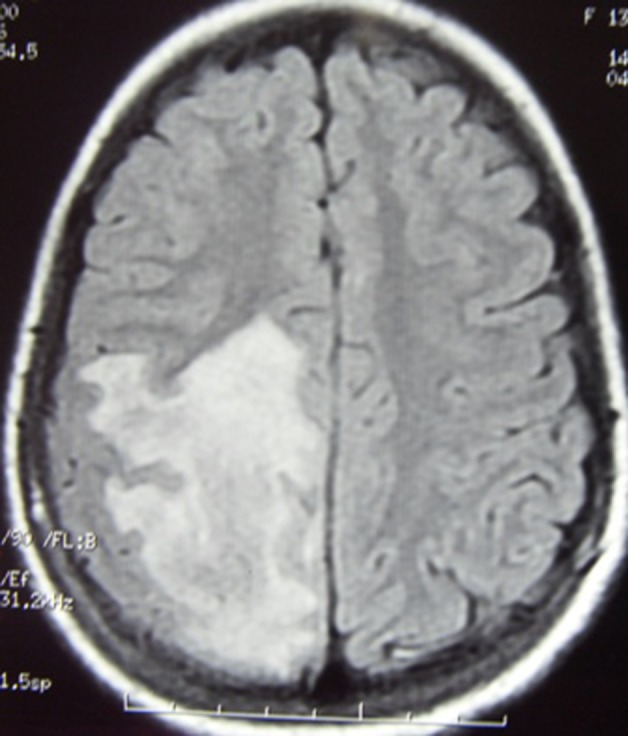
MRI T2 weighted flair image showing predominantly extensive white matter lesion in right parietal area.
Figure 2.
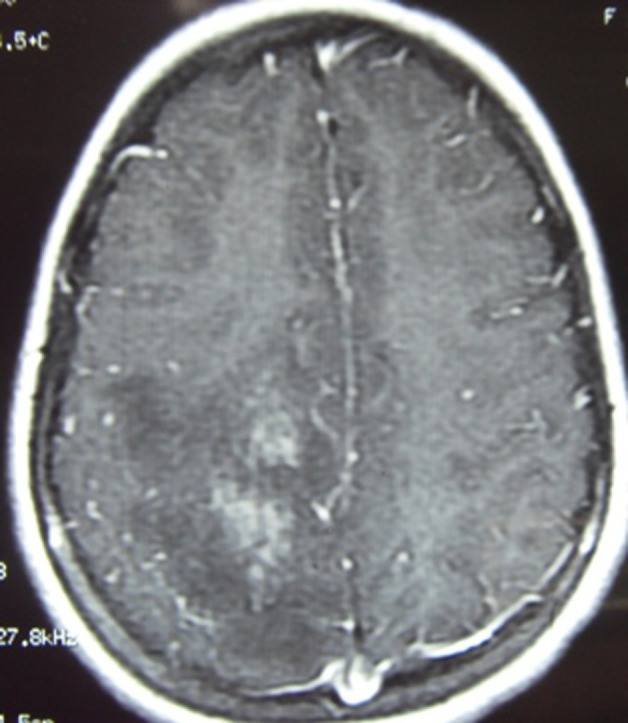
T1 contrast showing patchy enhancement.
Figure 3.
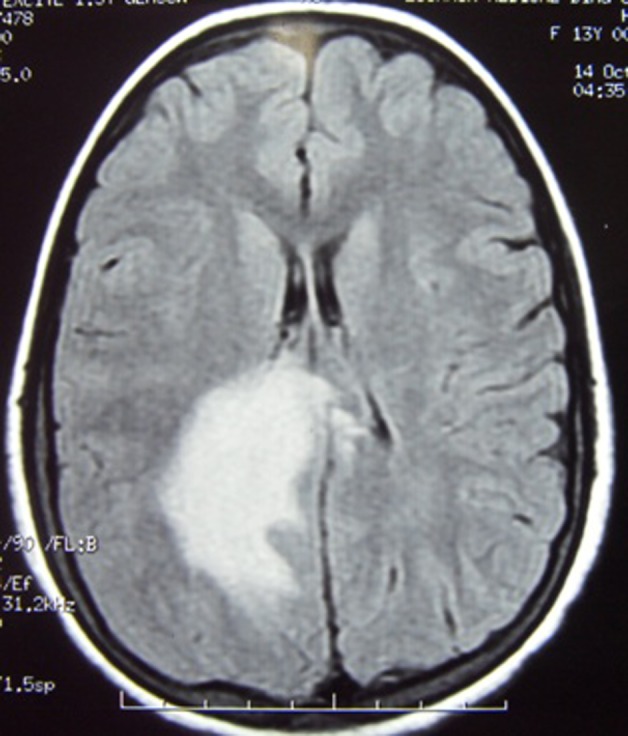
T2 flair image revealing tumefactive lesion with effacement of right lateral ventricle.
Investigations
At this point after 2½–3 months of onset of illness, the patient was evaluated for unexplained fever. The patient also developed arthralgia. Hb% was 7.8 g/dl; liver function test, renal function test and urinary examination were within normal limits. Ultrasound abdomen did not reveal any abnormality. Repeat x-ray chest was normal. Repeat MRI with contrast showed near complete resolution of lesion (figures 4 and 5). Other possibilities of connective tissue disorder and neurosarcoidosis were explored. Serum ACE was 17.8 units/litre (normal range 8–50 units/litre). ANA was positive in high titres.
Figure 4.
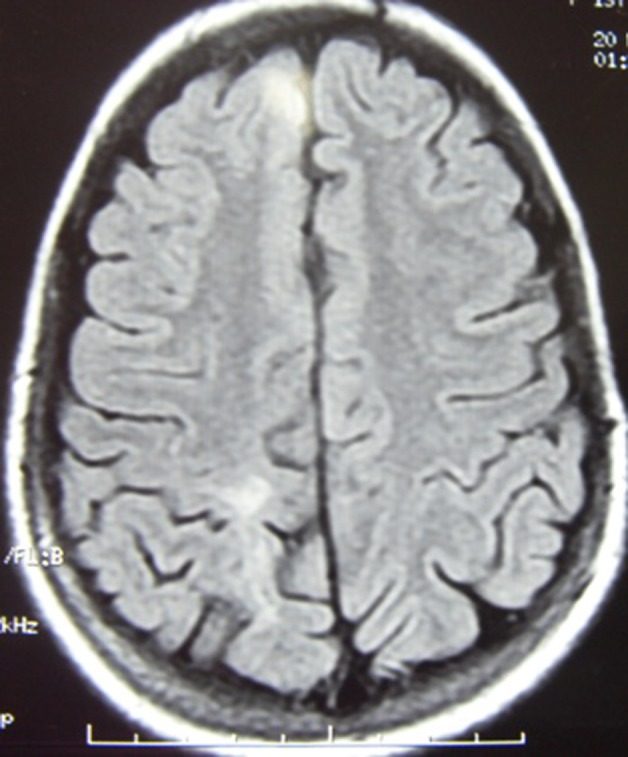
Repeat MRI T2 weighted flair showing resolution of lesion.
Figure 5.
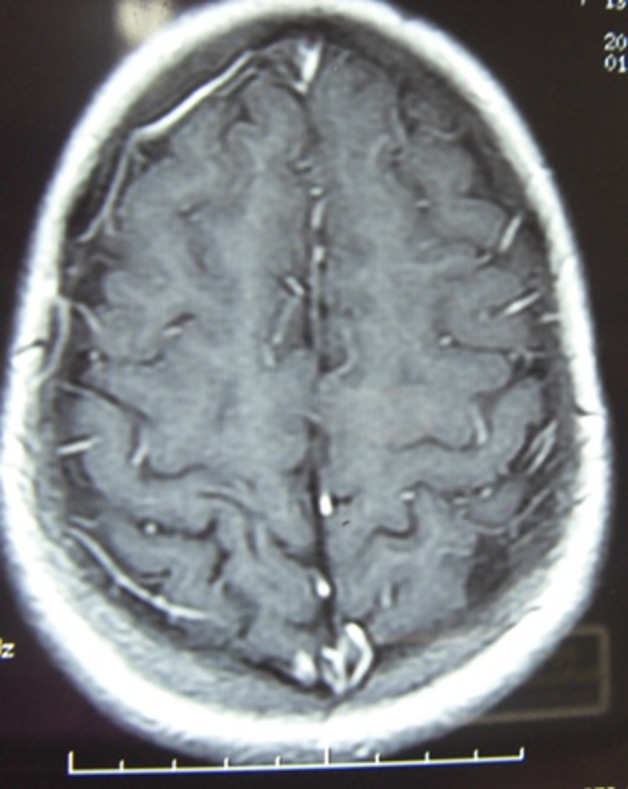
Repeat T1 contrast revealing almost disappearance of lesion.
Antidouble stranded DNA assay was 195.62 IU/ml (less than 35 IU/ml is negative). More than 55 IU/ml is highly positive for antibodies to double stranded DNA, which is virtually diagnostic of SLE. So final diagnosis of SLE was established and left-sided hemiparesis due to tumefactive demyelination was the initial manifestation of connective tissue disorder.
Differential diagnosis
Vasculitis, inflammatory granuloma, tumour.
Treatment
In initial phase, the patient was prescribed antituberculous chemotherapy with steroids as per guidelines. Finally as the patient’s diagnosis of SLE has been established, she started to receive antilupus therapy.
Outcome and follow-up
At follow-up after a year, the patient became totally asymptomatic and receiving disease modifying therapy under consultation of rheumatologist.
Discussion
The tumefactive demyelinating lesions are defined as a usually solitary demyelinating lesion larger than 2 cm, mimicking brain neoplastic lesions.2 Neuroimaging characteristics of tumefactive demyelinating lesions are large predominant white matter lesion, ill-defined borders, little mass effect, perilesional oedema, incomplete or open ring enhancement, vessel like structures on dynamic T2 weighted images and low relative cerebral blood volume.3 4
Our patient suffered from SLE, who initially presented with left-sided hemiparesis due to tumefactive demyelination. Neurological manifestations of SLE are vivid in nature ranging from serious manifestations (eg, acute confusional state, seizure disorder and stroke) to more subtle presentation, like mild cognitive dysfunction.
The prevalence of neuropsychiatric SLE is around 14% to 75%, reflecting variable diagnostic criteria and differences in selection of patients for study.5 However, neurological presentation at the onset of the disease is regarded to be rare, occurring only in 3%.6
A case of Parkinsonism as first manifestation of SLE has been reported in a 45-year-old woman, who presented with a strategic infarct of the right substantia nigra secondary to vasculitis. This was the pioneer case report in the literature secondary to vasculitis in a patient with SLE.7 A study has been done to find the relevance of cognitive dysfunction among patients with SLE who never displayed major neuropsychiatric manifestations. Prevalence of cognitive dysfunction was found to be 22.6% in patients with SLE as compared with 6.5% in control group.8
Jacobi et al described 37-year-old woman who presented with episodes of transverse myelitis and optic neuritis preceding confirmation of diagnosis of SLE. The diagnosis of neuromyelitis optica was based on clinical radiological evaluation and laboratory parameters. This case illustrates that Neuromyelitis optica could represent initial manifestation of SLE for many years.9
In a population based study of 43 patients suffering from SLE, abnormal MRI findings has been found to be more common in patients with SLE related to specific neuropsychiatric manifestations. These findings also supported the organic aetiology of cognitive dysfunction in SLE.10 Kano et al described limbic encephalitis in a well diagnosed patient of SLE. In this case malignancy, tumour markers and the antineural antibodies were negative. The author concluded that shared autoimmunity may cause both limbic encephalitis and SLE.11 Tumefactive demyelination is one subtype of pathological spectrum of central nervous system inflammatory demyelinating diseases. It can mimic lesion of multiple sclerosis or glial tumour. Neuroimagimng characteristics and histopathological studies help to make correct diagnosis of tumefactive demyelination.12
Recently a paper has been published which mentioned left homonymous hemianopsia as the leading symptom of a tumour-like demyelinating lesion of the right hemisphere mimicking brain tumour. They concluded that treating physicians encountering a cerebral lesion should suspect tumefactive MS, as this is of benign character and can be managed without exposing patients to the unnecessary risks of surgical procedure.13
Learning points.
-
▶
Various neuropsychiatric manifestations occur in patients suffering from SLE which can range from mild to serious life-threatening conditions.
-
▶
Sometimes neurological manifestations can be initial presentation of SLE which makes diagnostic difficulties.
-
▶
The association of tumefactive demyelination and SLE has not been previously mentioned in the literature.
-
▶
The patient suspected of tumefactive demyelination should also be evaluated for SLE, so appropriate treatment can be started at the earliest to have best clinical outcome.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Hahn BH. Systemic lupus erythematosus. In: Fauci AS, Braunwald E, Hauser SL, Longo DL, Jameson JL, Loscalzo J, eds. Harrison’s Principles of Internal Medicine. 14th Edition New York: McGraw Hill; 1998:1874–80 [Google Scholar]
- 2.Dagher AP, Smirniotopoulos J. Tumefactive demyelinating lesions. Neuroradiology 1996;38:560–5 [DOI] [PubMed] [Google Scholar]
- 3.Masdeu JC, Quinto C, Olivera C, et al. Open-ring imaging sign: highly specific for atypical brain demyelination. Neurology 2000;54:1427–33 [DOI] [PubMed] [Google Scholar]
- 4.Cha S, Pierce S, Knopp EA, et al. Dynamic contrast-enhanced T2*-weighted MR imaging of tumefactive demyelinating lesions. AJNR Am J Neuroradiol 2001;22:1109–16 [PMC free article] [PubMed] [Google Scholar]
- 5.McCune WJ, Golbus J. Neuropsychiatric lupus. Rheum Dis Clin North Am 1988;14:149–67 [PubMed] [Google Scholar]
- 6.The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum 1999;42:599–608 [DOI] [PubMed] [Google Scholar]
- 7.Orta Daniel SJ, Ulises RO. Stroke of the substance nigra and parkinsonism as first manifestation of systemic lupus erythematosus. Parkinsonism Relat Disord 2008;14:367–9 [DOI] [PubMed] [Google Scholar]
- 8.Olazarán J, López-Longo J, Cruz I, et al. Cognitive dysfunction in systemic lupus erythematosus: prevalence and correlates. Eur Neurol 2009;62:49–55 [DOI] [PubMed] [Google Scholar]
- 9.Jacobi C, Stingele K, Kretz R, et al. Neuromyelitis optica (Devic’s syndrome) as first manifestation of systemic lupus erythematosus. Lupus 2006;15:107–9 [DOI] [PubMed] [Google Scholar]
- 10.Ainiala H, Dastidar P, Loukkola J, et al. Cerebral MRI abnormalities and their association with neuropsychiatric manifestations in SLE: a population-based study. Scand J Rheumatol 2005;34:376–82 [DOI] [PubMed] [Google Scholar]
- 11.Kano O, Arasaki K, Ikeda K, et al. Limbic encephalitis associated with systemic lupus erythematosus. Lupus 2009;18:1316–9 [DOI] [PubMed] [Google Scholar]
- 12.Fallah A, Banglawala S, Ebrahim S, et al. Case Series: tumefactive demyelinating lesions: a diagnostic challenge. Can J Surg 2010;53:69–70 [PMC free article] [PubMed] [Google Scholar]
- 13.Evangelopoulos ME, Evangelopoulos DS, Potagas C, et al. Homonymous hemianopsia as the leading symptom of a tumor like demyelinating lesion: a case report. Cases J 2009;2:9366. [DOI] [PMC free article] [PubMed] [Google Scholar]


