Abstract
A 10-year-old boy presented with an atypical non-febrile septic arthritis/osteomyelitis. He was unresponsive to routine antibiotic treatment with flucloxacillin/gentamicin as the pain and fluid collection increased. Synovial fluid cultures are negative and gram stain remained negative. Only after PCR/16S ribosomal bacterial DNA amplification a Fusobacterium nucleatum could be detected, and antibiotic therapy switched to clindamycin with rapid response. Septic osteomyelitis and arthritis are relatively rare but important infections in children needing prompt treatment, and should be considered when a child complaints about joint or bone pain without prior recent trauma. Skin bacteria are the most prevalent causative organisms, whereas Fusobacteria or other anaerobic, Gram-negative microorganisms are very seldom encountered. If cultures remain negative and the patients responds insufficiently to empiric treatment, PCR/16S ribosomal bacterial DNA amplification can be useful to detect the causative microorganisms.
Background
Osteomyelitis and arthritis are causes of infection that are more common in children, compared with adults. If not recognised and treated in an early stage, they can cause significant mortality and morbidity. Staphylococcus aureus is the most common causative microorganism, whereas anaerobic, Gram-negative bacteria seldom cause septic arthritis of the hip in children. Therapy starts with empiric antibiotics, and is adjusted when cultures reveal the causative organism. If cultures fail to reveal a causative organism and empiric therapy appears ineffective, attention should be focused on rare microorganisms like anaerobic bacteria. Although these are mostly seen in immune compromised patients, they should be considered in patients with an atypical course of disease or patients who are not responding to empiric treatment.1
Case presentation
A 10-year-old boy initially presented in the emergency room with pain in the right groin and hip, and tenderness of the right knee. The pain had gradually increased over time and did not respond to oral analgesics. There had been no traumatic event. The boy had no fever, no other complaints including gastrointestinal, ear, nose, throat or respiratory complaints. Neither trauma or skin lesions nor surgical procedures including dental care had occurred previously.
He used an antalgic posture. Palpation of the hip and knee were not painful. Hip and knee movements were limited due to pain. No signs of herniation were present. X-ray of the hip showed no abnormalities. The child was initially diagnosed as having a tendinitis which was treated with non-steroidal anti-inflammatory analgesics and advice.
A few days later he revisited the emergency room, as despite the analgesics the pain in his hip and knee was increasing.
Investigations
Physical examination showed increased pain during hip extension, internal rotation and total external rotation. The hip was not red, warm or swollen. The pain in the knee was located on the lateral side, and increased in flexion and extension. No fever was present during the entire clinical course.
Laboratory results showed a C reactive protein (CRP) of 46 mg/l (n: <6 mg/l), an erythrocyte sedimentation rate (ESR) of 69 mm (n: <15 mm) and a leucocyte count of 7.4×109/l (n: 4.0–14.0×109/l).
A second x-ray of the hip showed a displaced right hip joint and swollen tissue. Sonography of the hips showed fluid collection and a distension of joint capsule. A bone scintigraphy showed no signs of an active osteomyelitis, making a transient synovitis of the hip the most likely diagnosis, and symptomatic treatment was continued.
However as complaints persisted over days, a MRI scan was made. This showed a fluid collection in the right hip (figure 1). Invasion of the ossal structures was suspected. Synovial fluid, aspirated under ultrasound guidance, appeared purulent and was sent for Gram stain and culture. The boy was admitted to the paediatric department for empiric treatment with intravenous flucloxacillin 800 mg four times per day, and gentamicin 180 mg daily.
Figure 1.
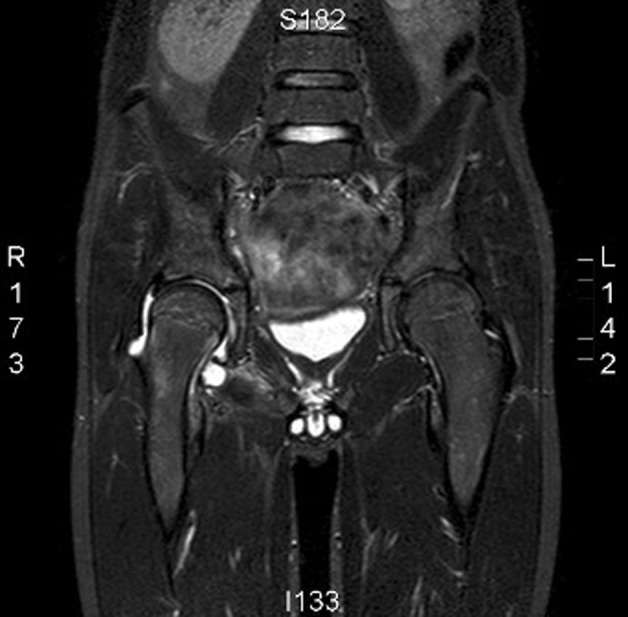
MRI showing fluid accumulation in the right hip and ossal abnormalities due to Fusobacterium nucleatum infection.
The clinical condition remained unchanged, and laboratory results showed an increase of CRP to 97 mg/l and ESR to 104 mm. Sonography showed a new fluid collection, which was aspirated and cultured. Both synovial fluid and blood cultures remained negative, after 7 days incubation. The abundant presence of granulocytes in the Gram stain, however, indicated a bacterial origin of the infection. For this reason a 16s sequence analysis directly on the synovial fluid, was performed which showed the presence of Fusobacterium nucleatum DNA.
Treatment
Treatment was switched to intravenous amoxicillin/clavulanate 1000/200 mg three times daily for 2 weeks, followed by oral clindamycin 300 mg three times daily for 6 weeks. The boy responded well, laboratory results normalised, and the boy was discharged in good condition.
Discussion
Difficulties in isolating anaerobic bacteria are common, as demonstrated in this patient. This case illustrates that amplification of the 16s ribosomal DNA region of bacteria (encoding the species specific structure of ribosomes) by PCR, followed by sequencing of its nucleotides and comparing this sequence to a 16s database, can be of additional value in determining the presence of bacteria. This has previously been described for detection of for example, Kingella Kingae, but to our knowledge not for Fusobacteria spp.2
PCR based methods are not hampered by the viability of bacteria since they detect DNA of both dead and viable bacteria. As obtaining an anaerobic blood culture is not a routine in children1 and Fusobacteria spp. are very sensitive to contact with oxygen, this may explain the negative routine cultures. Exposures of less than 1 h already has a dramatic effect on bacterial viability,3 and processing samples within 1 h after specimen collection is nearly impossible in routine clinical practice.
F nucleatum septic arthritis is extremely rare. Most reported cases of Fusobacterium infections involve adults. F nucleatum septic arthritis in combination with osteomyelitis of the pelvis has not been reported before.4 F nucleatum, a non-motile, non-sporulating, moderately long filamentous anaerobic Gram negative rod belonging to the Bacteroidaceae family, is a normal inhabitant of the oral cavity, gastrointestinal and female genital tract. Of all Fusobacterium spp., F nucleatum is responsible for the majority of invasive infections in humans. Fusobacteria have the ability to invade the human host as primary pathogen, unlike other anaerobic Gram negative bacteria.1 Fusobacterium spp. are virulent and have a synergistic potential with other anaerobic and aerobic bacteria, often resulting in polymicrobial infections.5 Most fusobacterial infections are located in the head, neck and upper respiratory tract. A fusobacterial infection located elsewhere, is reported to be in majority after orodental or pharyngeal infection, being the portal of entry. Lemierre disease is an example in which F necrophorum causes an oropharyngeal infection which is complicated by sepsis, thrombosis of the internal jugular vein and multiple metastatic infections, like septic arthritis.3 4 In this case, no recent oropharyngeal infection or dental portal of entry was found on clinical examination.
Treatment of first choice for a F nucleatum infection is most often amoxicillin in combination with clavunate, due to the potential β-lactamase production by F nucleatum, in combination with clindamycin for a duration of at least 4 weeks, or metronidazole. Clindamycin is also very effective in osteomyelitis, because of the good bone penetration, attaining a high bone to serum ratio. Most Fusobacterium spp. are resistant to erythromycin and rifampicin.5 6
Learning points.
-
▶
Consider PCR/16S ribosomal bacterial DNA amplification to detect the causative microorganisms in osteomyelitis/septic arthritis when cultures remain negative and empirical antibiotic treatment appears insufficient.
-
▶
Consider anaerobic Gram negative bacteria, when routine empiric antibiotic therapy fails in culture-negative osteomyelitis/septic arthritis.
-
▶
F nucleatum septic arthritis is extremely rare.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Huggan PJ, Murdoch DR. Fusobacterial infections: clinical spectrum and incidence of invasive disease. J Infect 2008;57:283–9 [DOI] [PubMed] [Google Scholar]
- 2.Verdier I, Gayet-Ageron A, Ploton C, et al. Contribution of a broad range polymerase chain reaction to the diagnosis of osteoarticular infections caused by Kingella kingae: description of twenty-four recent pediatric diagnoses. Pediatr Infect Dis J 2005;24:692–6 [DOI] [PubMed] [Google Scholar]
- 3.Hoffmann S, Justesen T. Effect of temperature, humidity and exposure to oxygen on the survival of anaerobic bacteria. J Med Microbiol 1980;13:609–12 [DOI] [PubMed] [Google Scholar]
- 4.Chryssagi AM, Brusselmans CB, Rombouts JJ. Septic arthritis of the hip due to Fusobacterium nucleatum. Clin Rheumatol 2001;20:229–31 [DOI] [PubMed] [Google Scholar]
- 5.Brook I. Fusobacterial infections in children. J Infect 1994;28:155–65 [DOI] [PubMed] [Google Scholar]
- 6.Brook I. Microbiology and management of joint and bone infections due to anaerobic bacteria. J Orthop Sci 2008;13:160–9 [DOI] [PubMed] [Google Scholar]


