Abstract
BACKGROUND & AIMS
Foxp3+ T regulatory cells (Tregs) help prevent autoimmunity, and increases in their numbers of functions could decrease the development of inflammatory bowel disease. Like other cells, Foxp3+ Tregs express histone/protein deacetylases (HDACs), which regulate chromatin remodeling and gene expression. We investigated whether disruption of a specific class IIa HDAC, HDAC9, activity in Tregs affects the pathogenesis of colitis in mice.
METHODS
We tested the effects of various HDAC inhibitors (HDACi) in models of colitis using wild-type mice. We also transferred Tregs and non-Treg cells from HDAC9−/− or wild-type mice to immunodeficient mice. HDAC9 contributions to the functions of Tregs were determined during development and progression of colitis.
RESULTS
Pan-HDACi, but not class I-specific HDACi, increased the functions of Foxp3+ Tregs, prevented colitis, and reduced established colitis in mice, indicating the role of class II HDACs in controlling Treg function. The abilities of pan-HDACi to prevent/reduce colitis were associated with increased numbers of Foxp3+ Tregs and their suppressive functions. Colitis was associated with increased local expression of HDAC9; HDAC9−/− mice resistant to development of colitis. HDAC9−/− Tregs expressed increased levels of the heat shock protein (HSP) 70, compared with controls. Immunoprecipitation experiments indicated an interaction between HSP70 and Foxp3. Inhibition of HSP70 reduced the suppressive functions of HDAC9−/− Tregs; Tregs that overexpressed HSP70 had increased suppressive functions.
CONCLUSIONS
Strategies to decrease HDAC9 expression or function in Tregs or to increase expression of HSP70 might be used to treat colitis and other autoimmune disorders.
The incidence of inflammatory bowel disease (IBD), including Crohn’s disease and ulcerative colitis, is currently between 70 and 150 cases per 100,000 individuals in the United States and is increasing in incidence.1 Although of unknown etiology, the development of IBD is likely markedly influenced by an individual’s genetic background, host immune responses, and the environment (including gut microbiota and exposure to toxins).1 Clinical and experimental data indicate that the development of IBD is mediated primarily by CD4+ T cells.2 Given continuous exposure of the gut to microbial and other antigens, the regulation of host inflammatory responses by thymic-derived Foxp3+ T regulatory cells (Tregs) is crucial to homeostasis.3–5 Hence, both humans and mice with defects in Foxp3 develop severe autoimmunity, including enteritis, and the adoptive transfer of Tregs can reverse established colitis and wasting disease in murine models.6
Many genes, including Foxp3,7,8 are regulated by epigenetic mechanisms such as acetylation, wherein acetyl groups are added to histone tails by histone acetyltransferases or removed by histone deacetylases (HDACs).6 HDACs are classified as class I (HDAC1–3, HDAC8), class IIa (HDAC4, 5, 7, and 9), class IIb (HDAC6 and 10), class III (SIRT1–7), and class IV (HDAC11).6 With exceptions, histone acetylation increases accessibility of transcriptional machinery and promotes gene transcription, whereas deacetylation leads to repression of gene transcription. The functions of numerous nonhistone proteins are also regulated by acetylation and deacetylation, eg, p53 is acetylated by CREB binding protein9 and deacetylated by HDAC1 and Sir2.10
HDAC inhibitors (HDACi) of the classical Zn-dependent HDACs (HDAC1–10) inhibit proliferation of tumor cells by inducing cell cycle arrest, differentiation, and/or apoptosis and are being trialed as anticancer agents.11 HDACi are typically active against the class I HDAC family alone (class I-selective HDACi) or block both class I and class II HDACs (pan-HDACi).11 Potent pan-HDACi such as Trichostatin-A (TsA) and its clinically approved derivative suberoylanilide hydroxamic acid (SAHA) are also being studied experimentally for potential anti-inflammatory effects. For example, SAHA use in mice reduced expression of proinflammatory cytokines and decreased mortality from acute graft-vs-host disease12 and impaired development of dextran sodium sulfate (DSS)-induced colitis.13
We recently showed that pan-HDACi such as TsA and SAHA increase the production and suppressive function of Tregs, and, in a survey of various murine disease models noted that, whereas TsA could ameliorate development of DSS colitis, the presence of Tregs was required for its efficacy.7 We have now further investigated the effects of HDACi on murine colitis, including the mechanisms by which HDACi use can alter Foxp3+ Treg functions. Our new data point to the importance of HDAC9 in regulating expression of heat shock protein 70 (HSP70) and controlling Foxp3+ Treg functions and may lead to new therapies for colitis.
Materials and Methods
Murine Colitis
HDAC9−/− C57BL/6 mice,14 inducible HSP70 transgenic (HSP70Tg) C57BL/6 mice,15 wild-type (WT) C57BL/6, and Rag1−/− C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME) were studied. Mice were housed in specific pathogen-free conditions and used for studies approved by the Institutional Animal Care and Use Committee of the Children’s Hospital of Philadelphia. We used 3 models of colitis to evaluate the effects of daily intraperitoneal (IP) injection of TsA (Alexis Biochemicals, San Diego, CA; 1 mg/kg), SAHA (Merck & Co, Rahway, NJ; 50 mg/kg), and MS275 (Sigma Chemicals, St. Louis, MO; 20 mg/kg) or control dimethyl sulfoxide (DMSO) and to study the role of HDAC9 in Treg cells. First, freshly prepared 4% DSS (wt/vol) (MP Biomedicals, Irvine, CA) was added daily for 5 days to pH-balanced tap water of study mice.16 Colitis was assessed by the daily monitoring of body weight, stool consistency, and fecal blood. Stool consistency was scored as 0 (hard), 2 (soft), or 4 (diarrhea). Fecal blood was scored as 0 (no blood), 2 (occult), or 4 (gross) using hemoccult cards. Second, for a preventative adoptive transfer model,17 CD4+CD25− T cells (5 × 105) isolated from HDAC9−/− or WT mice using magnetic beads (Miltenyi Biotec, Auburn, CA) to >95% purity (as shown by flow cytometry) were injected IP into Rag1−/− mice and monitored biweekly for clinical evidence of colitis. Third, for treatment in an adoptive transfer model, Rag1−/− mice were injected with CD4+CD25− cells (1 × 106, IP), and, once colitis developed, mice were injected with CD4+CD25+ cells from HDAC9−/− or WT mice (5 × 105 cells/mouse, IP) and monitored for continued weight loss and stool consistency. At cessation of study, H&E-stained paraffin sections of colons were used for histologic grading of colitis.18 We also tested whether HDAC9 affected the peripheral conversion of adoptively transferred CD45RBhigh cells. CD45RBhigh cells were isolated by fluorescence-activated cell sorting using CD4+CD25− lymphocytes from WT or HDAC9−/− mice; 1 × 106 cells were injected intravenously (IV) into RAG1−/− mice, and recipients were analyzed at weekly intervals (n = 4/group/time point).
Flow Cytometry of Colonic Intraepithelial and Lamina Propria Mononuclear Cells
Gut mononuclear cells were isolated as described,19 incubated with CD4-phycoerythrin (PE), CD8-fluorescein isothiocyanate (FITC), CD25-antigen-presenting cells (APC), and Foxp3-APC for 20 minutes at 4°C and analyzed by flow cytometry. For intracellular cytokine staining, cells were treated with GolgiStop (BD Pharmingen, San Jose, CA), stimulated for 4 hours with phorbol myristate acetate/ionomycin, stained with cell surface markers, fixed, permeabilized, and labeled with anticytokine monoclonal antibodies (mAbs).7
Treg Assays
Five × 104 carboxyfluoroscein succinimidyl ester (CFSE)-labeled CD4+CD25− (Teff) cells were stimulated with CD3 mAb in the presence of irradiated syngeneic APC and varying ratios of purified CD4+CD25+ WT or HDAC9−/− Tregs; suppression of proliferation was determined by the CFSE profile of dividing Teff cells at 72 hours.7
Quantitative Polymerase Chain Reaction, DNA Microarray, and Short Interfering RNA Knockdown
We performed quantitative polymerase chain reaction (qPCR) as described.7 DNA microarray studies were performed using CD4+CD25+ cells (>90% purity) isolated from WT or HDAC9−/− mice (3 mice/group) and GeneChip RG-U34A arrays (Affymetrix, Santa Clara, CA), according to manufacturer’s instructions. For Short Interfering RNA (siRNA) studies, CD4+CD25+ cells were transfected using lipofectamine 2000 and siRNA (Ambion, Austin, TX) specific for HDAC9 and Foxp3 or a negative control siRNA without specificity for rat, human, or mouse RNA sequences; transfected cells were evaluated using quantitative polymerase chain reaction (qPCR) and Treg suppression assays.
Immunoprecipitation
CD4+CD25+ Tregs were isolated using magnetic beads from WT C57BL/6 mice and lysed using RIPA buffer; HSP70 was immunoprecipitated using rabbit anti-HSP70 (Santa Cruz Biotechnology, Santa Cruz, CA) and a Millipore Immunoprecipitation Kit (“Catch and release v.2.0”; Millipore Corp, Billerica, MA); and the eluate was evaluated by Western blot using rat antimouse Foxp3 mAb (eBioscience, San Diego, CA; clone FJK-169).
Statistical Analysis
Data presented as mean ± SD were analyzed for statistical significance using χ2 and standard t test methods; P < .05 was considered significant.
Results
Differing Effects of Pan-HDACi and Class I-Specific HDACi Therapy on DSS Colitis
As part of a search for potential new therapies for colitis, we tested several HDACi compounds for their effects in the DSS-induced murine colitis model. Two pan-HDACi compounds, TsA and SAHA,11 but not MS275,20 a potent and long-acting HDAC class I-specific inhibitor, blocked development of colitis as shown by prevention of weight loss (Figure 1A) and associated bleeding (Figure 1B), diarrhea (Figure 1C), and histologic injury (Figure 1D). Pan-HDACi use decreased inflammatory cytokines and increased Foxp3 and anti-inflammatory cytokine messenger RNA (mRNA) and protein expression (Figure 1E and F). Both pan-HDACi increased the numbers and percentages of CD4+Foxp3+ cells in lymphoid tissues of DSS-treated mice (data not shown) and in vivo therapy with pan-HDACi but not a class I-specific HDACi enhanced Treg suppression (Figure 2).
Figure 1.
Contrasting effects of pan-HDACi and class I-specific HDACi therapy on colitis. Mice (n = 10/group) receiving DSS plus TsA, SAHA, MS275, or DMSO carrier alone were evaluated daily for (A) weight loss (mean ± SD), (B) bleeding, and (C) stool consistency. Mice receiving TsA or SAHA had less weight loss, stool blood, or diarrhea (P < .01) than mice treated with MS275 or DMSO. (D) H&E-stained colonic sections (original magnification, ×125) from mice receiving DSS and treatment for 10 days with TsA dissolved in DMSO or DMSO alone (representative of n = 6/group). (E) qPCR analysis of colons from normal mice or those receiving DSS or DSS plus TsA, SAHA, or MS75 at day 10 after initiation of DSS therapy; data (mean ± SD, n = 6/group) are expressed as fold increase compared with levels in normal WT colons and were normalized to 18S; Foxp3, P < .01 for TsA or SAHA vs DMSO or MS275; IL-10, P < .05 for TsA and P < .01 for SAHA vs control or MS275; IL-2,P < .005 for TsA or SAHA vs control or MS275; IFN-γ, P < .02 for TsA or SAHA vs control or MS275; TNF-α, P < .01 for TsA or SAHA vs control of MS275; and TGF-β, P < .05 for SAHA vs MS275; differences in TGF-β expression between DSS or TsA were not significant (P < .05). (F) Intracellular staining of lamina propria mononuclear cells on day 10 shows a decreased proportion of cells expressing TNF-α (P < .05) and an increased proportion of cells expressing IL-10 (P < .01) in mice receiving TSA or SAHA (representative of n = 6 mice/group).
Figure 2.
Contrasting effects of pan-HDACi and class I-specific HDACi therapy on Treg function in vivo. After 7 days of in vivo therapy in normal C57BL/6 mice using MS275 or SAHA, CD4+CD25+ cells were isolated using magnetic beads and added to cultures of CFSE-labeled CD4+CD25− T cells that were activated with CD3 mAb and irradiated antigen-presenting cells. The percentage of proliferating T cells is shown in each plot, and data are representative of 3 experiments.
Pan-HDACi Therapy Can Prevent or Cure T Cell-Induced Colitis
Because DSS can induce an attenuated colitis in T cell-deficient mice,21 the beneficial effects of pan-HDACi therapy on DSS colitis could reflect T cell-independent effects. We therefore undertook adoptive transfer of CD4+CD25− T cells into Rag1−/− mice to investigate the effects of pan-HDACi use on either development of colitis or the course of an existing colitis. For the prevention study, Rag1−/− mice were injected with 1 × 106 CD4+CD25− T cells and treated daily with TsA, SAHA, MS275, or DMSO carrier for 14 days postcell transfer. Pan-HDACi-treated mice did not develop colitis or weight loss, in contrast to MS275- or DMSO-treated mice (Figure 3A). Mesenteric lymph nodes isolated from adoptively transferred mice showed 12% of T cells in DMSO-treated mice were CD4+Foxp3+, whereas the proportions of CD4+Foxp3+ cells were almost doubled in mice treated with TsA (23%, Figure 3B) or SAHA (20%, data not shown). MS275-treated mice developed colitis and showed comparable numbers of CD4+Foxp3+ cells to DMSO-treated mice (data not shown). Finally, TsA-treated mice showed a modest increase in the proportion of lamina propria CD4+Foxp3+ cells (18%) as compared with DMSO-treated mice (11%, data not shown).
Figure 3.
Pan-HDACi therapy and prevention or treatment of T cell-induced colitis. Panels A and B show prevention data, and panels C and E involve treatment once colitis had developed. (A) Rag1−/− mice (mean ± SD, n = 8/group) adoptively transferred with 1 × 106 C57BL/6 CD4+CD25− cells and treated with TsA (yellow) or SAHA (red) did not develop colitis, in contrast to mice treated with MS275 (green, P < .03) or DMSO alone (blue, P < .01). (B) Flow cytometry of mesenteric LN cells harvested at 14 days showed increased Foxp3+ Tregs in TsA-treated mice; data representative of n = 8 mice/group. (C) Rag1−/− mice (mean ± SD, n = 8/group) adoptively transferred with 1 × 106 C57BL/6 CD4+CD25− cells developed clinical evidence of colitis and >20% weight loss by ~55 days posttransfer. In contrast to use of DMSO alone (blue), mice treated with TsA (red) showed clinical improvement (P < .005). (D) Flow cytometry of mesenteric LN cells harvested at 14 days after onset of therapy showed increase in Foxp3+ Tregs in TsA-treated mice; data representative of n = 8/group. (E and F) Representative histology (n = 8/group, Alcian blue; original magnification, ×125) after 3 weeks of therapy with DMSO (E) vs TsA (F), showing differential effects on leukocyte infiltration, goblet cell loss, and submucosa thickening.
For the treatment study, Rag1−/− mice were injected with 1 × 106 CD4+CD25− cells and followed until colitis developed at about 50 days, whereupon mice were treated with TsA or DMSO for 21 days. TsA-treated mice gradually regained their weight, and clinical features of colitis improved, in contrast to DMSO-treated mice that continued to lose weight and showed persistent diarrhea (Figure 3C). Mesenteric lymph nodes from TsA-treated mice again showed approximately twice as many CD4+Foxp3+ cells as in DMSO-treated mice (Figure 3D). Histologic analysis confirmed the beneficial effects of TsA on development of colitis. Whereas colons isolated from DMSO-treated mice had widespread mucosal inflammatory infiltrates, loss of goblet cells, and thickened submucosa (Figure 3E), TsA therapy led to a marked decrease in leukocyte infiltration, preservation of goblet cells, and lack of submucosal infiltration or thickening (Figure 3F). However, treatment of Rag−/− mice with TsA after the adoptive transfer of CD4+CD25− T cells from Scurfy mice, which have a frameshift mutation in the forkhead DNA binding domain in Foxp3, did not protect mice from development of colitis (Supplementary Figure 1). Hence, the efficacy of HDACi therapy in colitis is dependent on the presence of functionally competent Foxp3+ Tregs.
HDAC9 and Colitis
The contrasting effects of a pan-HDACi and a class I-specific HDACi on development of colitis, and on Treg numbers and function in vitro and in vivo, point to a role for class II HDACs in the pathogenesis of colitis. This was supported by assessment of HDAC mRNA expression in inflamed vs normal colons (Figure 4A). Whereas HDAC1 was not detected and smooth muscle-specific HDAC8 was increased by 3-fold, the ubiquitous class I HDACs, HDAC2, and HDAC3 were increased by only 2-fold in colons from DSS-treated mice. By contrast, whereas HDAC5 expression was unchanged, expression of the other class II HDACs was increased 4-fold for HDAC4, 8-fold for HDAC6 and HDAC7, and 12-fold for HDAC9.
Figure 4.
HDAC9 and colitis. (A) qPCR analysis of HDAC expression in colons of DSS-treated mice; data (mean ± SD, n = 6/group) expressed as fold increase over levels in normal colons (after normalization to 18S). (B) Rag−/− mice were injected with 1 × 106 WT CD4+CD25− naïve T cells, and weights (mean ± SD, n = 8/group) were measured at least twice weekly. Once colitis had developed, mice were injected with 5 × 105 CD4+CD25+ cells from naïve C57BL6 (red) or HDAC9−/− mice (green) or with control naïve CD4+CD25− cells (blue); **P < .005 for WT Tregs vs Teff cells and *P < .01 for HDAC9−/− vs WT Tregs. (C) Flow cytometric quantitation of splenocyte and mesenteric lymph node (MLN) cell numbers (mean ± SD, n = 4 mice/group) at 21 days after injection of CD4+CD25+ cells from HDAC9−/− vs WT Tregs (*P < .01). (D–F) Representative histology (n = 8/group) at 21 days posttransfer of indicated cell populations (Alcian blue; original magnification, ×100).
We have reported that HDAC9−/−, B6/129 mice were resistant to DSS-induced colitis,7 but whether this was due to lack of HDAC9 in a specific cell type in vivo, eg, Tregs, was not determined. We therefore assessed HDAC9 involvement in DSS colitis using mice that we had backcrossed for 8 generations to the C57BL/6 strain. In baseline studies, we found that, compared with WT C57BL/6 controls, Tregs isolated from HDAC9−/− mice had enhanced suppressive functions (Supplementary Figure 2). In addition, compared with WT C57BL/6 mice, colons from control, unmanipulated HDAC9−/− mice had increased expression of Foxp3 and interleukin (IL)-10 mRNA but unchanged levels of IL-2 and interferon (IFN)-γ mRNA (Supplementary Figure 3A). Consistent with these data, and unlike WT C57BL/6 mice, HDAC9−/− mice were remarkably resistant to the development of DSS colitis (Supplementary Figure 3B).
To assess directly the contribution of HDAC9 to Treg function during T cell-induced colitis, Rag1−/− mice were adoptively transferred with 1 × 106 CD4+CD25− cells isolated from the mesenteric lymph nodes of WT C57BL/6 mice. Once colitis developed, mice were injected IP with 5 × 105 CD4+CD25+ Tregs from WT or HDAC9−/− C57BL/6 mice or with the same number of CD4+CD25− T cells and followed for a further 3 weeks. HDAC9−/− Tregs were significantly more effective than WT Tregs in promoting weight gain and reducing diarrhea (Figure 4B). Consistent with these data, HDAC9−/− Tregs dampened the homeostatic proliferation of adoptively transferred CD4+ T cells in spleens and mesenteric lymph nodes more effectively than WT Tregs (Figure 4C). Histologic analysis showed that, whereas colons from mice receiving WT CD4+CD25− T cells displayed dense inflammatory infiltrates and loss of goblet cells (Figure 4D), mice receiving WT CD4+CD25− T cells plus WT Tregs had considerably less leukocyte infiltration (Figure 4E). The transfer of HDAC9−/− Tregs led to still further improvement with only minor colonic infiltrates and preservation of goblet cells (Figure 4F). Next, we evaluated the development of colitis using the adoptive transfer of CD4+CD25− cells from HDAC9−/− vs WT mice (Supplementary Figure 4). Both groups developed colitis, but mice injected with HDAC9−/− T cells developed colitis and associated weight loss more slowly than WT controls (P < .01 after 20 days onwards). These data from colitis models indicate that Foxp3+ Tregs lacking HDAC9 exhibit biologically relevant increased suppressive functions in vivo.
Knockdown of HDAC9 Enhances Treg Function
To assess any direct effects of HDAC9 on Foxp3 expression and Treg function, without the possibly confounding effects arising from the absence of HDAC9 during Treg development, we used siRNA to knockdown expression of HDAC9 in WT Tregs. HDAC9 knockdown, to ~10% of the HDAC9 levels expressed in control cells exposed to a scrambled siRNA sequence, increased Foxp3 expression by >6-fold and CTLA4 expression by 9-fold, whereas expression of PD-1 and other genes, including IFN-γ (data not shown), was unaffected (Figure 5A). HDAC9 siRNA also significantly enhanced Treg suppression whether either absolute numbers or percentages of CFSE-labeled effector T cells were considered (Figure 5B). The effects of siRNA knockdown of HDAC9 on Foxp3-dependent Treg function were less potent than that seen using HDAC9−/− Tregs, likely because of the inability to knock down HDAC9 expression in Tregs throughout the 72-hour in vitro suppression assays. However, these findings point to the value of selective targeting of HDAC9 as a potential mechanism to therapeutically enhance Treg function.
Figure 5.
HDAC9 knockdown and Foxp3+ Treg suppression. (A) qPCR analysis (mean ± SD, n = 4/group) of effects of HDAC9 knockdown on gene expression in resting and activated in naïve CD4+CD25+ cells; data normalized relative to 18S (*P < .05 and **P < .01 vs use of control siRNA). (B) Effects of HDAC9 knockdown on Treg function in vitro; data shown as number of proliferating cells (upper panel) and as percentage proliferating cells at each ratio (lower panel); data representative of 4 experiments (*P < .05 vs corresponding control siRNA value).
Increased Peripheral Conversion of HDAC9−/− CD4+CD25− T Cells to Foxp3+ Cells
Naïve CD4+CD25− Foxp3+ murine T cells cultured with IL-2 and TGF-β and undergoing T-cell receptor stimulation can become Foxp3+,22 and peripheral conversion of CD4+CD25− Foxp3− murine T cells can occur within the gut lamina propria under the influence of TGF-β and retinoic acid.23 Both types of conversion produce Foxp3+ cells that can suppress murine T-cell proliferation.22,23 Data in Figure 3 are consistent with local conversion following adoptive transfer of naive T cells to immunodeficient mice. We therefore assessed whether HDAC9 could affect peripheral conversion by comparing Foxp3 induction in CD3/CD28 mAb-stimulated WT and HDAC9−/− CD4+CD25− T cells cultured in vitro for 3 days in the presence of 10 U/mL of IL-2 and varying concentrations of TGF-β. No conversion was seen without TGF-β, but, at each concentration of TGF-β tested, HDAC9−/− cells converted to a greater extent than WT cells and in a TGF-β dose-dependent manner (Figure 6A).
Figure 6.
Conversion of HDAC9−/− vs WT CD4+CD25− cells. (A) WT and HDAC9−/− CD4+CD25− cells were cultured for 3 days with plate-bound CD3 and CD28 mAbs, plus IL-2 (10 U/mL) and increasing concentrations of TGF-α; the percentages of CD4+Foxp3+ cells are shown and are representative of 4 experiments. (B) WT and HDAC9−/− CD4+CD45RBhi CD4+CD25− cells (1 × 106 cells) were injected IP into Rag1−/− mice; spleen, mesenteric lymph node (MLN), and peripheral lymph node (LN) samplers harvested at the weeks indicated were analyzed by flow cytometry; data representative of 4 experiments.
To evaluate conversion in vivo, we injected Rag1−/− mice with purified CD4+CD45RBhi cells from HDAC9−/− and WT C57BL/6 mice. CD4+CD45RBhi cells were isolated by fluorescence-activated cell sorting and contained approximately 0.5%–1.0% Foxp3+ cells. We serially assessed Foxp3+ cell numbers by flow cytometry and immunohistochemistry.7 In contrast to WT controls, by 7 days posttransfer, Foxp3+ HDAC9−/− T cells began to develop in greater numbers in the colonic lamina propria (Supplementary Figure 5) and mesenteric lymph nodes (Figure 6B), followed by their spread to peripheral lymph nodes as seen by 14 days posttransfer (Figure 6B). However, by 1 month posttransfer, the numbers of Foxp3+ HDAC9−/− Tregs in lymphoid tissues were broadly equal to that of mice receiving transfer of WT cells, consistent with an ability of both HDAC9−/− and WT Tregs to regulate homeostatic development of Tregs by peripheral conversion.
HDAC9−/− Tregs Have Increased HSP70 Expression, Proliferation, and Cell Survival
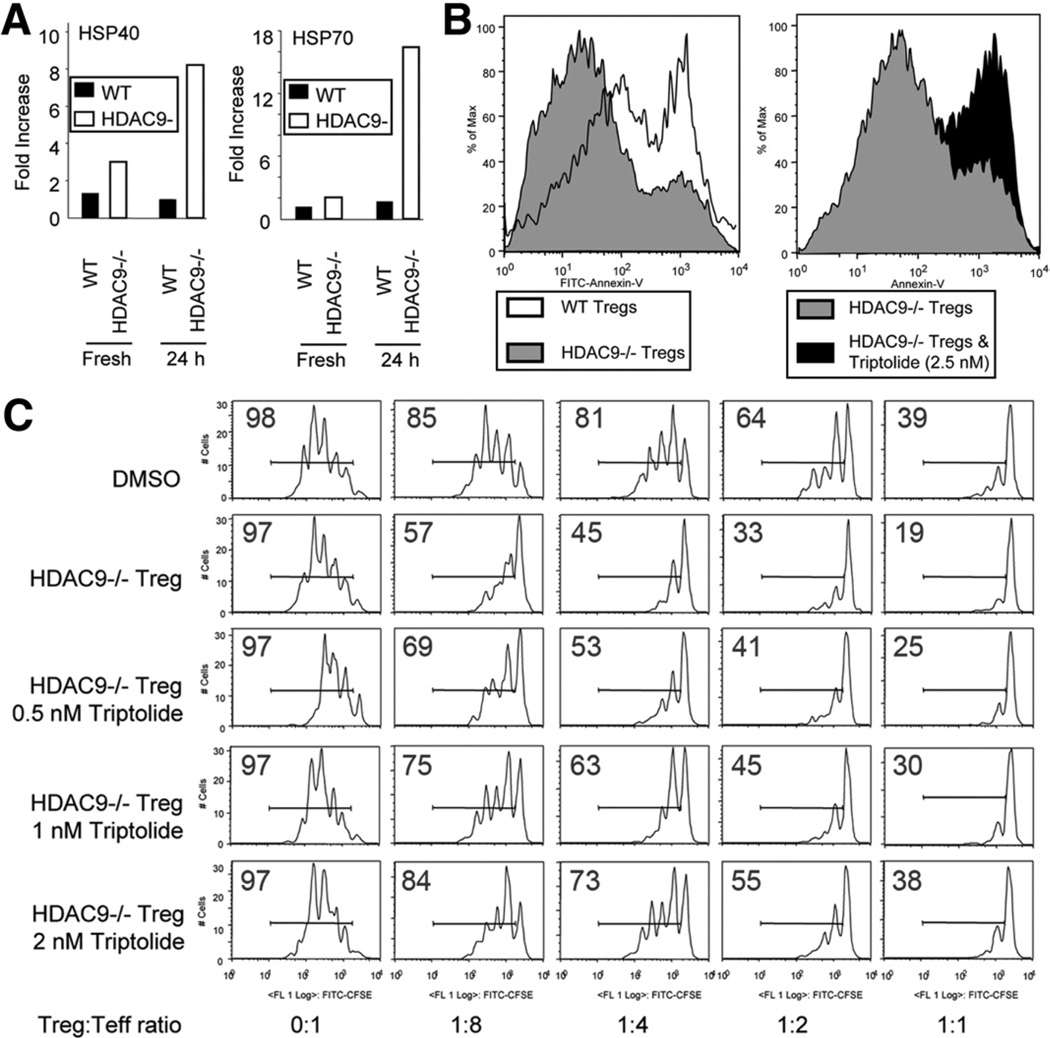
Microarray studies indicated that compared with WT Tregs, Tregs from HDAC9−/− C57BL/6 mice had increased levels of HSP40 and HSP70. These data were confirmed by qPCR studies in which baseline levels of HSP40 and HSP70 were approximately 2-fold greater in HDAC9−/− than WT Tregs, and, with T-cell receptor activation, HDAC9 expressed ~18-fold more HSP70 and 8-fold more HSP40 than WT Tregs (Figure 7A). To assess the biologic significance of HSP70 expression in Tregs, we tested addition of an HSP70 inhibitor, the diterpene triepoxide (Triptolide; Tocris Bioscience, Ellisville, MO),24 to Tregs stimulated in vitro for 24 hours with plate-bound CD3 mAb and IL-2. HDAC9−/− Tregs were more resistant than WT Tregs to development of apoptosis, but resistance was abrogated by even very low levels of Triptolide (Figure 7B). Triptolide also induced apoptosis of WT Tregs (Supplementary Figure 6). HSP70 was important to the enhanced suppressive function of HDAC9−/− vs WT Tregs because addition of Triptolide decreased HDAC9−/− Treg function in a dose-dependent manner to levels comparable with that of WT Tregs (Figure 7C). Besides altering the suppressive function of Tregs, inhibition of HSP70 may affect their survival. This was seen in our standard Treg assays by gating on the population of CD4+ cells that are CFSE negative. CFSE-negative WT cells showed decreased survival when compared with untreated HDAC9−/− Treg cells (data not shown), leading us to study further the ability of HDAC9−/− Tregs to survive and proliferate more than WT Tregs.
Figure 7.
Pharmacologic effects of HSP70 inhibition in Tregs. (A) qPCR analysis of HSP40 and HSP70 expression by resting or activated Tregs (using CD3/CD28 mAbs) from HDAC9−/− and WT mice; data shown as fold increase, normalized relative to 18S and representative of 3 experiments. (B) Flow cytometric analysis of WT or HDAC9−/− Treg cell apoptosis as shown by Annexin-V staining. Left panel shows Annexin-V staining of the cell populations after culture for 24 hours with CD3 mAb and IL-2. Right panel shows corresponding analysis of HDAC9−/− cells cultured with or without addition of Triptolide (data representative of 3 experiments). (C) Inhibitory effects of increasing concentrations of Triptolide on the functions of HDAC9−/− Tregs in vitro using a standard Treg suppression assay involving proliferation of CFSE-labeled T cells; percentage of CFSE+ cells in each well is indicated (data representative of 3 experiments).
Comparison of the resistance of activated WT vs HDAC9−/− Tregs to the development of apoptosis showed that HDAC9−/− Tregs had a major survival advantage, as seen in cultures of Tregs for up to 6 days in the presence of plate-bound CD3 mAb, IL-2, and TGF-β (Figure 8A). Increased Treg activity in vivo is commonly associated with greater numbers of Tregs, leading to a change in the Treg to Teff cell ratio. Similarly, enhanced Treg function in the standard Treg assay, in which fixed ratios of Tregs and proliferating T cells are studied, is thought to reflect increased production of inhibitory surface molecules and/or suppressive cytokines. Given evidence that Tregs from HDAC9−/− mice express high levels of HSP70, we tested whether HDAC9−/− Tregs were able to proliferate better in vitro than WT cells. We evaluated the ability of the CFSE-labeled Tregs to proliferate in vitro in response to soluble CD3 mAb plus APC, ie, under conditions like those of the suppression assay. Flow cytometric analysis at 48 hours showed negligible proliferation of Tregs in the absence of IL-2, whereas, in the presence of IL-2, HDAC9−/− Tregs proliferated ~4-fold more than WT (Figure 8B). We assessed Foxp3+ cell survival at the end of a standard Treg suppression assay by harvesting the cells after 3 days and analyzing the numbers of CD4+ Foxp3+ CFSE-labeled Tregs. The proportions of Foxp3+ HDAC9−/− Tregs were 1.5- to 2-fold increased over that of WT Tregs (Figure 8C), and this increase was also reflected following determination of absolute Treg cell numbers (data not shown). Hence, HDAC9−/− Tregs appear to have HSP70-dependent survival and/or proliferative advantages over their WT counterparts, both of which could contribute to their enhanced suppressive functions.
Figure 8.
HSP70 and Foxp3 expression in Tregs. (A) Comparison of Treg conversion at 3 and 6 days of culture of HDAC9−/− and WT T cells in presence of CD3 mAb (1 µg/mL), TGF-β (3 ng/mL), and IL-2 (5 U/mL). (B) Comparison of proliferation of WT and HDAC9−/− CFSE-labeled Tregs cultured for 3 days with CD3 mAb and irradiated APC, with or without IL-2 (5 U/mL) as indicated. (C) Assessment of Treg proliferation under Treg assay-like conditions. CFSE-labeled CD4+CD25+ T cells and unlabeled CD4+CD25− T cells (Teff) cells were stimulated for 72 hours with CD3 mAb (0.5 µg/mL) plus 4 × 105 irradiated APC; percentage of CFSE-labeled CD4+CD25+ T cells at each ratio of Teff to Treg is indicated. (D) Immunoprecipitation of HSP70 from WT Tregs leads to coprecipitation of Foxp3 (47 kilodaltons, lowermost molecular weight marker). (E) Increased coprecipitation of HSP70 and Foxp3 using HDAC9−/− vs WT Tregs and lack of coprecipitation of HSP70 and Foxp3 when using CD4+CD25− T cells. In panels A–E, data are representative of 3 experiments.
In biochemical studies, immunoprecipitation of HSP70 from Tregs led to pull down of Foxp3 (Figure 8D). Coprecipitation of HSP70 and Foxp3 was increased using HDAC9−/− vs WT Tregs, and no such coprecipitation was observed using WT CD4+CD25− T cells (Figure 8E). The data indicate an unexpected interaction of intracellular HSP70 and Foxp3 in Tregs, and this binding is increased in HDAC9−/− Tregs. Last, we assessed the effects of overexpression of HSP70 on Treg function using cells isolated from HSP70Tg mice. HSP70Tg Tregs proved more suppressive than their WT Treg counterparts using the standard suppression assay (Supplementary Figure 7). Hence, HSP70 is physically linked with Foxp3 and promotes the suppressive functions of Foxp3+ Tregs.
Discussion
Whereas biochemical studies have shown that the exposure of human and murine Tregs to pan-HDACi such as TsA or SAHA promotes Foxp3 acetylation, thereby increasing the binding of Foxp3 to DNA and enhancing Treg suppressive functions,7,8 the therapeutic consequences of this information are just beginning to be explored. We evaluated aspects of HDACi use in colitis models in the current study because HDACi therapy can protect against development of murine colitis7,13 and the beneficial effects of HDACi in murine DSS colitis are dependent on the presence of Foxp3+ Tregs.7 We now show that pan-HDACi but not class I-specific HDACi therapy enhances the conversion of conventional murine T cells to Foxp3+ Tregs and increases their suppressive functions. These data suggest a key role of one or more non-class I HDACs in controlling Treg functions. Because the HDACi tested do not target the class III HDACs, and the main class IIb HDAC (HDAC6) is primarily cytoplasmic, we focused on the class IIa HDACs (HDAC4, 5, 7, and 9) that shuttle from the nucleus to the cytoplasm upon phosphorylation.6 These findings are encouraging for efforts to develop HDACi to treat autoimmunity because class I HDACs are ubiquitous and vital for cell functions, such that their therapeutic targeting is largely restricted to oncology. By contrast, class IIa HDACs display tissue-specific patterns of expression, and class IIa HDAC-specific inhibitors are beginning to be identified;25,26 these may have less toxicity and hence more applicability in nononcology settings than pan-HDACi or class I-selective HDACi.
Of the 4 class IIa HDACs, HDAC9 is the one most highly expressed in Foxp3+ Tregs, and mice deficient in HDAC9 have increased numbers of Tregs.7 Moreover, on a per cell basis, Tregs of HDAC9−/− mice, whether on a mixed B6/1297 or, as shown here, on a pure C57BL/6 background, are more suppressive than WT Tregs. In the current study, we noted that class II HDACs were up-regulated to a greater extent in colitic mice than class I HDACs and that HDAC9−/− mice were more resistant to colitis than WT mice. These data might appear counterintuitive. However, HDAC9 normally suppresses Treg activation, but, upon T-cell receptor stimulation of Tregs, HDAC9 is phosphorylated and exported from the nucleus, allowing Foxp3 acetylation, DNA binding, and Treg suppressive functions to occur.7 Hence, targeting of HDAC9 expression in Tregs, by homologous recombination, siRNA knockdown, or pharmacologic means, appears a worthwhile new strategy for therapy of experimental colitis.
Microarray and confirmatory qPCR studies of WT and HDAC9−/− Tregs highlighted the significant up-regulation of HSP70, and its associated HSP subfamily member HSP40, in HDAC9−/− Tregs. HSP molecules are highly evolutionarily conserved and functionally act as molecular chaperones and folding catalysts. The closely related stress-induced HSP70 genes HSP70-1a and HSP70-1b are key to cellular responses to potentially harmful protein aggregates and protection against stress and apoptosis induced by heat, ischemia, oxidative stress, cytokines, and growth factor starvation.27 Previous genetic and murine data implicate HSP70 overexpression in protection from IBD28,29 but have not identified the cell types responsible for this protective effect. Our data indicate that HSP70 up-regulation by HDAC9−/− Tregs may be a prime mechanism for the increased resistance of HDAC9−/− mice to the development of colitis. In particular, HDAC9−/− Tregs are resistant to apoptosis, and blockade of HSP70 restores the susceptibility of HDAC9−/− Tregs to apoptosis and decreases their suppressive function. Conversely, overexpression of HSP70 enhances the suppressive functions of WT Tregs, similarly to that seen using HDAC9−/− Tregs. We also found a physical association of Foxp3 and HSP70 in Tregs. However, further studies will be required to clarify at several points. First, we do not yet know whether enhanced survival or proliferation plays a dominant role in mediating the increased potency of HDAC9−/− Tregs or whether these roles are equally important. Second, we do not yet know whether Foxp3 and HSP70 are directly linked or whether they coprecipitate as a result of both binding to one or more additional proteins. Third, whereas HSP70 may act as a chaperone for Foxp3, we do not yet know what other functions this HSP may serve in Treg cells.
We are currently studying the mechanism responsible for increased expression of HSP70 expression in HDAC9−/− Tregs. HSP70 is induced by HSF-1, and trimerization of HSF-1 is required for optimal HSP70 induction.30 Trimerization of HSF-1 and its DNA binding are modulated by acetylation of HSF-1.31 Further in vitro studies will be required to determine whether HDAC9 acts directly, or via recruitment of a class I HDAC with catalytic activity,32 to control HSF-1 acetylation or access of HSF-1 to chromatin. Similarly, we will need to determine whether HSP-70 acts by promoting the maturation of newly synthesized Foxp3 or via other effects. Last, in vivo studies, for example using DSS or other agents in Rag−/− mice adoptive transferred with Tregs lacking HDAC9, HSP70, or HDAC9 and HSP70, will be undertaken to explore further the effects of HDAC targeting and beneficial outcomes in these colitis models.
Collectively, our data point to a new mechanism for enhanced Treg suppression, namely the increased ability to survive and/or proliferate as a result of HDAC9 knockdown or deletion, and with the consequent induction of HSP70. We conclude that developing small molecule inhibitors or other strategies to decrease HDAC9 function, or to further enhance HSP70 expression, may be of therapeutic import for the management of IBD and potentially other autoimmune diseases.
Supplementary Material
Acknowledgments
Funding
Supported by the National Institutes of Health grants K08DK080189 (to E.F.d.Z.), R01AI073938 and P01AI073489 (to W.W.H.), and CDNHNF/NASPGHAN Fellow to Faculty Grant (to E.F.d.Z.).
Abbreviations used in this paper
- CFSE
carboxyfluoroscein succinimidyl ester
- HDAC
histone/protein deacetylase
- HDACi
HDAC inhibitor
- HSP
heat shock protein
- IBD
inflammatory bowel disease
- pPCR
quantitative polymerase chain reaction
- SAHA
suberoylanilide hydroxamic acid
- siRNA
small inhibitory RNA
- Treg
T regulatory cell
- TsA
Trichostatin-A
Footnotes
Supplementary Materials
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at doi:10.1053/j.gastro.2009.10.037.
Conflicts of interest
The authors disclose no conflicts.
References
- 1.Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 2002;347:911–920. doi: 10.1056/NEJMra020100. [DOI] [PubMed] [Google Scholar]
- 2.Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu Rev Immunol. 2002;20:495–549. doi: 10.1146/annurev.immunol.20.100301.064816. [DOI] [PubMed] [Google Scholar]
- 3.Singh B, Read S, Asseman C, et al. Control of intestinal inflammation by regulatory T cells. Immunol Rev. 2001;182:190–200. doi: 10.1034/j.1600-065x.2001.1820115.x. [DOI] [PubMed] [Google Scholar]
- 4.Mottet C, Uhlig HH, Powrie F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J Immunol. 2003;170:3939–3943. doi: 10.4049/jimmunol.170.8.3939. [DOI] [PubMed] [Google Scholar]
- 5.Duchmann R, Kaiser I, Hermann E, et al. Tolerance exists towards resident intestinal flora but is broken in active inflammatory bowel disease (IBD) Clin Exp Immunol. 1995;102:448–455. doi: 10.1111/j.1365-2249.1995.tb03836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoo CB, Jones PA. Epigenetic therapy of cancer: past, present and future. Nat Rev Drug Discov. 2006;5:37–50. doi: 10.1038/nrd1930. [DOI] [PubMed] [Google Scholar]
- 7.Tao R, de Zoeten EF, Ozkaynak E, et al. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat Med. 2007;13:1299–1307. doi: 10.1038/nm1652. [DOI] [PubMed] [Google Scholar]
- 8.Li B, Samanta A, Song X, et al. FOXP3 interactions with histone acetyltransferase and class II histone deacetylases are required for repression. Proc Natl Acad Sci U S A. 2007;104:4571–4576. doi: 10.1073/pnas.0700298104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gu W, Roeder RG. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell. 1997;90:595–606. doi: 10.1016/s0092-8674(00)80521-8. [DOI] [PubMed] [Google Scholar]
- 10.Juan LJ, Shia WJ, Chen MH, et al. Histone deacetylases specifically down-regulate p53-dependent gene activation. J Biol Chem. 2000;275:20436–20443. doi: 10.1074/jbc.M000202200. [DOI] [PubMed] [Google Scholar]
- 11.Johnstone RW. Histone-deacetylase inhibitors: novel drugs for the treatment of cancer. Nat Rev Drug Discov. 2002;1:287–299. doi: 10.1038/nrd772. [DOI] [PubMed] [Google Scholar]
- 12.Reddy P, Maeda Y, Hotary K, et al. Histone deacetylase inhibitor suberoylanilide hydroxamic acid reduces acute graft-vs-host disease and preserves graft-vs-leukemia effect. Proc Natl Acad Sci U S A. 2004;101:3921–3926. doi: 10.1073/pnas.0400380101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glauben R, Batra A, Fedke I, et al. Histone hyperacetylation is associated with amelioration of experimental colitis in mice. J Immunol. 2006;176:5015–5022. doi: 10.4049/jimmunol.176.8.5015. [DOI] [PubMed] [Google Scholar]
- 14.Zhang CL, McKinsey TA, Chang S, et al. Class II histone deacetylases act as signal-responsive repressors of cardiac hypertrophy. Cell. 2002;110:479–488. doi: 10.1016/s0092-8674(02)00861-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marber MS, Mestril R, Chi SH, et al. Overexpression of the rat inducible 70-kD heat stress protein in a transgenic mouse increases the resistance of the heart to ischemic injury. J Clin Invest. 1995;95:1446–1456. doi: 10.1172/JCI117815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Melgar S, Karlsson A, Michaelsson E. Acute colitis induced by dextran sulfate sodium progresses to chronicity in C57BL/6 but not in BALB/c mice: correlation between symptoms and inflammation. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1328–G1338. doi: 10.1152/ajpgi.00467.2004. [DOI] [PubMed] [Google Scholar]
- 17.Mudter J, Wirtz S, Galle PR, et al. A new model of chronic colitis in SCID mice induced by adoptive transfer of CD62L+ CD4+ T cells: insights into the regulatory role of interleukin-6 on apoptosis. Pathobiology. 2002;70:170–176. doi: 10.1159/000068150. [DOI] [PubMed] [Google Scholar]
- 18.de Jong D, Boot H, Taal B. Histological grading with clinical relevance in gastric mucosa-associated lymphoid tissue (MALT) lymphoma. Recent Results Cancer Res. 2000;156:27–32. doi: 10.1007/978-3-642-57054-4_4. [DOI] [PubMed] [Google Scholar]
- 19.Sato T, Kanai T, Watanabe M, et al. Hyperexpression of inducible costimulator and its contribution on lamina propria T cells in inflammatory bowel disease. Gastroenterology. 2004;126:829–839. doi: 10.1053/j.gastro.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 20.Hu E, Dul E, Sung CM, et al. Identification of novel isoform-selective inhibitors within class I histone deacetylases. J Pharmacol Exp Ther. 2003;307:720–728. doi: 10.1124/jpet.103.055541. [DOI] [PubMed] [Google Scholar]
- 21.Axelsson LG, Landstrom E, Goldschmidt TJ, et al. Dextran sulfate sodium (DSS) induced experimental colitis in immunodeficient mice: effects in CD4(+)-cell depleted, athymic and NK-cell depleted SCID mice. Inflamm Res. 1996;45:181–191. doi: 10.1007/BF02285159. [DOI] [PubMed] [Google Scholar]
- 22.Chen W, Jin W, Hardegen N, et al. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun CM, Hall JA, Blank RB, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Westerheide SD, Kawahara TL, Orton K, et al. Triptolide, an inhibitor of the human heat shock response that enhances stress-induced cell death. J Biol Chem. 2006;281:9616–9622. doi: 10.1074/jbc.M512044200. [DOI] [PubMed] [Google Scholar]
- 25.Mai A, Massa S, Pezzi R, et al. Class II (IIa)-selective histone deacetylase inhibitors. 1. Synthesis and biological evaluation of novel (aryloxopropenyl)pyrrolyl hydroxyamides. J Med Chem. 2005;48:3344–3353. doi: 10.1021/jm049002a. [DOI] [PubMed] [Google Scholar]
- 26.Chang S, Bezprozvannaya S, Li S, et al. An expression screen reveals modulators of class II histone deacetylase phosphorylation. Proc Natl Acad Sci U S A. 2005;102:8120–8125. doi: 10.1073/pnas.0503275102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daugaard M, Rohde M, Jaattela M. The heat shock protein 70 family: highly homologous proteins with overlapping and distinct functions. FEBS Lett. 2007;581:3702–3710. doi: 10.1016/j.febslet.2007.05.039. [DOI] [PubMed] [Google Scholar]
- 28.Nam SY, Kim N, Kim JS, et al. Heat shock protein gene 70-2 polymorphism is differentially associated with the clinical phenotypes of ulcerative colitis and Crohn’s disease. J Gastroenterol Hepatol. 2007;22:1032–1038. doi: 10.1111/j.1440-1746.2007.04927.x. [DOI] [PubMed] [Google Scholar]
- 29.Ohkawara T, Nishihira J, Takeda H, et al. Protective effect of geranylgeranylacetone on trinitrobenzene sulfonic acid-induced colitis in mice. Int J Mol Med. 2006;17:229–234. [PubMed] [Google Scholar]
- 30.Sorger PK. Heat shock factor and the heat shock response. Cell. 1991;65:363–366. doi: 10.1016/0092-8674(91)90452-5. [DOI] [PubMed] [Google Scholar]
- 31.Westerheide SD, Anckar J, Stevens SM, Jr, et al. Stress-inducible regulation of heat shock factor 1 by the deacetylase SIRT1. Science. 2009;323:1063–1066. doi: 10.1126/science.1165946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fischle W, Dequiedt F, Hendzel MJ, et al. Enzymatic activity associated with class II HDACs is dependent on a multiprotein complex containing HDAC3 and SMRT/N-CoR. Mol Cell. 2002;9:45–57. doi: 10.1016/s1097-2765(01)00429-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.