Abstract
A stereoselective halo-etherification of chiral enamides is described here. This work provides an approach to halogen containing cyclic ethers and reveals further mechanistic insights to the chemistry of chiral enamides.
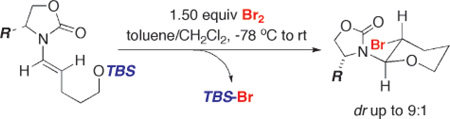
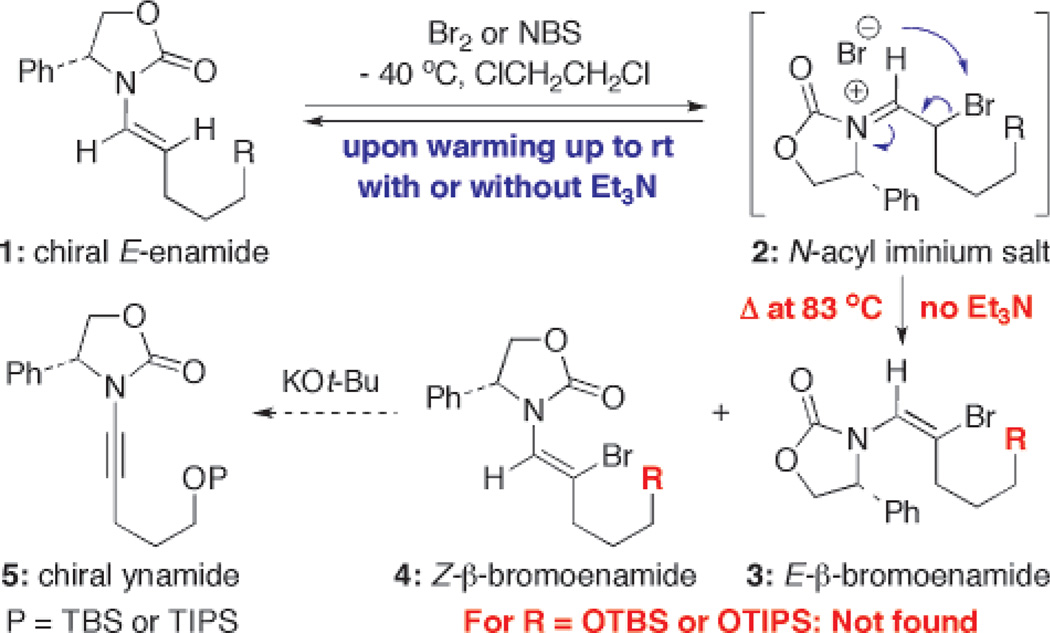
With elegant advances in metal-catalyzed N-alkenylations,1–4 chiral enamides should emerge as versatile building blocks for developing stereoselective synthetic methods.5–12 We encountered an interesting phenomenon involving enamides when we attempted years ago to transform enamides 1 to chiral ynamides13 via a sequence of bromination–elimination of the intermediate β-bromo-enamides 3 and 4 [Scheme 1].14 Two intriguing observations were made. First, the bromination behaved differently from a standard bromination of olefins. It was reversible with or without an amine base, and 3 and 4 were obtained only if the reaction was heated at ≥80 °C without base. Although upon its addition the bromine color disappeared rapidly in a colormetric titration manner, the color returned upon warming to rt. Second, when R is a TBSO or TIPSO group, the bromination led to a completely different product, thereby failing to access ynamides 5 via this protocol. While we suspected the N-acyl iminium salt intermediate 2 to be responsible for the reversibility issue [see blue arrows], we recently resolved the mystery product in the second observation. We reported here a stereoselective halo-etherification of chiral enamides in the synthesis of halogen containing cyclic ethers.
Scheme 1.
An Arrested Bromination–Elimination of Enamides
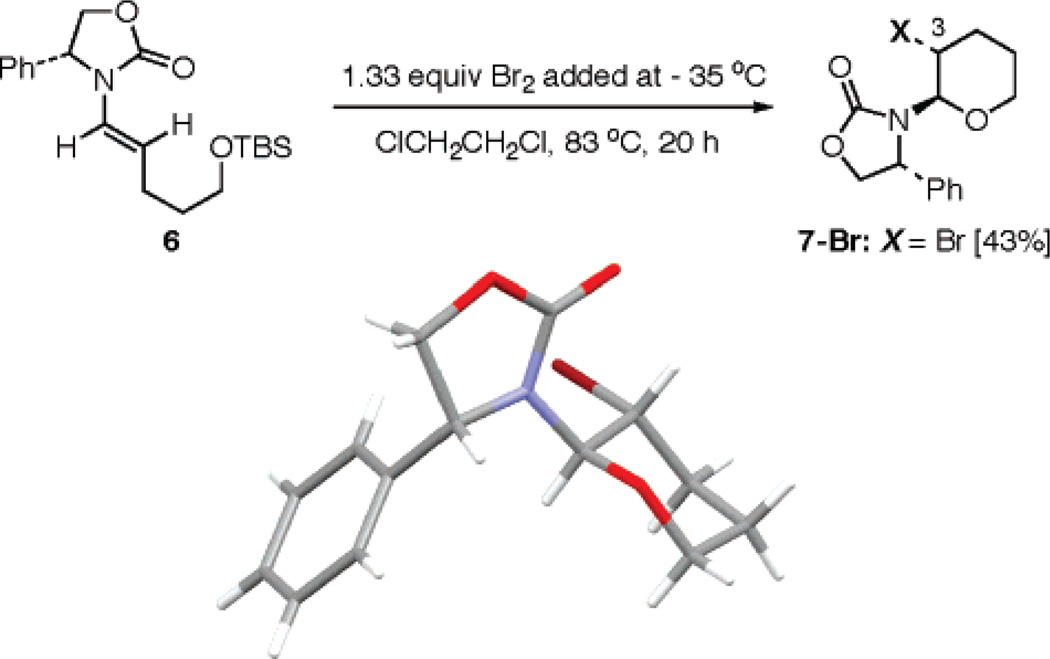
When chiral enamide 615 was subjected to bromination conditions in which 1.33 equiv of Br2 was added at −35 °C and the resulting mixture was heated at 83 °C in ClCH2-CH2Cl for 20 h, pyran 7-Br was isolated in 43% yield as a single isomer. Its relative stereochemistry was unambiguously assigned via single-crystal X-ray structure [Scheme 2]. No β-bromo-enamides related to 3 and 4 were found, and pyran 7-Br implies a bromine-promoted desilylative cyclization had taken place instead.
Scheme 2.
Observation of a Desilylative Bromo-Etherification
Given that halogen-induced etherifications16,17 involving enamides, especially chiral enamides,18,19 have only been sparsely explored, we examined this reaction in greater detail. As shown in Table 1, we were able to establish that (1) the bromo-etherification could take place readily at much lower temperatures with best yields obtained between −20 °C and rt with the diastereomeric ratio being independent of temperature [entries 1–6], (2) we could achieve chloro- and iodo-etherification using NCS/TBAC and ICl, respectively [entries 10 and 13], and (3) while I2 [entry 11] was not as useful, NXS [X = Br, Cl, and I] did not work at all [entries 7, 9, and 12]. When using NCS/TBAC and I2, we isolated a substantial amount of pyran 8 [entries 10 and 11], which is likely a result of adventitious HCl or HI promoted cyclization.
Table 1.
Temperatures and Choice of Activations
| entry | activation | solvent | temp [°C] |
time [h] |
yield [%]a |
pyrans | drb | 8c [%] |
|---|---|---|---|---|---|---|---|---|
| 1 | Br2 | toluened | −78 to rt | 12 | 92 | 7-Br | 6.9:1 | 0 |
| 2 | CH2Cl2 | −78 | 2 | 63 | 7-Br | 6.9:1 | 0 | |
| 3 | CH2Cl2 | −45e | 2 | 73 | 7-Br | 7.0:1 | 0 | |
| 4 | CH2Cl2 | −20e | 2 | 82 | 7-Br | 7.0:1 | 0 | |
| 5 | CH2Cl2 | 0e | 2 | 81 | 7-Br | 7.0:1 | 0 | |
| 6 | CH2Cl2 | rte | 1 | 82 | 7-Br | 6.9:1 | 0 | |
| 7 | NBS | CH2Cl2 | −78 to 40 | 24 | NR | 7-Br | 0 | |
| 8 | NBS + TBABf | CH2Cl2 | −45 to rt | 6 | 42 | 7-Br | 1:1.2 | 0 |
| 9 | NCS | toluened | −78 to 60 | 48 | NR | 7-Clg | 0 | |
| 10 | NCS + TBACh | CH2Cl2 | −45 to rt | 24 | 54 | 7-Cl | 6.8:1 | 30i |
| 11 | l2j | CH2Cl2 | −78 to 40 | 24 | 30 | 7-lk | 1:1.1 | 33l |
| 12 | NlS | CH2Cl2 | −78 to 40 | 24 | NR | 7−1 | 0 | |
| 13 | lCl | CH2Cl2 | −78 to rt | 2 | 70 | 7-l | 6.0:1 | 0 |
Isolated yields. NR: no reactions.
Ratios assigned using 1H and/or 13C NMR
X = H at C3. See Scheme 2 for the structure.
Br2 was added as a 0.5 M solution in CH2Cl2: toluene:CH2Cl2 is 3:1.
Br2 was added at −78 °C. The reaction was stirred at the temperature and time indicated after the addition.
TBAB: tetra-n-butyl ammonium bromide; NBS:TBAB:6 = 1.5:1.5:1. NBS and TBAB were premixed at −78 °C for 10 min.
X = Cl at C3.
TBAC: tetra-n-butyl ammonium chloride; NCS:TBAC:6 = 1.5: 1.5:1. NCS and TBAC were premixed at −78 °C for 10 min.
dr for 8 = 2:1.
4 Å MS was used.
X = I at C3.
dr for 8 = 3:1.
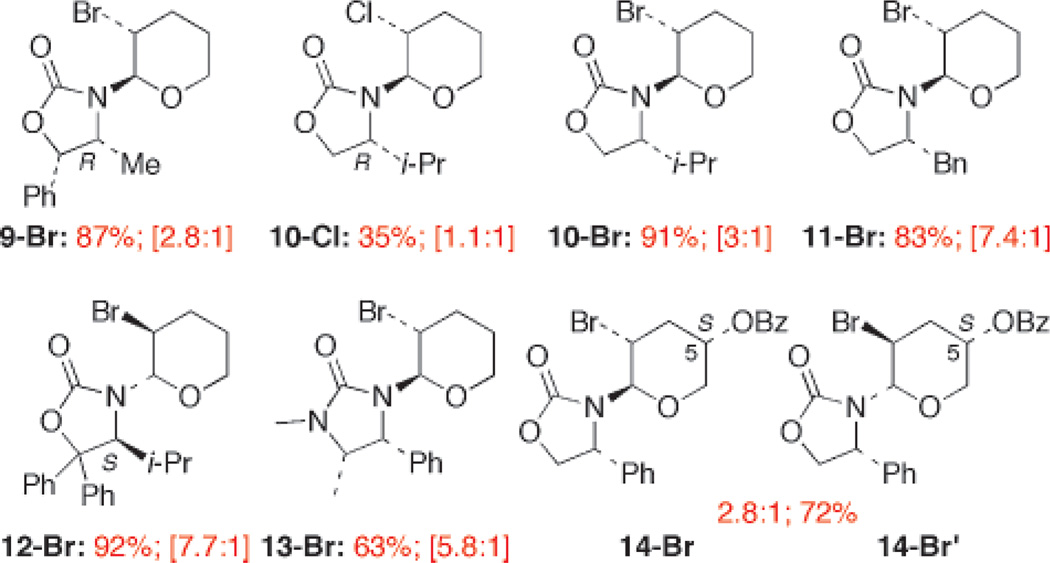
With these protocols in hand, we investigated the effect of the chiral auxiliary on the stereoselectivity. The diastereomeric ratio appears to be sensitive to the types of auxiliary used [Figure 1]. In comparison to Evans’ auxiliaries,20 Seebach’s auxiliary21 appears to be better giving 12-Br in a 7.7:1 ratio, although the ratio was lower when using Close’s auxiliary22 for the preparation of 13-Br. Interestingly, an additional substituent such as OBz [at C5] eroded the ratio 14-Br/14-Br′.
Figure 1.
Effect of the chiral auxiliary on stereoselectivity. All are isolated yields. Conditions: At −78 °C in toluene or CH2Cl2, added a respective halogen source: Br2 [0.5 M in CH2Cl2] and NCS/TBAC; stirred from −78 °C to rt for 0.5–2 h, except it is 24–48 h for the chlorination with NCS/TBAC.
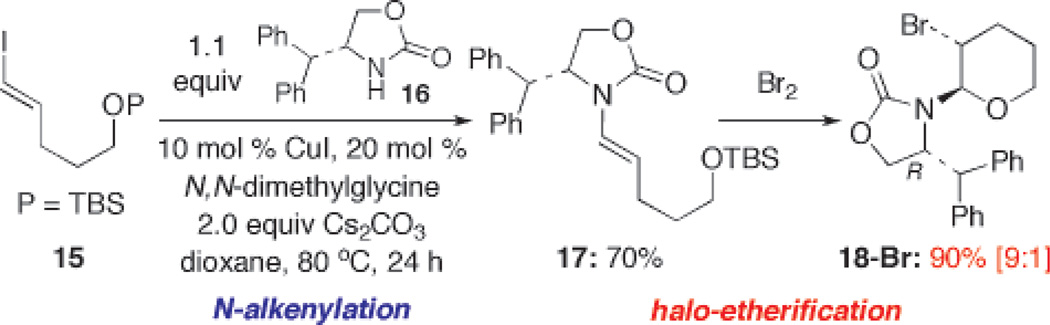
The best result came when using Sibi’s auxiliary. As shown in Scheme 3, in a sequence that is intended to demonstrate the synthetic potential of this method in conjunction with N-alkenylation via amidative cross-coupling,1,2 enamide 17 substituted with Sibi’s auxiliary was prepared employing Ma’s conditions.2c The ensuing bromination gave bromo-pyran 18-Br in 90% yield with a dr of 9:1.
Scheme 3.
A Sequential N-Alkenylation and Bromo-Etherification
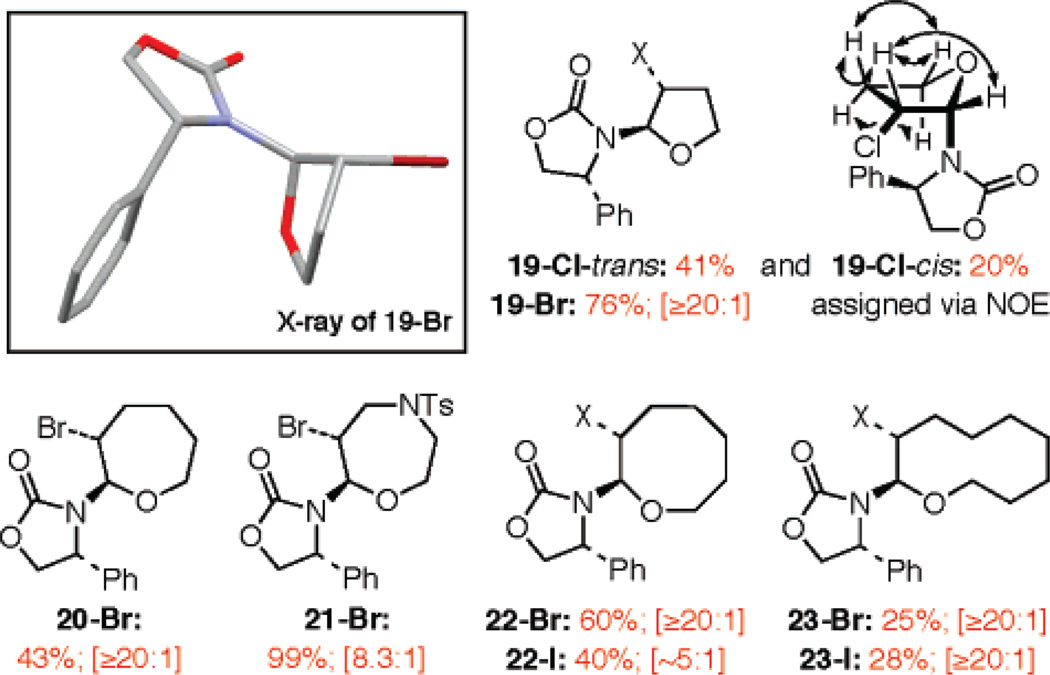
Given the prevalence of halogenated cyclic ethers among natural products, we next explored the formation of halogenated oxacycles with various ring sizes. As shown in Figure 2, we were able to construct 5-, 7-, 8-, as well as 10-membered rings, although yields suffered in the 10-membered-ring case. It is noteworthy that the ratios are all quite high in most of these examples, and for chloro-furan 19-Cl, the minor isomer is actually the cis, which was not seen in other cases.
Figure 2.
Halo-oxacycles of different ring sizes. All are isolated yields.
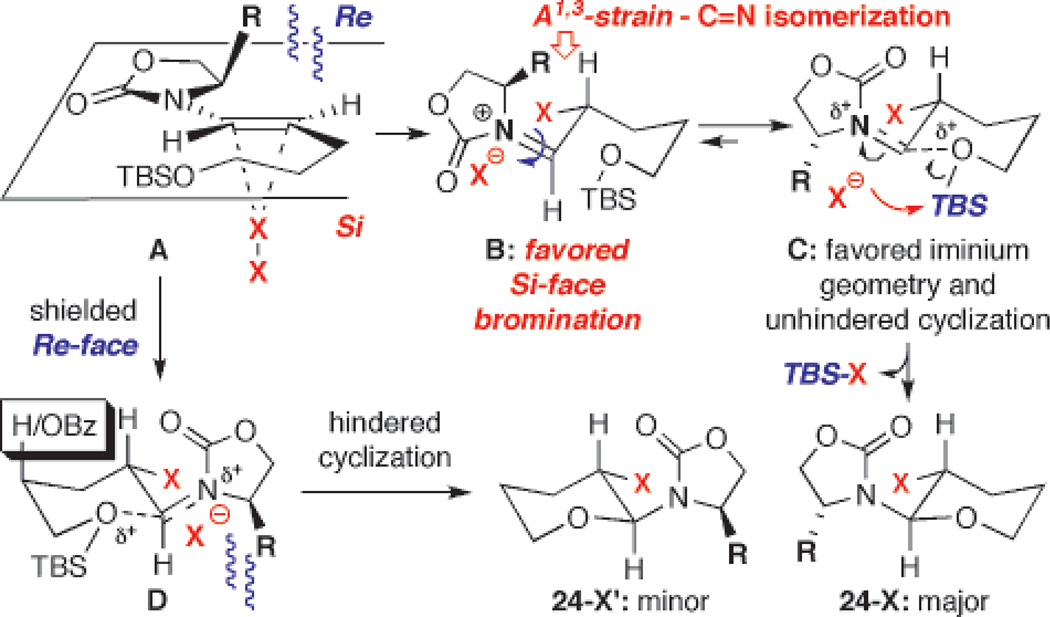
While synthetically this method represents a stereoselective approach for constructing chiral secondary halides, mechanistically, these halo-etherifications can provide insight to the chemistry of chiral enamides. On the basis of the observed stereochemical outcome, a proposed mechanistic model is shown in Scheme 4.
Scheme 4.
A Proposed Stereochemical Model
While the chiral oxazolidinone of the enamide plays a distinct role in providing the facial bias for the halogen to approach from the Si-face [see A],9,11a the desilylative cyclization through N-acyl iminium ion B represents a critical second step. An initial C=N bond isomerization [blue arrow] in B could occur to provide C in which the iminium C=N geometry is both devoid of allylic strain and favored for the ensuing cyclization. The cyclization is also likely initiated with complexation of the oxygen atom to the iminium carbon, and the loss of TBS-X would lead to the major diastereomer 24-X.
In contrast, the pathway leading to the minor isomer 24-X′ suffers from an unfavorable Re-face halogenation and a hindered cyclization through intermediate D. Support for this model arises from the halo-etherification in which the minor isomer 14-Br′ [Figure 1] was enriched relative to the reaction leading to 7-Br/7-Br′. The only difference is the presence of the OBz group [see the box]. This is in fact consistent with related cation intermediates where an OR substituent would prefer the axial position.24,25 This preference can only be accommodated in intermediate D [not shown but the OBz group would be equatorial in intermediate C en route to 14-Br].
In addition, the observations that NXS did not work and I2 was sluggish suggest that an effective desilylative cyclization through intermediate C likely depends upon not solely the nucleophilicity of the halide anion [versus succinimido anion: see Figure 3] since I−should be more nucleophilic than Br− or Cl−. Thus, we believe the proximity of the anion, or the “tightness” of the iminium ion pair, is also very important. In this regard, as the TBSO group approaches the iminium carbon, Br− or Cl− being a much tighter counter-anion to the iminium cation than I− provides a more facile desilylation.
Figure 3.
Nucleophilicity and the nature of iminium ion pairs in C.
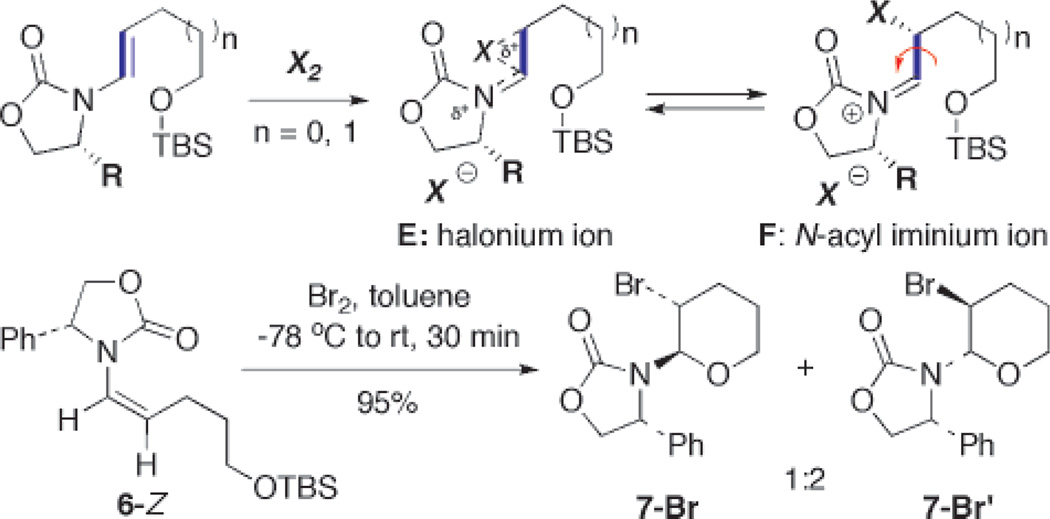
Another question concerns the involvement of the halonium intermediate E [Scheme 5] and its significance versus N-acyl iminium ion F. The isolation of 19-Cl-cis, arriving from its respective E-enamide, implies that halonium cation E is likely not important because isomerization of the C–C bond [in blue] is required to give 19-Cl-cis. A more definitive answer came when we brominated 6-Z3,15 and only obtained trans-bromo pyrans 7-Br and 7-Br′ in 95% yield with a 1:2 ratio26 in favor of the latter isomer. We detected no corresponding cis-products. The fact that the olefin geometry is not preserved again supports a free rotation of the C-C bond [in blue] occurring prior to the desilylative cyclization.
Scheme 5.
Bromination of a Z-Enamide
We have described here a stereoselective halo-etherification of chiral enamides, leading to the synthesis of halogenated cyclic ethers, which are prevalent among natural products. While this work provides a stereoselective entry to chiral secondary halides, these halo-etherifications also provide mechanistic insight to the chemistry of chiral enamides. Applications of this new synthetic method are currently underway.
Supplementary Material
Acknowledgment
Authors thank NIH-NIGMS [GM066055], PRF-AC [42106], and UW-Madison for financial support. The authors also thank Drs. Vic Young and Maren Pink [University of Minnesota] for solving X-ray structures.
Footnotes
Supporting Information Available: Experimental details, characterization data, X-ray structural analysis, and NMR spectral for all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.For reviews, see: Dehli JR, Legros J, Bolm C. Chem. Commun. 2005:973. doi: 10.1039/b415954c. Ley SV, Thomas AW. Angew. Chem. Int. Ed. 2003;42:5400. doi: 10.1002/anie.200300594. Hartwig JF. Angew. Chem. Int. Ed. 1998;37:2046. doi: 10.1002/(SICI)1521-3773(19980817)37:15<2046::AID-ANIE2046>3.0.CO;2-L. Wolfe JP, Wagaw S, Marcoux J-F, Buchwald SL. Acc. Chem. Res. 1998;31:805.
- 2.For recent enamide syntheses, see: Chechik-Lankin H, Livshin S, Marek I. Synlett. 2005:2098. Han C, Shen R, Su S, Porco JA., Jr Org. Lett. 2004;6:27. doi: 10.1021/ol0360041. Pan X, Cai Q, Ma D. Org. Lett. 2004;6:1809. doi: 10.1021/ol049464i. Brice JL, Meerdink JE, Stahl SS. Org. Lett. 2004;6:1845. doi: 10.1021/ol0494360. Jiang L, Job GE, Klapars A, Buchwald SL. Org. Lett. 2003;5:3667. doi: 10.1021/ol035355c. Shen R, Lin CT, Porco JA., Jr J. Am. Chem. Soc. 2002;124:5650. doi: 10.1021/ja026025a.
- 3.Zhang X, Zhang Y, Huang J, Hsung RP, Kurtz KCM, Oppenheimer J, Petersen ME, Sagamanova IK, Tracey MR. J. Org. Chem. 2006;71:4170. doi: 10.1021/jo060230h. [DOI] [PubMed] [Google Scholar]
- 4.Tracey MR, Hsung RP, Antoline JE, Kurtz KCM, Shen L, Slafer BW, Zhang Y. In: Science of Synthesis, Houben-Weyl Methods of Molecular Transformations. Weinreb SM, editor. Chapter 21.4. New York: Georg Thieme Verlag KG; 2005. [Google Scholar]
- 5.For reviews, see: Rappoport Z. The Chemistry of Enamines in The Chemistry of Functional Groups. New York: John Wiley and Sons; 1994. Whitesell JK, Whitesell MA. Synthesis. 1983:517. Hickmott PW. Tetrahedron. 1982;38:1975. Hickmott PW. Tetrahedron. 1982;38:3363.
- 6.For α-metalations of chiral enamides, see: Lander P, Hegedus LS. J. Am. Chem. Soc. 1994;116:8126.
- 7.For [2 + 2] cycloadditions of chiral enamides, see: Hegedus LS, Bates RW, Soderberg BC. J. Am. Chem. Soc. 1991;113:923. (b) For a review, see: Hegedus LS. Tetrahedron. 1997;53:4105. Riches AG, Wernersbach LA, Hegedus LS. J. Org. Chem. 1998;63:4691. For Paternò-Büchi [2 + 2], see: Bach T, Schröder J, Brandl T, Hecht J, Harms K. Tetrahedron. 1998;54:4507.
- 8.For Diels–Alder cycloadditions, see: Gohier F, Bouhadjera K, Faye D, Gaulon C, Maisonneuve V, Dujardin G, Dhal R. Org. Lett. 2007;9:211. doi: 10.1021/ol062626l. Crawly SL, Funk RL. Org. Lett. 2006;8:3995. doi: 10.1021/ol061461d. Palasz A. Org. Biomol. Chem. 2005;3:3207. doi: 10.1039/b504210k. Robiette R, Cheboub-Benchaba K, Peeters D, Marchand-Brynaert J. J. Org. Chem. 2003;68:9809. doi: 10.1021/jo0302049. Huang J, Xiong H, Hsung RP, Rameshkumar C, Mulder JA, Grebe TP. Org. Lett. 2002;4:2417. doi: 10.1021/ol020097p. Abbiati G, Clerici F, Gelmi ML, Gambini A, Pilati T. J. Org. Chem. 2001;66:6299. doi: 10.1021/jo010374q.
- 9.For epoxidations, see: Xiong H, Hsung RP, Shen L, Hahn JM. Tetrahedron Lett. 2002;43:4449. Adam W, Bosio SG, Wolff BT. Org. Lett. 2003;5:819. doi: 10.1021/ol0273194. Sivaguru J, Saito H, Poon T, Omonuwa T, Franz R, Jockusch S, Hooper C, Inoue Y, Adam W, Turro NJ. Org. Lett. 2005;7:2089. doi: 10.1021/ol0502230. Koseki Y, Kusano S, Ichi D, Yoshida K, Nagasaka T. Tetrahedron. 2000;56:8855.
- 10.For cyclopropanations of chiral enamides, see: Song Z, Lu T, Hsung RP, Al-Rashid ZF, Ko C, Tang Y. Angew. Chem. Int. Ed. 2007;46:4069. doi: 10.1002/anie.200700681. Voigt J, Noltemeyer M, Reiser O. Synlett. 1997:202. Akiba T, Tamura O, Hashimoto M, Kobayashi Y, Katoh T, Nakatani K, Kamada M, Hayakawa I, Terashima S. Tetrahedron. 1994;50:3905. Seebach D, Stucky G, Pfammatter E. Chem. Ber. 1989;122:2377. Seebach D, Stucky G. Angew. Chem., Int. Ed. 1988;27:1351.
- 11.For examples of cross-couplings, see: Mulder JA, Kurtz KCM, Hsung RP, Coverdale HA, Frederick MO, Shen L, Zificsak CA. Org. Lett. 2003;5:1547. doi: 10.1021/ol0300266. Naud S, Cintrat J-C. Synthesis. 2003:1391. Hoffmann RW, Brückner D. New J. Chem. 2001;25:369. Witulski B, Buschmann N, Bergsträβer U. Tetrahedron. 2000;56:8473.
- 12.For an enamide-yne cycloisomerization: Kim H, Lee C. J. Am. Chem. Soc. 2006;128:6336. doi: 10.1021/ja0619758.
- 13.For reviews on ynamides, see: Zificsak CA, Mulder JA, Hsung RP, Rameshkumar C, Wei L-L. Tetrahedron. 2001;57:7575. Mulder JA, Kurtz KCM, Hsung RP. Synlett. 2003:1379. Katritzky AR, Jiang R, Singh SK. Heterocycles. 2004;63:1455. (d) For a special issue dedicated to the chemistry of ynamides, see: Tetrahedron-Symposium-In-Print: Chemistry of Electron-Deficient Ynamines and Ynamides. Tetrahedron. 2006;62(Issue No.16)
- 14.Wei L-L, Mulder JA, Xiong H, Zificsak CA, Douglas CJ, Hsung RP. Tetrahedron. 2001;57:459. [Google Scholar]
- 15.See the **Supporting Information.
- 16.For reviews, see: Boivin TLB. Tetrahedron. 1987;43:3309. Cardillo G, Orena M. Tetrahedron. 1990;46:3321. Harding KE, Tiner TH. Comp. Org. Synh. 1991;4:363. Rousseau G, Homsi F. Chem. Soc. Rev. 1997;26:453. Robin S, Rousseau G. Tetrahedron. 1998;54:13681. Robin S, Rousseau G. Eur. J. Org. Chem. 2002:3099. Ranganathan S, Muraleedharan KM, Vaish NK, Jayaraman N. Tetrahedron. 2004;60:5273. French AN, Bissmire S, Wirth T. Chem. Soc. Rev. 2004;33:354. doi: 10.1039/b310389g. Tang Y, Oppenheimer J, Song Z, You L, Zhang X, Hsung RP. Tetrahedron. 2006;62:10785.
- 17.For some reviews on related metal-catalyzed cyclizations of olefins in constructions of hetereocycles, see: Nakamura I, Yamamoto Y. Chem. Rev. 2004;104:2127. doi: 10.1021/cr020095i. Beller M, Seayad J, Tillack A, Jiao H. Angew. Chem. Int. Ed. 2004;43:3368. doi: 10.1002/anie.200300616. Stahl SS. Angew. Chem. Int. Ed. 2004;43:3400. doi: 10.1002/anie.200300630.
- 18.For some examples on halo-etherfication and halo-lactonization of enamides, see: Rudler H, Denise B, Xu Y, Parlier A, Vaissermann J. Eur. J. Org. Chem. 2005:3724. Rudler H, Denise B, Parlier A, Daranb J. Chem. Commun. 2002;9:940. doi: 10.1039/b201780f. Martín-López MJ, Bermejo-González F. Tetrahedron. 1998;54:12379. Williams R, Milreis GF. Tetrahedron Lett. 1990;31:4297. Shin C, Sato Y, Yoshimura J. Tetrahedron Lett. 1981;22:2401.
- 19.For oxidative etherifications of enamides, see: Rameshkumar C, Xiong H, Tracey MR, Berry CR, Yao LJ, Hsung RP. J. Org. Chem. 2002;67:1339. doi: 10.1021/jo011048d. McComas CC, Perales JB, Vranken DLV. Org. Lett. 2002;4:2337. doi: 10.1021/ol026015e. For oxidative lactonizations of enamides, see: Stolowich NJ, Wang J, Spencer JB, Santander PJ, Roessner CA, Scott AI. J. Am. Chem. Soc. 1996;118:1657. Ihara M, Noguchi K, Fukumoto K, Kametani T. Tetrahedron. 1985;41:2109.
- 20.Heathcock CH. Aldrichim. Acta. 1990;23:99. [Google Scholar]
- 21.Hintermann T, Seebach D. Helv. Chim. Acta. 1998;81:2093. [Google Scholar]
- 22.Close WJ. J. Org. Chem. 1950;15:1131. [Google Scholar]
- 23.Sibi MP, Porter NA. Acc. Chem. Res. 1999;32:163. [Google Scholar]
- 24.Romero JAC, Tabacco SA, Woerpel KA. J. Am. Chem. Soc. 2000;122:168. [Google Scholar]
- 25.Hosokawa S, Kirschbaum B, Isobe M. Tetrahedron Lett. 1998;39:1917. [Google Scholar]
- 26.This reversal in ratio is consistent with the mechanistic model in which the same favored, Si-face bromination of 6-Z would lead to an N-acyl iminium intermediate that is slow to cyclize, whereas the hindered Re-face bromination gives the respective N-acyl iminium intermediate that is more favored for the desilylative cyclization.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.