Abstract
Objective: Evaluate predictors of vaginal colonization with lactobacilli after treatment for bacterial vaginosis (BV). Methods. Vaginal fluid specimens from women with BV underwent qPCR for Lactobacillus crispatus, L. jensenii, and L. iners pre- and posttreatment. Results. Few women with BV were colonized with L. crispatus (4/44, 9%) or L. jensenii (1/44, 2%), though all had L. iners. One month posttreatment 12/44 (27%) had L. crispatus, 12/44 (27%) L. jensenii, and 43/44 (98%) L. iners. Presence of L. jensenii posttreatment was associated with cure (Risk Ratio (RR) 1.67; 95% CI 1.09–2.56); L. crispatus showed a similar trend (RR 1.41; 95% CI 0.89–2.24, P = 0.14). Receptive oral sex was associated with 2.2-log10 lower concentration of L. crispatus (95% CI −4.38, −.02), and digital-vaginal sex with 2.6-log10 lower concentration (95% CI −4.87, −.33). Conclusion. One month after BV treatment, few women established colonization with L. crispatus or L. jensenii. Few behaviors were associated with colonization.
1. Introduction
Bacterial vaginosis (BV) is the most common cause of vaginal discharge in reproductive age women [1], is present in approximately 29% of women in the United States [2], and is characterized by vaginal colonization with anaerobic bacterial species along with loss of lactobacilli. The clinical sequelae of BV are significant—a nearly two-fold-increased risk of HIV-1 acquisition [3, 4], preterm delivery [5, 6], and pelvic inflammatory disease (PID) [7]—and affect millions of women worldwide each year, making BV a significant health problem. Treatment with antibiotics has a cure rate of 50–80% [8, 9] but recurrence within 1 to 3 months is common (30–52%) [10–12].
Hydrogen peroxide (H2O2-) producing species of vaginal lactobacilli are associated with decreased rates of BV [13, 14], and better reproductive health outcomes [15, 16] compared to non-H2O2-producing species. Lactobacillus crispatus is the most common vaginal H2O2-producing Lactobacillus species [17, 18]. L. jensenii is another frequently isolated H2O2-producing species [18, 19]. Some hypothesize that the recurrence rate of BV is high because these protective lactobacilli do not recolonize the vagina after antibiotic treatment aimed at eradicating BV-associated anaerobes, and so leave an ecologic void that is quickly refilled by opportunistic organisms. In one study, only 40% of women were recolonized with any H2O2-producing species of lactobacilli 30 days after oral metronidazole treatment and 57% were recolonized 30 days after vaginal clindamycin [20]. Most women are colonized with a single dominant species of Lactobacillus [21], but it is unclear if this is because there is competition between species for the vaginal niche. A majority of women studied in the US are colonized with Lactobacillus iners [22, 23], a fastidious species that does not commonly produce H2O2 and that has been associated with increased risk of abnormal vaginal microbiota in pregnant women [24]. Little is known about the effect of L. iners on a woman's ability to colonize with beneficial H2O2-producing species.
Presence of H2O2-producing lactobacilli [14, 25, 26], specifically L. crispatus [24], has been associated with decreased risk of abnormal vaginal microbiota and BV; thus, recolonization with these species after treatment for BV is likely an important marker of vaginal health. We undertook this nested cohort study to evaluate the effect of sexual behavior on vaginal recolonization with two hydrogen peroxide producing Lactobacillus species, L. crispatus and L. jensenii, one month after treatment for BV.
2. Methods
2.1. Study Population and Design
We conducted an analysis of women diagnosed with and treated for BV while enrolled in an observational cohort study in Seattle, WA. As previously described, participants were recruited through advertisements, media, and community referral, and had to be ≥16 years old and report having had sex with at least one woman in the previous year, a group with relatively high BV prevalence [27]. Study visits were scheduled every three months for a year, with additional visits for vaginal symptoms and/or 4 weeks after treatment for BV. At each visit, participants completed a computer-assisted self-interview (CASI) that collected information about demographics, sexual practices, medical, and reproductive history. The study was approved by the University of Washington Institutional Review Board and all participants provided informed consent at enrollment. Participants underwent pelvic examination with collection of vaginal swabs for saline microscopy, Gram stain, and bacterial culture. A separate foam swab was collected by rolling along the vaginal wall and was then frozen at −80°C for use in molecular assays. Women diagnosed with BV by Amsel's clinical criteria [28] were treated with vaginal metronidazole gel, 37.5 mg nightly for 5 nights, and assessed at a followup visit after 4 weeks. Vaginal fluid Gram stains were scored using the criteria outlined by Nugent et al. [29], however, treatment success was defined solely as absence of BV by Amsel's criteria.
We included all participants who were diagnosed with BV during the study and whose followup visits occurred 3 to 8 weeks after treatment. Only the first BV-positive visit was included for participants who were diagnosed with BV more than once. The study was conducted between October 2003 and December 2006, but between 3/2/2004 and 12/8/2005, only women with vaginal pH > 4.5 at the followup visit had samples taken follow-up (due to limitations in study funding). Because of this differential assessment and the resulting potential bias, all women whose followup visits fell within this time period were excluded. Participants whose samples did not have enough material to complete all PCR assays were also excluded.
2.2. Molecular Assays
Frozen vaginal swabs from the BV-positive visit and a followup visit within 3–8 weeks were processed as previously described [30]. All extracted DNA was tested in a quantitative PCR assay using primers targeting the human 18S rRNA gene to validate that successful DNA extraction occurred. An internal amplification control PCR using exogenous DNA from a jellyfish gene was used to test for presence of PCR inhibitors [31].
Vaginal fluid samples were then subjected to taxon-directed 16S rRNA gene quantitative PCR assays for the detection and quantification of L. crispatus, L. jensenii, and L. iners [30, 32]. Each assay has previously been validated and proven sensitive (to a level of 1–10 DNA copies/reaction) and specific (does not detect other bacteria at a concentration of 106 copies/reaction). The assays use a TaqMan format, and are run on an ABI 7500 Thermocycler (Applied Biosystems, Foster City, CA) or Eppendorf Mastercycler ep Realplex thermal cycler (Eppendorf, Westbury, NY).
2.3. Statistical Analysis
The primary outcome of interest was presence or absence of L. crispatus or L. jensenii after treatment for BV. In secondary analyses, we assessed the relationship between sexual behaviors and quantities of bacteria, expressed as 16S rDNA gene copies/vaginal swab and log transformed. Univariate log binomial regression was used to assess the relationship between presence or absence of either L. crispatus or L. jensenii and (a) different behaviors, and (b) presence and quantity of L. iners. Univariate linear regression was used to assess the relationship between sexual behaviors and quantity of L. crispatus or L. jensenii in the subset of women who were colonized. Given the relatively small number of women, we did not perform multivariate analyses.
3. Results
A total of 336 women were enrolled in the observational cohort. Of these, 136 (40%) were diagnosed with BV during the study: 96 at enrollment, and 40 at a routine study visit or a nonscheduled visit with symptomatic BV. Eleven women never returned for followup, 58 women had followup visits that fell during the period of exclusion, and 23 did not have adequate sample remaining for all of the assays and were excluded, leaving 44 women available for this analysis.
3.1. Baseline Characteristics
The 44 women had a mean age of 25 ± 3 years and were primarily white (35/44; 80%). Half of the visits occurred during the proliferative phase of the menstrual cycle, and half in the luteal phase. The majority of women had only female partners (31/44; 70%), while smaller percentages had male only (4/44; 9%), partners of both genders (4/44; 9%) or no sexual partner in the last 3 months (5/44; 11%). All women had BV by Amsel's criteria, and 98% (43/44) also had BV by Nugent's score, which was significantly different than women excluded from this substudy, of whom only 85% (78/92) had BV by Nugent's score (P = .02). This was the only characteristic that differed between women in the substudy and those that were excluded. Women in the substudy were as likely to complete antibiotic treatment for BV as women in the larger cohort (89% versus 90%; P = .95).
At diagnosis, 29/44 (66%) women reported having had receptive oral-vaginal contact in the previous 90 days. Slightly more reported digital-vaginal sex (82%), while fewer reported toy-vaginal sex (36%) during that same time. Only 8 (18%) women reported sexual contact with a male partner in the 3 months prior to BV diagnosis, 7 of whom reported having penile-vaginal sex during that time. All 44 women were colonized with L. iners at BV diagnosis, while few were colonized with L. crispatus (4/44, 9%) or L. jensenii (1/44, 2%).
3.2. Posttreatment Characteristics
Nearly all women (43/44; 98%) were colonized with L. iners after treatment. Only 12/44 (27%) were colonized with L. crispatus and 12/44 (27%) with L. jensenii. Of those, six women were colonized with both species, and six each with only one of the two species. Posttreatment, 18 women (41%) still met Amsel's criteria for BV and were considered to have failed treatment, all of whom also had a Nugent score ≥7. Among these women, only 3 (16.7%) were colonized with L. crispatus and 2 (11.1%) with L. jensenii. Of the 26 women who achieved cure, a slightly higher percentage (but still a minority) were colonized with L. crispatus (9/26; 35%) and L. jensenii (10/26; 38%). Presence of L. crispatus at diagnosis or followup trended towards association with cure (Risk Ratio 1.41; 95% CI .89, 2.24; P = .14), but this was not statistically significant. Women colonized with L. jensenii after treatment had significantly higher rates of treatment success (RR 1.67; 95% CI 1.09, 2.56; P = .02).
Of the four women colonized with L. crispatus at BV diagnosis, one achieved cure and had higher concentrations after treatment, while 3 failed treatment, and had lower (n = 2) or undetectable (n = 1) concentrations. The one woman colonized with L. jensenii at BV diagnosis no longer had detectable colonization after treatment, and also failed treatment (Figure 1). Among colonized women, mean Log10 concentration of L. crispatus after treatment was 6.1 ± 1.9 gene copies/mL and for L. jensenii was 6.0 ± .7 gene copies/mL. All 44 women were colonized with high quantities of L. iners at the BV diagnosis visit (mean Log10 copies 6.5 ± .9), and the quantity did not change significantly at the followup visit (mean Log10 copies 6.7 ± 1.1; P = .40).
Figure 1.
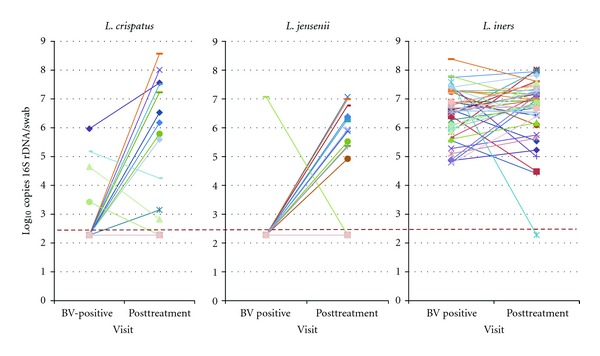
Change in concentrations of three different species of lactobacilli, as measured by species-specific quantitative PCR, 4 weeks after treatment for bacterial vaginosis with vaginal metronidazole. Each line represents an individual patient and her concentration of each bacterium before and after treatment. The dotted line represents the lower limit of detection of the qPCR assay.
Between the visit at which BV was diagnosed and treatment provided, and subsequent followup (median 33 days, IQR 28–37), 21/44 women (48%) reported oral-vaginal sex, 26 (59%) digital-vaginal sex, 8 (18%) penile-vaginal sex, and 9 (20%) toy-vaginal sex. Among all 44 women, no interim sexual behaviors were associated with presence or absence of either L. jensenii or L. crispatus at the followup visit or with treatment failure (Table 1). Among women who were colonized with L. jensenii or L. crispatus at the followup visit, we examined whether behaviors reported in the interim period between treatment and followup at 32 days were associated with quantity of bacteria detected at the followup visit (Table 2). In the subset of 12 women establishing colonization by followup, report of digital-vaginal sex was significantly associated with 2.6-Log10 lower concentrations of L. crispatus (95% CI −4.87, −.33). Report of receptive oral sex was associated with 2.2-Log10 lower concentrations of L. crispatus (95% CI −4.38, −.02). No behaviors were associated with quantity of L. jensenii detected at that visit.
Table 1.
Univariate association between reported sexual behaviors during treatment and followup and presence of L. crispatus or L. jensenii at the posttreatment visit.
| Presence of L. crispatus (n = 44) | Presence of L. jensenii (n = 44) | BV diagnosis by Amsel's at followup (n = 18) | ||
|---|---|---|---|---|
| Sexual behavior during followup period | N | Prevalence ratio | Prevalence ratio | Prevalence ratio |
|
| ||||
| Number of partners | 12 | Reference | Reference | Reference |
| 0 | ||||
| 1 | 26 | 1.62 (.39, 6.65) | 1.12 (.34, 3.46) | 1.1 (.50, 2.44) |
| 2+ | 6 | 3.0 (.67, 13.4) | 1.33 (.30, 5.96) | .4 (.06, 2.70) |
| Oral vaginal sex | 21 | 2.19 (.77, 6.22) | 1.53 (.57, 4.1) | 1.10 (.54, 2.23) |
| Digital-vaginal sex | 26 | 2.08 (.65, 6.63) | 2.08 (.65, 6.63) | .87 (.43, 1.76) |
| Toy-vaginal sex | 9 | 1.30 (.44, 3.82) | .78 (.21, 2.94) | 1.11 (.48, 2.56) |
| Penile-vaginal sex | 8 | .9 (.24, 3.34) | .9 (.24, 3.34) | .9 (.34, 2.38) |
| Use of vaginal lubricant | 11 | 2.42 (.66, 8.93) | .30 (.04, 2.21) | .73 (.30, 1.78) |
Table 2.
Association between reported sexual behaviors during treatment and followup and quantity of L. crispatus or L. jensenii in women who were colonized at the posttreatment visit.
| Sexual behavior during followup period | L. crispatus | L. jensenii | ||
|---|---|---|---|---|
| N | Log10 difference in 16S rRNA copies/mL | N | Log10 difference in 16S rRNA gene copies/mL | |
| Number of partners | ||||
| 0 | 2 | Reference | 3 | Reference |
| 1 | 7 | −1.66 (−4.87, 1.55) | 7 | −.03 (−1.21, 1.16) |
| 2+ | 3 | −3.13 (−6.52, .25) | 2 | −.16 (−1.73, 1.40) |
| Oral vaginal sex | 8 | −2.20 (−4.38, −.02) | 7 | −.11 (−1.05, .83) |
| Digital-vaginal sex | 9 | −2.60 (−4.87, −.33) | 9 | −.06 (−1.13, 1.02) |
| Toy-vaginal sex | 3 | −1.65 (−4.32, 1.03) | 2 | −.15 (−1.39, 1.09) |
| Penile-vaginal sex | 2 | .69 (−2.67, 4.04) | 2 | −.14 (−1.39, 1.10) |
| Use of vaginal lubricant | 4* | −2.92 (−6.09, .24) | 1* | −.53 (−2.07, 1.0) |
*Missing data for 5 women.
4. Discussion
In this cohort of women reporting sex with women, rates of vaginal colonization with two species of commensal H2O2-producing lactobacilli four weeks after treatment for bacterial vaginosis were low. Though colonization was infrequent, women who were able to establish colonization achieved high concentrations of each of these bacteria.
For clinicians and affected women, the high rate of BV recurrence after antibiotic treatment is exceedingly frustrating [10–12]. Several groups have evaluated whether adding probiotic compounds containing lactobacilli to treatment improves outcomes, but results have been mixed [33–36]. In a study of healthy women treated with vaginal probiotic capsules containing L. crispatus, participants who reported penile-vaginal sex between treatment and followup were less likely to establish colonization with the probiotic strain [37]. We hypothesized that sexual activity in the month after treatment may inhibit vaginal colonization with beneficial lactobacilli, possibly through reinoculation with BV-associated bacteria from vulvar or rectal reservoirs, which might increase risk for BV recurrence.
In the parent study of nearly 350 women from which this nested case control study was derived, we demonstrated that women cured of BV had higher rates of colonization by L. crispatus after treatment (42%) than women with persistent BV (26%; P = .0003); data on L. jensenii were not available [30]. A different study obtained vaginal swabs for culture and found that by 4 weeks after treatment with vaginal metronidazole 59% of women were colonized with hydrogen peroxide producing lactobacilli [20]. Other studies used Nugent score to characterize shifts of the vaginal bacteria, and reported that as many as 66% of treated women had at least some lactobacilli at 21–30 days after treatment [38], though H2O2 production was not measured. Our group previously measured posttreatment quantity of L. crispatus in a cohort of pregnant women using PCR and found that only 9/53 (17%) of women had detectable levels 4–6 weeks after treatment [39].
Few studies have evaluated behavioral predictors of colonization with lactobacilli. In women with BV, those who report more sexual partners are less likely to be colonized with H2O2-producing lactobacilli [40]. In our cohort, women colonized with L. crispatus who reported digital-vaginal and/or oral-vaginal sex had lower quantities of this bacterium. Although it did not reach statistical significance, we saw a paradoxical opposite trend in the risk related to these behaviors for vaginal colonization with L. crispatus or L. jensenii, suggesting that women with more partners, or reporting more frequent oral-vaginal or digital-vaginal sex, were more likely to be colonized. One possible explanation is that women colonized by L. crispatus and L. jensenii more likely achieved cure of BV, thus reducing the likelihood of vaginal symptoms that might deter them from engaging in sex. This observation highlights the difficulty in studying the complex relationships between sexual behaviors and the dynamic nature of vaginal microbiology—temporal associations are difficult to ascertain unless both outcomes are measured frequently (ideally, daily).
The main limitation of this study is the small sample size, which reduced our power to detect potential associations between behaviors and colonization with specific lactobacilli. A significant number of participants with BV did not have a posttreatment sample, which limited our ability to examine the entire study group. Participants selected for this substudy were similar to the larger cohort except for having higher Nugent scores at diagnosis, which may partially explain their high rate of treatment failure. This cohort is composed primarily of women who have sex exclusively with women, and our results may differ from those obtained in a cohort of primarily heterosexual women. However, this allowed us to study the effect of several different types of sexual behavior on the vaginal microbiota. The population had well-characterized information about sexual activity during the treatment period, and a very high rate of followup (92%). Our quantitative PCR analysis allowed detection of small quantities of bacteria and analysis of changes in quantity of bacteria after treatment with respect to sexual behaviors.
5. Conclusions
Vaginal colonization with H2O2-producing lactobacilli 4 weeks after treatment for BV was uncommon, suggesting that there is a window of vulnerability during which women may be more susceptible to reinfection or recurrence. While no sexual behaviors were found to impact presence of colonization, quantity of L. crispatus was decreased in women reporting digital-vaginal and oral-vaginal contact. Quantity of L. jensenii was not affected by any reported sexual behaviors. This suggests that some species of commensal lactobacilli may be more sensitive to the effect of sexual activity on the vaginal environment.
Acknowledgments
This study was supported by National Institute of Allergy and Infectious Diseases Grant RO1 AI052228 (J. M. Marrazzo). Dr. C. Mitchell was supported by the Women's Reproductive Health Research Award, and the UW ITHS Tuition Support Degree Program Award.
References
- 1.Eschenbach DA, Hillier S, Critchlow C, Stevens C, DeRouen T, Holmes KK. Diagnosis and clinical manifestiations of bacterial vaginosis. American Journal of Obstetrics and Gynecology. 1988;158(4):819–828. doi: 10.1016/0002-9378(88)90078-6. [DOI] [PubMed] [Google Scholar]
- 2.Koumans EH, Sternberg M, Bruce C, et al. The prevalence of bacterial vaginosis in the United States, 2001–2004; associations with symptoms, sexual behaviors, and reproductive health. Sexually Transmitted Diseases. 2007;34(11):864–869. doi: 10.1097/OLQ.0b013e318074e565. [DOI] [PubMed] [Google Scholar]
- 3.Martin HL, Richardson BA, Nyange PM, et al. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. Journal of Infectious Diseases. 1999;180(6):1863–1868. doi: 10.1086/315127. [DOI] [PubMed] [Google Scholar]
- 4.Atashili J, Poole C, Ndumbe PM, Adimora AA, Smith JS. Bacterial vaginosis and HIV acquisition: a meta-analysis of published studies. Journal of Acquired Immune Deficiency Syndromes. 2008;22(12):1493–1501. doi: 10.1097/QAD.0b013e3283021a37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hillier SL, Nugent RP, Eschenbach DA, et al. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. The New England Journal of Medicine. 1995;333(26):1737–1742. doi: 10.1056/NEJM199512283332604. [DOI] [PubMed] [Google Scholar]
- 6.Hay PE, Lamont RF, Taylor-Robinson D, Morgan DJ, Ison C, Pearson J. Abnormal bacterial colonisation of the genital tract and subsequent preterm delivery and late miscarriage. British Medical Journal. 1994;308(6924):295–298. doi: 10.1136/bmj.308.6924.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wiesenfeld HC, Hillier SL, Krohn MA, et al. Lower genital tract infection and endometritis: insight into subclinical pelvic inflammatory disease. Obstetrics and Gynecology. 2002;100(3):456–463. doi: 10.1016/s0029-7844(02)02118-x. [DOI] [PubMed] [Google Scholar]
- 8.Klebanoff MA, Hauth JC, MacPherson CA, et al. Time course of the regression of asymptomatic bacterial vaginosis in pregnancy with and without treatment. American Journal of Obstetrics and Gynecology. 2004;190(2):363–370. doi: 10.1016/j.ajog.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 9.Joesoef MR, Schmid GP, Hillier SL. Bacterial vaginosis: review of treatment options and potential clinical indications for therapy. Clinical Infectious Diseases. 1999;28(supplement 1):S57–S65. doi: 10.1086/514725. [DOI] [PubMed] [Google Scholar]
- 10.Boris J, Pahlson C, Larsson PG. Six years observation after successful treatment of bacterial vaginosis. Infectious Diseases in Obstetrics and Gynecology. 1997;5(4):297–302. doi: 10.1155/S1064744997000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradshaw CS, Morton AN, Hocking J, et al. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. Journal of Infectious Diseases. 2006;193(11):1478–1486. doi: 10.1086/503780. [DOI] [PubMed] [Google Scholar]
- 12.Myer L, Kuhn L, Denny L, Wright TC., Jr. Recurrence of symptomatic bacterial vaginosis 12 months after oral metronidazole therapy in HIV-positive and -negative women. Journal of Infectious Diseases. 2006;194(12):1797–1799. doi: 10.1086/509625. [DOI] [PubMed] [Google Scholar]
- 13.Hawes SE, Hillier SL, Benedetti J, et al. Hydrogen peroxide-producing lactobacilli and acquisition of vaginal infections. Journal of Infectious Diseases. 1996;174(5):1058–1063. doi: 10.1093/infdis/174.5.1058. [DOI] [PubMed] [Google Scholar]
- 14.Hillier SL, Krohn MA, Klebanoff SJ, Eschenbach DA. The relationship of hydrogen peroxide-producing lactobacilli to bacterial vaginosis and genital microflora in pregnant women. Obstetrics and Gynecology. 1992;79(3):369–373. doi: 10.1097/00006250-199203000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Eckert LO, Moore DE, Patton DL, Agnew KJ, Eschenbach DA. Relationship of vaginal bacteria and inflammation with conception and early pregnancy loss following in-vitro fertilization. Infectious Disease in Obstetrics and Gynecology. 2003;11(1):11–17. doi: 10.1155/S1064744903000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilks M, Wiggins R, Whiley A, et al. Identification and H2O2 production of vaginal Lactobacilli from pregnant women at high risk of preterm birth and relation with outcome. Journal of Clinical Microbiology. 2004;42(2):713–717. doi: 10.1128/JCM.42.2.713-717.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antonio MAD, Hawes SE, Hillier SL. The identification of vaginal Lactobacillus species and the demographic and microbiologic characteristics of women colonized by these species. Journal of Infectious Diseases. 1999;180(6):1950–1956. doi: 10.1086/315109. [DOI] [PubMed] [Google Scholar]
- 18.Martin R, Suarez JE. Biosynthesis and degradation of H2O2 by vaginal lactobacilli. Applied and Environmental Microbiology. 2010;76(2):400–405. doi: 10.1128/AEM.01631-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vallor AC, Antonio MAD, Hawes SE, Hillier SL. Factors associated with acquisition of, or persistent colonization by, vaginal lactobacilli: role of hydrogen peroxide production. Journal of Infectious Diseases. 2001;184(11):1431–1436. doi: 10.1086/324445. [DOI] [PubMed] [Google Scholar]
- 20.Agnew KJ, Hillier SL. The effect of treatment regimens for vaginitis and cervicitis on vaginal colonization by lactobacilli. Sexually Transmitted Diseases. 1995;22(5):269–273. doi: 10.1097/00007435-199509000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Zhou X, Brown CJ, Abdo Z, et al. Differences in the composition of vaginal microbial communities found in healthy Caucasian and black women. ISME Journal. 2007;1(2):121–133. doi: 10.1038/ismej.2007.12. [DOI] [PubMed] [Google Scholar]
- 22.Fredricks DN, Fiedler TL, Marrazzo JM. Molecular identification of bacteria associated with bacterial vaginosis. The New England Journal of Medicine. 2005;353(18):1899–1911. doi: 10.1056/NEJMoa043802. [DOI] [PubMed] [Google Scholar]
- 23.Spear GT, Gilbert D, Landay AL, et al. Pyrosequencing of the genital microbiotas of HIV-seropositive and -seronegative women reveals lactobacillus iners as the predominant lactobacillus species. Applied and Environmental Microbiology. 2011;77(1):378–381. doi: 10.1128/AEM.00973-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verstraelen H, Verhelst R, Claeys G, De Backer E, Temmerman M, Vaneechoutte M. Longitudinal analysis of the vaginal microflora in pregnancy suggests that L. crispatus promotes the stability of the normal vaginal microflora and that L. gasseri and/or L. iners are more conducive to the occurrence of abnormal vaginal microflora. BMC Microbiology. 2009;9, article 116 doi: 10.1186/1471-2180-9-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eschenbach DA, Davick PR, Williams BL, et al. Prevalence of hydrogen peroxide-producing Lactobacillus species in normal women and women with bacterial vaginosis. Journal of Clinical Microbiology. 1989;27(2):251–256. doi: 10.1128/jcm.27.2.251-256.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hillier SL, Krohn MA, Rabe LK, Klebanoff SJ, Eschenbach DA. The normal vaginal flora, H2O2-producing lactobacilli, and bacterial vaginosis in pregnant women. Clinical Infectious Diseases. 1993;16(supplement 4):S273–S281. doi: 10.1093/clinids/16.supplement_4.s273. [DOI] [PubMed] [Google Scholar]
- 27.Marrazzo JM, Thomas KK, Fiedler TL, Ringwood K, Fredricks DN. Relationship of specific vaginal bacteria and bacterial vaginosis treatment failure in women who have sex with women. Annals of Internal Medicine. 2008;149(1):20–28. doi: 10.7326/0003-4819-149-1-200807010-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amsel R, Totten PA, Spiegel CA. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. American Journal of Medicine. 1983;74(1):14–22. doi: 10.1016/0002-9343(83)91112-9. [DOI] [PubMed] [Google Scholar]
- 29.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. Journal of Clinical Microbiology. 1991;29(2):297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fredricks DN, Fiedler TL, Thomas KK, Mitchell CM, Marrazzo JM. Changes in vaginal bacterial concentrations with intravaginal metronidazole therapy for bacterial vaginosis as assessed by quantitative PCR. Journal of Clinical Microbiology. 2009;47(3):721–726. doi: 10.1128/JCM.01384-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khot PD, Ko DL, Hackman RC, Fredricks DN. Development and optimization of quantitative PCR for the diagnosis of invasive aspergillosis with bronchoalveolar lavage fluid. BMC Infectious Diseases. 2008;8, article 73 doi: 10.1186/1471-2334-8-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Srinivasan S, Liu C, Mitchell CM, et al. Temporal variability of human vaginal bacteria and relationship with bacterial vaginosis. Plos ONE. 2010;5(4) doi: 10.1371/journal.pone.0010197. Article ID e10197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hummelen R, Changalucha J, Butamanya NL, Cook A, Habbema JDF, Reid G. Lactobacillus rhamnosus GR-1 and L. reuteri RC-14 to prevent or cure bacterial vaginosis among women with HIV. International Journal of Gynecology and Obstetrics. 2010;111(3):245–248. doi: 10.1016/j.ijgo.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 34.Larsson PG, Stray-Pedersen B, Ryttig KR, Larsen S. Human lactobacilli as supplementation of clindamycin to patients with bacterial vaginosis reduce the recurrence rate; a 6-month, double-blind, randomized, placebo-controlled study. BMC Women’s Health. 2008;8, article 3 doi: 10.1186/1472-6874-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinez RCR, Franceschini SA, Patta MC, et al. Improved cure of bacterial vaginosis with single dose of tinidazole (2 g), Lactobacillus rhamnosus GR-1, and Lactobacillus reuteri RC-14: a randomized, double-blind, placebo-controlled trial. Canadian Journal of Microbiology. 2009;55(2):133–138. doi: 10.1139/w08-102. [DOI] [PubMed] [Google Scholar]
- 36.Mastromarino P, Macchia S, Meggiorini L, et al. Effectiveness of Lactobacillus-containing vaginal tablets in the treatment of symptomatic bacterial vaginosis. Clinical Microbiology and Infection. 2009;15(1):67–74. doi: 10.1111/j.1469-0691.2008.02112.x. [DOI] [PubMed] [Google Scholar]
- 37.Antonio MAD, Meyn LA, Murray PJ, Busse B, Hillier SL. Vaginal colonization by probiotic lactobacillus crispatus CTV-05 is decreased by sexual activity and endogenous lactobacilli. Journal of Infectious Diseases. 2009;199(10):1506–1513. doi: 10.1086/598686. [DOI] [PubMed] [Google Scholar]
- 38.Nyirjesy P, McIntosh MJ, Gattermeir DJ, Schumacher RJ, Steinmetz JI, Joffrion JL. The effects of intravaginal clindamycin and metronidazole therapy on vaginal lactobacilli in patients with bacterial vaginosis. American Journal of Obstetrics and Gynecology. 2006;194(5):1277–1282. doi: 10.1016/j.ajog.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 39.Mitchell CM, Hitti JE, Agnew KJ, Fredricks DN. Comparison of oral and vaginal metronidazole for treatment of bacterial vaginosis in pregnancy: impact on fastidious bacteria. BMC Infectious Diseases. 2009;9, article 89 doi: 10.1186/1471-2334-9-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beigi RH, Wiesenfeld HC, Hillier SL, Straw T, Krohn MA. Factors associated with absence of H2O2-producing Lactobacillus among women with bacterial vaginosis. Journal of Infectious Diseases. 2005;191(6):924–929. doi: 10.1086/428288. [DOI] [PubMed] [Google Scholar]


