Abstract
Limited data are available on inappropriate shocks in Korean patients implanted with an implantable cardioverter-defibrillator (ICD). We investigated the impact of inappropriate shocks on clinical outcomes. This retrospective, single-center study included 148 patients treated between October 1999 and June 2011. The primary outcome was a composite event of all-cause mortality or hospitalization for any cardiac reason. The median follow-up duration was 29 months (interquartile range: 8 to 53). One or more inappropriate shocks occurred in 34 (23.0%) patients. A history of atrial fibrillation was the only independent predictor of inappropriate shock (hazard ratio [HR]: 4.16, 95% confidence interval [CI]: 1.89-9.15, P < 0.001). Atrial fibrillation was the most common cause of inappropriate shock (67.7%), followed by supraventricular tachycardia (23.5%), and abnormal sensing (8.8%). A composite event of all-cause mortality or hospitalizations for any cardiac reason during follow-up was not significantly different between patients with or without inappropriate shock (inappropriate shock vs no inappropriate shock: 35.3% vs 35.4%, adjusted HR: 1.06, 95% CI: 0.49-2.29, P = 0.877). Inappropriate shocks do not affect clinical outcomes in patients implanted with an ICD, although the incidence of inappropriate shocks is high.
Keywords: Implantable Cardioverter-Defibrillator, Inappropriate Shock
INTRODUCTION
Implantable cardioverter-defibrillator (ICD) therapy reduces mortality in survivors of sudden cardiac arrest and patients at high risk for cardiovascular disease (1-5). Despite the proven survival benefits of an ICD, a common adverse effect is inappropriate shocks. Inappropriate shocks occur following atrial arrhythmias with rapid ventricular conduction or sensing errors and can be painful, impair the quality of life, cause psychiatric disturbances, and be potentially arrhythmogenic (6, 7). Recent studies on the relationship between inappropriate shocks and clinical outcomes have shown mixed results (8, 9). Therefore, we investigated the impact of inappropriate shock on long-term clinical outcomes in patients implanted with an ICD as primary and secondary prevention of sudden cardiac death.
MATERIALS AND METHODS
Study population
The medical records of 148 patients implanted with an ICD between October 1999 and June 2011 at Samsung Medical Center in Seoul, Korea were analyzed retrospectively. Any additional information was collected by contacting general practitioners, reviewing hospital records, and conducting telephone interviews. The primary outcome was a composite event of all-cause mortality or hospitalization for cardiac causes. The secondary outcome was all-cause mortality during follow-up.
ICD implantation
The indication for ICD implantation was secondary prevention in patients who had experienced aborted sudden cardiac death, sustained ventricular tachyarrhythmia, or presumed tachyarrhythmic syncopal attacks. The indication for ICD implantation in all other patients was primary prevention. All defibrillator systems were implanted in the pectoral region. The ICDs were manufactured by St. Jude Medical, Inc. (St. Paul, MN, USA), Medtronic, Inc (Minneapolis, MN, USA) or Guidant Corp (Indianapolis, IN, USA). We often attempted to tailor the therapy based on electrophysiology studies, a patient's arrhythmia history, or both (patient-specific tailored programming). The ICDs can be programmed to provide different therapies for tachyarrhythmias in up to three heart rate zones. The ICD can deliver bursts of antitachycardia pacing, cardioversion, or defibrillation in each therapy zone. Although a variety of algorithms exist, antitachycardia pacing was usually delivered at a slightly faster rate (a cycle length 10% to 12% shorter) than the rate of the detected tachycardia. Devices often delivered synchronized cardioversion for tachyarrhythmias in this range (heart rate below 160 or 180 beats/min), and usually delivered unsynchronized shocks for very rapid ventricular arrhythmias (heart rate > 180 or 200 beats/min). Furthermore, most devices offered 3 algorithms intended to minimize inappropriate shocks: 1) "stability", detecting irregularity in cycle length of the tachyarrhythmia; 2) "sudden onset", monitoring the cycle length for the sudden or abrupt onset of a high ventricular rate rather than a gradually increasing heart rate (10); and 3) "QRS morphology", comparing the electrograms during the tachycardia to the baseline QRS shape, duration, and polarity. The dual-chamber devices provided additional algorithms evaluating the atrial rate (11). The ICD programming, including such discriminator usage, was left to the discretion of the operators.
Follow-up
After ICDs were implanted, patients were followed in our outpatient ICD clinic. The devices were interrogated, and the complete data set (including intracardiac electrograms) was recorded. The delivered therapy was adjudicated by a trained electrophysiologist. Appropriate ICD therapy was defined as either cardioversion, defibrillation of ventricular tachycardia (VT), or defibrillation of ventricular fibrillation (VF) based on the stored electrogram. An inappropriate shock was defined as an episode, starting with a shock not delivered for VT or VF and ending when the ICD detected sinus rhythm. The rhythm triggering therapy was categorized as atrial fibrillation or atrial flutter, supraventricular including sinus tachycardia, or abnormal sensing.
Statistical analysis
All values are presented as the mean ± standard deviation or median with interquartile range. Comparisons between continuous variables were tested using the t-test or Mann-Whitney U test, as appropriate. Categorical data were tested using the chi-square test or Fisher's exact test, as appropriate. Event-free survival was estimated by the Kaplan-Meier method and compared using the log-rank test. Adjusted hazard rates were compared by multivariable Cox proportional-hazards regression analysis. Covariates that were statistically significant by univariate analysis or that were clinically relevant were considered candidate variables for the multivariate models. The relationship between inappropriate shocks and clinical outcomes was assessed using a Cox proportional-hazards model adjusting for the following covariates: age > 70 yr, previous coronary bypass surgery, history of atrial fibrillation, left ventricular ejection fraction < 40%, serum creatinine ≥ 1.5 (mg/dL), interim appropriate shocks, interim inappropriate shocks, and device type. All tests were two-tailed, and P < 0.05 was considered statistically significant. All analyses were performed with SPSS software version 18.0 (SPSS Inc., Chicago, IL, USA).
Ethics statement
The institutional review board at Samsung Seoul Hospital approved this study (IRB No. 2009-09-047). All of subjected patients gave written informed consent for the ICD implantation procedure.
RESULTS
Baseline characteristics and etiology of inappropriate shocks
A total of 148 patients with an ICD were identified from the ICD database at Samsung Medical Center. Of these, 34 (23.0%) patients experienced one or more episodes of inappropriate ICD shocks. Baseline clinical characteristics are provided in Table 1. Overall, 34 patients were implanted with an ICD for primary prevention and 114 patients for secondary prevention of sudden cardiac death. The two groups had largely similar clinical characteristics, except significantly fewer inappropriate shock recipients had prior coronary bypass surgery than nonrecipients (0 vs 13.2%, P = 0.023). During the follow-up period, 5 (15.6%) and 58 patients (50.0%) with an ICD for primary and secondary prevention, respectively, had an appropriate shock (P < 0.001). Six (18.8%) and 28 patients (24.1%) with an ICD for primary and secondary prevention, respectively, had an inappropriate shock (P = 0.521). Atrial fibrillation or atrial flutter was the most common cause of inappropriate shock (67.7%), followed by supraventricular tachycardia including sinus tachycardia (23.5%), and then abnormal sensing (8.8%). Of these, two patients suffered inappropriate shocks because of T-wave oversensing and one patient experienced inappropriate shocks because of oversensing due to noise. We analyzed the heart rate at the time of a patient's first inappropriate shock for atrial fibrillation or atrial flutter, and supraventricular tachycardia including sinus tachycardia, but not for shocks due to abnormal sensing. The mean ventricular rate that triggered inappropriate shock for atrial fibrillation or atrial flutter was 179 ± 28 beats/min, and for supraventricular tachycardia including sinus tachycardia it was 172.4 ± 23.2 beats/min.
Table 1.
Baseline patient characteristics
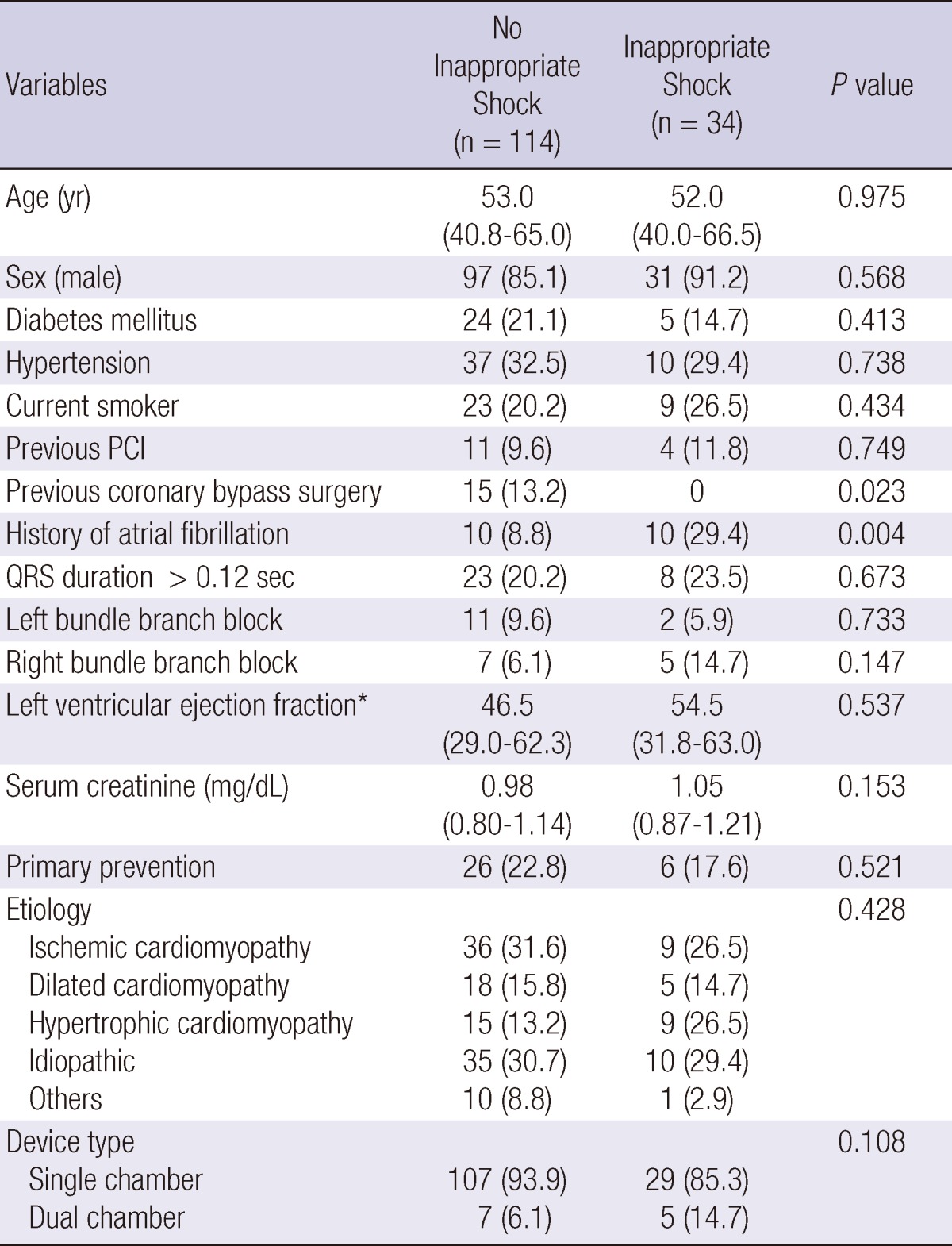
Values are median with interquartile range or No. (%). Others = There were 6 Brugada syndrome, 1 long QT syndrome, 1 arrhythmogenic right ventricle dysplasia, 1 Ebstein anormaly, 1 valvular heart disease in no appropriate shock group and 1 ARVD in appropriate shock group. PCI, percutaneous coronary intervention; ARVD, arrhythmogenic right ventricular dysplasia.
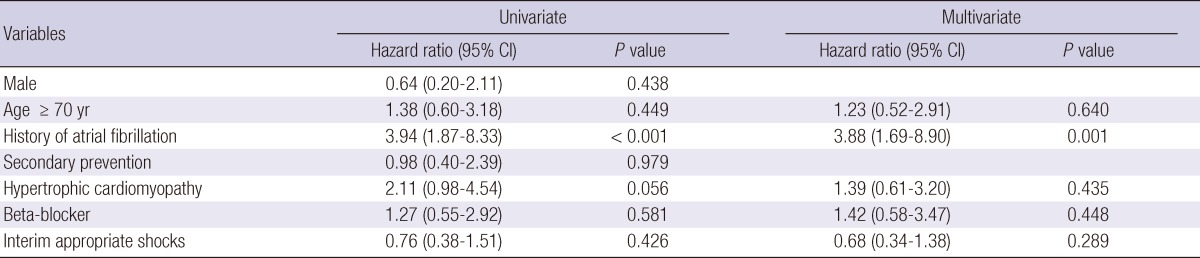
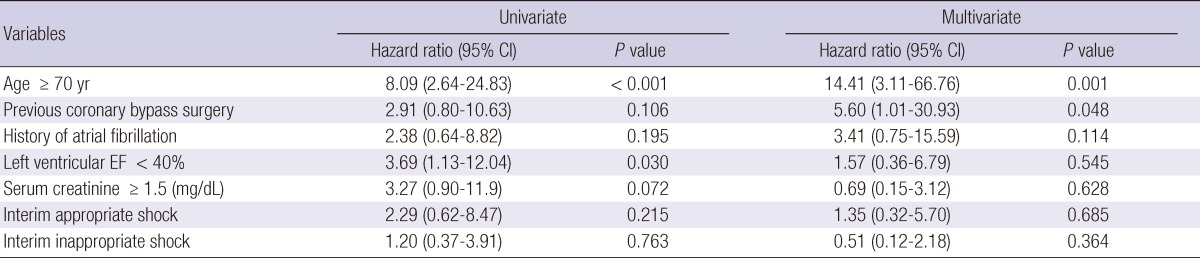
Predictors of inappropriate shock and mortality during follow-up
Cox regression analysis was performed to identify predictors of inappropriate shock (Table 2). Sex, age ≥ 70 yr, secondary prevention, hypertrophic cardiomyopathy, use of beta-blocker, and interim appropriate shock did not influence inappropriate shock in the total patient population. The only significant univariate predictor of inappropriate shock was a history of atrial fibrillation. In the multivariate Cox regression model, the only independent predictor of inappropriate shock was a history of atrial fibrillation. Cox regression analysis was also performed to recognize predictors of mortality (Table 3). Previous coronary bypass surgery, history of atrial fibrillation, serum creatinine ≥ 1.5 (mg/dL), interim appropriate shock, and interim inappropriate shock did not influence mortality in the total patient population. The significant univariate predictors of mortality were age ≥ 70 yr and left ventricular ejection fraction < 40%. In the multivariate Cox regression model, the independent predictors for mortality were age ≥ 70 yr and previous coronary bypass surgery.
Table 2.
Predictors of ≥ 1 inappropriate shocks
Adjusted covariates include age > 70 yr, history of atrial fibrillation, hypertrophic cardiomyopathy, use of beta-blocker at discharge, and interim appropriate shocks. CI, confidence interval.
Table 3.
Predictors of all-cause mortality
Adjusted covariates include age > 70 yr, previous coronary bypass surgery, history of atrial fibrillation, left ventricular ejection fraction < 40%, serum creatinine ≥ 1.5 (mg/dL), interim appropriate shocks, interim inappropriate shock, and device type. CI, confidence interval; EF, ejection fraction.
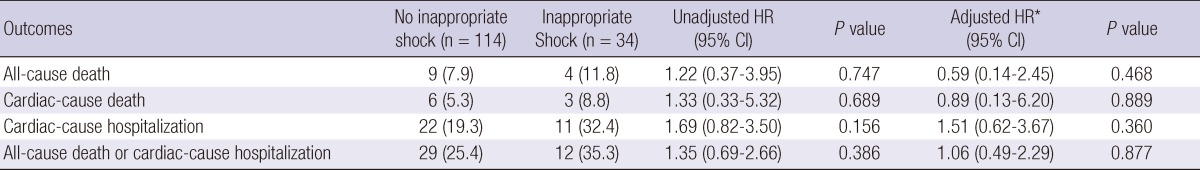
Clinical outcomes
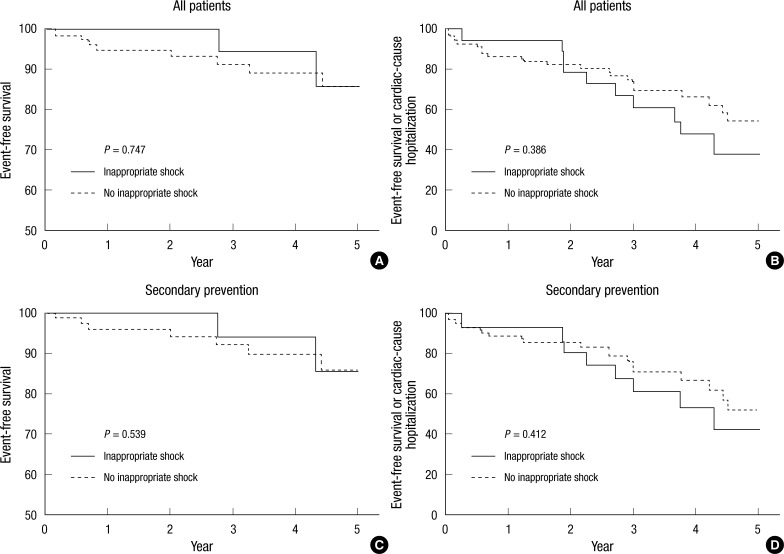
The median follow-up duration was 29 months (interquartile range: 8 to 53). Table 4 and Fig. 1 show the cumulative clinical outcomes of the study population during the follow-up period. The composite event of all-cause mortality or hospitalization for any cardiac cause was high in the inappropriate shock group, but the difference between groups was not statistically significant (25.4% for no inappropriate shock vs 35.3% for inappropriate shock, adjusted hazard ratio [HR]: 1.06, 95% confidence interval [CI]: 0.49-2.29, P = 0.877). During the follow-up period, there were no significant differences between groups for all-cause mortality (7.9% vs 11.8%, adjusted HR: 0.59, 95% CI: 0.14-2.45, P = 0.468), death due to cardiac causes (5.3% vs 8.8%, adjusted HR: 0.89, 95% CI: 0.13-6.20, P = 0.889), and hospitalization due to cardiac causes (19.3% vs 32.4%, adjusted HR: 1.51, 95% CI: 0.62-3.67, P = 0.360). In patients implanted for secondary prevention, the incidences of combined all-cause mortality and hospitalization due to a cardiac cause, as well as all-cause mortality only between the two groups did not differ significantly (P = 0.412 for combined all-cause mortality or hospitalization due to a cardiac cause, P = 0.539 for all-cause mortality only).
Table 4.
Clinical outcomes in inappropriate shock group compared with no inappropriate shock during follow-up period.
Values are No. (%). *Adjusted covariates include age > 70 yr, previous coronary bypass surgery, history of atrial fibrillation, left ventricular ejection fraction < 40%, serum creatinine ≥ 1.5 (mg/dL), interim appropriate shocks, interim inappropriate shock, and device type. CI, confidence interval; HR, hazard ratio.
Fig. 1.
Kaplan-Meier curves of inappropriate shock versus no inappropriate shock. (A) All-cause mortality in inappropriate shock (solid) versus no inappropriate shock (dashed). (B) A composite event of all-cause mortality or hospitalization for any cardiac reason in inappropriate shock versus no inappropriate shock. (C) All-cause mortality in inappropriate shock versus no inappropriate shock in patients for second prevention of sudden cardiac death. (D) A composite event of all-cause mortality or hospitalization for any cardiac reason in inappropriate shock versus no inappropriate shock in patients for second prevention of sudden cardiac death.
DISCUSSION
We investigated the predictors and impacts of inappropriate shocks on long-term clinical outcome in patients implanted with an ICD for primary and secondary prevention of sudden cardiac death using a retrospective registry in Korea. The only predictor of inappropriate shock was a history of atrial fibrillation. Inappropriate shocks did not affect mortality and/or hospitalization for cardiac causes during the follow-up period, although the incidence of inappropriate shocks was high.
Large-scale studies have estimated the incidence of inappropriate shock in patients implanted with an ICD to range from 10% to 24% (8, 12-14). Similarly, 34 patients (23.0%) in our study experienced at least one inappropriate shock. Of these, 18 (52.9%) had experienced two or more inappropriate shocks. The most common cause of inappropriate shocks is atrial fibrillation or atrial flutter, and several studies have demonstrated that a history of atrial fibrillation is a consistent clinical predictor of inappropriate shock (12, 13, 15). Similarly, 66.7% of inappropriate shocks in our study were due to atrial fibrillation or atrial flutter. Additionally, multivariate analysis showed that a history of atrial fibrillation was the only significant predictor of inappropriate shocks. In contrast to previous studies, young age, the absence of coronary artery disease, and the use of beta-blockers were not predictors of inappropriate shocks in our study. In younger patients, inappropriate shocks were mainly caused by abnormal sensing and sinus tachycardia (16). In the current study, young age may not have been a predictor of inappropriate shock because only 8.8% patients had inappropriate shocks by abnormal sensing.
Although the impact of inappropriate shock on clinical outcome is unclear, it reduces quality of life due to pain and psychological morbidity (17, 18). Strategies to reduce inappropriate therapy using device programming rely on the ability to distinguish supraventricular and atrial arrhythmias from VT. Advanced algorithms, multiple sensing leads, and improved device programming should reduce the occurrence of inappropriate shock (19-22). In this study, ICDs were reprogrammed in 15 patients with an inappropriate shock. Of these, only one patient experienced additional inappropriate shocks.
In the Multicenter Automatic Defibrillator Implantation Trial-II (MADIT-II), Daubert et al. (13) reported that increased mortality in patients experiencing inappropriate shocks might be caused by adverse direct mechanical, arrhythmic, or hemodynamic effects of the shocks themselves, such as fatal proarrhythmia. In contrast to this and other reports (23, 24), inappropriate shocks had no impact on clinical outcome in our study. Dichtl et al. (8) also found that inappropriate shocks did not affect survival in a large number of patients with an ICD. Thus, further large-cohort studies with a long follow-up are needed to provide more accurate data on the relationship between inappropriate shock and clinical outcome.
Our study has several limitations. First, it was nonrandomized, retrospective, and observational study, so confounding factors might have affected the results. Although we performed risk adjustments for potential confounding factors, we were not able to correct for unmeasured variables. Second, as all patients were from a single center, our observations and conclusions cannot necessarily be generalized. Third, the relatively small size of our study population may have influenced our results. Lastly, the relationship between inappropriate shocks and quality of life could not be assessed because quality of life data were not collected.
In conclusion, inappropriate shocks do not affect clinical outcomes in ICD recipients, although the incidence of inappropriate shocks is high, mainly due to atrial fibrillation. A long-term and large-scale randomized trial is needed to validate the clinical findings in this study.
References
- 1.Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, Levine JH, Saksena S, Waldo AL, Wilber D, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. N Engl J Med. 1996;335:1933–1940. doi: 10.1056/NEJM199612263352601. [DOI] [PubMed] [Google Scholar]
- 2.Kadish A, Dyer A, Daubert JP, Quigg R, Estes NA, Anderson KP, Calkins H, Hoch D, Goldberger J, Shalaby A, et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350:2151–2158. doi: 10.1056/NEJMoa033088. [DOI] [PubMed] [Google Scholar]
- 3.Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 4.Kim YH, Kim JS. Clinical characteristics in patients with implantable cardioverter-defibrillator (ICD) Korean Circ J. 2004;34:395–404. [Google Scholar]
- 5.Song PS, Kim JS, Shin DH, Park JW, Bae KI, Lee CH, Jung DC, Ryu DR, On YK. Electrical storms in patients with an implantable cardioverter defibrillator. Yonsei Med J. 2011;52:26–32. doi: 10.3349/ymj.2011.52.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schron EB, Exner DV, Yao Q, Jenkins LS, Steinberg JS, Cook JR, Kutalek SP, Friedman PL, Bubien RS, Page RL, et al. Quality of life in the antiarrhythmics versus implantable defibrillators trial: impact of therapy and influence of adverse symptoms and defibrillator shocks. Circulation. 2002;105:589–594. doi: 10.1161/hc0502.103330. [DOI] [PubMed] [Google Scholar]
- 7.Lüderitz B, Jung W, Deister A, Marneros A, Manz M. Patient acceptance of the implantable cardioverter defibrillator in ventricular tachyarrhythmias. Pacing Clin Electrophysiol. 1993;16:1815–1821. doi: 10.1111/j.1540-8159.1993.tb01816.x. [DOI] [PubMed] [Google Scholar]
- 8.Dichtl W, Wolber T, Paoli U, Brüllmann S, Stühlinger M, Berger T, Spuller K, Strasak A, Pachinger O, Haegeli LM, et al. Appropriate therapy but not inappropriate shocks predict survival in implantable cardioverter defibrillator patients. Clin Cardiol. 2011;34:433–436. doi: 10.1002/clc.20910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cevik C, Perez-Verdia A, Nugent K. Implantable cardioverter defibrillators and their role in heart failure progression. Europace. 2009;11:710–715. doi: 10.1093/europace/eup091. [DOI] [PubMed] [Google Scholar]
- 10.Higgins SL, Lee RS, Kramer RL. Stability: an ICD detection criterion for discriminating atrial fibrillation from ventricular tachycardia. J Cardiovasc Electrophysiol. 1995;6:1081–1088. doi: 10.1111/j.1540-8167.1995.tb00385.x. [DOI] [PubMed] [Google Scholar]
- 11.Kühlkamp V, Dörnberger V, Mewis C, Suchalla R, Bosch RF, Seipel L. Clinical experience with the new detection algorithms for atrial fibrillation of a defibrillator with dual chamber sensing and pacing. J Cardiovasc Electrophysiol. 1999;10:905–915. doi: 10.1111/j.1540-8167.1999.tb01261.x. [DOI] [PubMed] [Google Scholar]
- 12.van Rees JB, Borleffs CJ, de Bie MK, Stijnen T, van Erven L, Bax JJ, Schalij MJ. Inappropriate implantable cardioverter-defibrillator shocks: incidence, predictors, and impact on mortality. J Am Coll Cardiol. 2011;57:556–562. doi: 10.1016/j.jacc.2010.06.059. [DOI] [PubMed] [Google Scholar]
- 13.Daubert JP, Zareba W, Cannom DS, McNitt S, Rosero SZ, Wang P, Schuger C, Steinberg JS, Higgins SL, Wilber DJ, et al. Inappropriate implantable cardioverter-defibrillator shocks in MADIT II: frequency, mechanisms, predictors, and survival impact. J Am Coll Cardiol. 2008;51:1357–1365. doi: 10.1016/j.jacc.2007.09.073. [DOI] [PubMed] [Google Scholar]
- 14.Germano JJ, Reynolds M, Essebag V, Josephson ME. Frequency and causes of implantable cardioverter-defibrillator therapies: is device therapy proarrhythmic? Am J Cardiol. 2006;97:1255–1261. doi: 10.1016/j.amjcard.2005.11.048. [DOI] [PubMed] [Google Scholar]
- 15.Bhavnani SP, Coleman CI, White CM, Clyne CA, Yarlagadda R, Guertin D, Kluger J. Association between statin therapy and reductions in atrial fibrillation or flutter and inappropriate shock therapy. Europace. 2008;10:854–859. doi: 10.1093/europace/eun128. [DOI] [PubMed] [Google Scholar]
- 16.Kleemann T, Becker T, Doenges K, Vater M, Senges J, Schneider S, Saggau W, Weisse U, Seidl K. Annual rate of transvenous defibrillation lead defects in implantable cardioverter-defibrillators over a period of >10 years. Circulation. 2007;115:2474–2480. doi: 10.1161/CIRCULATIONAHA.106.663807. [DOI] [PubMed] [Google Scholar]
- 17.Keren A, Sears SF, Nery P, Shaw J, Green MS, Lemery R, Gollob MH, Amyotte B, Birnie DH. Psychological adjustment in ICD patients living with advisory fidelis leads. J Cardiovasc Electrophysiol. 2011;22:57–63. doi: 10.1111/j.1540-8167.2010.01867.x. [DOI] [PubMed] [Google Scholar]
- 18.Marcus GM, Chan DW, Redberg RF. Recollection of pain due to inappropriate versus appropriate implantable cardioverter-defibrillator shocks. Pacing Clin Electrophysiol. 2011;34:348–353. doi: 10.1111/j.1540-8159.2010.02971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lo R, Al-Ahmad A, Hsia H, Zei PC, Wang PJ. Optimal Programming of ICDs for Prevention of Appropriate and Inappropriate Shocks. Curr Treat Options Cardiovasc Med. 2008;10:408–416. doi: 10.1007/s11936-008-0032-y. [DOI] [PubMed] [Google Scholar]
- 20.Swerdlow CD, Gunderson BD, Ousdigian KT, Abeyratne A, Sachanandani H, Ellenbogen KA. Downloadable software algorithm reduces inappropriate shocks caused by implantable cardioverter-defibrillator lead fractures: a prospective study. Circulation. 2010;122:1449–1455. doi: 10.1161/CIRCULATIONAHA.110.962407. [DOI] [PubMed] [Google Scholar]
- 21.Auricchio A, Meijer A, Kurita T, Schloss E, Brinkman K, Claessens-van Ooijen M, Sterns L. Safety, efficacy, and performance of new discrimination algorithms to reduce inappropriate and unnecessary shocks: the PainFree SST clinical study design. Europace. 2011;13:1484–1493. doi: 10.1093/europace/eur133. [DOI] [PubMed] [Google Scholar]
- 22.Gold MR, Ahmad S, Browne K, Berg KC, Thackeray L, Berger RD. Prospective comparison of discrimination algorithms to prevent inappropriate ICD therapy: primary results of the Rhythm ID Going Head to Head Trial. Heart Rhythm. 2012;9:370–377. doi: 10.1016/j.hrthm.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 23.Moss AJ, Greenberg H, Case RB, Zareba W, Hall WJ, Brown MW, Daubert JP, McNitt S, Andrews ML, Elkin AD. Long-term clinical course of patients after termination of ventricular tachyarrhythmia by an implanted defibrillator. Circulation. 2004;110:3760–3765. doi: 10.1161/01.CIR.0000150390.04704.B7. [DOI] [PubMed] [Google Scholar]
- 24.Poole JE, Johnson GW, Hellkamp AS, Anderson J, Callans DJ, Raitt MH, Reddy RK, Marchlinski FE, Yee R, Guarnieri T, et al. Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med. 2008;359:1009–1017. doi: 10.1056/NEJMoa071098. [DOI] [PMC free article] [PubMed] [Google Scholar]