Abstract
Penicillium marneffei may cause life-threatening systemic fungal infection in immune-compromised patients and it is endemic in Southeast Asia. A 39-yr-old HIV-infected male, living in Laos, presented with fever, cough, and facial vesiculopapular lesions, which had been apparent for two weeks. CT scans showed bilateral micronodules on both lungs; Pneumocystis jirovecii was identified by bronchoscopic biopsy. Despite trimethoprim-sulfamethoxazole and anti-tuberculosis medications, the lung lesions progressed and the facial lesions revealed central umbilications. Biopsy of the skin lesions confirmed disseminated penicilliosis, with the culture showing P. marneffei hyphae and spores. The P. marneffei was identified by rRNA PCR. A review of the bronchoscopic biopsy indicated penicilliosis. The patient completely recovered after being prescribed amphotericin-B and receiving antiretroviral therapy. This is the first case of penicilliosis in a Korean HIV-infected patient. It is necessary to consider P. marneffei when immunocompromised patients, with a history of visits to endemic areas, reveal respiratory disease.
Keywords: Penicillium marneffei, HIV/AIDS, Korean, Disseminated Infection
INTRODUCTION
Penicillium marneffei can cause a life-threatening systemic fungal infection in human immunodeficiency virus (HIV)-infected patients, and is prevalent in Southeast Asia, including Thailand, northeastern India, southern China, Hong Kong, Vietnam, and Taiwan. In 1956, the first P. marneffei isolation was reported in the bamboo rat's liver (1) and the first human infection was in a 61-yr-old American missionary who lived in Southeast Asia and suffered from Hodgkin's lymphoma in 1973 (2). In 1988, the first disseminated P. marneffei infection case was reported in a HIV-infected patient (3). P. marneffei might cause systemic infection in an immunocompetent host, but most of the reported cases of disseminated infection were related to HIV-infected patients.
In endemic areas, the P. marneffei infection is regarded as an acquired immune deficiency syndrome (AIDS) marker, or an "AIDS defining illness," following tuberculosis and cryptococcosis in frequency (4). However, as overseas travel increases, sporadic cases of P. marneffei infection have been reported among Americans, Europeans, Australians, Japanese, and Africans who resided in or visited the endemic areas (5).
Here we report on the first case of P. marneffei in a Korean HIV-infected patient who had been living in the endemic area. The infection was confirmed as penicilliosis through fungal culture and molecular analysis.
CASE DESCRIPTION
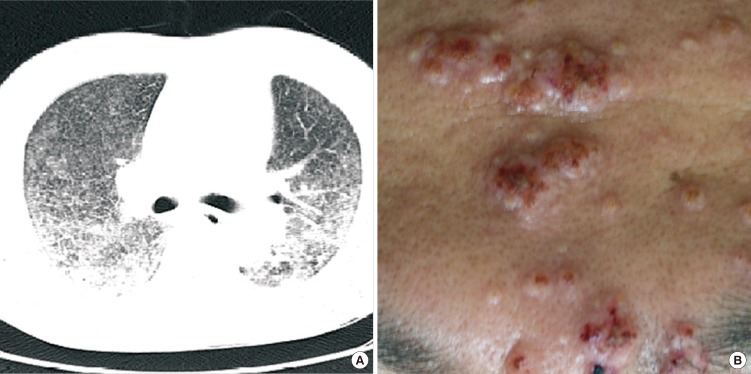
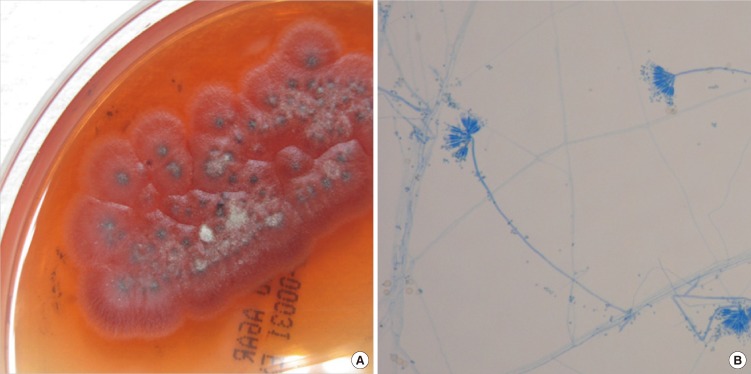
A 39-yr-old male patient visited the emergency room on May 24, 2010 with fever, cough, and multiple papules that had been on his face for two weeks. He had been managing a farm in Laos for four years. He was told that he had been infected with HIV and miliary tuberculosis was suspected after a chest computed tomography (CT) scan at a hospital in Thailand. The patient denied any history of homosexual contact. Upon arrival at the emergency room, his mental status was alert and the diffuse vesiculopapular lesions were easily observed on face. His vital signs were: blood pressure of 151/90 mmHg, a body temperature of 37.1℃, a heart rate of 116 beats per minute, and a respiration rate of 27 breaths per minute. His oxygen saturation was 90% and arterial blood gas analysis revealed pH 7.46, PaCO2 34 mmHg, PaO2 55 mmHg, and HCO3 24 mM on room air. His breathing sounds were coarse with crackles in the bilateral lung field. Upon laboratory examination, his complete blood cell counts were: white blood cell count 4,100/µL (neutrophil 92.0%, lymphocytes 4.8%), hemoglobin 11.4 g/dL, and platelet 134,000/µL, while erythrocyte sedimentation rate and C-reactive protein were 114 mm/hr and 90 mg/L, respectively. Peripheral CD4+ T lymphocyte count and HIV-RNA viral load were 7 cells/µL and 457,392 copies/mL. A chest X-ray revealed diffuse reticulonodular opacities in the entire lung field. Chest CT scans revealed bilaterally distributed micronodules and consolidation with a ground-glass opacity pattern on a dependent portion of his lungs (Fig. 1A). Suspecting tuberculosis, anti-tuberculosis medications of isoniazid, rifampin, ethambutol, and pyrazinamide were started. In addition, the results of the trans-bronchoscopic lung biopsy were initially reported as Pneumocystis jirovecii and trimethoprim-sulfamethoxazole was added to his regimen. One week after admission, vesiculopapular facial lesions exhibited central hemorrhagic changes (Fig. 1B) and spread to the neck, trunk, and upper extremities. A skin biopsy was performed, which revealed massive dermatophytosis and fungal abscess. Deoxycholate amphotericin-B (0.7 mg/kg) was started and antiretroviral therapy (ART) with abacavir, lamivudine, and efavirenz was also implemented for the treatment of HIV infection. Considering the typical skin lesions and fungal organisms in the biopsy, a disseminated P. marneffei infection was suspected. Fungal culture of skin tissue obtained by biopsy revealed colonies of green-gray belts with red pigmentation in Sabouraud dextrose agar plate (Fig. 2A) and mycelia was seen in the microscopic examination after staining with Lactophenol cotton blue by touching clear scotch tape to the colony (Fig. 2B). To identify the fungal species, we performed sequencing of the internal transcribed spacer (ITS) and 26S rRNA gene regions. The ITS region (including the 5.8S rRNA gene) and the 26S rRNA gene D1/D2 domains were amplified with the primer pairs of pITS-F/pITS-R and NL1/NL4, respectively (6). Sequence similarity searches were performed using basic local alignment search tool (BLAST), which revealed a complete (100%) match with P. marneffei. After 10 days of amphotericin-B administration, the patient exhibited clinical and radiological improvement. The amphotericin-B was changed to oral itraconazole at 400 mg/day after 14 days of the amphotericin-B treatment. After eight weeks, the dose of itraconazole was modified to 200 mg/day and was maintained for an additional six months. The patient continues to visit an outpatient clinic without any specific problems.
Fig. 1.
Clinical features of disseminated P. marneffei infection. (A) Initial chest CT findings. Bilaterally distributed multiple small nodules and consolidation with ground-glass opacity pattern were observed in dependent portions. (B) Skin lesions one week after admission. Multiple erythematous papular lesions in forehead revealed central umbilication.
Fig. 2.
Penicillium marneffei cultured at 25℃ on Sabouraud dextrose agar plate. (A) Gross findings of culture. Colonies revealed distinctive red diffusible pigment and the surface was powdery and gray-green with a white border. (B) Microscopic findings. Lactophenol cotton blue stain preparation from colony revealed metulae and conidia of Penicillium marneffei.
DISCUSSION
We reported the first case of P. marneffei in a Korean, HIV-infected patient who had lived in a P. marneffei endemic area. Primary human infections of P. marneffei have been known to be acquired by inhalation of spores from soil which are then disseminated to the skin, the reticuloendothelial system and the gut by hematogenous spread and other organ systems. Penicilliosis in HIV-infected patients may be misdiagnosed as Mycobacterium tuberculosis because multiple nodules and mediastinal lymphadenopathy are radiologic findings of penicilliosis (7, 8). Fungal cultures or direct staining of infected tissues is required to differentiate penicilliosis from tuberculosis.
In addition, diffuse alveolar shadows have been observed in about 10% of patients infected with P. marneffei (4, 9), which may reveal intense parenchymal opacification like acute respiratory distress syndrome. Therefore, penicilliosis may also be misdiagnosed as Pneumocystis jirovecii pneumonia (PCP) (10). PCP rarely reveals pleural effusions, while P. marneffei infection sometimes accompany pleural effusion. Sputum examination may also be helpful to differentiate PCP from a P. marneffei infection (11, 12).
In our case, an initial chest CT scan showed bilaterally distributed micronodules and consolidation with a diffuse ground-glass opacity pattern and a trans-bronchoscopic lung biopsy result was initially misinterpreted as Pneumocystis jirovecii. Therefore, we suspected a pulmonary tuberculosis and PCP co-infection. Clinical improvement, however, was not obvious despite anti-tuberculosis medication and trimethoprim-sulfamethoxazole administration. This led us to suspect the P. marneffei infection, considering typical skin lesions revealed fungal organisms in the biopsy and an aggravated pulmonary infiltration.
Unlikely pulmonary presentations, skin lesions are relatively characteristic in penicilliosis. The proportion of patients with skin lesion has been reported between 28% and 71% in disseminated penicilliosis (4, 9). Skin lesions present as multiple flesh-colored, dome-shaped papules in the early stage, similar to molluscum contagiosum (13). However, simple papules observed on the face, trunk, neck and extremities frequently ulcerate over time and change with central necrotic umbilication (5, 14). Molluscum contagiosum resolves by itself in six months to two years in HIV-infected patients in accordance with immune function improvement after ART. But skin lesions of P. marneffei are not expected to improve without active antifungal treatment.
P. marneffei mainly affects people with impaired cellular immunity (i.e. HIV-infected patients) (15) and the severity and clinical manifestations depend on the patient's immunity (16). In patients with normal immunity, P. marneffei mostly causes mild and localized infections, but it can cause severe disseminated infections with generalized lymphadenopathy and persistent fever in immunocompromised HIV-infected patients (5). It is known that the prognosis of penicilliosis can be improved if immunity could be recovered during the antifungal treatment in immunocompromised patients (9, 15).
There have been two case reports of P. marneffei infection in the Republic of Korea. One case was a continuous ambulatory peritoneal dialysis-associated peritonitis which revealed improvement after catheter removal and antifungal therapy (17). The second case was a disseminated P. marneffei infection which occurred after a visit to China to receive a liver transplant, and that area of China is a P. marneffei endemic area (18). This patient was taking immunosuppressive therapy of tacrolimus and mycophenolate. Lymphocytopenia, which is considered a marker of impaired cellular immunity, was observed in both the liver transplant recipient (lymphocyte count 410 cells/µL) and our HIV-infected patient (lymphocyte count 197 cells/µL, CD4+ T lymphocyte count 7 cells/µL). While both patients also exhibited a disseminated P. marneffei infection, the liver transplant recipient died due to refractory viral and bacterial infections, despite intensive treatment. In previous reports, the mortality rate of patients with P. marneffei infection was reported as 13.5% (7/52) in HIV-infected patients and 60% (6/10) in non-HIV infected patients (9, 15). In our case, the patient exhibited a good clinical outcome although he initially presented severe disseminated penicilliosis. We suppose that recovery depends upon the immune recovery related with ART and is similar to findings of previous studies which revealed a better outcome in HIV-infected penicilliosis patients compared to the non-HIV-infected patients.
The authors experienced the first case of P. marneffei in a Korean HIV-infected patient. We suppose that it is important to consider P. marneffei when immunocompromised patients with histories of visits to areas of endemicity reveal respiratory disease, generalized lymphadenopathy, and central umbilicated vesicular skin lesions.
References
- 1.Capponi M, Sureau P, Segretain G. Penicilliosis de Rhizomys sinensis. Bull Sco Pathol Exot Filiales. 1956;49:418–421. [PubMed] [Google Scholar]
- 2.DiSalvo AF, Ficking AM, Ajello L. Infection caused by Penicillium marneffei: description of first natural infection in man. Am J Clin Pathol. 1973;60:259–263. doi: 10.1093/ajcp/60.2.259. [DOI] [PubMed] [Google Scholar]
- 3.Piehl MR, Kaplan RL, Haber MH. Disseminated penicilliosis in a patient with acquired immunodeficiency syndrome. Arch Pathol Lab Med. 1988;112:1262–1264. [PubMed] [Google Scholar]
- 4.Supparatpinyo K, Khamwan C, Baosoung V, Nelson KE, Sirisanthana T. Disseminated Penicillium marneffei infection in southeast Asia. Lancet. 1994;344:110–113. doi: 10.1016/s0140-6736(94)91287-4. [DOI] [PubMed] [Google Scholar]
- 5.Yap FB, Thevarajah S, Asmah J. Penicillium marneffei infection in an African man. Dermatol Online J. 2010;16:2. [PubMed] [Google Scholar]
- 6.Kim MN, Shin JH, Sung H, Lee K, Kim EC, Ryoo N, Lee JS, Jung SI, Park KH, Kee SJ, et al. Candida haemulonii and closely related species at 5 university hospitals in Korea: identification, antifungal susceptibility, and clinical features. Clin Infect Dis. 2009;48:e57–e61. doi: 10.1086/597108. [DOI] [PubMed] [Google Scholar]
- 7.Lu PX, Zhu WK, Liu Y, Chen XC, Zhan NY, Liu JQ, Zang J, Yang GD, Ye RX, Cai LS. Acquired immunodeficiency syndrome associated disseminated Penicillium marneffei infection: report of 8 cases. Chin Med J. 2005;118:1395–1399. [PubMed] [Google Scholar]
- 8.Zhao DW, Zhang T, Ma DQ, Wang W, Yuan CW, Duan Y. Disseminated Penicillium marneffei infection in acquired immunodeficiency syndrome: a case report. Chin Med J. 2005;118:1054–1056. [PubMed] [Google Scholar]
- 9.Wu TC, Chan JW, Ng CK, Tsang DN, Lee MP, Li PC. Clinical presentations and outcomes of Penicillium marneffei infections: a series from 1994 to 2004. Hong Kong Med J. 2008;14:103–109. [PubMed] [Google Scholar]
- 10.Thomas CF, Jr, Limper AH. Pneumocystis pneumonia. N Engl J Med. 2004;350:2487–2498. doi: 10.1056/NEJMra032588. [DOI] [PubMed] [Google Scholar]
- 11.King LJ, Padley SP. Imaging of the thorax AIDS. Imaging. 2002;14:60–76. [Google Scholar]
- 12.Kuhlman JE, Kavuru M, Fisherman EK, Siegelman SS. Pneumocystis carinii pneumonia: spectrum of parenchymal CT findings. Radiology. 1990;175:711–714. doi: 10.1148/radiology.175.3.2343118. [DOI] [PubMed] [Google Scholar]
- 13.Chiewchanvit S, Mahanupab P, Hirunsri P, Vanittanakom N. Cutaneous manifestations of disseminated Penicillium marneffei mycosis in five HIV-infected patients. Mycoses. 1991;34:245–249. doi: 10.1111/j.1439-0507.1991.tb00652.x. [DOI] [PubMed] [Google Scholar]
- 14.Ho KK. Penicillium marneffei infection in a chinese man with HIV infection. Hong Kong Dermatol Venereol Bull. 2000:28–30. [Google Scholar]
- 15.Wong SS, Wong KH, Hui WT, Lee SS, Lo JY, Cao L, Yuen KY. Differences in clinical and laboratory diagnostic characteristics of Penicilliosis marneffei in human immunodeficiency virus (HIV)- and non-HIV-infected patients. J Clin Microbiol. 2001;39:4535–4540. doi: 10.1128/JCM.39.12.4535-4540.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vanittanakom N, Cooper CR, Jr, Fisher MC, Sirisanthana T. Penicillium marneffei infection and recent advances in the epidermiology and molecular biology aspects. Clin Microbiol Rev. 2006;19:95–110. doi: 10.1128/CMR.19.1.95-110.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han SH, Choi HY, Lee SC, Goo YS, Chang KH, Kang SW, Choi KH, Kim JM, Lee HY, Han DS, et al. A case of Penicilium marneffei CAPD peritonitis. Korean J Nephrol. 2002;21:680–685. [Google Scholar]
- 18.Seo JY, Ma YE, Lee JH, Lee ST, Ki CS, Lee NY. A case of disseminated Penicillium marneffei infection in a liver transplant recipient. Korean J Lab Med. 2010;30:400–405. doi: 10.3343/kjlm.2010.30.4.400. [DOI] [PubMed] [Google Scholar]