Abstract
Objective:
To examine the risk of fracture in patients with multiple sclerosis (MS) compared with population-based controls.
Methods:
A population-based cohort study was performed in the Dutch PHARMO Record Linkage System (1998–2008). Patients with MS (n = 2,415) were matched by year of birth, sex, and practice to up to 6 patients without MS (controls). We used Cox proportional hazards models to estimate the hazard ratio (HR) of fracture in MS. Time-dependent adjustments were made for age, history of disease, and drug use.
Results:
During follow-up, there were 59 fractures among patients with MS (2.4%) and 227 fractures among controls (1.8%). Patients with MS had a 1.7-fold increased risk of osteoporotic fracture (HR 1.73 [95% confidence interval (CI) 1.18–2.53]) and a 4-fold increased risk of hip fracture (HR 4.08 [95% CI 2.21–7.56]). The risk of osteoporotic fracture was significantly greater for patients with MS who had been prescribed antidepressants (HR 3.25 [95% CI 1.77–5.97]) or hypnotics/anxiolytics (HR 3.40 [95% CI 2.06–5.63]) in the previous 6 months, compared with controls.
Conclusions:
Increased awareness of the risk of hip fracture is warranted in patients with MS, especially in those who have recently been prescribed antidepressants or hypnotics/anxiolytics.
Multiple sclerosis (MS) is an inflammatory autoimmune disorder of the CNS, characterized by acute focal demyelination and axonal loss. It is one of the most common causes of neurologic disability in young adults1 and affects approximately 1.3 million people worldwide.2
Patients with MS often have multiple risk factors for fracture,3 including low bone mineral density (BMD)4–8 and an increased risk of falling.9–11 The increased risk of falling may be caused by poorer postural balance, impaired vision, disability, or spasticity.9,11,12 Explanations for low BMD in MS include vitamin D deficiency,4–6,13 immobility,6,7 a low level of daily activity,4,14 and the use of glucocorticoids (GCs).4,7,15 Furthermore, it has been recognized that inflammatory autoimmune disease may promote local and systemic bone destruction by osteoclasts and inhibit bone formation by osteoblasts.16 The acute inflammation associated with MS might therefore lead to bone loss.5
To our knowledge, there has previously been only one study that quantified the risk of fracture in patients with MS.17 The aim of this study was to estimate the risk of fracture in patients with MS compared with population-based controls.
METHODS
Data source.
Information for this study was retrieved from the Dutch PHARMO Record Linkage System (RLS). The PHARMO RLS is a large, patient-centric data network including multiple linked observational databases designed for safety and outcomes research of drugs which collates patient records in 48 geographically defined areas in the Netherlands (covering a demographic region of 3 million inhabitants). For this source population, we have used pharmacy dispensing data linked to the national hospitalization registry of the Netherlands.18,19 Drug exposure information includes drug name, type of prescriber, dispensing date, amount dispensed, dosage instructions, and cost. Hospitalizations in the national hospitalization registry are coded with a discharge diagnosis, or a diagnosis for a day care admission (e.g., to receive IV administered drugs such as natalizumab) according to the International Classification of Diseases (ICD-9). In case a hospitalization is due to an external cause, such as an accident or poisoning, hospitals in the Netherlands are required to add a supplementary code to the code from one of the main chapters of ICD-9, indicating the nature of the condition (E800–E999). A previous study using PHARMO RLS data has demonstrated a high level of data validity with respect to the reporting of hip fractures (>89% of fractures were confirmed).20
Study population and design.
We conducted a retrospective follow-up study on patients with MS and population-based controls. Our case population included all patients aged 18 years or older with at least one hospitalization with a recorded diagnosis of MS (ICD-9-CM: 340) during the period of PHARMO RLS data collection (January 1998–December 2008). The index date for the patients with MS was defined as the date of the first diagnosis of MS after enrolment in PHARMO RLS. Each patient with MS was subsequently matched by year of birth, sex, and practice to up to 6 randomly selected controls from the general population (i.e., patients without a recorded diagnosis of MS in the national hospitalization registry at any time during enrolment). Controls were given the index date of their corresponding matched case.
Cases and controls were then followed from their index date to the end of data collection, the date of transfer of the patient out of the practice area, or the patient's death, whichever came first.
Study outcomes.
The records of all study participants were inspected for the occurrence of fractures after the index date. Fractures were classified according to ICD-9 categories 800–829. A clinical osteoporotic fracture was defined as a fracture of the radius/ulna, vertebrae, femur, hip, humerus, pelvis, or ribs. In addition, the supplementary classification of external causes was used to identify which fractures were coded with a fall (ICD-9 categories E880–886 or E888). For any, osteoporotic and hip fracture, we considered 2 additional endpoints: fractures with a recorded fall and fractures without a recorded fall.
Bias and confounding.
The follow-up period for each participant was divided into 30-day intervals. The presence of risk factors was determined by reviewing the computerized records for any evidence of each risk factor before the start of an interval.
Potential confounders that were determined at baseline were sex and history of fracture. For a time-dependent analysis we considered age, a history of chronic diseases (asthma, congestive heart failure, rheumatoid arthritis, cerebrovascular disease,21 noninfectious enteritis and colitis, renal failure, dementia, and epilepsy), as well as a history of falling 1 year to 3 months before the start of each 30-day interval. Any prescriptions for oral GCs,22,23 statins, antiarrhythmics (excluding digoxin and sotalol), antidiabetics, antidepressants,24 antipsychotics,25 hypnotics/anxiolytics,26 anticonvulsants,27 estrogen containing hormone replacement therapy,28 calcium, vitamin D, bisphosphonates, opioids, or asthma medication 6 months before the start of an interval were also considered as potential confounders. Table e-1 on the Neurology® Web site at www.neurology.org shows ICD-9 and Anatomic Therapeutic Chemical (ATC) codes.
Statistical analysis.
Cox proportional hazards models were used to provide an estimate of the relative risk (hazard ratio [HR]) of fracture among patients with MS, adjusted for any potential confounders that changed the HR >1% in an age/sex-adjusted analysis. Analyses were stratified by duration of disease (time since first diagnosis recorded in PHARMO RLS) and drug use in the previous 6 months. We determined the proportion of patients with at least 1 prescription for vitamin D, calcium, or a bisphosphonate during follow-up. All statistical analyses were conducted using SAS® 9.1/9.2 software.
Standard protocol approvals, registrations, and patient consents.
Ethical approval for the study was obtained from the PHARMO institute.
RESULTS
The study population included 2,415 patients with a hospitalization for MS and 12,641 population-based controls. Baseline characteristics of the participants are given in table 1. Of all patients with MS, 72.2% were female and the mean age at index date (first recorded diagnosis of MS in our dataset) was 43.6 years. Of all patients with MS, 59 experienced a fracture during follow-up. Of these, 39 were osteoporotic fractures and 20 concerned other types of fracture, which included fractures of the face bones (n = 2), fractures of the hand (n = 2), fractures of tibia/fibula (n = 6), and fractures of the foot/ankle (n = 10).
Table 1.
Baseline characteristics of patients with MS and controls
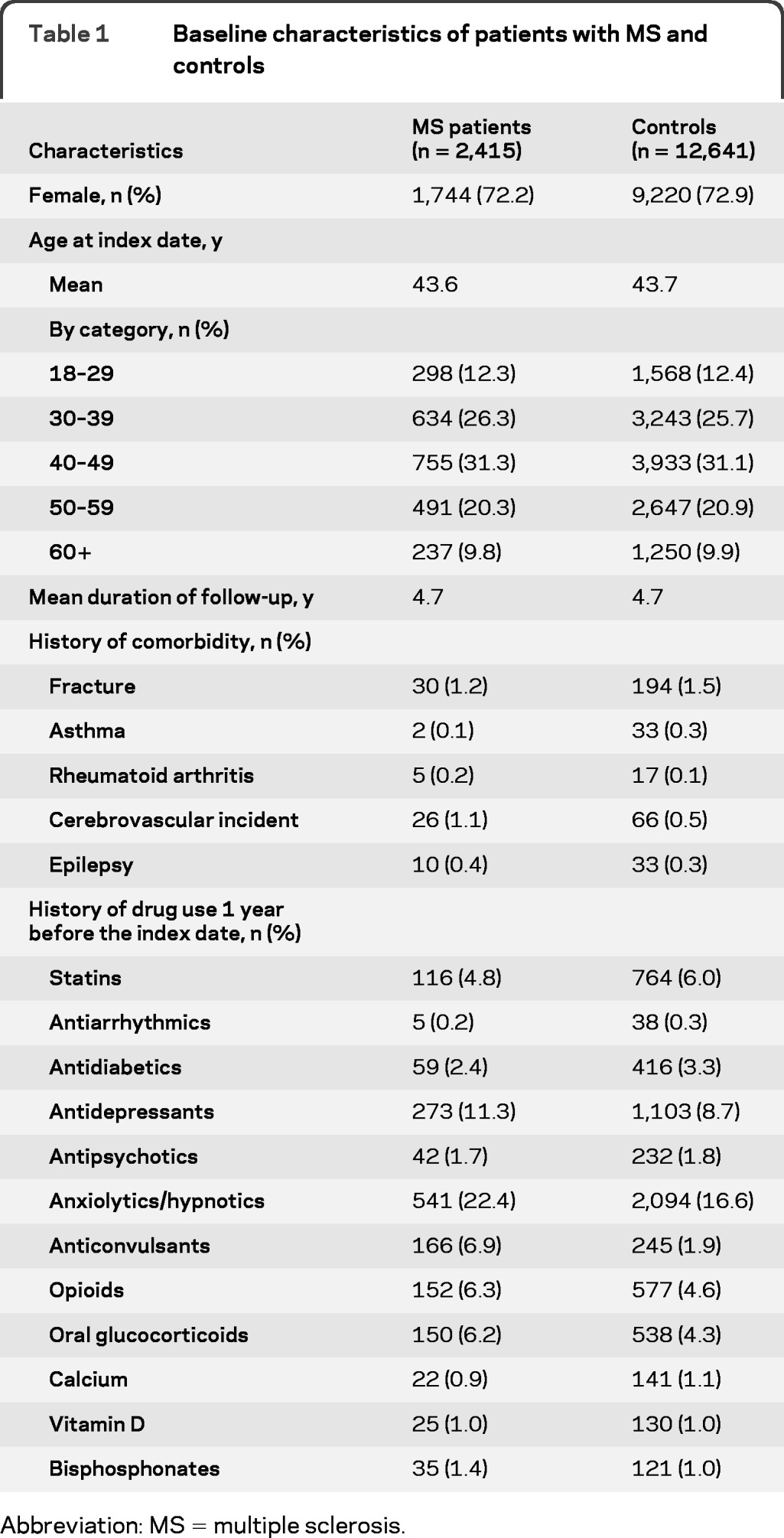
Abbreviation: MS = multiple sclerosis.
Table 2 reveals that patients with MS had a 4-fold increased risk of hip fracture compared with controls. The risk of osteoporotic fracture was increased 1.7-fold. Risks of any, osteoporotic, and hip fracture were significantly increased when the fracture had occurred in conjunction with a fall. However, for fractures without a recorded fall, risks were not significantly increased. Furthermore, the risk of any fracture was increased in women with MS vs women without MS, but not in men with MS vs men without MS.
Table 2.
Risk of fracture in patients with MS (n = 2,415) compared with controls (n = 12,641), by type of fracture

Abbreviations: CI = confidence interval; HR = hazard ratio; MS = multiple sclerosis
Adjusted for age, sex, the use of antidepressants, anticonvulsants, and bisphosphonates in the previous 6 months.
Adjusted for age, sex, the use of statins, antidepressants, hypnotics/anxiolytics, anticonvulsants, bisphosphonates, opioids in the previous 6 months.
Adjusted for age, sex, the use of antidepressants, anticonvulsants, opioids in the previous 6 months.
Adjusted for age, sex, the use of hypnotics/anxiolytics in the previous 6 months.
Adjusted for age, sex.
Subgroup too small to estimate HR.
Male patients with MS vs male controls.
Female patients with MS vs female controls.
The risk of osteoporotic fracture was significantly greater (more than 3 times increased) for patients with MS who had been prescribed antidepressants or hypnotics/anxiolytics in the previous 6 months, compared with all controls (table 3). In addition, a longer duration of disease appeared to be associated with a greater risk of osteoporotic fracture. A comparison between patients with MS and controls who both used the same type of medication yielded slightly different HRs than the results in table 3: osteoporotic fracture risk among patients with MS who had been prescribed antidepressants compared to “control” patients who had been prescribed antidepressants yielded adjusted HR 2.52 (95% confidence interval [CI] 1.15–5.52), while prescription of hypnotics/anxiolytics among patients with MS revealed an adjusted HR of 4.06 (95% CI 2.13–7.75) compared to “control” patients who had been prescribed this medication (data not shown). When we compared patients with MS who had used antidepressants and controls who also had used antidepressants with nonexposed patients, we found a significant difference between exposed patients with MS and exposed controls (p = 0.006). For the use of hypnotics/anxiolytics, the difference between patients with MS and controls was significant as well (p < 0.001).
Table 3.
Risk of osteoporotic fracture in patients with MS (n = 2,415) compared with controls (n = 12,641), by history of drug use and duration of disease
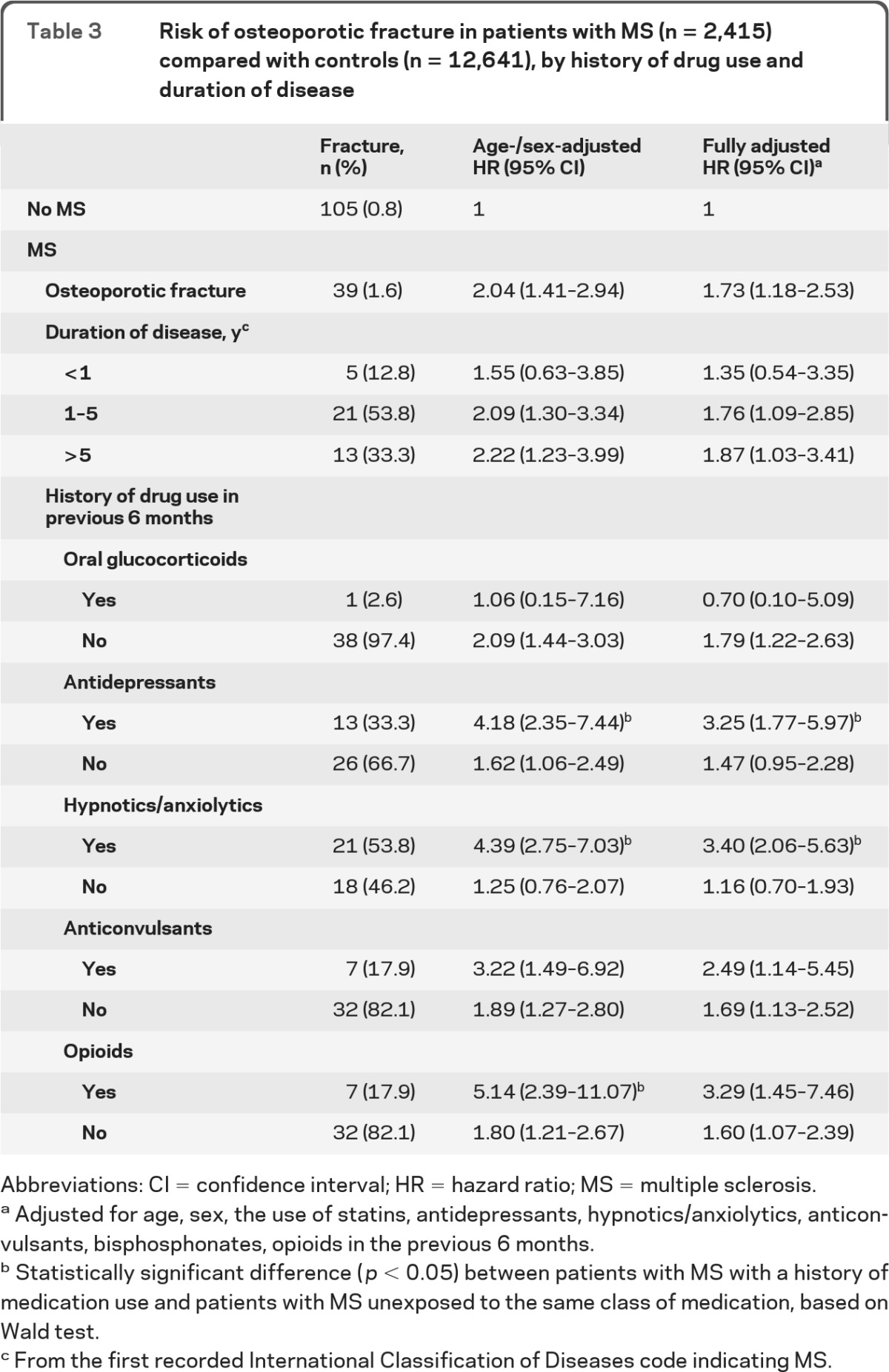
Abbreviations: CI = confidence interval; HR = hazard ratio; MS = multiple sclerosis.
Adjusted for age, sex, the use of statins, antidepressants, hypnotics/anxiolytics, anticonvulsants, bisphosphonates, opioids in the previous 6 months.
Statistically significant difference (p < 0.05) between patients with MS with a history of medication use and patients with MS unexposed to the same class of medication, based on Wald test.
From the first recorded International Classification of Diseases code indicating MS.
A small proportion of the patients with MS had been prescribed calcium (3.1%), vitamin D (3.8%, sometimes in combination with calcium), or a bisphosphonate (4.8%) at least once during follow-up. In controls these proportions were 2.3%, 3.0%, and 2.8%, respectively.
DISCUSSION
In this study, we found that patients with MS had a 4-fold greater risk of hip fracture than controls. Overall, we found that the risk of osteoporotic fracture was increased 1.7-fold, and that fracture risk was significantly greater for patients with MS who had recently been prescribed antidepressants or hypnotics/anxiolytics, compared with controls.
Our results are in line with those from other studies that have reported on fracture risk17 or low BMD in patients with MS.4–8 Using data from the General Practice Research Database (UK), we have previously shown that patients with MS were at an increased risk of fracture.17 In that study, patients with MS (n = 5,565, 5.7 years of follow-up from their first MS diagnosis) had an almost 3-fold increased risk of hip fracture and a risk of osteoporotic fracture that was increased 1.4-fold, compared with controls. A cohort study4 reported that at baseline patients with MS had had significantly more fractures than age-matched controls and BMD in patients with MS was almost 1 SD lower in the spine and 1 to 1.6 SD lower in the femoral neck. However, the authors do not provide duration of disease. A Turkish study6 examined patients with MS (mean duration of disease 7.3 years) and found that BMD at the lumbar spine in MS was nearly 1 SD lower than in controls. Similarly, a cross-sectional study showed that BMD values were significantly lower in patients with MS (mean disease duration 8.4 years in women and 10.4 years in men) at the lumbar spine and femoral sites, compared with controls.8 A reduced BMD at either the lumbar spine or the femoral neck was found5 in 80% of male patients with MS with a mean disease duration of 17.0 years. Of these men, 43% had osteopenia and 37% had osteoporosis. In women with MS, 38% had osteopenia and 44% had osteoporosis. In this study, we found that the risk of fracture was increased in female patients with MS, but not in male patients with MS. This may be explained by lower bone mass in women with MS.3
The increased risk of hip fracture that we found in association with MS may be caused by different underlying mechanisms. It is generally accepted that MS increases the risk of falling,9–11 due to the symptoms of the disease including imbalance, muscle weakness, blurred vision, and dizziness. In our study, we found that the risk of fracture for patients with MS was particularly elevated when considering fractures related to a fall. A Swedish study9 found that the odds of falling were doubled for each whole step on the Expanded Disability Status Scale score for patients with scores between 3.5 and 6.0. Another study11 reported that never or occasional use of a wheelchair approximately doubled the risk of falling compared with the use of a wheelchair all the time. Thus, the association between disability and risk of fracture is unclear. Intuitively, the more disabled a patient becomes, the greater might be their risk of falling. When a patient starts using a wheelchair, however, their risk might actually decline. Certainly, immobility has been linked with a reduced BMD, probably due to increased renal calcium losses, decreased gastrointestinal calcium absorption, and increased bone turnover.7 Other causes of low BMD in patients with MS include the inflammatory nature of MS,29 the use of GCs,4,7,15 and low vitamin D levels.6 These are discussed next.
MS is an inflammatory autoimmune disorder and it has been shown that inflammatory autoimmune disease often leads to bone loss.16 Among the supposed mechanisms of bone loss are the increased destruction by osteoclasts, both locally and systemically, as well as the inhibition of bone formation by osteoblasts.
GC-induced osteoporosis can also be explained by systemic or direct effects on bone cells.30 It has been demonstrated that patients who receive several courses of high-dose GCs (daily dose >15 mg and cumulative exposure >1 g in prednisolone equivalents) have a substantially increased risk of fracture.23 Patients with MS who experience a relapse are often prescribed a short course of methylprednisolone, either orally or IV, with a dosage that corresponds to 1.5–3.3 g prednisone equivalents. However, in our study, the number of osteoporotic fractures among recent GC users was too low to assess the role of GCs on fracture risk and information on the use of IV GCs was not available. Nevertheless, it is possible that the use of GCs might contribute to the increased risk of osteoporotic fracture observed.
It has been shown that vitamin D deficiency is common in patients with MS.4–6 Vitamin D deficiency has been associated with low BMD6 and, in addition, low vitamin D levels may modulate the clinical course of MS.31,32 In our study, we found that only a small percentage of patients with MS had been prescribed vitamin D and we were unable to capture other sources of vitamin D (e.g., through over the counter use, diet, or sunlight exposure). Therefore we cannot comment on its association with fracture risk. Nevertheless, in case vitamin D proves to have a positive effect on the course of disease and increased BMD, treatment may provide beneficial effects on 2 clinically relevant aspects of MS.
In this study, among recent users of hypnotics/anxiolytics, we found significantly more osteoporotic fractures associated with MS, a finding that corroborates reports from elsewhere.33 In addition, we found that in patients prescribed antidepressants, osteoporotic fracture risk was increased for patients with MS, a finding also reported elsewhere.24 One of the possible mechanisms by which antidepressant use may contribute to fracture risk is the effect that antidepressants could have on the microarchitecture of bone.34 Also, the use of antidepressants has been associated with an increased risk of falling35 and depression itself might also increase fracture risk.36
Strengths of our study include its reasonable sample size and the duration of follow-up. This is one of the first studies to estimate the risk of fracture in patients with MS compared with healthy controls using a large population-based cohort. Moreover, we had detailed longitudinal information on drug prescribing, which enabled us to stratify by exposure to medication.
Our study has several limitations. Data on smoking and body mass index (BMI) were not available. In our study, the absence of data on smoking and BMI may have led to an overestimation of the association between MS and fracture risk.37,38 Information on the degree of disability in patients with MS was lacking too, as well as information on the course of each patient's disease and data on medication that has been administered in hospitals. Because patients with MS in our study were included based on a hospital registration, there may have been a selection bias toward patients with more severe MS. Therefore, there may have been patients with MS in our cohort who had longstanding MS before their start of follow-up, although the mean length of their disease (at first recorded ICD code) has probably decreased during the period of data collection. Over the last decade, the number of day care admissions for Dutch patients with MS has increased approximately 4-fold, from 2.6/10,000 person-years in 1998, to up to 9.4/10,000 person-years in 2008,39 probably because of the availability of new treatment options such as natalizumab. A small proportion of our control patients may have had MS, because we could only identify patients with MS from the registry of inpatient and day care admissions. This may have slightly decreased the HR toward the null. Since PHARMO RLS only comprises information on inpatient diagnoses, there may have been an underregistration of fractures of extremities. Not every patient with such a fracture may be hospitalized, but treated as an outpatient instead. This may have led to a nondifferential misclassification and thus an underestimation of a true association between MS and these fracture types. Furthermore, we cannot be certain whether the increased risk of fracture that we found with exposure to antidepressants and hypnotics/anxiolytics was associated with use of the medication or worsening of MS. Although the control population in our study (which was pharmacy–based) may have slightly differed from the controls in our UK study (which were general practitioner–based), this has probably not substantially influenced our pivotal results, because they were similar. The ICD and ATC codes that were used for the potential confounding variables have not been validated.
The identification of patients with MS using one recorded ICD code indicating MS in PHARMO RLS has not been validated. We have however analyzed data from the United Kingdom and Denmark. The study population from the United Kingdom has been described elsewhere.17 Work on the Danish study population has been accepted for publication but not published yet. In brief, we identified all patients from the nationwide Danish MS Registry (from 1949 onwards)40 and linked their data to the national hospitalization registry using a unique personal identifier. The linked dataset comprised 13,841 patients with MS. In the United Kingdom, 80.7% of all MS diagnoses that were recorded in the national hospital registry were also recorded in the UK General Practice Research Database. In Denmark, 99.5% of all MS diagnoses in the national hospital registry were also recorded in the national MS Registry. The chance of a false-negative was 31% in the UK data (probable patients with MS from the GPRD study who had no diagnosis in the national hospital registry) and 4.1% in Denmark (patients who were registered with MS in the MS Registry, but had no diagnosis in the national hospital registry). For a false-positive, chances were 8.5% in the UK data (patients who had a recorded MS diagnosis in the hospital registry, but were not probable patients in GPRD) and 2.3% in Denmark (recorded MS diagnosis in the hospital registry, but not in the MS Registry).
Overall, we found that patients with MS had a substantially increased risk of hip fracture and that the risk of osteoporotic fracture was greater for patients with MS who had recently been prescribed hypnotics/anxiolytics or antidepressants. Fracture risk assessment may be indicated in patients with MS.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Dr. Helen Seaman for assistance in improving the readability of this manuscript.
GLOSSARY
- ATC
Anatomic Therapeutic Chemical
- BMD
bone mineral density
- BMI
body mass index
- CI
confidence interval
- GC
glucocorticoid
- HR
hazard ratio
- ICD
International Classification of Diseases
- MS
multiple sclerosis
- RLS
Record Linkage System
Footnotes
Editorial, page 1902
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Marloes T. Bazelier: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, statistical analysis. Tjeerd-Pieter van Staa: analysis or interpretation of data, study supervision. Bernard Uitdehaag: drafting/revising the manuscript, analysis or interpretation of data. Cyrus Cooper: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data, study supervision. Hubert Leufkens: drafting/revising the manuscript, study supervision. Peter Vestergaard: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data, statistical analysis, study supervision. Ron Herings: drafting/revising the manuscript, acquisition of data. Frank de Vries: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data, statistical analysis, study supervision, obtaining funding.
DISCLOSURE
The Department of Pharmacoepidemiology and Clinical Pharmacology, Utrecht Institute for Pharmaceutical Sciences, employing authors Marloes Bazelier, Tjeerd-Pieter van Staa, Hubert Leufkens, and Frank de Vries, has received unrestricted funding for pharmacoepidemiological research from GlaxoSmithKline, Novo Nordisk, the private-public funded Top Institute Pharma (www.tipharma.nl, includes co-funding from universities, government, and industry), the Dutch Medicines Evaluation Board, and the Dutch Ministry of Health. Tjeerd van Staa also works for the General Practice Research Database (GPRD), UK. The GPRD is owned by the UK Department of Health and operates within the Medicines and Healthcare products Regulatory Agency (MHRA). GPRD is funded by the MHRA, Medical Research Council, various universities, contract research organizations and pharmaceutical companies. Bernard Uitdehaag has received honoraria for consultancy from Novartis, Merck Serono, and Synthon. Cyrus Cooper, Peter Vestergaard, and Ron Herings report no disclosures. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Compston A, Coles A. Multiple sclerosis. Lancet 2002; 359: 1221– 1231 . [DOI] [PubMed] [Google Scholar]
- 2.Dua T, Romani P. Atlas Multiple Sclerosis Resources in the World 2008. In: Library WHO, ed. Available at: http://www.who.int/mental_health/neurology/Atlas_MS_WEB.pdf Accessed November 26, 2010
- 3.Marrie RA, Cutter G, Tyry T, Vollmer T. A cross-sectional study of bone health in multiple sclerosis. Neurology 2009; 73: 1394– 1398 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cosman F, Nieves J, Komar L, et al. Fracture history and bone loss in patients with MS. Neurology 1998; 51: 1161– 1165 . [DOI] [PubMed] [Google Scholar]
- 5.Weinstock-Guttman B, Gallagher E, Baier M, et al. Risk of bone loss in men with multiple sclerosis. Mult Scler 2004; 10: 170– 175 . [DOI] [PubMed] [Google Scholar]
- 6.Ozgocmen S, Bulut S, Ilhan N, Gulkesen A, Ardicoglu O, Ozkan Y. Vitamin D deficiency and reduced bone mineral density in multiple sclerosis: effect of ambulatory status and functional capacity. J Bone Miner Metab 2005; 23: 309– 313 . [DOI] [PubMed] [Google Scholar]
- 7.Formica CA, Cosman F, Nieves J, Herbert J, Lindsay R. Reduced bone mass and fat-free mass in women with multiple sclerosis: effects of ambulatory status and glucocorticoid use. Calcif Tissue Int 1997; 61: 129– 133 . [DOI] [PubMed] [Google Scholar]
- 8.Tüzün S, Altintaş A, Karacan I, Tangürek S, Saip S, Siva A. Bone status in multiple sclerosis: beyond corticosteroids. Mult Scler 2003; 9: 600– 604 . [DOI] [PubMed] [Google Scholar]
- 9.Nilsagård Y, Lundholm C, Denison E, Gunnarsson LG. Predicting accidental falls in people with multiple sclerosis: a longitudinal study. Clin Rehabil 2009; 23: 259– 269 . [DOI] [PubMed] [Google Scholar]
- 10.Peterson EW, Cho CC, von Koch L, Finlayson ML. Injurious falls among middle aged and older adults with multiple sclerosis. Arch Phys Med Rehabil 2008; 89: 1031– 1037 . [DOI] [PubMed] [Google Scholar]
- 11.Finlayson ML, Peterson EW, Cho CC. Risk factors for falling among people aged 45 to 90 years with multiple sclerosis. Arch Phys Med Rehabil 2006; 87: 1274– 1279 . [DOI] [PubMed] [Google Scholar]
- 12.Cattaneo D, De Nuzzo C, Fascia T, Macalli M, Pisoni I, Cardini R. Risks of falls in subjects with multiple sclerosis. Arch Phys Med Rehabil 2002; 83: 864– 867 . [DOI] [PubMed] [Google Scholar]
- 13.Kärkkäinen MK, Tuppurainen M, Salovaara K, et al. Does daily vitamin D 800 IU and calcium 1000 mg supplementation decrease the risk of falling in ambulatory women aged 65–71 years? A 3-year randomized population-based trial (OSTPRE-FPS). Maturitas 2010; 65: 359– 365 . [DOI] [PubMed] [Google Scholar]
- 14.Schwid SR, Goodman AD, Puzas JE, McDermott MP, Mattson DH. Sporadic corticosteroid pulses and osteoporosis in multiple sclerosis. Arch Neurol 1996; 53: 753– 757 . [DOI] [PubMed] [Google Scholar]
- 15.Dovio A, Perazzolo L, Osella G, et al. Immediate fall of bone formation and transient increase of bone resorption in the course of high-dose, short-term glucocorticoid therapy in young patients with multiple sclerosis. J Clin Endocrinol Metab 2004; 89: 4923– 4928 . [DOI] [PubMed] [Google Scholar]
- 16.Schett G, David JP. The multiple faces of autoimmune-mediated bone loss. Nat Rev Endocrinol 2010; 6: 698– 706 . [DOI] [PubMed] [Google Scholar]
- 17.Bazelier MT, van Staa TP, Uitdehaag BM, et al. The risk of fracture in patients with multiple sclerosis: the UK general practice research database. J Bone Miner Res 2011; 26: 2271– 2279 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herings RM. PHARMO: a record linkage system for postmarketing surveillance of prescription drugs in the Netherlands, in the Department of Pharmacoepidemiology and Therapy. Utrecht: Utrecht University; 1993:232 [Google Scholar]
- 19. [Accessed January 27, 2011]. Available at: www.pharmo.nl.
- 20.Herings RM, Stricker BH, de Boer A, Bakker A, Sturmans F, Stergachis A. Current use of thiazide diuretics and prevention of femur fractures. J Clin Epidemiol 1996; 49: 115– 119 . [DOI] [PubMed] [Google Scholar]
- 21.Pouwels S, Lalmohamed A, Leufkens B, et al. Risk of hip/femur fracture after stroke: a population-based case-control study. Stroke 2009; 40: 3281– 3285 . [DOI] [PubMed] [Google Scholar]
- 22.Van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C. Use of oral corticosteroids and risk of fractures. J Bone Miner Res 2000; 15: 993– 910 . [DOI] [PubMed] [Google Scholar]
- 23.De Vries F, Bracke M, Leufkens HG, Lammers JW, Cooper C, Van Staa TP. Fracture risk with intermittent high-dose oral glucocorticoid therapy. Arthritis Rheum 2007; 56: 208– 214 . [DOI] [PubMed] [Google Scholar]
- 24.van den Brand MW, Samson MM, Pouwels S, et al. Use of anti-depressants and the risk of fracture of the hip or femur. Osteoporos Int 2009; 20: 1705– 1713 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pouwels S, van Staa TP, Egberts AC, Leufkens HG, Cooper C, de Vries F. Antipsychotic use and the risk of hip/femur fracture: a population-based case-control study. Osteoporos Int 2009; 20: 1499– 1506 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herings RM, Stricker BH, de Boer A, Bakker A, Sturmans F. Benzodiazepines and the risk of falling leading to femur fractures: dosage more important than elimination half-life. Arch Intern Med 1995; 155: 1801– 1807 . [PubMed] [Google Scholar]
- 27.Petty SJ, O'Brien TJ, Wark JD. Anti-epileptic medication and bone health. Osteoporos Int 2007; 18: 129– 142 . [DOI] [PubMed] [Google Scholar]
- 28.Huot L, Couris CM, Tainturier V, Jaglal S, Colin C, Schott AM. Trends in HRT and anti-osteoporosis medication prescribing in a European population after the WHI study. Osteoporos Int 2008; 19: 1047– 1054 . [DOI] [PubMed] [Google Scholar]
- 29.Hearn AP, Silber E. Osteoporosis in multiple sclerosis. Mult Scler 2010; 16: 1031– 1043 . [DOI] [PubMed] [Google Scholar]
- 30.Canalis E, Delany AM. Mechanisms of glucocorticoid action in bone. Ann NY Acad Sci 2002; 966: 73– 81 . [DOI] [PubMed] [Google Scholar]
- 31.Goldberg P, Fleming MC, Picard EH. Multiple sclerosis: decreased relapse rate through dietary supplementation with calcium, magnesium and vitamin D. Med Hypoth 1986; 21: 193– 200 . [DOI] [PubMed] [Google Scholar]
- 32.Ascherio A, Munger KL, Simon KC. Vitamin D and multiple sclerosis. Lancet Neurol 2010; 9: 599– 612 . [DOI] [PubMed] [Google Scholar]
- 33.Landi F, Onder G, Cesari M, Barillaro C, Russo A, Bernabei R; Silver Network Home Care Study Group Psychotropic medications and risk for falls among community-dwelling frail older people: an observational study. J Gerontol A Biol Sci Med Sci. 2005; 60: 622– 626 . [DOI] [PubMed] [Google Scholar]
- 34.Bliziotes M, Eshleman A, Burt-Pichat B, et al. Serotonin transporter and receptor expression in osteocytic MLO-Y4 cells. Bone 2006; 39: 1313– 1321 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thapa PB, Gideon P, Cost TW, Milam AB, Ray WA. Antidepressants and the risk of falls among nursing home residents. N Engl J Med 1998; 339: 875– 882 . [DOI] [PubMed] [Google Scholar]
- 36.Wu Q, Liu J, Gallegos-Orozco JF, Hentz JG. Depression, fracture risk, and bone loss: a meta-analysis of cohort studies. Osteoporos Int 2010; 21: 1627– 1635 . [DOI] [PubMed] [Google Scholar]
- 37.Nortvedt MW, Riise T, Maeland JG. Multiple sclerosis and lifestyle factors: the Hordaland Health Study. Neurol Sci 2005; 26: 334– 339 . [DOI] [PubMed] [Google Scholar]
- 38.Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int 2008; 19: 385– 397 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Centraal Bureau voor de Statistiek, 19 May 2011. Available at: statline.cbs.nl Accessed July 14, 2011
- 40.Koch-Henriksen N, Rasmussen S, Stenager E, Madsen M. The Danish Multiple Sclerosis Registry: history, data collection and validity. Dan Med Bull 2001; 48: 91– 94 . [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


