Abstract
Objective:
To assess progesterone treatment of intractable seizures in women with partial epilepsy.
Methods:
This randomized, double-blind, placebo-controlled, phase III, multicenter, clinical trial compared the efficacy and safety of adjunctive cyclic natural progesterone therapy vs placebo treatment of intractable seizures in 294 subjects randomized 2:1 to progesterone or placebo, stratified by catamenial and noncatamenial status. It compared treatments on proportions of ≥50% responders and changes in seizure frequency from 3 baseline to 3 treated menstrual cycles.
Results:
There was no significant difference in proportions of responders between progesterone and placebo in the catamenial and noncatamenial strata. Prespecified secondary analysis showed that the level of perimenstrual seizure exacerbation (C1 level) was a significant predictor of responders for progesterone but not placebo. With increasing C1 levels, responders increased from 21% to 57% with progesterone vs 19% to 20% with placebo. Reductions in seizure frequency correlated with increasing C1 levels for progesterone but not placebo, progressing from 26% to 71% for progesterone vs 25% to 26% for placebo. A prespecified clinically important separation between progesterone and placebo responders (37.8% vs 11.1%; p = 0.037) was realized among 21.4% of women who had C1 level ≥3.
Conclusion:
There was no difference in the primary outcome of ≥50% responder rates between progesterone vs placebo for catamenial or noncatamenial groups. Post hoc findings suggest that the level of perimenstrual seizure exacerbation is a significant predictor of responder rate with progesterone and that progesterone may provide clinically important benefit for a subset of women with perimenstrually exacerbated seizures.
Classification of evidence:
This study provides Class III evidence that cyclic progesterone is ineffective in women with intractable partial epilepsy. Post hoc analysis identified a subset of women with higher levels of perimenstrual seizure exacerbation that were responsive to treatment.
There are compelling reasons to investigate the potential role of reproductive steroids in the treatment of intractable seizures in women with epilepsy. Seizures do not occur randomly.1,2 They tend to cluster.1 Seizure clusters often occur with a periodicity.3 Women with partial epilepsy have a recurrent pattern of seizures that corresponds to the monthly cycle interval of the menstrual cycle as demonstrated by a mathematical waveform (cosinor) analysis.3 In cases where seizure periodicity aligns with the menstrual cycle, the term “catamenial epilepsy” applies.2 The existence of catamenial epilepsy draws support from findings in some large clinical studies.4,5 Catamenial epilepsy likely relates to 3 factors: 1) some reproductive steroids have neuroactive properties,6–9 2) the serum concentrations of neuroactive reproductive steroids vary across the menstrual cycle,2 and 3) some brain substrates that are susceptible to epilepsy show sensitive structural and electrophysiologic responses to these steroids.10–14 Catamenial epilepsy suggests that hormones affect seizure occurrence and, therefore, hormones might have a role in treatment.
The purpose of this investigation was to determine if cyclic adjunctive progesterone supplement is superior to placebo in the treatment of intractable seizures in women with and without catamenial epilepsy.
METHODS
Subjects.
The women, 13–45 years old, had intractable seizures, i.e., persistent seizures despite trials of ≥2 antiepileptic drugs (AEDs) at therapeutic levels, EEG documentation of focal paroxysmal onset, ≥2 seizures/month during the 3 months prior to enrollment, and stable AED treatment for ≥1 month. All had monthly menses with intervals of 23–35 days. Women were excluded if they had a progressive neurologic or systemic disorder or >2-fold elevation in liver enzyme levels. None took major tranquilizers or used hormonal contraception during the 3 months prior to enrollment.
Standard protocol approvals, registrations, and patient consents.
This study was approved by the Institutional Review Boards of all 15 participating hospital sites. Written informed consent was obtained from all patients or parents/guardians in the case of minors. The trial was conducted under Food and Drug Administration–approved IND for the investigation of progesterone treatment of epilepsy. The study is listed at http://clinicaltrials.gov/under ClinicalTrials.gov identifier: NCT00029536.
Study design.
This was a phase III, randomized, double-blind, placebo-controlled, multicenter trial to compare short-term efficacy and safety of adjunctive cyclic natural progesterone therapy vs placebo for intractable seizures in women with partial epilepsy. Women charted seizures and menses for 3 baseline and treatment menstrual cycles. Following baseline, women were classified into catamenial or noncatamenial stratum, based on baseline phase seizure pattern and frequency data using Herzog et al.5 criteria. Randomization was carried out separately for the 2 strata, 2:1 to progesterone or placebo. Treatment consisted of identical progesterone 200 mg or placebo lozenges, taken 3 times daily on days 14–28 of treatment cycles. The detailed description is in appendix e-1 on the Neurology® Web site at www.neurology.org.
Sample size.
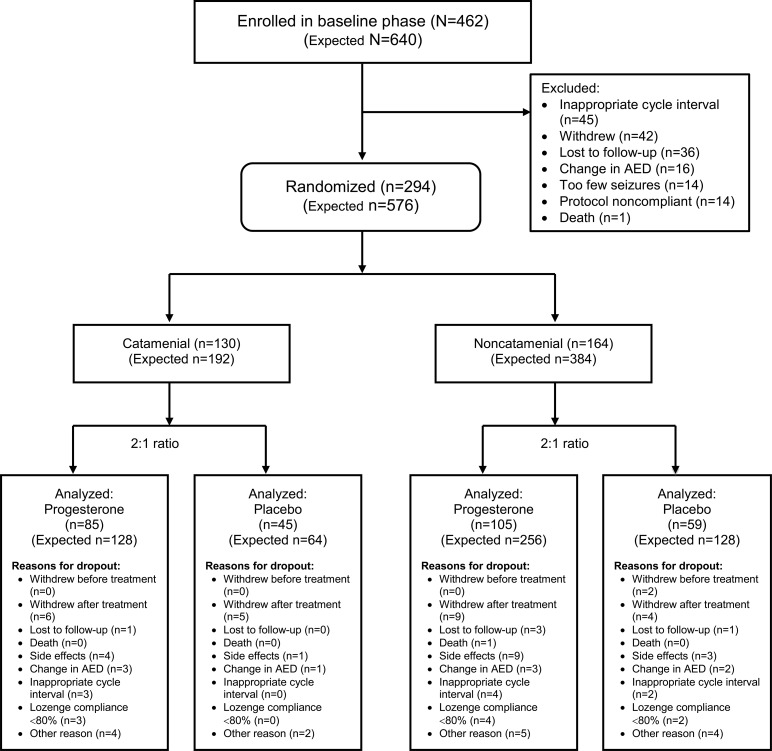
The trial was designed to populate 2 treatment groups in each stratum. A sample size of 192 randomized women was determined to be necessary for the catamenial stratum to demonstrate a significant difference between progesterone and placebo treatments at α level = 0.05 and power of 0.80 for the clinically meaningful outcome that 35% of progesterone vs 15% of placebo-treated women would show a 50% reduction in seizure frequency. The 15% placebo responder rate was based on past AED trials.15,16 Enrollment of 640 women was determined to be necessary to obtain 192 randomized women for the catamenial stratum, considering that one-third would meet the criteria for catamenial designation and allowing for 10% dropouts during baseline (figure 1).
Figure 1. Consolidated standards of reporting trials (CONSORT) flow chart.
This is a diagram of the stratification and 2:1 randomization of subjects into 4 treatment groups, including the actual analyzed and originally expected numbers. AED = antiepileptic drug.
Outcome measures.
The primary outcome was percent of responders for all seizures combined during treatment as compared to baseline. Secondary outcomes were 1) percent of women who showed ≥50% reduction in average daily seizure frequency (ADSF) for the most severe seizure type (MSST) and individual seizure types (secondary generalized motor seizures [SGMS], complex partial seizures [CPS], simple partial seizures [SPS]), 2) percent of women who became free of seizures, and 3) change in ADSF for all seizures combined, the MSST, and individual seizure types.
Statistical analysis.
Analyses were conducted using the intention-to-treat paradigm that included all available data on all randomized subjects. Women who dropped out of the treatment phase after randomization and before completion of 2 treatment cycles were considered to be nonresponders. Responder rates and seizure-free rates were presented as proportions. Seizure frequencies were presented as ADSF. Changes in ADSF had skewed distributions and were compared as proportions rather than absolute numbers. Outcomes were compared between treatment groups separately for each stratum. Continuous variables were compared using independent t test for normally distributed data and Wilcoxon rank sum test for otherwise distributed data. Proportions were compared using χ2 analysis or Fisher exact test. Correlations were determined using Pearson or Spearman bivariate correlational analysis. All significance results are presented as 2-sided p values.
A prespecified stepwise binary logistic regression analysis was carried out on all randomized subjects to identify factors that predicted responders (binary categorical dependent variable) for each treatment regardless of catamenial designation from a set of mixed categorical and continuous demographic (age, BMI, race), epilepsy (laterality, focality, MSST), antiepileptic drug (category), and catameniality (pattern [perimenstrual, C1; periovulatory, C2; entire luteal phase, C3] and level) predictor variables. A multivariate stepwise linear regression analysis identified factors that predicted changes in ADSF from the same set of predictor variables.
Patterns and levels of catamenial seizure exacerbation were established using baseline phase data. They were designated as per our previous definitions5 that compared ADSF during the phase of exacerbation with comparator phases: C1 pattern: ADSF on days −3 to 3 compared to the midfollicular (days 4 to 9) and midluteal phase (days −12 to −4) phases combined; C2 pattern: ADSF on days 10 to −13 compared to the midfollicular and midluteal phases combined; C3 pattern: ADSF on days 10 to 3 compared to the midfollicular phase. Catamenial levels represent the multiples of ADSF during the phase of exacerbation compared to the comparator phases.
Planned interim analyses for futility and efficacy were carried out separately for each stratum (appendix e-1).
RESULTS
Disposition of subjects.
The trial enrolled 462 subjects and randomized 294 (figure 1). Randomization was stopped at 164 subjects for the noncatamenial stratum and 130 subjects for the catamenial stratum when futility analyses showed that the blinded conditional power of the comparison for the primary outcome for that stratum dropped below 50%.
The progesterone and placebo groups in the 2 strata were comparable for all demographic, seizure, and AED characteristics except for SGMS that occurred more often in women randomized to progesterone than to placebo in the catamenial stratum (78.6% vs 55.6%, p = 0.006) (table 1).
Table 1.
Demographic and seizure characteristics
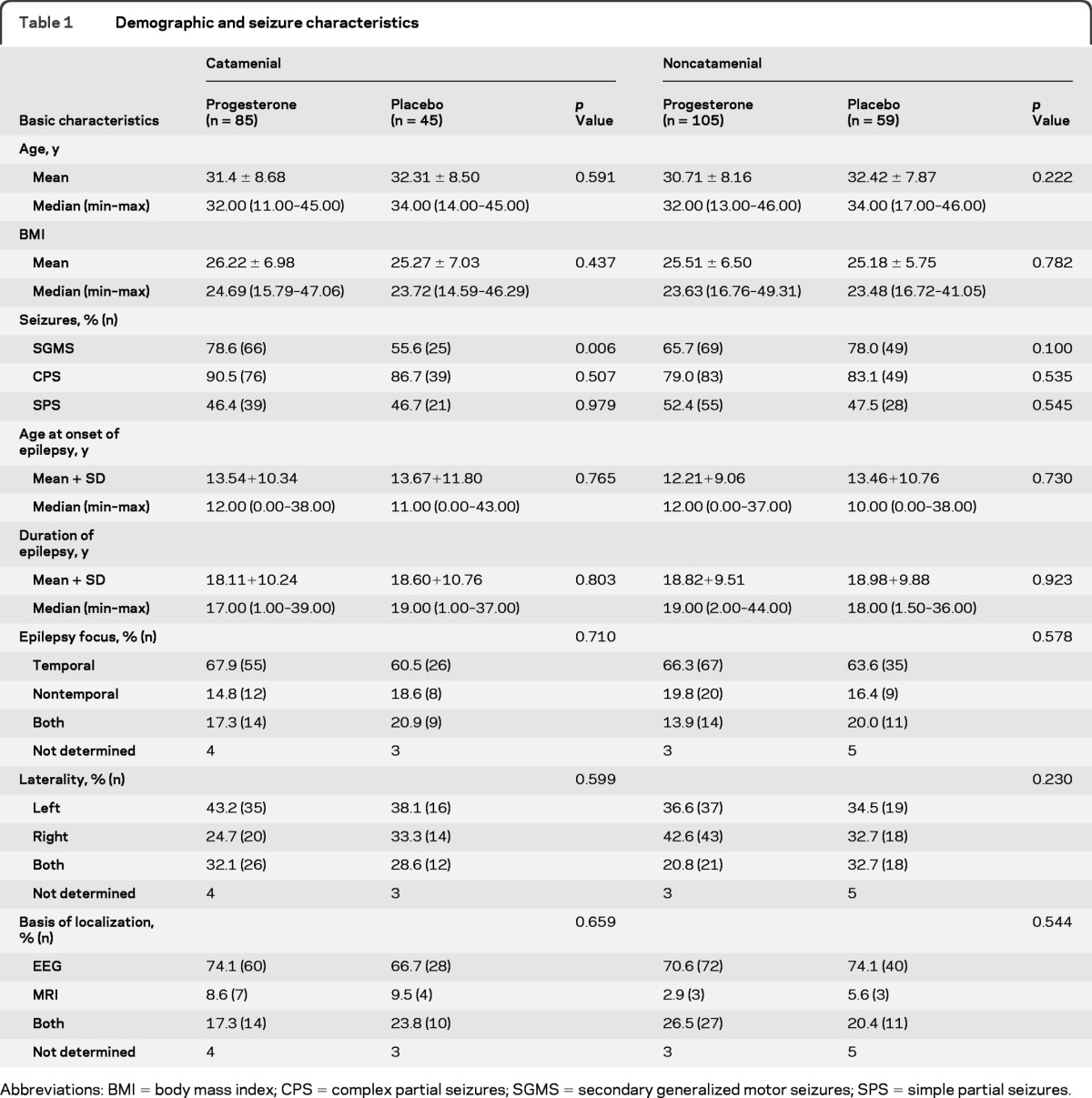
Abbreviations: BMI = body mass index; CPS = complex partial seizures; SGMS = secondary generalized motor seizures; SPS = simple partial seizures.
During the baseline phase, 168 of the 462 women (36.4%) dropped out. The most common reason was inappropriate menstrual cycle length (9.7%) (figure 1). During the treatment phase, there were no differences in exit rates overall or for any particular reason between progesterone and placebo in either stratum (figure 1).
Seizure outcomes: Proportion of responders.
There were no significant differences in the proportions of responders for all seizures combined between progesterone and placebo-treated women in the catamenial (progesterone: 18/79, 22.8% vs placebo: 9/45, 20.0%) and noncatamenial (20/99, 20.2% vs 10/52, 19.2%) strata (table 2). There were no significant differences in proportions of responders for the MSST between progesterone and placebo in the catamenial (24/79, 30.4% vs 11/45, 24.4%) and noncatamenial (22/99, 22.2% vs 15/52, 28.8%) strata (table 2). Proportions of responders for each seizure type considered individually did not differ significantly between progesterone and placebo treatments for either stratum.
Table 2.
Percent of subjects with ≥50% reduction in average daily seizure frequency and percent change in average daily seizure frequency with treatment
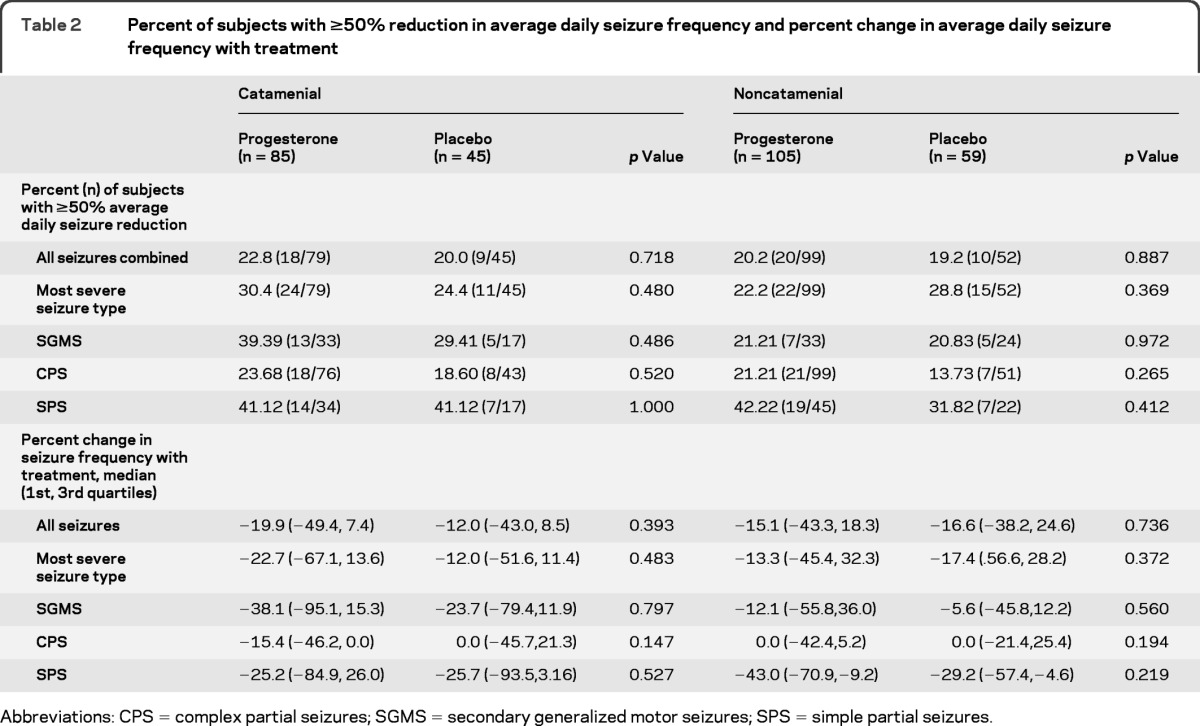
Abbreviations: CPS = complex partial seizures; SGMS = secondary generalized motor seizures; SPS = simple partial seizures.
There were no significant differences for proportions of women with complete elimination of all seizures: catamenial progesterone (3/79, 3.8%), catamenial placebo (0/45, 0%), noncatamenial progesterone (0/99, 0%), noncatamenial placebo (2/52, 3.8%), or for the proportions of women with complete elimination of their MSST: catamenial progesterone (11/79, 13.9%), catamenial placebo (3/45, 6.7%), noncatamenial progesterone (6/99, 6.1%), noncatamenial placebo (6/52, 11.5%).
Seizure outcomes: Reduction in seizure frequency.
There were no significant differences between treatments with respect to proportional changes in ADSF for all seizures combined, MSST, or each seizure type considered separately (table 2).
Changes in progesterone and AED levels.
Progesterone-treated subjects had significantly higher serum progesterone levels during the treatment phase than during the baseline phase for both the catamenial (median [1st, 3rd quartiles] = 44.4 [28.9, 87.9] vs 9.2 [5.1, 13.7], p < 0.0001) and noncatamenial strata (49.6 [27.0, 88.2] vs 12.0 [7.5, 14.0], p < 0.0001). There was no significant difference for placebo-treated subjects (table e-1). Comparisons of AED serum levels between baseline and treatment with progesterone or placebo for the 5 most commonly used AEDs (carbamazepine, levetiracetam, lamotrigine, topiramate, phenytoin) showed no significant difference in either stratum (table e-2). More detailed results will be reported elsewhere.
Predictors of progesterone treatment efficacy: Responders.
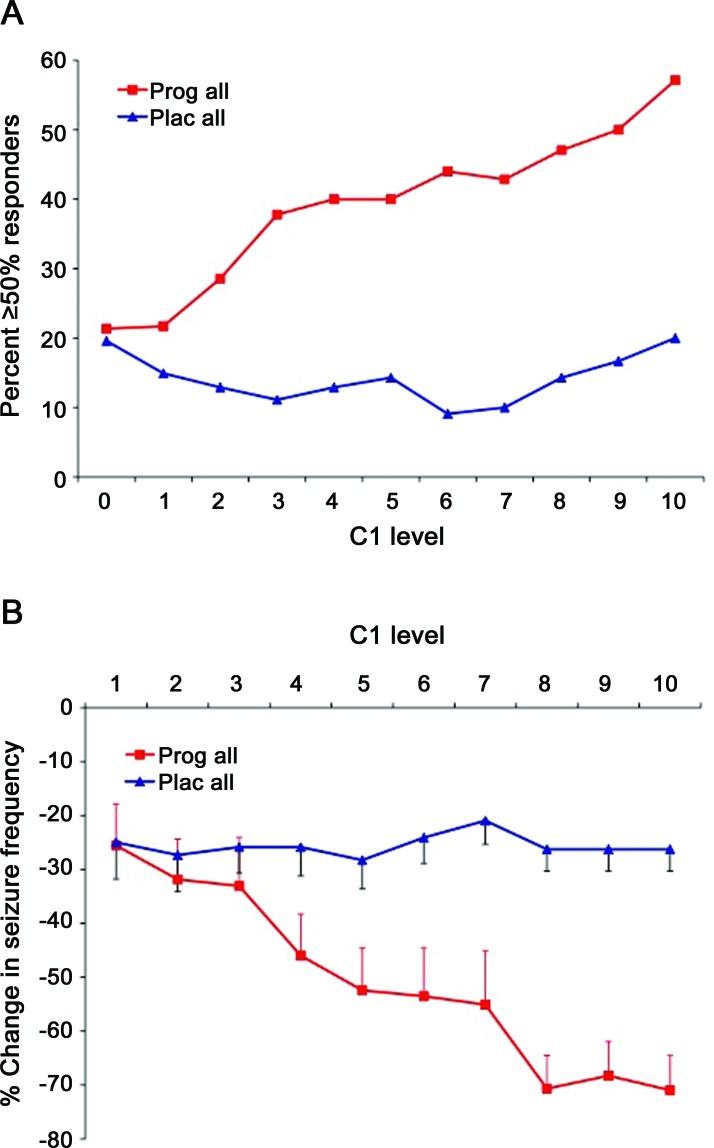
Logistic regression analysis identified C1 level (p = 0.001) and C2 pattern (p = 0.027) as significant predictors of progesterone responders. It showed significant interaction between C1 level and treatment (p = 0.003). As C1 levels increased from 1 to 10, responder rates increased from 21.3% to 57.1% with progesterone treatment (r = 0.271, p = 0.0005) vs 19.6% to 20.0% with placebo (r = 0.032, p = 0.757) (figure 2A).
Figure 2. Responder rates with progesterone and placebo treatment vs perimenstrual (C1) catamenial level of seizure exacerbation.
(A) Percent ≥50% responders in relation to C1 level: progesterone vs placebo. This is a plot of ≥50% responders vs the level of perimenstrual seizure exacerbation (C1 level). C1 levels were determined during baseline and are expressed as multiples of the combined midfollicular and midluteal seizure frequencies. Each level includes all women who had seizure exacerbation greater than or equal to that specific level of catameniality. With increasing C1 levels, the rate of ≥50% responders increased from 21.3% to 57.1% with progesterone treatment as compared to an increase of only 19.6% to 20.0% with placebo treatment. The anticipated primary outcome that 35% of catamenial progesterone–treated vs 15% of placebo-treated women would show a ≥50% reduction in seizure frequency is realized at C1 level ≥3 where 37.8% of progesterone-treated as compared to 11.1% of placebo-treated women were ≥50% responders (p = 0.0372). In comparison to the responder rate of the combined placebo group, the progesterone responder rates are significantly greater at each C1 level ≥3. (B) Percent change in average daily seizure frequency in relation to C1 level: progesterone vs placebo. With increasing C1 levels from 1 to 10, the percent reduction in average daily seizure frequency (ADSF) (mean ± SEM) progressed from 25.5% to 71.0% for progesterone as compared to 25.0% to 26.2% for placebo. Separation between the treatments reached significance at C1 levels ≥4. In comparison to change in ADSF in the combined placebo group, the changes in ADSF in progesterone-treated subjects are significant at each C1 level ≥3.
The difference in responder rates between progesterone and placebo was not significant when considering women with C1 level ≥1.69, the selected cutoff level for designation to the catamenial stratum (progesterone: 27.3% vs placebo: 14.3%; p = 0.1313). Note, however, that the anticipated primary outcome that ≥35% of catamenial progesterone vs ≤15% of placebo-treated women would show a 50% reduction in seizure frequency was realized at C1 level ≥3 where 37.8% of progesterone as compared to 11.1% of placebo-treated women were responders (p = 0.0372). At each C1 level ≥3, the progesterone responder rates were significantly greater than the responder rate of the combined placebo group.
An estimation of the proportion of women who might respond to progesterone depends not only on the responder rate at any given C1 level but also on the proportion of women who have that level of seizure exacerbation. Baseline data showed that 63 of the 294 women, i.e., 21.4%, had C1 level ≥3, the level which showed the anticipated significant and clinically important separation between the rates of progesterone and placebo responders (table 3).
Table 3.
Percent of women with various levels of perimenstrual seizure exacerbation
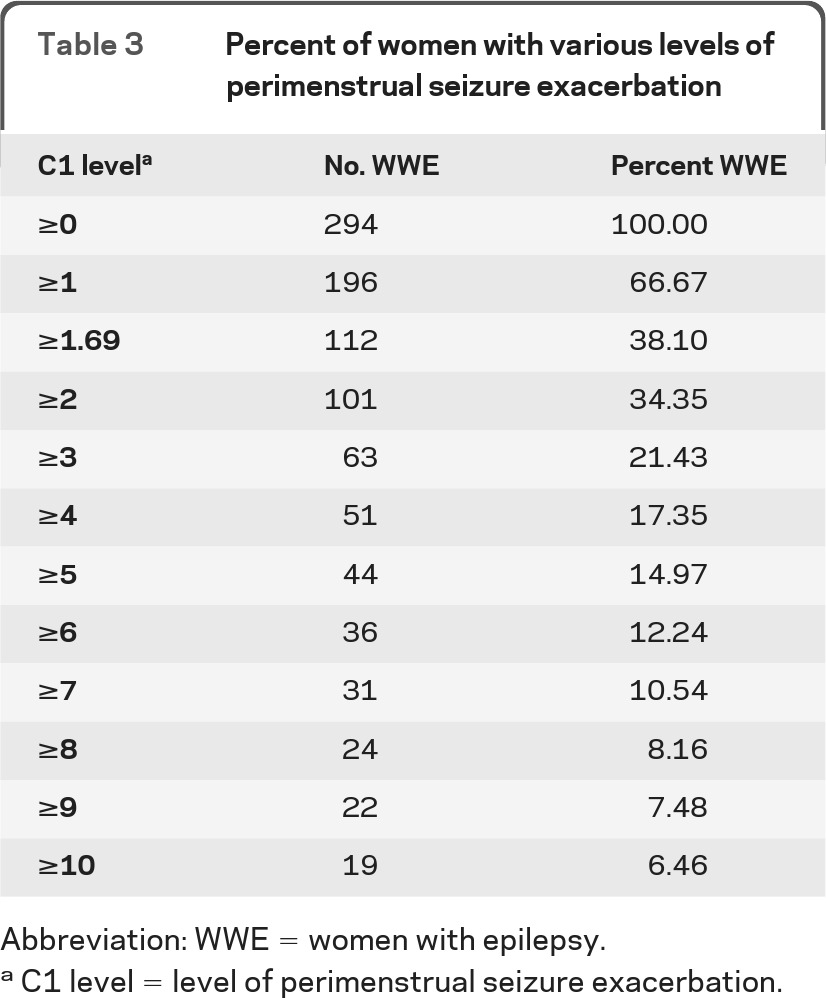
Abbreviation: WWE = women with epilepsy.
C1 level = level of perimenstrual seizure exacerbation.
The other predictor of responders with progesterone was the C2 pattern (p = 0.027). There was no significant interaction between C2 pattern and type of treatment (p = 0.262). C2 pattern did not show a difference in responders between progesterone and placebo (1/28, 3.6% vs 0/14, 0%; Fisher exact test p = 1.000). Rather, women whose seizures showed a C2 pattern had a responder rate that was substantially lower on progesterone than women with either the C1 (1/28, 3.6% vs 15/47, 31.9%; Fisher exact test p = 0.0033) or C3 (1/28, 3.6% vs 11/51, 21.6%; Fisher exact test p = 0.0475) pattern but did not differ significantly from women on placebo (1/28, 3.6% vs 0/14, 0.0%; Fisher exact test p = 1.0000).
C1 level was the only significant predictor of progesterone responders for the MSST (p = 0.005). There was significant interaction between C1 level and treatment (p = 0.008). There were also significant interactions between C1 level and treatment for some individual seizure types: SGMS (p = 0.034) and CPS (p = 0.016) but not SPS (p = 0.709). Progesterone responder rates increased with C1 levels for the MSST from 24% to 64% (r = 0.254, p = 0.001) as well as for SGMS from 31% to 56% (r = 0.256, p = 0.038) and CPS, 29% to 63% (r = 0.211, p = 0.012) but not SPS (r = 0.023, p = 0.839). There was no significant correlation between C1 levels and percent placebo responders.
Predictors of progesterone treatment efficacy: Change in average daily seizure frequency.
Multivariate stepwise linear regression identified C1 level (p < 0.001), C2 pattern (p = 0.021), and epilepsy laterality (p = 0.039) as significant predictors of changes in ADSF. There was significant interaction between C1 level and treatment (univariate analysis of variance F = 7.537, p = 0.001). There was no significant interaction with treatment for C2 pattern (p = 0.191) or epilepsy laterality (p = 0.098). With increasing C1 levels from 1 to 10, the percent reduction in ADSF progressed from −25.5% to −71.1% for progesterone (r = −0.269, p < 0.001) vs −25.0% to −26.3% for placebo (r = −0.085, p = 0.407) (figure 2B). Separation between treatments at each C1 level reached significance at C1 levels ≥4. At each C1 level ≥3, the changes in ADSF in progesterone-treated subjects were significantly greater than the change in ADSF in the combined placebo group.
C1 level was the only significant predictor for percent change in ADSF for the MSST (p = 0.016). Again, there was a significant interaction between treatment and C1 level (p = 0.005) and ADSF decreased with progesterone (r = −0.207, p = 0.006) but not placebo (r = −0.019, p = 0.922).
Serious adverse events.
There were 22 serious adverse events (SAEs) among the 462 women, 13 during baseline and 9 during treatment. Of the 9 during the treatment phase, 6 occurred on progesterone and 3 on placebo, commensurate with the 2:1 randomization. The most common SAE (12/22) was hospitalization for seizures: baseline phase 7, treatment phase 5, progesterone 2, placebo 3. Two subjects died, 1 in baseline and 1 during treatment. The baseline death was by suicide, committed after the initial visit. The treatment phase death was attributed to sudden unexplained death in epilepsy and considered unlikely to be related to progesterone treatment. The 8 other SAEs had singular occurrence. Five (nausea, fever and vomiting, cellulitis, insomnia, AED overdose) occurred during baseline and 3 (stomach flu, thyroid carcinoma, blurred vision) on treatment, all on progesterone but considered unlikely to be related to progesterone.
Adverse events.
There were no significant differences in proportions of occurrences of any adverse event between the 2 treatment groups within either stratum. Adverse events that occurred with a frequency of ≥5% in any of the 4 treatment groups are reported in table e-3.
DISCUSSION
The findings show that cyclic progesterone is comparable to placebo in the treatment of intractable seizures in women with partial epilepsy. Post hoc analysis identified a subset of women with perimenstrual seizure exacerbation who were responsive to treatment.
The principal positive findings are based on a prespecified secondary analysis. The level of perimenstrual catameniality (C1 level) is a predictor of the efficacy of progesterone treatment. There was a significant interaction between C1 level and treatment. With increasing C1 levels, responder rates increased progressively from 21.3% to 57.1% for progesterone vs only 19.6% to 20.0% with placebo. Changes in ADSF progressed from −25.5% to −71.0% for progesterone vs only −25.0% to −26.3% for placebo. There was also significant interaction between C1 level and progesterone treatment for the MSST, SGMS, and CPS but not SPS. These findings of the secondary analysis require formal confirmation in an investigation of the derived hypotheses.
The separation between responder rates for all seizures combined for progesterone (27.3%) vs placebo (14.3%) treatments was not significant at C1 level ≥1.69, the cutoff level selected for designation to the catamenial stratum. At C1 level ≥3, however, the separation (37.8% vs 11.1%) was significant (p = 0.0372) and achieved the anticipated clinically important separation goal of the trial.
Failure of the trial to prove the principal hypothesis may relate to the design that attempted to treat 3 patterns of catamenial epilepsy which likely differ in pathophysiology with a single treatment regimen.2,5 Specifically, cyclic progesterone supplement may have greater efficacy where progesterone withdrawal (C1 pattern), rather than estrogen surge (C2- peri-ovulatory pattern) or low midluteal progesterone levels (C3 pattern), are causally implicated. The design also assumed that the mathematically determined cutoff for catamenial designation would match the cutoff for significant progesterone response.5 The absence of a significant difference between progesterone and placebo responders at the C1 cutoff level of ≥1.69 and finding of a significant difference at a clinically important level at C1 level ≥3 may suggest that there is a difference between the catamenial level that mathematically best distinguishes hormonally sensitive seizures5 and the level that distinguishes progesterone responders at a statistically significant and clinically important level. Alternatively, a larger sample size may be required, i.e., 235 progesterone and 118 placebo-treated subjects to show the demonstrated C1 ≥1.69 progesterone responder rate of 27.3% vs placebo rate of 14.2% with p ≤ 0.05 and power of 0.80.
Progesterone is a naturally occurring hormone with a long history of clinical use for the treatment of reproductive disorders, e.g., inadequate luteal phase cycles, infertility, and postmenopausal hormone replacement. Its safe use during pregnancy has been well documented. This novel use of cyclic progesterone supplement as a treatment for women with epilepsy showed a favorable short-term safety profile that did not differ significantly from placebo in the frequency and types of adverse events. The profile is also favorable in comparison to AEDs.
The practical applications are that the detection of a ≥3-fold level of perimenstrual seizure exacerbation by charting of seizures and menses might suggest a favorable response to treatment with adjunctive cyclic progesterone supplement. Periovulatory seizure exacerbation may not respond to progesterone or may require the start of treatment earlier in the cycle than day 14. Since an earlier start to progesterone supplement may disrupt the menstrual cycle and lead to intermenstrual bleeding, there may be a role for the use of synthetic GABAergic steroids, analogues of allopregnanolone such as ganaxolone that are devoid of reproductive hormonal properties and, therefore, can be used throughout the cycle as well as in men.17 Alternative and perhaps more effective approaches to suppression of the preovulatory estrogen surge include parenteral treatment with depomedroxyprogesterone18 or GnRH analog with or without hormonal supplement.19–21 Investigations of these parenteral treatments, however, have been limited to small open-label trials. Finally, the role of progesterone treatment in primarily generalized epilepsy, especially juvenile myoclonic epilepsy, that is more common in women and tends to develop in adrenarchal and pubertal years, remains to be investigated.
Supplementary Material
GLOSSARY
- ADSF
average daily seizure frequency
- AED
antiepileptic drug
- CPS
complex partial seizures
- MSST
most severe seizure type
- SAE
serious adverse event
- SGMS
secondary generalized motor seizures
- SPS
simple partial seizures.
Footnotes
Supplemental data at www.neurology.org
Contributor Information
Andrew G. Herzog, Beth Israel Deaconess Medical Center, Principal Investigator.
Kristen M. Fowler, Beth Israel Deaconess Medical Center, Clinical Trials Specialist.
Sarah D. Smithson, Beth Israel Deaconess Medical Center, clinical coordinator.
Donald L. Schomer, Beth Israel Deaconess Medical Center, co-investigator.
Edward B Bromfield, Brigham & Women's, Site Investigator.
Barbara A. Dworetzky, Brigham & Women's, Site Investigator.
Sonia Replansky, Brigham & Women's, Site Coordinator.
Katherine Gleason, Brigham & Women's, Site Coordinator.
Alison Pack, Columbia, Site Investigator.
Alison Randall, Columbia, Site Investigator.
Cynthia Harden, Cornell, Site Investigator.
Blagovast Nikolov, Cornell Site Coordinator.
Barbara Jobst, Dartmouth, Site Investigator.
Gregory L. Holmes, Dartmouth, Site Investigator.
Emily Clough, Dartmouth, Site Coordinator.
Tracy Ostler, Dartmouth, Site Coordinator.
Page B. Pennell, Emory, Site Investigator.
Kimford Meador, Emory, Site Investigator.
Melanee Newman, Emory, Site Coordinator.
Gregory L. Krauss, Johns Hopkins, Site Investigator.
Peter W. Kaplan, Hopkins, Site Investigator.
Faith Mugai, Johns Hopkins, Site Coordinator.
Amanda Cole, Johns Hopkins, Site Coordinator.
Eva Andermann, Montreal Neurological Institute, Site Investigator.
Frederick Andermann, Montreal Neurological Institute, Site Investigator.
Suha Mercho, Montreal Neurological Institute, Site Coordinator.
Teresa Tran, MINCEP, Site Investigator.
Sabina Gapany, MINCEP, Site Coordinator.
Michael R. Sperling, Thomas Jefferson, Site Investigator.
Joyce D. Liporace, Thomas Jefferson, Site Investigator.
Sevie Shuman, Thomas Jefferson, Site Coordinator.
Gwendolyn Manney, Thomas Jefferson, Site Coordinator.
Laura A. Kalayjian, USC, Site Investigator.
Christianne N. Heck, USC, Site Investigator.
Sandra Oviedo, USC, Site Coordinator.
Guadalupe Corral-Leyva, USC, Site Coordinator.
Marianna Spanaki, Henry Ford, Site Investigator.
Tricia Ting, University of Maryland, Site Investigator.
Karen Callison, University of Maryland, Site Coordinator.
Nathan B. Fountain, University of Virginia, Charlottesville, Site Investigator.
Mark Quigg, University of Virginia, Charlottesville, Site Investigator.
Cheryl Frye, University of Albany, Director of Neuroesteroid Assay Lab.
Joseph Massaro, Harvard Clinical Research Institute, Biostatistician.
Brian Marquis, Freedom Drug, Boston, Research Pharmacist
AUTHOR CONTRIBUTIONS
Dr. Herzog: study concept or design, acquisition of data, analysis or interpretation of data, drafting/revising the manuscript study supervision, obtaining funding. Ms. Fowler: study concept or design, acquisition of data, analysis or interpretation of data, drafting/revising the manuscript, obtaining funding. Ms. Smithson: acquisition of data, analysis or interpretation of data, drafting/revising the manuscript. Dr. Kalyajian: acquisition of data, analysis or interpretation of data, drafting/revising the manuscript. Dr. Heck: acquisition of data, analysis or interpretation of data, drafting/revising the manuscript. Dr. Sperling: acquisition of data, analysis or interpretation of data, drafting/revising the manuscript. Dr. Liporace: acquisition of data, analysis or interpretation of data, drafting/revising the manuscript. Dr. Harden: acquisition of data, analysis or interpretation of data, drafting/revising the manuscript. Dr. Dworetzky: acquisition of data, analysis or interpretation of data, drafting/revising the manuscript. Dr. Pennell: acquisition of data, analysis or interpretation of data, drafting/revising the manuscript. Dr. Massaro: statistical analysis, analysis or interpretation of data, drafting/revising the manuscript.
DISCLOSURE
Dr. Herzog was the principal investigator on this research that was supported by NIH NINDS R01 39466. He also received support for investigator-initiated research projects funded by GlaxoSmithKline and Abbott as well as the Epilepsy Foundation. Ms. Fowler, Ms. Smithson, and Dr. Kalyajian report no disclosures. Dr. Heck has received research support from Boston Scientific and Neuropace. Dr. Sperling has received research support from NIH, UCB Pharma, Eisai, Vertex Pharmaceuticals, Marinus, Novartis, Medtronics, Neuropace, Lundbeck, and Sunovion, has consulted for Vertex and Sunovion, and served on the speaker's bureau of UCB Pharma. Dr. Liporace receives a grant from NIH and is on the speaker's bureau for UCB Pharma from 2009–2011. Dr. Harden was on the speaker's bureau for GlaxoSmithKline, UCB Pharma, and Lundbeck Pharmaceuticals. She has served as a consultant to Novartis and Upsher-Smith. She has received grant support from Forest Pharmaceuticals, the Milken Family Foundation, and the Epilepsy Foundation. Dr. Dworetzky has an educational grant from the AAN. Dr. Pennell receives grant support from the NIH, Milken Family Foundation, and Epilepsy foundation, and serves on the Board of Directors for the Epilepsy Research Foundation, the Epilepsy Foundation, and the American Epilepsy Society. Dr. Massaro was paid for statistical consultation on this project through Harvard Clinical Research Institute, the academic CRO that oversaw the data management and biostatistical services for this study. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Tauboll E, Lundervold A, Gjerstad L. Temporal distribution of seizures in epilepsy. Epilepsy Res 1991; 8: 153– 165 . [DOI] [PubMed] [Google Scholar]
- 2.Herzog AG. Catamenial epilepsy: definition, prevalence, pathophysiology and treatment. Seizure 2008; 17: 151– 159 . [DOI] [PubMed] [Google Scholar]
- 3.Quigg M, Smithson SD, Fowler KM, Sursal T, Herzog AG, Progesterone Trial Study Group Laterality and location influence catamenial seizure expression in women with partial epilepsy. Neurology 2009; 73: 223– 227 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laidlaw J. Catamenial epilepsy. Lancet 1956; 271: 1235– 1237 . [DOI] [PubMed] [Google Scholar]
- 5.Herzog AG, Klein P, Ransil BJ. Three patterns of catamenial epilepsy. Epilepsia 1997; 38: 1082– 1088 . [DOI] [PubMed] [Google Scholar]
- 6.Harrison NL, Majewska MD, Harrington JW, Barker JL. Structure-activity relationships for steroid interactions with the γ-aminobutyric acidA receptor complex. J Pharmacol Exp Ther 1987; 241: 346– 353 . [PubMed] [Google Scholar]
- 7.Kokate TG, Svensson BE, Rogawski MA. Anticonvulsant activity of neurosteroids: correlation with g-aminobutyric acid-evoked chloride current potentiation. J Pharmacol Exp Ther 1994; 270: 1223– 1229 . [PubMed] [Google Scholar]
- 8.Frye CA. The neurosteroid 3a-5a-THP has antiseizure and possible neuroprotective effects in an animal model of epilepsy. Brain Res 1995; 696: 113– 120 . [DOI] [PubMed] [Google Scholar]
- 9.Lawrence C, Martin BS, Sun C, Williamson J, Kapur J. Endogenous neurosteroid synthesis modulates seizure frequency. Ann Neurol 2010; 67: 689– 693 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woolley CS, McEwen BS. Estradiol regulates hippocampal dendritic spine density via an N-methyl-D-aspartate receptor-dependent mechanism. J Neurosci 1994; 14: 7680– 7687 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scharfman HE, Mercurio TC, Goodman JH, Wilson MA, MacLusky NJ. Hippocampal excitability increases during the estrous cycle in the rat: a potential role for brain-derived neurotrophic factor. J Neurosci 2003; 23: 11641– 11652 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reddy DS. Neurosteroids: endogenous role in the human brain and therapeutic potentials. Prog Brain Res 2010; 186: 113– 137 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABAA receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci 2005; 8: 797– 804 . [DOI] [PubMed] [Google Scholar]
- 14.Quigg M, Smithson SD, Fowler KM, Sursal T, Herzog AG, Progesterone Trial Study Group Laterality and location influence catamenial seizure expression in women with partial epilepsy. Neurology 2009; 73: 223– 227 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Betts T, Waegemans T, Crawford P. A multicentre, double-blind, parallel group study to evaluate the tolerability and efficacy of two oral doses of levetiracetam, 2000 mg daily and 4000 mg daily, without titration in patients with refractory epilepsy. Seizure 2000; 9: 80– 87 . [DOI] [PubMed] [Google Scholar]
- 16.Barcs G, Walker EB, Elger CE, et al. Oxcarbazepine placebo-controlled, dose-ranging trial in refractory partial epilepsy. Epilepsia 2000; 41: 1597– 1607 . [DOI] [PubMed] [Google Scholar]
- 17. Marinus Pharmaceuticals March 3, 2009 announcement. Available at: http://www.marinuspharma.com/nr_positive_results.html March 3, 2009
- 18.Mattson RH, Cramer JA, Caldwell BV, Siconolfi BC. Treatment of seizures with medroxyprogesterone acetate: preliminary report. Neurology 1984; 34: 1255– 1258 . [DOI] [PubMed] [Google Scholar]
- 19.Bauer J, Wildt L, Flugel D, Stefan H. The effect of a synthetic GnRH analogue on catamenial epilepsy: a study in ten patients. J Neurol 1992; 239: 284– 286 . [DOI] [PubMed] [Google Scholar]
- 20.Haider Y, Barnett DB. Catamenial epilepsy and goserelin. Lancet 1991; 338: 1530 . [DOI] [PubMed] [Google Scholar]
- 21.Reid B, Gangar KF. Catamenial epilepsy and goserelin. Lancet 1992; 339: 253 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.