Abstract
Objective:
The widely reported associations between various nutrients and cognition may occur through many biologic pathways including those of β-amyloid (Aβ). However, little is known about the possible associations of dietary factors with plasma Aβ40 or Aβ42. The aim of the current study was to evaluate the association between nutrient intake and plasma Aβ levels.
Methods:
In this cross-sectional study, plasma Aβ40 and Aβ42 and dietary data were obtained from 1,219 cognitively healthy elderly (age >65 years), who were participants in a community-based multiethnic cohort. Information on dietary intake was obtained 1.2 years, on average, before Aβ assay. The associations of plasma Aβ40 and Aβ42 levels and dietary intake of 10 nutrients were examined using linear regression models, adjusted for age, gender, ethnicity, education, caloric intake, apolipoprotein E genotype, and recruitment wave. Nutrients examined included saturated fatty acid, monounsaturated fatty acid, ω-3 polyunsaturated fatty acid (PUFA), ω-6 PUFA, vitamin E, vitamin C, β-carotene, vitamin B12, folate, and vitamin D.
Results:
In unadjusted models that simultaneously included all nutrients, higher intake of ω-3 PUFA was associated with lower levels of Aβ40 (β = −24.7, p < 0.001) and lower levels of Aβ42 (β = −12.3, p < 0.001). In adjusted models, ω-3 PUFA remained a strong predictor of Aβ42 (β = −7.31, p = 0.02), whereas its association with Aβ40 was attenuated (β = −11.96, p = 0.06). Other nutrients were not associated with plasma Aβ levels.
Conclusions:
Our data suggest that higher dietary intake of ω-3 PUFA is associated with lower plasma levels of Aβ42, a profile linked with reduced risk of incident AD and slower cognitive decline in our cohort.
There is increasing evidence to suggest that diet may play an important role in preventing or delaying the onset of Alzheimer disease (AD).1 We have previously reported that a Mediterranean-type diet and a dietary pattern explaining the maximum variation of 7 AD-related nutrients were associated with lower risk of prevalent AD, incident AD, incident mild cognitive impairment (MCI), or MCI conversion to AD2–5 in a New York population. However, the potential biologic mechanisms for the relation between diet and AD have not been well addressed.
One important pathologic hallmark of AD is β-amyloid (Aβ) peptide (mainly Aβ40 and Aβ42) deposition in the brain, resulting in formation of plaques. Although the brain burden of Aβ has been considered to be the most direct marker of AD pathology and has been well associated with clinical manifestations of AD severity, it is not easy or practical to measure in epidemiologic studies. In contrast, plasma Aβ is relatively easy to obtain and minimally invasive. In addition, it has been suggested that a dynamic equilibrium between central and peripheral pools of Aβ might exist; thus, the changes in Aβ42 content in blood over a longer period of time may reflect Aβ deposition in the brain.6 Several large-scale studies have found that plasma levels of Aβ peptides have predictive value for AD or cognitive decline.7–12
In the present study, we aimed to examine whether dietary intake of nutrients was associated with plasma Aβ levels in a cross-sectional analysis of an elderly (65 years or older) New York population.
METHODS
Study design.
We performed a cross-sectional study to examine the association between dietary intake of nutrients and plasma Aβ levels.
Study setting and participants.
Washington Heights/Hamilton Heights Columbia Aging Project (WHICAP) is a large-scale community-based project with the aim of understanding the antecedents, biologic risk factors, genetics, and course of cognitive aging and dementia. Participants in the cohort were identified from a probability sample of Medicare beneficiaries aged 65 or older, residing in northern Manhattan,2–4 stratified by age and ethnicity.
The initial sample for this study included 2,778 participants of the WHICAP-II cohort, which represents a combination of continuing members of the subcohort originally recruited in 1992 (n = 604) and members of a new subcohort recruited in 1999 (n = 2,174). Both subcohorts used similar sampling, assessments and study procedures.2,3 In brief, at entry, a physician elicited each participant's medical and neurologic history and conducted a standardized physical and neurologic examination. Each participant also underwent a structured in-person interview including an assessment of health and function and a neuropsychological battery.13 Participants were followed at intervals of approximately 1.5 years, repeating the baseline examination and consensus diagnosis.2,3 The diagnosis of any type of dementia or its absence was based on standard research criteria14 and was established using all available information including medical records gathered at the initial and follow-up assessments at a consensus conference of physicians, neurologists, neuropsychologists, and psychiatrists. The diagnosis was made blind to diet information.
Because dementia itself may affect participants' dietary habits or participants' report of dietary habits, we excluded participants with prevalent dementia (n = 345) from the initial study sample of 2,778. We further excluded participants with no dietary assessments (n = 189). An additional 1,025 participants were excluded from the analysis because their Aβ levels were not measured. Thus, the analytic sample for the current study included a total of 1,219 nondemented participants with available dietary evaluations and Aβ levels, representing a subset (approximately half) of the study population used in our previous studies.2,3,5
Standard protocol approvals, registrations, and patient consents.
The Columbia University Institutional Review Board has reviewed and approved this project. All individuals provided written informed consent.
Outcome variable: plasma Aβ40 and Aβ42.
Details on methods of Aβ measurements have been previously reported by our group.7,9,10,15 In brief, a 10-mL sample of venous blood (tripotassium EDTA) was collected at baseline after standard handling procedures. Plasma samples had since been stored, within 2 hours after collection, at −70°C. Laboratory personnel were blinded to the cognitive status of the samples. Plasma levels of Aβ40 and Aβ42 were measured in duplicate by using a combination of monoclonal antibody 6E10 (specific to an epitope present on 1−16 amino acid residues of Aβ) and rabbit antisera R165 (vs Aβ42) and R162 (vs Aβ40) in a double-antibody sandwich ELISA as described previously.7,9 The detection limit for these assays was 9 pg/mL for Aβ40 and 10 pg/mL for Aβ42. All the samples of the same recruitment wave (which is controlled for in the analysis) were tested in a single day, limiting the day-to-day variation of our assays. The mean of the within-assay coefficient of variation was 4.6% for Aβ40 and 9.3% for Aβ42. The test-retest reliability of the measurement of plasma Aβ40 and Aβ42 was excellent (Cronbach α coefficient = 0.91).7
Exposure variable: dietary intake of nutrients.
Dietary data regarding average food consumption over the prior year were obtained using the 61-item version of Willett's semiquantitative food frequency questionnaire (SFFQ) (Channing Laboratory, Cambridge, MA), administered by trained interviewers in English or Spanish. Our dietary questionnaires were sent to Channing Laboratory from where dietary data (both consumption frequency for foods and consumption amount for nutrients) were returned in electronic format. The daily dietary consumption amount of a nutrient was computed by summing up the nutrient amount contained in each food (i.e., the daily consumption frequency of food portion multiplied by the nutrient content of the specified portion). Previous studies have reported reasonable validity and reliability of the SFFQ in other populations16–21 and in our study population.22–24
The nutrient intakes from foods and from supplements were separately estimated, and only the nutrient intake from foods was used in the current analysis. We regressed caloric intake (measured in kilocalories) and calculated the derived residuals for each nutrient.25 Similarly to our previous report,5 in the current study, we focused on 10 nutrients that are most consistently reported to be related to AD or cognitive function according to the previous literature: ω-3 polyunsaturated fatty acid (PUFA), ω-6 PUFA, monounsaturated fatty acid (MUFA), saturated fatty acid (SFA), β-carotene, vitamin C, vitamin E, vitamin B12, folate, and vitamin D.26
Dietary information was collected on average 1.2 (SD 3.1) years before the collection of blood samples used for Aβ assessments.
Potential confounders.
Age (years), education (years), and caloric intake (kcal) were used as continuous variables. Ethnic group was based on self-report using the format of the 1990 census. Participants were then assigned to 1 of 4 groups: African American (black non-Hispanic), Hispanic, white (non-Hispanic), or other. Ethnicity was used as a dummy variable with white (non-Hispanic) as the reference. Gender was used as a dichotomous variable with male as the reference. Apolipoprotein E (APOE) genotypes were used as a dichotomous variable: absence (as reference) vs presence of either 1 or 2 ϵ4 alleles. Recruitment wave was included in the regression models as a dichotomous variable to indicate either the 1992 or the 1999 subcohort. Alcohol intake was used as a dichotomous variable: mild-moderate alcohol consumption (>0−<30 g/day) (as reference) vs either no (0 g/day) or more than moderate (≥30 g/day) consumption.
Statistical analyses.
We compared demographic characteristics, clinical characteristics, memory status, and dietary intakes of nutrients according to the tertiles of Aβ peptides, using χ2 tests for categorical variables and analysis of variance for continuous variables. For nutrients found to be associated with Aβ peptides, we examined their associations with participants' demographic-clinical characteristics using similar analytical methods.
We used generalized linear regression models to evaluate the association between nutrients and plasma Aβ peptides. Models were initially unadjusted and then adjusted for age, sex, education, recruitment wave, ethnicity, caloric intake, and APOE genotype. The results from analyses with log-transformed and nontransformed Aβ levels were similar; we present the result for the nontransformed Aβ variables. Although we used caloric intake-adjusted residuals for nutrient variables, we also included caloric intake as a covariate in the models.25 Finally, in a fully adjusted model, we additionally adjusted for alcohol drinking, use of medication (medications affecting blood vessel thrombus formation propensity including warfarin, heparin, aspirin, and antiplatelet and antidiabetic agents including insulin and oral hypoglycemic),12,27 and intake of nutrients from supplements (except for MUFA and SFA, for which no supplements were consumed). For nutrients that appeared to be associated with Aβ levels, we further examined their food sources by examining the correlations between foods and the nutrients.
We performed a series of supplementary analyses. Because dementia in preclinical stages may influence the report of dietary intake, we performed sensitivity analysis to further exclude participants who might be at the preclinical stages of dementia, i.e., participants with incident dementia who progressed to dementia over the course of the follow-up. We also performed supplementary analyses on 3 major subtypes of ω-3 PUFAs, including docosahexaenoic acid (DHA, 22:6), eicosapentaenoic acid (20:5), and α-linolenic acid (18:3). Finally, because literature reports suggest that benefits of some PUFAs are contingent on the APOE ϵ4 status,28–30 we examined effect modification by APOE for ω-3 PUFA in adjusted models that included a multiplicative term between the ω-3 PUFA and APOE.
All analyses were conducted using PASW Statistics (IBM, Chicago, IL). All p values were based on 2-sided tests. The significance level was set at 0.05 for all tests.
RESULTS
Missing data analysis.
Participants who were included in the analysis (n = 1,219) and participants who were excluded from the analyses because of missing diet or Aβ levels (n = 1,214) did not differ in age, gender, education, race, or APOE genotype (data not shown).
Clinical, demographic, and dietary characteristics and plasma Aβ level.
Participants with higher plasma levels of Aβ peptide (either Aβ40 or Aβ42) were older and less educated and had lower intakes of ω-3 PUFA, ω-6PUFA, and MUFA (table 1 for Aβ40 and table 2 for Aβ42). Plasma Aβ was not related to other clinical, demographic, or nutritional variables. Table e-1 on the Neurology® Web site at www.neurology.org shows the average scores of selected neuropsychological tests of the participants.
Table 1.
Demographic, clinical, and dietary characteristics of participants by Aβ40 tertiles
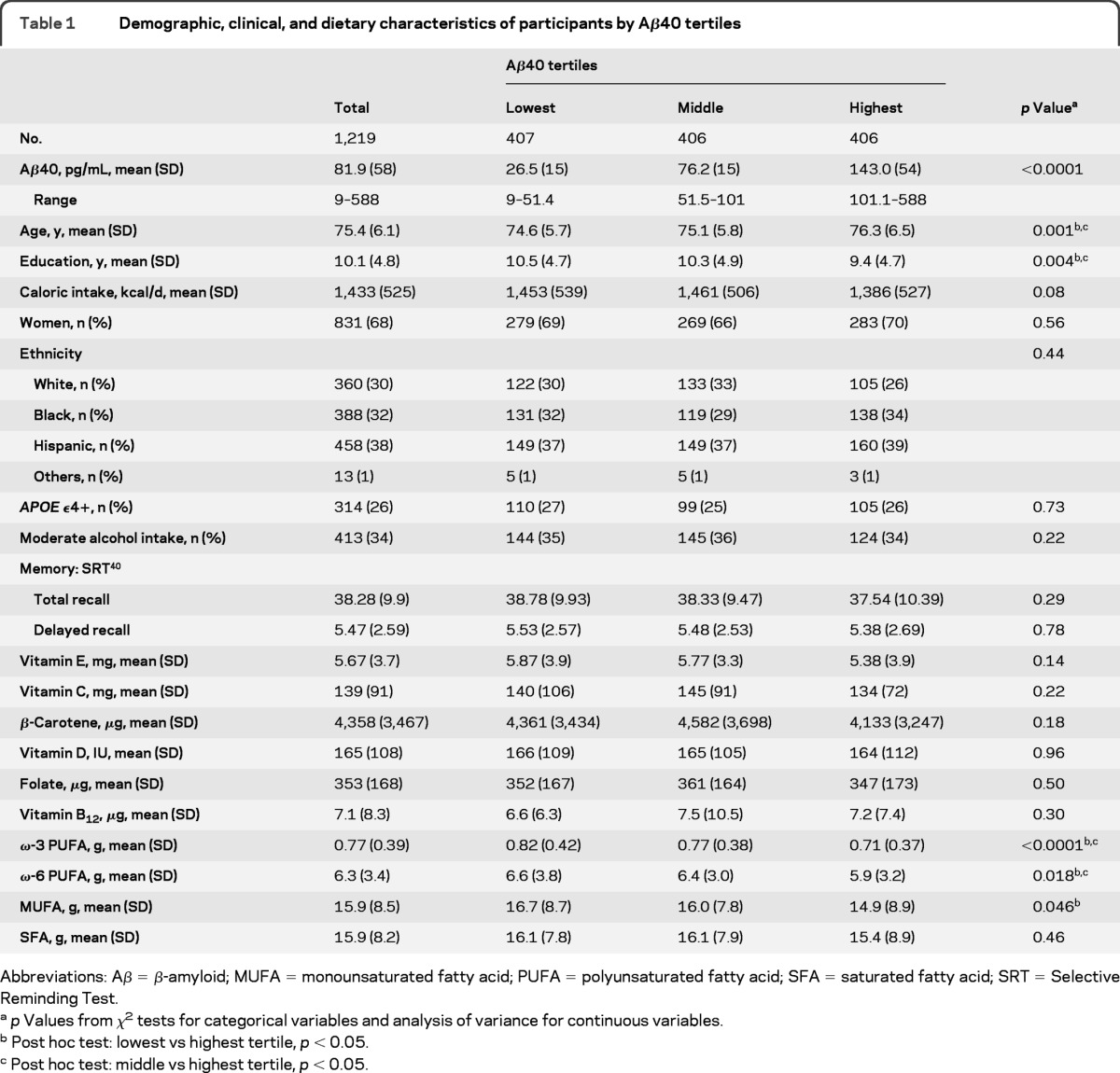
Abbreviations: Aβ = β-amyloid; MUFA = monounsaturated fatty acid; PUFA = polyunsaturated fatty acid; SFA = saturated fatty acid; SRT = Selective Reminding Test.
p Values from χ2 tests for categorical variables and analysis of variance for continuous variables.
Post hoc test: lowest vs highest tertile, p < 0.05.
Post hoc test: middle vs highest tertile, p < 0.05.
Table 2.
Demographic, clinical, and dietary characteristics of participants by Aβ42 tertiles
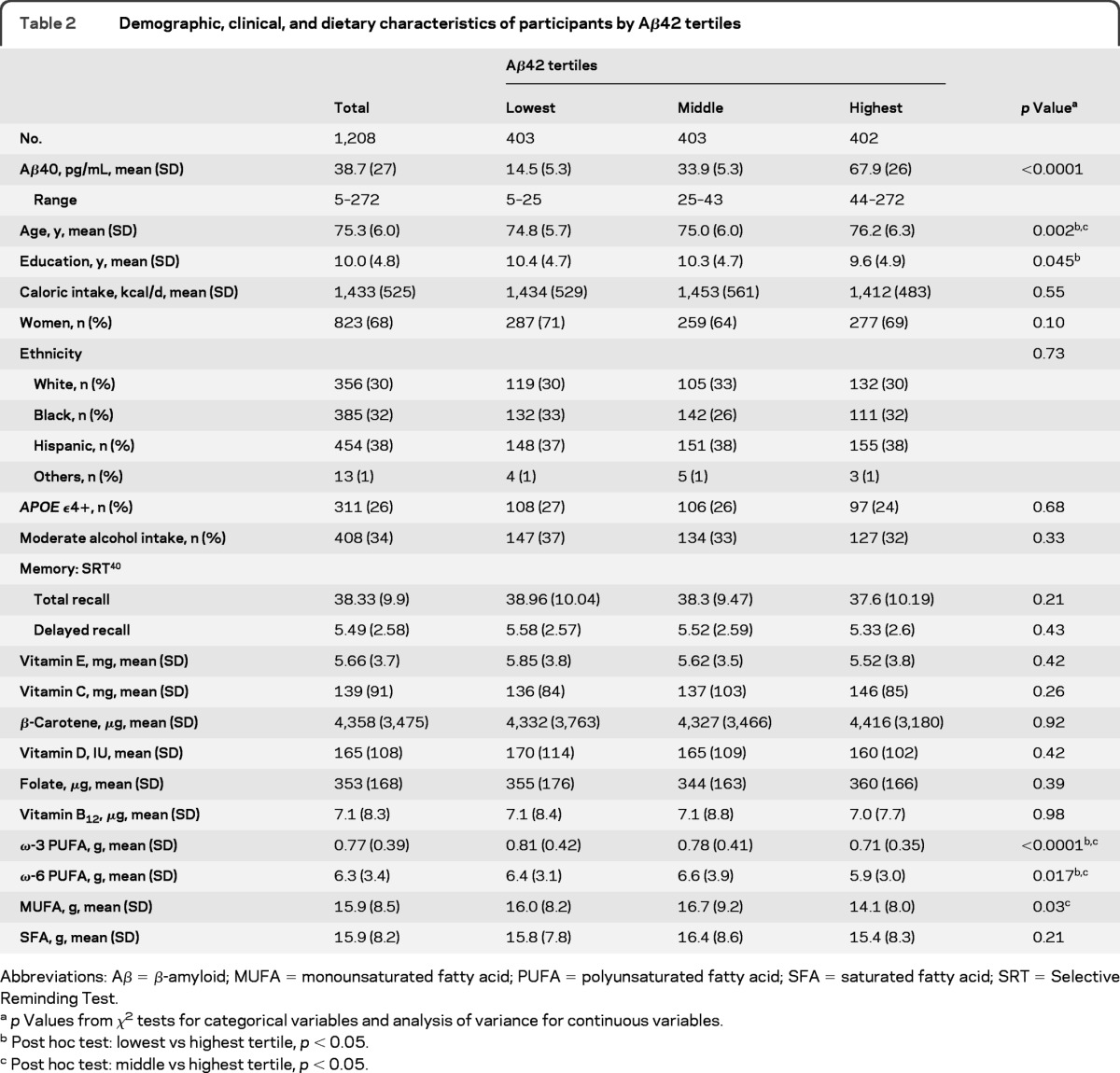
Abbreviations: Aβ = β-amyloid; MUFA = monounsaturated fatty acid; PUFA = polyunsaturated fatty acid; SFA = saturated fatty acid; SRT = Selective Reminding Test.
p Values from χ2 tests for categorical variables and analysis of variance for continuous variables.
Post hoc test: lowest vs highest tertile, p < 0.05.
Post hoc test: middle vs highest tertile, p < 0.05.
Clinical, demographic, and Aβ characteristics and nutrient intake.
Participants with higher ω-3 PUFA, higher ω-6 PUFA, or higher MUFA intakes had higher education, were more likely to be white or black and less likely to be Hispanic, and had lower levels of Aβ42 (table 3). Participants with higher ω-3 PUFA also had lower levels of Aβ40 (table 3).
Table 3.
Demographic and clinical characteristics of participants by nutrients intake tertiles
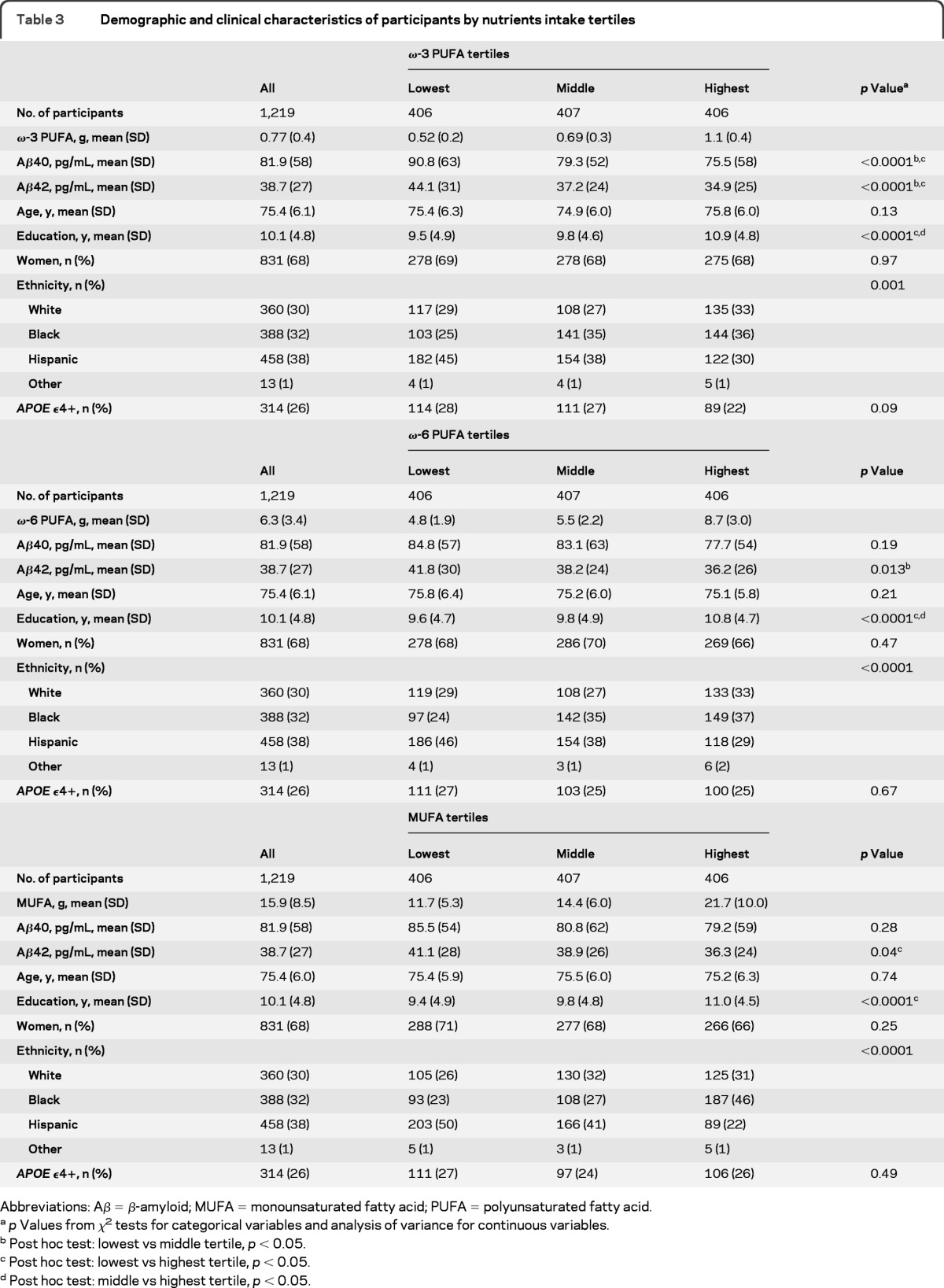
Abbreviations: Aβ = β-amyloid; MUFA = monounsaturated fatty acid; PUFA = polyunsaturated fatty acid.
p Values from χ2 tests for categorical variables and analysis of variance for continuous variables.
Post hoc test: lowest vs middle tertile, p < 0.05.
Post hoc test: lowest vs highest tertile, p < 0.05.
Post hoc test: middle vs highest tertile, p < 0.05.
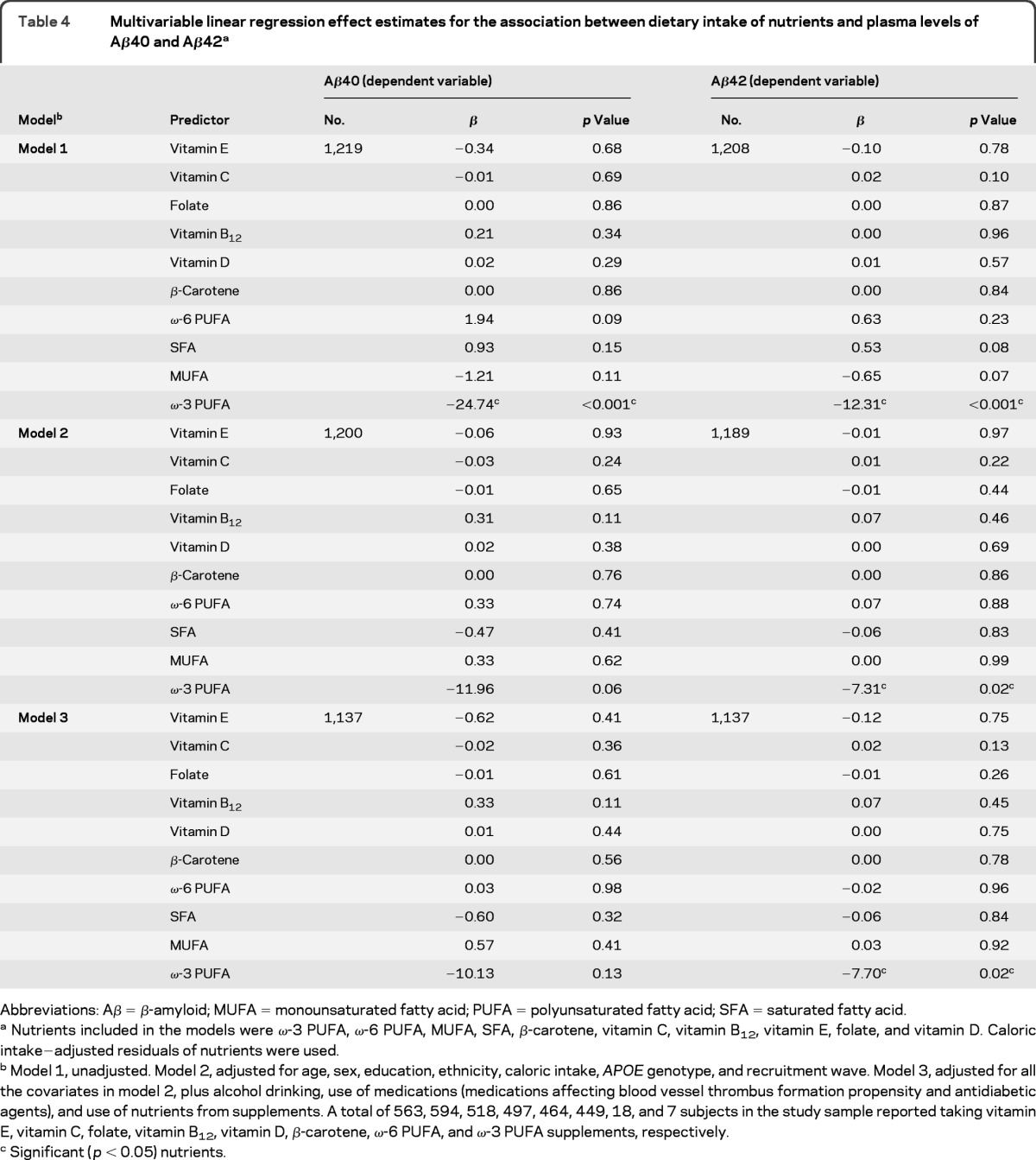
Nutrients and Aβ peptides.
In the unadjusted models, higher intake of ω-3 PUFA was significantly associated with reduced plasma levels of both Aβ40 and Aβ42 (table 4, model 1). The association of higher intake of ω-3 PUFA with lower levels of Aβ42 remained significant after multivariable adjustment, whereas its association with Aβ40 was attenuated (table 4, model 2). In the fully adjusted model, ω-3 PUFA intake remained significantly associated with Aβ42 (table 4, model 3). None of the other nutrients was associated with Aβ peptides in these models. The ω-3 PUFA was mainly from salad dressing, fish, poultry, margarine, and nuts, with correlation coefficients of 0.53, 0.44, 0.30, 0.19, and 0.09, respectively.
Table 4.
Multivariable linear regression effect estimates for the association between dietary intake of nutrients and plasma levels of Aβ40 and Aβ42a
Abbreviations: Aβ = β-amyloid; MUFA = monounsaturated fatty acid; PUFA = polyunsaturated fatty acid; SFA = saturated fatty acid.
Nutrients included in the models were ω-3 PUFA, ω-6 PUFA, MUFA, SFA, β-carotene, vitamin C, vitamin B12, vitamin E, folate, and vitamin D. Caloric intake−adjusted residuals of nutrients were used.
Model 1, unadjusted. Model 2, adjusted for age, sex, education, ethnicity, caloric intake, APOE genotype, and recruitment wave. Model 3, adjusted for all the covariates in model 2, plus alcohol drinking, use of medications (medications affecting blood vessel thrombus formation propensity and antidiabetic agents), and use of nutrients from supplements. A total of 563, 594, 518, 497, 464, 449, 18, and 7 subjects in the study sample reported taking vitamin E, vitamin C, folate, vitamin B12, vitamin D, β-carotene, ω-6 PUFA, and ω-3 PUFA supplements, respectively.
Significant (p < 0.05) nutrients.
Supplementary analyses.
A total of 128 participants developed incident dementia after a mean 3.8 (SD = 2.2) years of follow-up. Among the remaining 1,091 participants, higher intake of ω-3 PUFA remained associated with lower levels of Aβ peptides (β = −14.3, p = 0.04 for Aβ40 and β = −7.39, p = 0.02 for Aβ42)in models adjusted for age, sex, education, recruitment wave, ethnicity, caloric intake, and APOE genotype, whereas other nutrients were not associated with Aβ peptide levels (data not shown). In models adjusted for age, sex, education, recruitment wave, ethnicity, caloric intake, and APOE genotype, none of the subtypes of ω-3 PUFA was significantly associated with the level of Aβ (data not shown), suggesting that the overall intake of ω-3 PUFAs might play a more important role. There seemed to be no significant interaction between ω-3 PUFA and APOE status on plasma level of Aβ40 (p = 0.67) or on Aβ42 (p = 0.39).
DISCUSSION
In this cross-sectional study of a group of elderly participants without dementia, we found that higher dietary intake of ω-3 PUFA was associated with decreased plasma Aβ42 levels, independent of age, gender, ethnicity, education, and APOE genotype. For this population, the ω-3 PUFA was most likely from salad dressing, fish, poultry, margarine, and nuts.
Data from transgenic animal models of AD have consistently demonstrated reduced Aβ production/accumulation after treatment with ω-3 PUFA.31–34 In one study, DHA-enriched diets significantly reduced total detergent-insoluble Aβ by more than 70% compared with low-DHA or control chow diets.32 Image analysis of brain sections revealed that overall plaque burden was reduced by 40.3%.32 Other investigators reported that dietary supplementation with DHA in the 3xTg-AD mouse model of AD reduced the intraneuronal accumulation of Aβ.33 In a different study, 6-month-old APP/PS1 mice were fed either a typical Western diet chow containing 1% cholesterol or a DHA-enriched diet for 12 months.34 The mice fed with the DHA-enriched diet had increased regional cerebral blood volume, indicating a larger circulation in the brain, probably due to vasodilatation and a decreased amount of vascular Aβ deposition.34 Thus, our data are consistent with results observed in these animal studies, pointing to a beneficial role of dietary ω-3 PUFA in brain pathology.
In humans, a recent clinical trial evaluated the effect of ω-3 PUFA supplementation on the CSF level of Aβ42 in patients with mild to moderate AD.35 No effect on the CSF Aβ42 levels was noted after treatment with ω-3 PUFA supplement for 6 months (compared with the placebo-treated group).35 However, the null results might have been partly due to the few participants (n = 35), the short period of treatment (6 month), and the inclusion of participants who already have dementia.35 Future clinical trials with larger sample sizes and longer follow-up period are warranted. Alternatively, our study supports the potential of using an observational study design to examine the effects of ω-3 PUFA, in particular those of dietary source, on circulating levels of Aβ.
The results of the present study are also consistent with previous reports from the WHICAP population. We have reported a lower risk of incident AD, incident MCI, and progression from MCI to AD among participants who adhered more to a Mediterranean-type diet, a diet characterized by high intake of fish (a main dietary source of ω-3 PUFA).2–4 We also found that a dietary pattern characterized by (among other nutrients) high ω-3 PUFA was associated with a nearly 40% reduced risk of incident AD.5 Recently, we found that increasing intake of ω-3 PUFAs was associated with a 20−30% lower risk of dementia.36 In this cohort, higher baseline Aβ40 or Aβ42 levels are associated with increased risk of incident AD7,9,15 and faster decline in multiple cognitive domains.10 Taken together, our results support the hypothesis that ω-3 PUFA could be associated with AD at least partially via its association with Aβ; i.e., a higher dietary intake of ω-3 PUFA might lead to lower plasma levels of Aβ42 (and possibly Aβ40)and a subsequent lower risk of AD.
We detected no persistent association for other nutrients, including ω-6 PUFA, MUFA, SFA, antioxidants (vitamin E, vitamin C, and β-carotene), homocysteine-related B vitamins (vitamin B12 and folate), or vitamin D, suggesting that these nutrients might have little or no association with Aβ-related mechanisms. Thus, their potential associations with AD or cognitive decline might involve other pathways, such as antioxidative, vascular, anti-inflammatory, or metabolic. Alternatively, in contrast with ω-3 PUFAs, which have been shown to correlate well with plasma fatty acids biomarkers,37,38 it is possible that the SFFQ nutrient estimates for other nutrients are not reflecting their respective blood levels very well, providing another explanation for the failure to reject the null hypothesis for these nutrients.
Some limitations need to be considered when these results are interpreted. The present study has a cross-sectional design; thus, causal inferences cannot be drawn. Our outcome is not a stable trait but rather a moving target with temporal dynamic changes, because previous studies have consistently shown that blood Aβ levels change during the process of AD development.7,9,12 A single assessment of Aβs may be susceptible to short-term variation. There could also be measurement error of nutrients from the SFFQ, which could bias our results if reporting error is associated with the Aβ measurements. We tried to minimize this possibility by excluding participants with prevalent dementia and subsequently those with incident dementia. Estimation of nutrient intake from a SFFQ may be different and potentially less accurate than nutrient levels measured in blood, which may have more direct biologic relevance.39 However, plasma nutrients can be affected by many other factors such as metabolic rate and genetic factors, are reflective of instantaneous time points (and less of long-term habitual intake of foods), are subject to other types of measurement errors, and are often technically and financially difficult to measure in large-scale epidemiologic studies such as ours. We focused on dietary intake of nutrients in our study because findings regarding dietary intakes can be directly translated into a lifestyle modification measure. We analyzed 10 selected nutrients and may have missed potential associations that other unanalyzed nutrients might have with Aβ peptides. Although ω-3 PUFAs have been shown to correlate well with plasma fatty acid biomarkers37,38 and they remained significantly associated with Aβ42 after controlling for multiple factors, we could not completely rule out the possibility of this association being due to residual confounding. Results from this population may not be directly generalizable to other populations. Finally, because Aβ physiology is not fully understood, the associations of nutrients with plasma Aβ may not be directly interpreted as associations with brain Aβ.
Our study has several strengths. This study includes a large and ethnically diverse community-based population. We used a previously validated instrument to collect dietary data.22 Exclusion of participants with prevalent dementia (and incident dementia in supplementary analyses) allowed us to examine the relationship between dietary nutrient intake and the Aβ levels free from the influence of cognitive impairment in reporting of dietary habits. Measures for multiple potential confounding factors have been carefully recorded and adjusted for in the analyses.
In the current study, we found that higher dietary ω-3 PUFA intake was associated with lower plasma Aβ42 level, suggesting that the potential beneficial effects of ω-3 PUFA intake on AD and cognitive function in the literature might be at least partly explained by an Aβ-related mechanism.
Supplementary Material
GLOSSARY
- Aβ
β-amyloid
- AD
Alzheimer disease
- DHA
docosahexaenoic acid
- MCI
mild cognitive impairment
- MUFA
monounsaturated fatty acid
- PUFA
polyunsaturated fatty acid
- SFA
saturated fatty acid
- SFFQ
semiquantitative food frequency questionnaire
- WHICAP
Washington Heights/Hamilton Heights Columbia Aging Project
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Dr. Gu: contributed to drafting/revising the manuscript, study concept or design, analysis or interpretation of data, and statistical analysis. Dr. Schupf: contributed to drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data, and study supervision or coordination. Dr. Cosentino: contributed to drafting/revising the manuscript, study concept or design, analysis or interpretation of data, and study supervision or coordination. Dr. Luchsinger: contributed to drafting/revising the manuscript, study concept or design, analysis or interpretation of data, and study supervision or coordination. Dr. Scarmeas: contributed to drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data, study supervision or coordination, and obtaining funding.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1. Luchsinger JA, Noble JM, Scarmeas N. Diet and Alzheimer's disease. Curr Neurol Neurosci Rep 2007; 7: 366– 372 [DOI] [PubMed] [Google Scholar]
- 2. Scarmeas N, Stern Y, Tang MX, Mayeux R, Luchsinger JA. Mediterranean diet and risk for Alzheimer's disease. Ann Neurol 2006; 59: 912– 921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Scarmeas N, Luchsinger JA, Schupf N, et al. Physical activity, diet, and risk of Alzheimer disease. JAMA 2009; 302: 627– 637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Scarmeas N, Stern Y, Mayeux R, Manly JJ, Schupf N, Luchsinger JA. Mediterranean diet and mild cognitive impairment. Arch Neurol 2009; 66: 216– 225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gu Y, Nieves JW, Stern Y, Luchsinger JA, Scarmeas N. Food combination and Alzheimer disease risk: a protective diet. Arch Neurol 2010; 67: 699– 706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. DeMattos RB, Bales KR, Cummins DJ, Paul SM, Holtzman DM. Brain to plasma amyloid-β efflux: a measure of brain amyloid burden in a mouse model of Alzheimer's disease. Science 2002; 295: 2264– 2267 [DOI] [PubMed] [Google Scholar]
- 7. Mayeux R, Honig LS, Tang MX, et al. Plasma Aβ40 and Aβ42 and Alzheimer's disease: relation to age, mortality, and risk. Neurology 2003; 61: 1185– 1190 [DOI] [PubMed] [Google Scholar]
- 8. van Oijen M, Hofman A, Soares HD, Koudstaal PJ, Breteler MM. Plasma Aβ(1-40) and Aβ(1-42) and the risk of dementia: a prospective case-cohort study. Lancet Neurol 2006; 5: 655– 660 [DOI] [PubMed] [Google Scholar]
- 9. Schupf N, Tang MX, Fukuyama H, et al. Peripheral Aβ subspecies as risk biomarkers of Alzheimer's disease. Proc Natl Acad Sci USA 2008; 105: 14052– 14057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cosentino SA, Stern Y, Sokolov E, et al. Plasma β-amyloid and cognitive decline. Arch Neurol 2010; 67: 1485– 1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yaffe K, Weston A, Graff-Radford NR, et al. Association of plasma β-amyloid level and cognitive reserve with subsequent cognitive decline. JAMA 2011; 305: 261– 266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Seppala TT, Herukka SK, Hanninen T, et al. Plasma Aβ42 and Aβ40 as markers of cognitive change in follow-up: a prospective, longitudinal, population-based cohort study. J Neurol Neurosurg Psychiatry 2010; 81: 1123– 1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stern Y, Andrews H, Pittman J, et al. Diagnosis of dementia in a heterogeneous population: development of a neuropsychological paradigm-based diagnosis of dementia and quantified correction for the effects of education. Arch Neurol 1992; 49: 453– 460 [DOI] [PubMed] [Google Scholar]
- 14. American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders, 3rd ed. Washington, DC: American Psychiatric Press; 1987. [Google Scholar]
- 15. Mayeux R, Tang MX, Jacobs DM, et al. Plasma amyloid β-peptide 1-42 and incipient Alzheimer's disease. Ann Neurol 1999; 46: 412– 416 [DOI] [PubMed] [Google Scholar]
- 16. Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 1985; 122: 51– 65 [DOI] [PubMed] [Google Scholar]
- 17. Ma J, Folsom AR, Shahar E, Eckfeldt JH. Plasma fatty acid composition as an indicator of habitual dietary fat intake in middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) Study Investigators. Am J Clin Nutr 1995; 62: 564– 571 [DOI] [PubMed] [Google Scholar]
- 18. Sun Q, Ma J, Campos H, Hankinson SE, Hu FB. Comparison between plasma and erythrocyte fatty acid content as biomarkers of fatty acid intake in US women. Am J Clin Nutr 2007; 86: 74– 81 [DOI] [PubMed] [Google Scholar]
- 19. Hunter DJ, Rimm EB, Sacks FM, et al. Comparison of measures of fatty acid intake by subcutaneous fat aspirate, food frequency questionnaire, and diet records in a free-living population of US men. Am J Epidemiol 1992; 135: 418– 427 [DOI] [PubMed] [Google Scholar]
- 20. Jacques PF, Sulsky SI, Sadowski JA, Phillips JC, Rush D, Willett WC. Comparison of micronutrient intake measured by a dietary questionnaire and biochemical indicators of micronutrient status. Am J Clin Nutr 1993; 57: 182– 189 [DOI] [PubMed] [Google Scholar]
- 21. Tangney CC, Bienias JL, Evans DA, Morris MC. Reasonable estimates of serum vitamin E, vitamin C, and β-cryptoxanthin are obtained with a food frequency questionnaire in older black and white adults. J Nutr 2004; 134: 927– 934 [DOI] [PubMed] [Google Scholar]
- 22. Luchsinger JA, Tang MX, Siddiqui M, Shea S, Mayeux R. Alcohol intake and risk of dementia. J Am Geriatr Soc 2004; 52: 540– 546 [DOI] [PubMed] [Google Scholar]
- 23. Luchsinger JA, Tang MX, Shea S, Mayeux R. Caloric intake and the risk of Alzheimer disease. Arch Neurol 2002; 59: 1258– 1263 [DOI] [PubMed] [Google Scholar]
- 24. Luchsinger JA, Tang MX, Shea S, Mayeux R. Antioxidant vitamin intake and risk of Alzheimer disease. Arch Neurol 2003; 60: 203– 208 [DOI] [PubMed] [Google Scholar]
- 25. Willett W, Stampfer M. Implications of total energy intake for epidemiological analyses. In: Willett W.ed. Nutritional Epidemiology. New York: Oxford University Press; 1998: 273− 301 [Google Scholar]
- 26. Buell JS, Dawson-Hughes B, Scott TM, et al. 25-Hydroxyvitamin D, dementia, and cerebrovascular pathology in elders receiving home services. Neurology 2010; 74: 18– 26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Blasko I, Jellinger K, Kemmler G, et al. Conversion from cognitive health to mild cognitive impairment and Alzheimer's disease: prediction by plasma amyloid β 42, medial temporal lobe atrophy and homocysteine. Neurobiol Aging 2008; 29: 1– 11 [DOI] [PubMed] [Google Scholar]
- 28. Barberger-Gateau P, Raffaitin C, Letenneur L, et al. Dietary patterns and risk of dementia: the Three-City cohort study. Neurology 2007; 69: 1921– 1930 [DOI] [PubMed] [Google Scholar]
- 29. Huang TL, Zandi PP, Tucker KL, et al. Benefits of fatty fish on dementia risk are stronger for those without APOE ϵ4. Neurology 2005; 65: 1409– 1414 [DOI] [PubMed] [Google Scholar]
- 30. Morris MC, Evans DA, Bienias JL, et al. Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Arch Neurol 2003; 60: 940– 946 [DOI] [PubMed] [Google Scholar]
- 31. Puskas LG, Kitajka K, Nyakas C, Barcelo-Coblijn G, Farkas T. Short-term administration of omega 3 fatty acids from fish oil results in increased transthyretin transcription in old rat hippocampus. Proc Natl Acad Sci USA 2003; 100: 1580– 1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lim GP, Calon F, Morihara T, et al. A diet enriched with the omega-3 fatty acid docosahexaenoic acid reduces amyloid burden in an aged Alzheimer mouse model. J Neurosci 2005; 25: 3032– 3040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Green KN, Martinez-Coria H, Khashwji H, et al. Dietary docosahexaenoic acid and docosapentaenoic acid ameliorate amyloid-β and tau pathology via a mechanism involving presenilin 1 levels. J Neurosci 2007; 27: 4385– 4395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hooijmans CR, Rutters F, Dederen PJ, et al. Changes in cerebral blood volume and amyloid pathology in aged Alzheimer APP/PS1 mice on a docosahexaenoic acid (DHA) diet or cholesterol enriched typical Western diet (TWD). Neurobiol Dis 2007; 28: 16– 29 [DOI] [PubMed] [Google Scholar]
- 35. Freund-Levi Y, Hjorth E, Lindberg C, et al. Effects of omega-3 fatty acids on inflammatory markers in cerebrospinal fluid and plasma in Alzheimer's disease: the OmegAD study. Dement Geriatr Cogn Disord 2009; 27: 481– 490 [DOI] [PubMed] [Google Scholar]
- 36. Gustafson D, Bäckman K, Gu Y, et al. Dietary fatty acids (FA) and dementia: observations from the Washington Heights and Inwood Columbia Aging Project. Alzheimer Dement 2011; 7: S296– S297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bowman GL, Shannon J, Ho E, et al. Reliability and validity of food frequency questionnaire and nutrient biomarkers in elders with and without mild cognitive impairment. Alzheimer Dis Assoc Disord 2011; 25: 49– 57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Arsenault LN, Matthan N, Scott TM, et al. Validity of estimated dietary eicosapentaenoic acid and docosahexaenoic acid intakes determined by interviewer-administered food frequency questionnaire among older adults with mild-to-moderate cognitive impairment or dementia. Am J Epidemiol 2009; 170: 95– 103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schaefer EJ, Bongard V, Beiser AS, et al. Plasma phosphatidylcholine docosahexaenoic acid content and risk of dementia and Alzheimer disease: the Framingham Heart Study. Arch Neurol 2006; 63: 1545– 1550 [DOI] [PubMed] [Google Scholar]
- 40. Buschke H, Fuld PA. Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology 1974; 24: 1019– 1025 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


