Abstract
Objective:
To develop multivariate models for prediction of early motor deficit improvement in acute stroke patients with focal extremity paresis, using admission clinical and imaging data.
Methods:
Eighty consecutive patients with motor deficit due to first-ever unilateral stroke underwent CT perfusion (CTP) within 9 hours of symptom onset. Limb paresis was prospectively assessed using admission and discharge NIH Stroke Scale (NIHSS) scoring. CTP scans were coregistered to the MNI-152 brain space and subsegmented to 146 pairs of cortical/subcortical regions based on preset atlases. Stepwise multivariate binary logistic regressions were performed to determine independent clinical and imaging predictors of paresis improvement.
Results:
The rates of early motor deficit improvement were 18/49 (37%), 15/42 (36%), 8/25 (32%), and 7/23 (30%) for the right arm, right leg, left arm, and left leg, respectively. Admission NIHSS was the only independent clinical predictor of early limb motor deficit improvement. Relative CTP values of the inferior frontal lobe white matter, lower insular cortex, superior temporal gyrus, retrolenticular portion of internal capsule, postcentral gyrus, precuneus parietal gyri, putamen, and caudate nuclei were also independent predictors of motor improvement of different limbs. The multivariate predictive models of motor function improvement for each limb had 84%–92% accuracy, 79%–100% positive predictive value, 75%–94% negative predictive value, 83%–88% sensitivity, and 80%–100% specificity.
Conclusions:
We developed pilot multivariate models to predict early motor functional improvement in acute stroke patients using admission NIHSS and atlas-based location-weighted CTP data. These models serve as a “proof-of-concept” for prospective location-weighted imaging prediction of clinical outcome in acute stroke.
One of the first questions asked by stroke patients and their families at admission is if and how soon they can expect improvement in their functional deficits. The ability to quantify the likelihood of such improvement could therefore be of great clinical interest.
Important prognostic variables in current clinical practice include the admission NIH Stroke Scale (NIHSS) score and admission “core infarct” lesion volume on magnetic resonance diffusion-weighted imaging (DWI). However, admission infarct volume and clinical stroke severity alone can only predict 30% to 50% of the variance in motor impairment improvement; thus a predictive model may also include information regarding the infarction location, structural integrity of descending motor pathways, and cortical activation in fMRI studies.1–3
The accuracy of such prognostication might be improved with the addition of kinetic cerebral perfusion parameters to predictive models. Whereas acute DWI lesions are highly specific for infarction, perfusion scans can provide complementary information by detecting regions of severely impaired blood flow with high probability of infarction. The precise spatial localization of cerebral hypoperfusion can substantially contribute to the accuracy of predictive models of stroke outcome, especially when used in combination with other clinical information.2
In present study, we combined admission clinical and topographic hemodynamic imaging data to develop prognostic models for prediction of early functional improvement in acute stroke patients presenting with single extremity motor deficits. An automated location-weighted atlas-based methodology was used to quantify the effects of the complex spatial pattern of admission cerebral perfusion deficits on early functional outcome.
METHODS
Standard protocol approvals, registrations, and patient consent.
This study received approval from our Institutional Review Board and was compliant with the Health Insurance Portability and Accountability Act. All patients provided informed written consent.
Patients.
We retrospectively reviewed the prospectively collected database of all consecutive patients admitted to our stroke unit between December 2006 and April 2008. Subjects were included if they had a first-ever unilateral ischemic stroke within the anterior circulation territory; presented with upper or lower contralateral limb paresis, without preexisting motor deficit; and underwent admission CTP scan within 9 hours of symptom onset.
Each patient's motor function, at the time of both admission and discharge, was determined based on prospectively acquired NIHSS scoring, with specific attention to the extremity motor scores (components 5 and 6, based on a 0–4 scale). Data were collected as part of the Specialized Program of Translational Research in Acute Stroke (SPOTRIAS) patient registry at our hospital.
All patients presenting with paresis of a given extremity were dichotomized into 2 groups based on decrease in component motor score at discharge: those with, and those without, clinically detectable improvement.
Image acquisition.
All patients underwent admission noncontrast CT scanning, followed by CT angiography (CTA) and CTP on the same 64-detector helical scanner (Light Speed; GE Medical Systems, Milwaukee, WI). Dynamic CTP was performed as a 66-second biphasic cine series scanning 2 consecutive slabs of 8 contiguous 5-mm-thick sections.4 All CTP series were transferred to a GE Advantage workstation (General Healthcare, Milwaukee, WI) for postprocessing of CTP maps including cerebral blood flow (CBF), cerebral blood volume (CBV), and mean transit time (MTT) series using delay-corrected deconvolution-based commercial CT perfusion software (CT Perfusion 4, General Healthcare, Milwaukee, WI) as described previously.5
Image analysis (CTP and CTA).
All CTP maps were automatically coregistered to MNI-152 brain space with FLIRT version 5.5 (FMRIB's Linear Image Registration Tool, Oxford Centre for Functional Magnetic Resonance Imaging of the Brain, Oxford, UK).5 Briefly, all coregistered CTP maps were parcellated into 146 pairs of mirrored cortical and subcortical areas based on preset Talairach and JHU atlases supplied with FSL 4.1.2 (Oxford Centre for Functional Magnetic Resonance Imaging of the Brain). For each brain region, the relative CTP values (rCBF, rCBV, and rMTT) were calculated as the ratio of stroke side mean value divided by contralateral mirror region mean value.
For CTA data analysis, we stratified patients based on the presence of admission proximal cerebral artery occlusions (either distal/terminal internal carotid artery [ICA] or proximal [M1 or M2] middle cerebral artery [MCA] occlusion).6 The presence of proximal cerebral artery occlusion was defined as a binary variable assigned “1” in case of occlusion and “0” in the absence of arterial occlusion. Arterial occlusion status was determined based on the final clinical reports of the admission CTA examinations in the permanent medical record.
Statistical analysis.
For each limb, separate univariate and multivariate statistical analyses were performed to determine the predictors of paresis improvement, using similar methodology to earlier published studies.5 Repeated-measures analysis of variance was performed to statistically confirm the within-subject reduction of component motor NIHSS score of patients with improvement vs those without improvement of motor function.
Patients' clinical characteristics and CTA findings were compared between those with and those without motor improvement using appropriate univariate analyses (χ2, Wilcoxon, or t tests). For the full set of CTP parameters per region (rCBV, rCBF, rMTT), receiver operating characteristic (ROC) curve analyses were performed, and the area under the ROC curves (AUC) were calculated. To determine the independent predictors of early improvement, stepwise binary multiple logistic regression models were used. For each limb, we included in the multivariate model only those clinical variables that were significantly different between the 2 groups based on the univariate analyses, and only those CTP variables with ROC AUC >0.75 for prediction of early improvement.5
Using multivariate binary logistic regression, one can calculate a unitless arbitrary number (v) for each patient that can be applied to a standardized formula to calculate the probability of early paresis improvement. Using this v parameter value, the accuracy of each model could then be determined based on the ROC AUC.
All data are reported as frequency (percent), median (range), or mean ± SEM. Statistical analysis was performed using SPSS-17 (SPSS Inc., Chicago, IL) and STATA (version 10, 2001; College Station, TX).
RESULTS
Patients.
A total of 80 consecutive patients were included: the mean age was 71.3 ± 1.7 years, 46 (58%) were female, and 52 (65%) had left hemispheric ischemic stroke. Patients were discharged from the hospital within 3 to 23 days (median, 6 days) after stroke. A total of 55/80 (69%) had major proximal cerebral artery occlusions, 21 (26%) had no visible major intracranial vessel occlusion, and the remainder had small distal vessel occlusions. Thirty patients (37.5%) received IV tissue plasminogen activator (tPA) and 8 (10%) underwent intra-arterial (IA) endovascular treatment following IV tPA, of whom 5 had complete and 2 had partial recanalization. There was a significant before-after decrease in component motor score of patients with improvement compared to those without early improvement of motor function (p < 0.0001, in all 4 limbs).
Predicting right arm motor deficit improvement.
Forty-nine patients presented with right arm paresis. Patients with early improvement were significantly younger (p = 0.01) and less likely to have major proximal cerebral artery occlusion (p = 0.013); both total admission NIHSS score (p = 0.002) and component #5b (p = 0.034) were significantly higher in those without any improvement (table 1).
Table 1.
Comparison of clinical/CTA characteristics of patients with and without motor improvement (univariate analysis)a
Abbreviations: CTA = CT angiography; CTP = CT perfusion; IA = intra-arterial; NIHSS = NIH Stroke Scale.
All data are reported as frequency (%), median (range), or mean ± SEM.
Significant.
Component 5 (upper limb) and 6 (lower limb) of NIHSS test battery determined the degree of limb paresis based on a 0–4 scale (0 = no drift; 1 = drift; 2 = some effort against gravity; 3 = no effort against gravity; 4 = no movement).
The binary logistic regression analysis (table 2) showed that the only independent predictors of early motor improvement were the admission NIHSS score and the rMTT values of both the left superior parietal lobule gray matter (GM) (Brodmann area [BA] 7) and the retrolenticular portion of the internal capsule (figure). Using the v parameter derived from the binary logistic regression model, we could predict early right arm paresis improvement with 88% sensitivity and 84% specificity (ROC AUC = 0.86). This corresponded to a 79% positive predictive value (PPV) and a 90% negative predictive value (NPV).
Table 2.
Coefficients for the independent clinical and imaging predictors of motor improvement, based on the multivariate binary logistic regression model for each limba
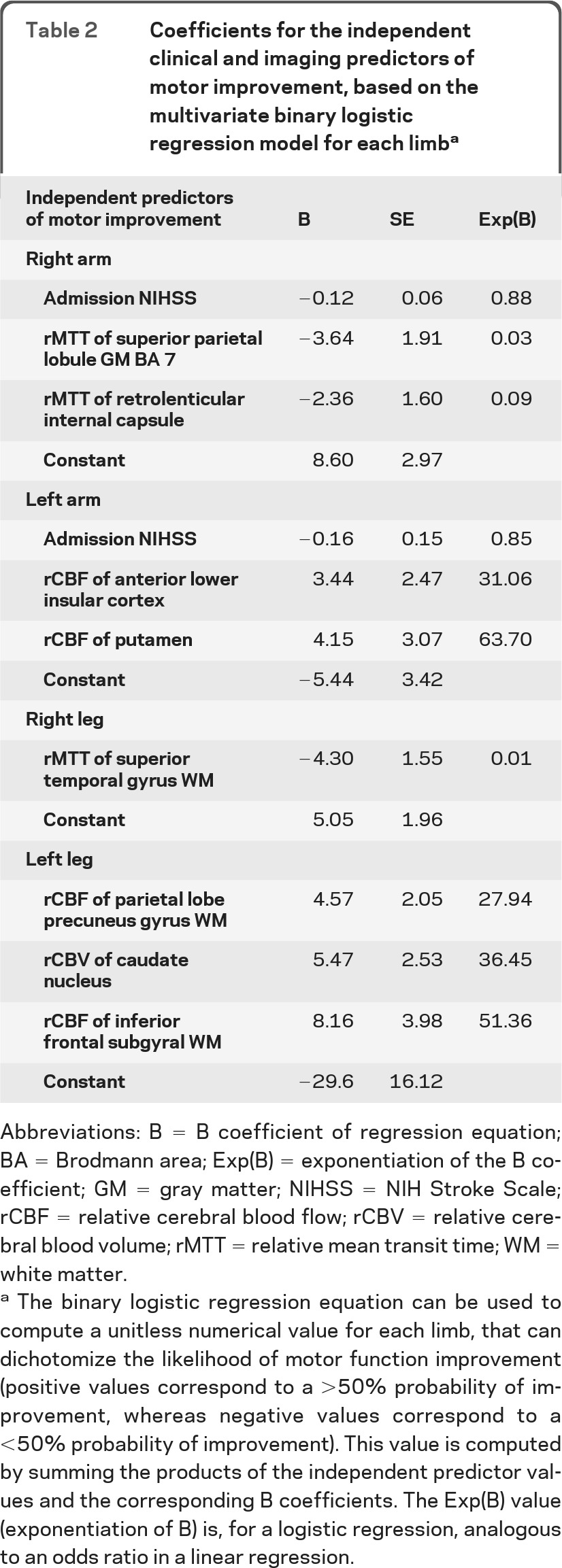
Abbreviations: B = B coefficient of regression equation; BA = Brodmann area; Exp(B) = exponentiation of the B coefficient; GM = gray matter; NIHSS = NIH Stroke Scale; rCBF = relative cerebral blood flow; rCBV = relative cerebral blood volume; rMTT = relative mean transit time; WM = white matter.
The binary logistic regression equation can be used to compute a unitless numerical value for each limb, that can dichotomize the likelihood of motor function improvement (positive values correspond to a >50% probability of improvement, whereas negative values correspond to a <50% probability of improvement). This value is computed by summing the products of the independent predictor values and the corresponding B coefficients. The Exp(B) value (exponentiation of B) is, for a logistic regression, analogous to an odds ratio in a linear regression.
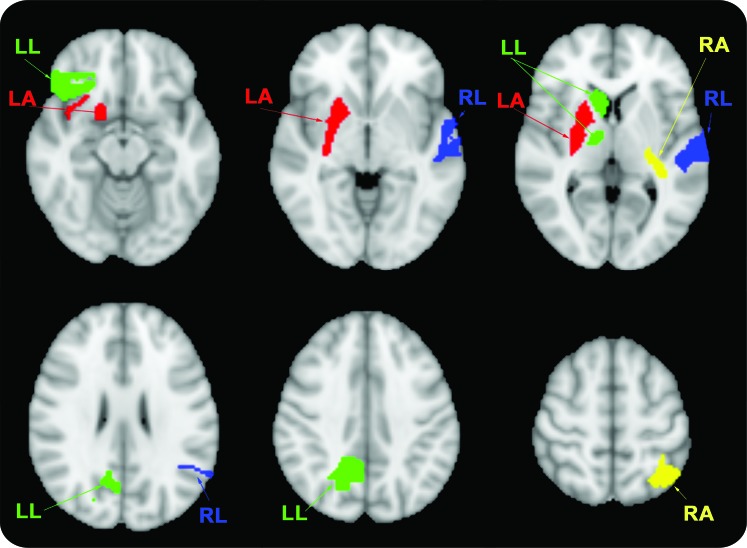
Figure. Graphic representation of cortical regions that were independent predictors of motor improvement in patients with limb paresis.
Right arm (RA, yellow): relative mean transit time (rMTT) of both the left superior parietal lobule gray matter and retrolenticular internal capsule; left arm (LA, red): relative cerebral blood flow (rCBF) of both the right lower anterior insular cortex and putamen; right leg (RL, blue): rMTT of the left superior temporal gyrus white matter (WM); left leg (LL, green): relative cerebral blood volume (rCBV) of right caudate nucleus, rCBF of parietal lobe precuneus WM, and rCBF of inferior frontal lobe WM.
Predicting left arm motor deficit improvement.
Twenty-five patients presented with left arm paresis. In univariate analysis, only admission NIHSS was significantly different between patients with left arm paresis improvement vs those without (p = 0.033, table 1).
In the binary logistic regression (table 2), the only independent predictors of left arm motor improvement were admission NIHSS score and the rCBF of both the right putamen and the anterior lower insular cortex (figure). Using the v parameter derived from the binary logistic regression model, we could predict early left arm motor function improvement with 86% sensitivity and 94% specificity (ROC AUC = 0.90, 86% PPV, and 89% NPV).
Predicting right leg motor deficit improvement.
Forty-two patients presented with right leg paresis. In patients with motor improvement the incidence of cerebral artery occlusion on admission CTA (p = 0.005), mean age (p = 0.005), admission total NIHSS score (p = 0.001), and NIHSS right leg motor component score (p = 0.023) were significantly lower (table 1).
In the binary logistic regression (table 2), the only independent predictor of right leg motor improvement was the rMTT of the left superior temporal gyrus WM (figure). This CTP parameter alone predicted improvement with 85% sensitivity and 80% specificity (ROC AUC = 0.84, 88% PPV, and 75% NPV).
Predicting left leg motor deficit improvement.
Twenty-three patients presented with left leg paresis. There was no significant difference in the univariate analysis between the admission clinical and CTA characteristics of those with and without improvement (table 1).
In the binary logistic regression model (table 2), the independent predictors of improvement were the rCBF of the parietal lobe precuneus WM, the rCBV of the caudate nucleus, and the rCBF of the inferior frontal subcortical WM (figure). Using the v parameter derived from the binary logistic regression model, we could predict early motor function improvement with 83% sensitivity and 100% specificity (ROC AUC = 0.92, 100% PPV, and 94% NPV).
DISCUSSION
In our study, we developed multivariate regression models for the prediction of early motor improvement in stroke patients with limb paresis, using routine admission clinical and CTP imaging data. These models could predict early improvement of paresis with 84%–92% accuracy. These prediction models not only have the potential to contribute prognostic information to the clinical or research setting, but also could enhance acute therapeutic triage. Specifically, our results have the potential for rapid and accurate stratification of patients, identifying those likely to do well independent of therapy (for whom the risk of treatment may outweigh the benefit—“too good to treat”), vs those likely to do poorly despite therapy (“too bad to treat”), for whom early transfer to an inpatient rehabilitation center may be most beneficial.7
We developed an automated method for location-weighted analysis of CTP scans, with minimal operator-dependent bias, and highly reproducible results. This image analysis approach could help to optimize the generalizability of predictive models. In addition, our results confirm that multivariate models integrating both clinical and imaging parameters can improve prognostication of early stroke outcome. However, long-term stroke recovery is complex, and there are many contributing factors, including potentially modifiable ones.3 Although many variables (including muscle mass, aerobic fitness prior to stroke, level of poststroke fatigue, and depression) play a role in long-term rehabilitation, early functional recovery in ischemic stroke is presumably largely attributable to reperfusion of eloquent brain regions. Therefore, integrative long-term predictive models might also include prestroke functional status, motor-evoked potentials in the early poststroke phase, genotyping, and neurophysiologic assessment of neural plasticity.3,8
In acute stroke, the observed clinical deficits may in part be attributable to dysfunctional, critically ischemic brain outside the region of the acute infarct “core.” Dynamic contrast perfusion scanning (CTP or magnetic resonance perfusion [MRP]) can detect such hypoperfused regions that not only are well correlated with the presenting physical examination, but that also reflect potentially salvageable still viable ischemic brain tissues (i.e., at-risk penumbra), although it should be noted that perfusion map parameters only reflect correlates of cerebral oxygenation, and should not be interpreted as cerebral oxygenation itself. CTP scans, compared to MRP, are more practical in terms of availability, speed, and cost. In addition, the linear relation between contrast concentration and attenuation in CT imaging makes perfusion quantification more reliable compared to that of MRP techniques.9
Previous studies have also suggested that the precise neuroanatomic localization of such dysfunctional regions could substantially improve the accuracy of the correlation between imaging findings and the clinical deficit. Indeed, it was shown that the incorporation of infarct location data into volume-based models of functional outcome significantly improved the correlation between imaging-derived severity scores and the observed NIHSS scores.2
In our study, rather than modeling tissue outcome per se, as in much of the perfusion imaging literature (“core” vs “penumbra” vs “benign oligemia”),4 we determined the recoverable function of the stroke patient. We could determine the relationship between specific spatial patterns of brain ischemia, and the probability of subsequent motor improvement.
Our multivariate models suggest that the perfusion status of specific cerebral regions is well correlated with single limb motor deficit improvement in stroke patients, although these areas may not contribute to motor function directly, and may be different for different limbs, as discussed below. Indeed, CTP parameters of some cerebral regions not directly contiguous with the corticospinal tract were among the independent predictors of motor deficit improvement that we identified. Similarly, an fMRI-based motor improvement pattern in stroke patients within 24–48 hours after stroke that included small clusters of voxels in the ipsilesional postcentral gyrus and cingulate cortex was recently reported.1 It has also been shown that infarction of the striatum, corona radiata, external capsule, posterior limb of the internal capsule, and middle frontal gyrus contribute to estimating the likelihood of motor deficit in stroke patients.10
Notably, previous studies reported that paresis was caused not only by damage to the precentral motor strip or its descending fibers but also by premotor, parietal, and striatothalamic lesions.11,12 Indeed, the insular ribbon may serve as an example of how local blood flow deficits may predict more global stroke outcome. The insula is highly sensitive to hypoperfusion.13 Severe insular ribbon ischemia is not only associated with a low probability of aphasia improvement,5 but also a high probability of infarct growth.14 We therefore speculate that severe admission hypoperfusion of the insula may be a “canary in the coal mine” for poor global stroke outcome.
In addition, diffusion tensor imaging studies have reported significant differences in fractional anisotropy of the retrolenticular portion of internal capsule in stroke patients with poorer motor skill recovery compared to controls.15 The superior parietal lobule (BA 7) and the parietal precuneus are also involved in remembering and executing the correct order of task components and visuomotor function.16,17 Moreover, the left superior temporal and bilateral inferior frontal gyri play a role in ideation of voluntary simple movement.18
The right-left asymmetry in our findings might simply be an epiphenomenon reflecting differences in vascular embolic distribution between the right and left circulations in our relatively small cohort. However, this difference could also be attributable to the variable sensitivity of different CTP parameters in different brain locations (cortical vs deep).13 In addition, there is a growing literature reporting right-left asymmetry in topographic predictive models of stroke patients.10,13 That some of the clinical variables listed in the univariate analysis of table 1, including age and motor score, were significantly different in patients with right compared to left hemispheric stroke might also be due to the smaller number of patients with right hemispheric stroke in our cohort. Patient handedness and true functional/anatomic asymmetry might have also contributed to our findings.19,20 Unfortunately, data regarding handedness were not available in our cohort. However, it is plausible that symptoms due to cerebral ischemia are more apparent if language or dominant hand function is affected, mostly in left hemispheric strokes, whereas right hemispheric strokes are usually associated with neglect, which reduces awareness of such deficits. This phenomenon could lead to a spatially heterogenous distribution of stroke lesions between patients with right vs left hemispheric stroke.
In addition to CTP variables, admission NIHSS was an independent predictor of functional improvement in our study. In our series, total admission NIHSS was a stronger predictor of clinical outcome than the specific motor component of the test (i.e., NIHSS items 5 and 6). Moreover, right-hemispheric lesion volumes have a smaller effect on NIHSS scores than left hemispheric lesion volumes.21 This further underscores why different models should be developed for left vs right hemispheric stroke.
One limitation in our study was the lack of patient evaluation with a specialized test of motor function for each limb, such as the Fugl-Meyer. Although total NIHSS score may be a more robust predictor of clinical improvement as a global measure of stroke severity, a more detailed and dedicated motor function test battery score may have performed better as a predictor of individual limb recovery. As another caveat, patients' discharge dates, rather than fixed time frame intervals, were used as the clinical endpoint in our study, which also may have diluted the strength of our correlations.
Another limitation of our study is that the correlation between a specific brain region and clinical outcome may be unapparent if voxels in that region were not sufficiently scanned due to artifact, patient positioning, or hardware-related coverage limitations. There is also the caveat that with coregistration and segmentation of brain scans based on spatial coordinates of an “idealized” brain image and preset atlases (such MNI-125), at least a small degree of misregistration is unavoidable. The standardized atlases used for our brain parcellation were developed based on cerebral cortex cytoarchitecture (BAs) and deep subcortical DTI tractography.22 The presumption that these subregions best distinguish areas of different function/perfusion remains controversial. A voxel-based analysis might avoid this drawback; however, voxel-based studies are even more subject to problems of variability.23,24
We were able to develop accurate multivariate atlas-based models for prediction of motor improvement in acute stroke patients presenting with single limb paresis, based on localization of admission CTP hypoperfused regions and NIHSS examination. Accurate functional prediction is important for determining appropriate rehabilitation strategies and discussing expectations of functional improvement with patients and their families; in the future, such models may help identify patients with clinical penumbra who are potential targets for therapy. These preliminary models can serve as a “proof-of-concept” for prospective location-weighted imaging prediction of early clinical improvement in acute stroke.
Supplementary Material
Glossary
GLOSSARY
- AUC
area under the receiver operating characteristic curve
- BA
Brodmann area
- CBF
cerebral blood flow
- CBV
cerebral blood volume
- CTA
CT angiography
- CTP
CT perfusion
- DWI
diffusion-weighted imaging
- GM
gray matter
- IA
intra-arterial
- ICA
internal carotid artery
- MCA
middle cerebral artery
- MRP
magnetic resonance perfusion
- MTT
mean transit time
- NIHSS
NIH Stroke Scale
- NPV
negative predictive value
- PPV
positive predictive value
- ROC
receiver operating characteristic
- tPA
tissue plasminogen activator
Footnotes
Editorial, page 1811
AUTHOR CONTRIBUTIONS
Seyedmehdi Payabvash helped with the design/conceptualization of the study, analysis/interpretation of the data, and drafting/revising the manuscript. Leticia C.S. Souza helped with analysis/interpretation of the data. Shervin Kamalian helped with analysis/interpretation of the data. Yifei Wang helped with analysis/interpretation of the data. John Passanese helped with analysis/interpretation of the data and drafting/revising the manuscript. Shahmir Kamalian helped with analysis/interpretation of the data. Steve Fung helped with analysis/interpretation of the data. Elkan F. Halpern helped with analysis/interpretation of the data. Pamela W. Schaefer helped with drafting/revising the manuscript. R. Gilberto Gonzalez helped with drafting/revising the manuscript. Karen L. Furie helped with drafting/revising the manuscript. Michael H. Lev helped with the design/conceptualization of the study, analysis/interpretation of the data, and drafting/revising the manuscript.
DISCLOSURE
Dr. Payabvash, Dr. Souza, Dr. Kamalian, Dr. Wang, Dr. Passanese, Dr. Kamalian, Dr. Fung, Dr. Halpern, Dr. Schaefer, Dr. Gonzalez, and Dr. Furie report no disclosures. Dr. Lev receives research support and/or is consultant to GE Healthcare, and Millennium Pharmaceuticals. Go to Neurology.org for full disclosures.
REFERENCES
- 1. Marshall RS, Zarahn E, Alon L, Minzer B, Lazar RM, Krakauer JW. Early imaging correlates of subsequent motor recovery after stroke. Ann Neurol 2009; 65: 596– 602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Menezes NM, Ay H, Wang Zhu M, et al. The real estate factor: quantifying the impact of infarct location on stroke severity. Stroke 2007; 38: 194– 197 [DOI] [PubMed] [Google Scholar]
- 3. Stinear C. Prediction of recovery of motor function after stroke. Lancet Neurol 2010; 9: 1228– 1232 [DOI] [PubMed] [Google Scholar]
- 4. Kamalian S, Kamalian S, Maas MB, et al. CT cerebral blood flow maps optimally correlate with admission diffusion-weighted imaging in acute stroke but thresholds vary by postprocessing platform. Stroke 2011; 42: 1923– 1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Payabvash S, Kamalian S, Fung S, et al. Predicting language improvement in acute stroke patients presenting with aphasia: a multivariate logistic model using location-weighted atlas-based analysis of admission CT perfusion scans. AJNR Am J Neuroradiol 2010; 31: 1661– 1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Torres-Mozqueda F, He J, Yeh IB, et al. An acute ischemic stroke classification instrument that includes CT or MR angiography: the Boston Acute Stroke Imaging Scale. AJNR Am J Neuroradiol 2008; 29: 1111– 1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang H, Camicia M, Terdiman J, Hung YY, Sandel ME. Time to inpatient rehabilitation hospital admission and functional outcomes of stroke patients. PM R 2011; 3: 296– 304 [DOI] [PubMed] [Google Scholar]
- 8. Hendricks HT, van Limbeek J, Geurts AC, Zwarts MJ. Motor recovery after stroke: a systematic review of the literature. Arch Phys Med Rehabil 2002; 83: 1629– 1637 [DOI] [PubMed] [Google Scholar]
- 9. Konstas AA, Wintermark M, Lev MH. CT perfusion imaging in acute stroke. Neuroimaging Clin N Am 2011; 21: 215– 238 [DOI] [PubMed] [Google Scholar]
- 10. Phan TG, Chen J, Donnan G, Srikanth V, Wood A, Reutens DC. Development of a new tool to correlate stroke outcome with infarct topography: a proof-of-concept study. Neuroimage 2010; 49: 127– 133 [DOI] [PubMed] [Google Scholar]
- 11. Feys H, Hetebrij J, Wilms G, Dom R, De Weerdt W. Predicting arm recovery following stroke: value of site of lesion. Acta Neurol Scand 2000; 102: 371– 377 [DOI] [PubMed] [Google Scholar]
- 12. Kunesch E, Binkofski F, Steinmetz H, Freund HJ. The pattern of motor deficits in relation to the site of stroke lesions. Eur Neurol 1995; 35: 20– 26 [DOI] [PubMed] [Google Scholar]
- 13. Payabvash S, Souza LC, Wang Y, et al. Regional ischemic vulnerability of the brain to hypoperfusion: the need for location specific computed tomography perfusion thresholds in acute stroke patients. Stroke 2011; 42: 1255– 1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cheng B, Golsari A, Fiehler J, Rosenkranz M, Gerloff C, Thomalla G. Dynamics of regional distribution of ischemic lesions in middle cerebral artery trunk occlusion relates to collateral circulation. J Cereb Blood Flow Metab 2011; 31: 36– 40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schaechter JD, Fricker ZP, Perdue KL, et al. Microstructural status of ipsilesional and contralesional corticospinal tract correlates with motor skill in chronic stroke patients. Hum Brain Mapp 2009; 30: 3461– 3474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Caminiti R, Ferraina S, Johnson PB. The sources of visual information to the primate frontal lobe: a novel role for the superior parietal lobule. Cereb Cortex 1996; 6: 319– 328 [DOI] [PubMed] [Google Scholar]
- 17. Nair DG, Fuchs A, Burkart S, Steinberg FL, Kelso JA. Assessing recovery in middle cerebral artery stroke using functional MRI. Brain Inj 2005; 19: 1165– 1176 [DOI] [PubMed] [Google Scholar]
- 18. Caffarra P, Gardini S, Vezzadini G, Bromiley A, Venner A. The ideation of movement is supported by fronto-temporal cortical regions involved in the retrieval of semantic knowledge. Acta Biomed 2010; 81: 21– 29 [PubMed] [Google Scholar]
- 19. Good CD, Johnsrude I, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. Cerebral asymmetry and the effects of sex and handedness on brain structure: a voxel-based morphometric analysis of 465 normal adult human brains. Neuroimage 2001; 14: 685– 700 [DOI] [PubMed] [Google Scholar]
- 20. Zemke AC, Heagerty PJ, Lee C, Cramer SC. Motor cortex organization after stroke is related to side of stroke and level of recovery. Stroke 2003; 34: e23– e28 [DOI] [PubMed] [Google Scholar]
- 21. Fink JN, Selim MH, Kumar S, et al. Is the association of National Institutes of Health Stroke Scale scores and acute magnetic resonance imaging stroke volume equal for patients with right- and left-hemisphere ischemic stroke? Stroke 2002; 33: 954– 958 [DOI] [PubMed] [Google Scholar]
- 22. Wakana S, Caprihan A, Panzenboeck MM, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage 2007; 36: 630– 644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kimberg DY, Coslett HB, Schwartz MF. Power in Voxel-based lesion-symptom mapping. J Cogn Neurosci 2007; 19: 1067– 1080 [DOI] [PubMed] [Google Scholar]
- 24. Dronkers NF, Wilkins DP, Van Valin RD, Jr, Redfern BB, Jaeger JJ. Lesion analysis of the brain areas involved in language comprehension. Cognition 2004; 92: 145– 177 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.