Abstract
Objective:
Sudden unexpected death in epilepsy (SUDEP) poses a poorly understood but considerable risk to people with uncontrolled epilepsy. There is controversy regarding the significance of postictal generalized EEG suppression as a biomarker for SUDEP risk, and it remains unknown whether postictal EEG suppression has a neurologic correlate. Here, we examined the profile of autonomic alterations accompanying seizures with a wrist-worn biosensor and explored the relationship between autonomic dysregulation and postictal EEG suppression.
Methods:
We used custom-built wrist-worn sensors to continuously record the sympathetically mediated electrodermal activity (EDA) of patients with refractory epilepsy admitted to the long-term video-EEG monitoring unit. Parasympathetic-modulated high-frequency (HF) power of heart rate variability was measured from concurrent EKG recordings.
Results:
A total of 34 seizures comprising 22 complex partial and 12 tonic-clonic seizures from 11 patients were analyzed. The postictal period was characterized by a surge in EDA and heightened heart rate coinciding with persistent suppression of HF power. An increase in the EDA response amplitude correlated with an increase in the duration of EEG suppression (r = 0.81, p = 0.003). Decreased HF power correlated with an increase in the duration of EEG suppression (r = −0.87, p = 0.002).
Conclusion:
The magnitude of both sympathetic activation and parasympathetic suppression increases with duration of EEG suppression after tonic-clonic seizures. These results provide autonomic correlates of postictal EEG suppression and highlight a critical window of postictal autonomic dysregulation that may be relevant in the pathogenesis of SUDEP.
Sudden unexpected death in epilepsy (SUDEP) is the leading cause of death directly related to epilepsy and is particularly prevalent in patients with pharmacologically intractable epilepsy.1–3 The pathophysiology of SUDEP is poorly understood, but the autonomic nervous system is suspected to be involved.4 SUDEP mainly occurs in the context of a generalized tonic-clonic seizure (GTCS),5–7 but it remains unclear why in some cases a particular GTCS becomes fatal unlike all other GTCS in the past. One possibility is that the intensity of a seizure, including its physiologic impact and duration, is a key factor in initiating a lethal cycle of events.4 A previous study revealed that the duration of postictal generalized EEG suppression (PGES) was significantly prolonged in earlier recorded GTCS of patients who later died of SUDEP and found the risk of SUDEP to increase in direct proportion to duration of PGES.8 However, another study demonstrated an association between PGES and GTCS but found no correlation between the occurrence or duration of PGES and SUDEP.9 Here, we sought to determine whether PGES has a neurologic correlate such as impaired autonomic function by performing high-resolution characterization of both sympathetic and parasympathetic alterations associated with seizures.
METHODS
Standard protocol approvals, registrations, and patient consents.
The institutional review boards of MIT and Children's Hospital Boston approved this study. We recruited patients with epilepsy who were admitted to the long-term video-EEG monitoring unit for presurgical evaluation or invasive monitoring for possible epilepsy surgery. Typically patients stayed for 3−7 days in the long-term monitoring unit. All participants (or their caregivers) provided written informed consent. We excluded patients without recorded complex partial seizures (CPS) or GTCS during monitoring. We also excluded seizures that were not preceded by a seizure-free interval of at least 60 minutes.
Recordings.
EEG recordings were performed using conventional scalp EEG electrodes (10–20 system) with a sampling rate of 256 Hz. In 2 patients, recordings were obtained from implanted intracranial electrodes at 500 Hz. EKG recordings were monitored concurrently from a modified lead II with adhesive electrodes placed below the clavicles. Sympathetic-mediated electrodermal activity (EDA) was recorded at 20 Hz using a custom-built wrist-worn skin conductance biosensor10 and synchronized with the video/EEG/EKG recordings by generating technical artifacts at the beginning and end of each session for offline realignment.
EEG/EDA/EKG analysis.
Two board-certified clinical neurophysiologists, blinded to EDA, heart rate variability, and clinical information, examined the video and EEG recordings. Seizure type, ictal EEG localization, EEG seizure onset and offset, clinical seizure onset, and duration of PGES were determined. PGES was scored in accordance with Lhatoo et al.8 and was defined as the immediate postictal (within 30 seconds), generalized lack of EEG activity larger than 10 μV in amplitude, excluding muscle, movement, breathing, and electrode artifacts. Duration of PGES was defined until electrical brain activity >10 μV resumed.
EDA recordings were analyzed using custom-written software in MATLAB (MathWorks Inc.). For each seizure, the corresponding peri-ictal EDA recording from 60 minutes before EEG seizure onset up to 120 minutes afterward was segmented. To obtain the time profile of EDA alterations, a 1-minute moving average window with zero overlap was applied to the preictal and postictal segments. The interbeat interval (R-R interval) time series was extracted from the EKG recordings and analyzed using the smoothed pseudo-Wigner-Ville time-frequency distribution. The parasympathetic-mediated high-frequency (HF) spectral component was extracted from the smoothed pseudo-Wigner-Ville distribution by integrating the spectral powers between 0.15 and 0.4 Hz. The time profile of HF power alterations (figure 3, A and B) was obtained using a 1-minute moving average window with no overlap that was applied to the preictal and postictal segments. Further information is provided in the e-Methods on the Neurology® Web site at www.neurology.org.
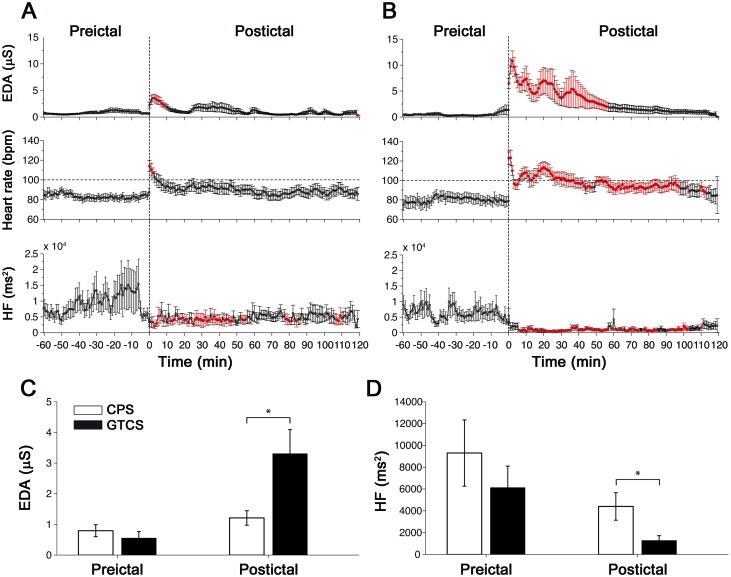
Figure 3. Autonomic footprints of epileptic seizures.
High-resolution profiles of autonomic alterations computed every minute during a peri-ictal period of 3 hours for (A) complex partial seizures (CPS) and (B) secondarily generalized tonic-clonic seizures (GTCS). Each postictal measurement epoch was sequentially compared with the baseline level taken as the average of the entire 60-minute preictal period. Epochs in red indicate statistical significance after accounting for multiple comparisons using the false discovery rate controlling procedure (p < 0.05; paired, two-sided Wilcoxon signed rank test). (C) Electrodermal activity (EDA) during the preictal and postictal period in CPS and GTCS. (D) High-frequency (HF) power during the preictal and postictal periods in CPS and GTCS.
Statistical analysis.
Statistical analyses were performed using the MATLAB statistics toolbox. Within seizure type, comparisons of preictal and postictal measurements were performed using paired, two-sided Wilcoxon signed rank tests (WSRTs). To account for multiple comparisons, the resulting p values were adjusted using the false discovery rate controlling procedure.11 Comparisons between seizure types were made using unpaired, two-sided Mann-Whitney-Wilcoxon (MWW) tests. The strength and direction of the linear relationship between the different pairs of variables were quantified using the Pearson correlation coefficient. Each test was performed at a significance level of 0.05. Data are expressed as mean ± SE.
RESULTS
We included 11 patients with refractory epilepsy in this study, all of whom were candidates for epilepsy surgery (clinical information are summarized in table e-1). In addition to combined video-EEG and EKG monitoring, we successfully recorded more than 1,176 hours of EDA data with an average of 4.5 days/patient. Examples of the long-term EDA recordings are shown in figure 1 and figure e-1. A total of 34 seizures comprising 22 CPS and 12 secondarily GTCS met the inclusion criteria. The seizures occurred at different times during the day and night and during both awake and sleep states (figure e-2).
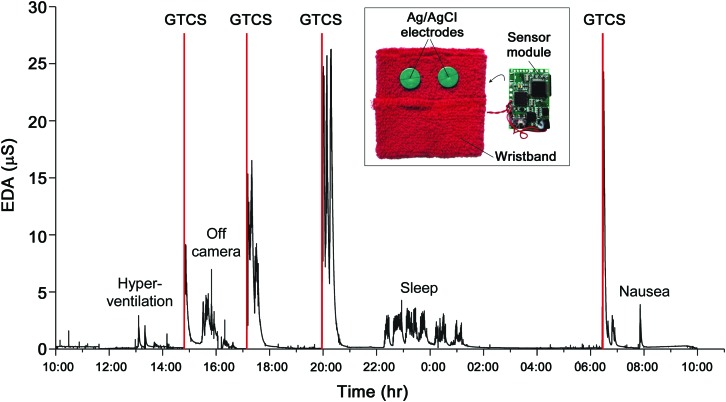
Figure 1. Long-term electrodermal activity (EDA) recordings obtained from a wearable biosensor.
EDA was measured as skin conductance in units of micro-Siemens. In this example of a 24-hour continuous EDA recording from a single patient, 4 secondarily generalized tonic-clonic seizures (GTCS) were captured. Vertical red lines denote EEG seizure onset. Inset: The wearable biosensor consists of a wristband with an integrated sensor module that can be worn comfortably for long periods of time and during daily activities. The underside of the wristband is shown to reveal standard dry Ag/AgCl electrodes used for EDA measurements.
Critical window of autonomic dysregulation.
First, we examined the time courses of autonomic alterations in CPS and GTCS. EKG recordings were not included for 6 CPS and 1 GTCS because of short postictal recordings (<30 minutes), missing data, and signal corruption by artifacts. Examples of changes in autonomic activity from a single seizure of each type are shown in figure 2.
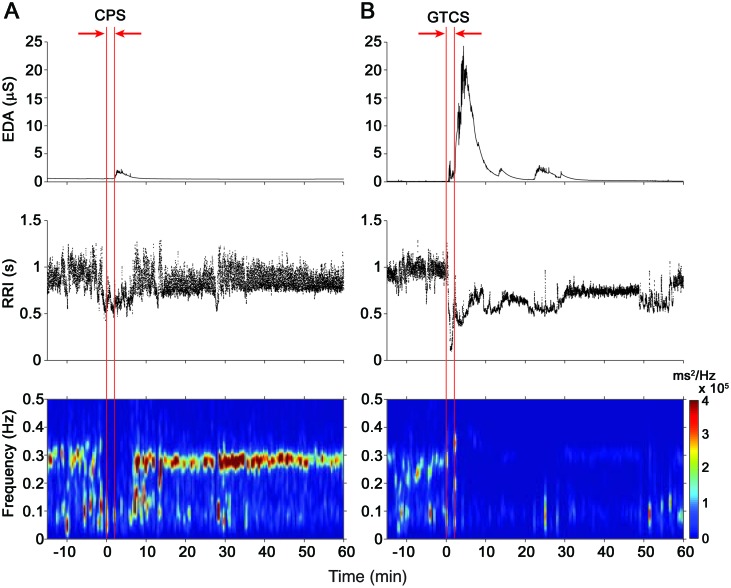
Figure 2. Changes in autonomic activity after individual epileptic seizures.
Examples of alterations in electrodermal activity (EDA) and R-R intervals (RRI) along with time-frequency mapping of the RRI during peri-ictal segments of 75 minutes. (A) A small increase in EDA is observed with a decrease in RRI (i.e., increase in heart rate) during this complex partial seizure (CPS). There is also a brief reduction of the high-frequency spectral component (0.15–0.4 Hz) of the RRI during the postictal period that reappears after approximately 5 minutes. (B) A large surge in EDA is visible after this secondarily generalized tonic-clonic seizure (GTCS) accompanied by a drop in RRI. Note the reduction in RRI variability during the postictal period and the dramatic reduction of the high-frequency power. Vertical red lines denote EEG seizure onset and offset.
In all 12 (100%) GTCS, we observed an EDA response defined in this work as an increase in EDA greater than 2 SD above baseline, whereas 19 of 22 (86%) CPS exhibited an EDA response (table e-2). The latency from EEG seizure onset to EDA response onset was not different between the 2 seizure types (p > 0.1; MWW test); the median latency for all seizures was 33.25 seconds. The amplitude of the EDA response was not different between GTCS arising in sleep and during wakefulness (p = 0.88; MWW test).
We observed a period of autonomic disturbance after CPS (figure 3A). Sympathetic-mediated EDA was significantly higher for the first 9 minutes before returning to baseline (p < 0.05; WSRT). Postictal heart rate was elevated for 3 minutes (p < 0.05; WSRT). Parasympathetic-modulated HF power was persistently lower for approximately 55 minutes with sporadic brief periods of reduction afterward (p < 0.05; WSRT).
Strikingly, we found a period of severe and uninterrupted autonomic dysregulation after GTCS. This period was characterized by significant elevation of EDA for 56 minutes (p < 0.05; WSRT) and increased heart rate lasting approximately 100 minutes (p < 0.05; WSRT) with persistent tachycardia for 40 minutes and suppressed HF power unrelieved for approximately 100 minutes (p < 0.05; WSRT). These findings suggest that the early postictal phase appeared to be dominated by sympathetic overactivation coupled with parasympathetic (vagal) suppression, whereas the later phase seemed to be dominated by impaired vagal recovery.
Comparison between CPS and GTCS.
Preictal levels (averaged over 60 minutes) of EDA and HF power were not different between seizure types. However, postictal EDA (averaged over 120 minutes) was significantly higher in GTCS than in CPS (p = 0.004; MWW) (figure 3C), suggesting much higher sympathetic activation. In addition, postictal HF power was significantly lower in GTCS (p = 0.033; MWW) (figure 3D), suggesting much lower vagal influence. Therefore, autonomic activity after GTCS was affected more severely, and these alterations lasted longer compared with that after CPS.
Magnitude of autonomic alterations in GTCS is strongly correlated with PGES.
An example of PGES following a GTCS along with changes in EDA is shown in figure 4. Changes in EDA for each individual GTCS are shown in figure e-3. The duration of PGES could not be measured reliably in one seizure because of corruption of the EEG signal by movement artifacts. PGES was observed in 9 of 11 (81.8%) of the GTCS for which we had EEG recordings.
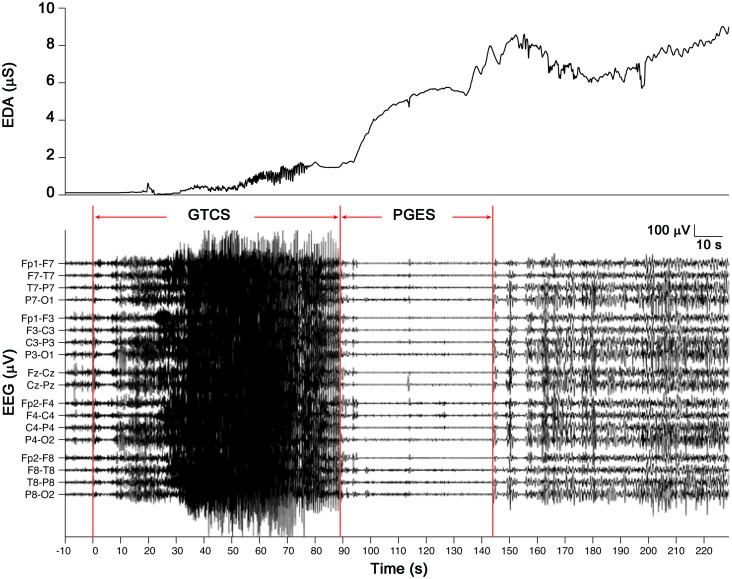
Figure 4. Postictal generalized EEG suppression (PGES) after a secondarily generalized tonic-clonic seizure (GTCS).
An example of a tonic-clonic seizure abruptly terminated and replaced by flattening of EEG signals in all channels is shown. The rise in electrodermal activity (EDA) begins during the seizure event but increases most notably during the postictal phase.
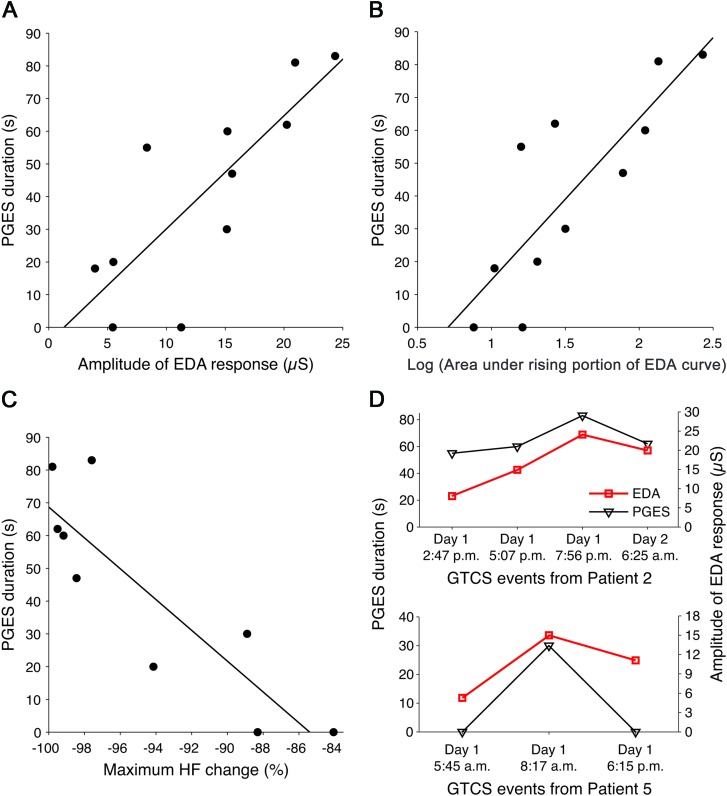
Across GTCS as a group, an increase in the EDA response amplitude was correlated with an increase in the duration of PGES (r = 0.81, p = 0.003) (figure 5A). Similarly, an increase in the log-transformed area under the EDA curve was correlated with the duration of PGES (r = 0.75, p = 0.008). Increases in the log-transformed area under the rising portion of the EDA response showed strong correlation (r = 0.83, p = 0.002) (figure 5B). Decreased HF power (the maximal percentage change) correlated with an increase in the duration of PGES (r = −0.87, p = 0.002) (figure 5C). Notably, PGES was not dependent on the total seizure duration (p > 0.3) or on the duration of the generalized motor phase (p > 0.3). There was no association between any of the above autonomic parameters and seizure duration (p > 0.14) or duration of the motor phase (p > 0.2), suggesting that the autonomic changes were specific for PGES.
Figure 5. Relationship between degree of postictal autonomic disturbance and postictal generalized EEG suppression (PGES) in secondarily generalized tonic-clonic seizures (GTCS).
Scatterplots of PGES duration vs (A) electrodermal activity (EDA) response amplitude, (B) log-transformed area under the rising portion of the EDA curve, and (C) maximum percent high-frequency (HF) power change. (D) The association between EDA response amplitude and PGES in GTCS on a patient-specific level showed close agreement in trends. Patient 2 had 4 GTCS events (top), and patient 5 had 3 GTCS events (bottom).
We also examined the relation between PGES and autonomic variables on a patient-specific level (within patients who had multiple GTCS) to see how sensitive the autonomic measures might be as an indicator of PGES. We observed that the trends in PGES and amplitude of EDA response amplitude after all 4 GTCS from patient 2 were in close agreement (figure 5D, top). This similarity in trends was also present for all 3 GTCS in patient 5 (figure 5D, bottom). The 2 GTCS from patient 3 could not be compared because one had the EEG corrupted by motion.
DISCUSSION
The present study quantified both sympathetic and parasympathetic autonomic disturbance after GTCS and showed a significant correlation of these alterations with the duration of PGES. We found that autonomic activity after GTCS was affected more severely than that after CPS, and this impairment lasted up to 100 minutes. This critical period appears to involve sympathetic overactivation evidenced by a surge in EDA, coupled with vagal suppression indicated by suppressed HF power in the early phase and impaired vagal recovery in the later phase. Importantly, the degree of both sympathetic activation and parasympathetic suppression increased approximately linearly with duration of PGES.
Although many studies have analyzed baseline (interictal) alterations of autonomic function in patients with epilepsy,12 data on ictal and specifically postictal autonomic alterations are limited despite evidence that they are among the most dangerous effects of seizures.13 Prior studies on postictal autonomic modulation limited heart rate variability analysis to a single measurement taken during the early14,15 postictal period (<15 minutes) and in one patient during the late16 postictal period (5–6 hours). Thus, little is known about the time course of autonomic disturbance after seizures. Moreover, decoupling the relative influence of parasympathetic or sympathetic system is challenging because heart rate, like blood pressure and respiration rate, is controlled by both branches of the autonomic nervous system.17 Although there is a consensus that HF power reflects vagal modulation of the heart rate, there is no clear cardiac measure of sympathetic modulation that can be easily uncoupled. Claims that power in the low-frequency range (LF) (0.04–0.15 Hz) reflects primarily sympathetic modulation of heart rate or that the LF/HF ratio reflects the sympathovagal balance are highly controversial because β-blockade does not reduce but rather increases LF power,18 and direct cardiac sympathetic blockage via epidural anesthesia has no effect on it.19 In contrast, EDA provides a sensitive index of sympathetic activity alone.20
The findings here expand current knowledge by presenting continuous, minute-by-minute profiles of both sympathetic and parasympathetic modulation up to 2 hours after CPS and GTCS. GTCS induced much higher and prolonged sympathetic activation and greater reduction of cardiac vagal influence than CPS. This difference in both the degree and duration of autonomic dysregulation may in part explain why GTCS are a major risk factor for SUDEP. The high-resolution time course revealed 2 phases of postictal autonomic disturbance after GTCS. The first phase involved a prolonged sympathetic surge in EDA lasting approximately 65 minutes. This time course is in close agreement with changes in plasma concentrations of catecholamines that are maximally elevated in the first 10 minutes after GTCS and decline over 60 minutes to normal levels.21 Taken together, these findings support the notion of a generalized sympathetic neural activation. Conversely, the persistent low HF power and delayed recovery in heart rate even after sympathetic levels returned to baseline suggest impaired vagal recovery in the second phase. Experimental22 and clinical studies23–25 have consistently shown that decreased vagal activity is associated with increased risk for cardiac sudden death in subjects without epilepsy. Augmented vagal activity is protective against lethal arrhythmias,26,27 and decreased vagal modulation leading to an increase in ventricular automaticity could in turn make the heart susceptible to arrhythmias. A previous case study described a patient who underwent repeated measures of HF power, which progressively deteriorated before SUDEP.28 The autonomic profiles uncovered in this study indicate that there exists a critical window of disordered autonomic regulation after seizures, especially GTCS, which may lead to increased vulnerability of a patient for sudden death.
Diffuse flattening of the EEG was observed in 7 patients with epilepsy who died suddenly during monitoring.8,29–33 In these patients, the fatal seizure was abruptly interrupted and replaced by severe suppression of the EEG that failed to recover. Recording PGES or autonomic dysfunction during SUDEP alone may be insufficient to prove cause. An important issue raised by Surges et al.9 is “whether these postictal EEG patterns have a neurologic correlate, such as impaired consciousness, cognitive and behavioral disturbances, or altered autonomic function. The latter could lead to SUDEP.” Our data show strong correlations between PGES and both sympathetic and vagal alterations, providing indirect evidence that PGES is indeed associated with the severity of autonomic disturbance. A comparison of autonomic changes in GTCS with and without PGES would provide stronger evidence for autonomic changes as a marker of PGES. Although we only had a few GTCS recordings, we found that GTCS with prolonged PGES (>20 seconds) had significantly higher sympathetic activation and greater vagal reduction than GTCS with short PGES (figure e-4). Every case of a GTCS with EDA response amplitude greater than 15 μS exhibited prolonged PGES. The relationship between PGES, autonomic function, and SUDEP is not fully understood. One possibility is that prolonged PGES excessively inhibits different neuronal systems, such as parasympathetic centers or circuits, and results in unbridled sympathetic activation. In turn, the dysregulation of the central autonomic network may impair its ability to compensate for seizure-related respiratory or cardiovascular dysfunction, leading to SUDEP. Further studies investigating the relationship between these autonomic alterations and the occurrence and type of apnea, as well as its duration or severity, may provide further information.
These findings must be interpreted in the setting of data acquisition of the present study. First, because of the exploratory nature of the study, relatively few patients and seizures were included. Despite SUDEP and PGES being more commonly described in an older population, children have a higher frequency of epilepsy and we observed the same phenomenon of PGES in this pediatric cohort. In accounting for other known risk factors for SUDEP including frequent GTCS, taking multiple antiepileptic drugs, and candidacy for surgery, the patients in our study were at high risk for SUDEP. This may explain why the rate of PGES we observed was higher (81.8% in GTCS) than previous studies in children34 (31.6% in GTCS) and adults8,9 (40−65% in GTCS).
The current results provide a promising outlook for patients with epilepsy: The possibility of autonomic biomarkers of seizure intensity, PGES, or possible SUDEP risk is attractive for ambulatory monitoring. By using autonomic parameters as surrogate measures (e.g., sympathetically mediated EDA or parasympathetically mediated HF power) for PGES, long-term studies can be performed to examine the reliability of PGES as a predictor for SUDEP. For example, large-scale, longitudinal home studies in which patients are asked to wear the wrist-worn EDA biosensor along with concurrent ambulatory EEG and EKG monitoring may allow a better characterization of the relationship between autonomic dysfunction, PGES, and SUDEP. With the onboard tri-axis accelerometer and wireless transceiver, it is also possible to develop an automated convulsive seizure detector with 94% sensitivity and one false alarm rate per 24 hours that can alert caregivers.35 The changes in EDA associated with GTCS appear different from EDA patterns observed during daily activities collected over 1 week in a natural home environment.10 Nonetheless, our present data were obtained in an inpatient setting, and further studies are necessary to evaluate the utility of EDA for home-based ambulatory seizure monitoring. Autonomic parameters such as the amplitude of the EDA response might also provide caregivers with information regarding the severity of the seizure and aid in identifying potentially dangerous seizures that require immediate medical attention. Supervision and attention to recovery after a seizure may be important in SUDEP prevention because most deaths are unwitnessed.36 The findings presented here might help discriminate when a postictal period is likely to be life-threatening.
SUDEP in most cases is triggered by a GTCS, but it remains unknown what physiologic contributory factors make this particular seizure fatal unlike all other seizures in the past. Our data reveal a window of significantly disordered autonomic regulation after GTCS that may create or contribute to conditions for sudden death. The findings in this study identify a strong correlation between the degree of autonomic dysregulation and duration of PGES after a GTCS for both sympathetic and parasympathetic nervous systems. Because prolonged PGES was observed in patients who subsequently died of SUDEP, it will be of great interest to establish whether or not these autonomic footprints could provide reliable biomarkers to identify patients with epilepsy who are at risk of SUDEP.
Supplementary Material
ACKNOWLEDGMENT
The authors thank L. Fleming for handling institutional review board issues, M.C. Sabtala, S. Manganaro, and the long-term monitoring unit staff for assisting in data collection, E.H. Park for coordinating data download and transfer, and G. Abazi and H. Mustafa for obtaining patient consent.
GLOSSARY
- CPS
complex partial seizures
- EDA
electrodermal activity
- GTCS
generalized tonic-clonic seizures
- HF
high-frequency
- LF
low-frequency
- MWW
Mann-Whitney-Wilcoxon
- PGES
postictal generalized EEG suppression
- SUDEP
sudden unexpected death in epilepsy
- WSRT
Wilcoxon signed rank test
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Dr. Poh, Dr. Loddenkemper, and Dr. Picard wrote the manuscript. Dr. Poh and Dr. Picard initiated the study. Dr. Poh, Dr. Loddenkemper, Dr. Madsen, and Dr. Picard designed the study. Dr. Poh and N.C. Swenson built the wrist-worn sensors. Dr. Poh, Dr. Loddenkemper, N.C. Swenson, and S. Goyal conducted the study. Dr. Poh and N.C. Swenson performed computational analysis of autonomic signals. Dr. Loddenkemper and Dr. Reinsberger performed EEG analysis. All authors participated in editing the manuscript.
DISCLOSURE
Dr. Poh reports no disclosures. Dr. Loddenkemper is supported by the Epilepsy Foundation of America. Dr. Reinsberger is supported by the Epilepsy Foundation of America. N.C. Swenson, S. Goyal, and Dr. Madsen report no disclosures. Dr. Picard is Co-founder, Chief Scientist, and Chairman of Affectiva, Inc. The wrist-worn sensors used in this study were completely designed and built at MIT. Affectiva, Inc subsequently licensed the technology and has commercialized the sensors. Go to Neurology.org for full disclosures.
REFERENCES
- 1. Tomson T, Nashef L, Ryvlin P. Sudden unexpected death in epilepsy: current knowledge and future directions. Lancet Neurol 2008; 7: 1021– 1031 [DOI] [PubMed] [Google Scholar]
- 2. Sillanpaa M, Shinnar S. Long-term mortality in childhood-onset epilepsy. N Engl J Med 2010; 363: 2522– 2529 [DOI] [PubMed] [Google Scholar]
- 3. Surges R, Thijs RD, Tan HL, Sander JW. Sudden unexpected death in epilepsy: risk factors and potential pathomechanisms. Nat Rev Neurol 2009; 5: 492– 504 [DOI] [PubMed] [Google Scholar]
- 4. So EL. What is known about the mechanisms underlying SUDEP? Epilepsia 2008; 49: 93– 98 [DOI] [PubMed] [Google Scholar]
- 5. Nilsson L, Farahmand BY, Persson PG, Thiblin I, Tomson T. Risk factors for sudden unexpected death in epilepsy: a case-control study. Lancet 1999; 353: 888– 893 [DOI] [PubMed] [Google Scholar]
- 6. Walczak TS, Leppik IE, D'Amelio M, et al. Incidence and risk factors in sudden unexpected death in epilepsy: a prospective cohort study. Neurology 2001; 56: 519– 525 [DOI] [PubMed] [Google Scholar]
- 7. Langan Y, Nashef L, Sander JW. Case-control study of SUDEP. Neurology 2005; 64: 1131– 1133 [DOI] [PubMed] [Google Scholar]
- 8. Lhatoo SD, Faulkner HJ, Dembny K, Trippick K, Johnson C, Bird JM. An electroclinical case-control study of sudden unexpected death in epilepsy. Ann Neurol 2010; 68: 787– 796 [DOI] [PubMed] [Google Scholar]
- 9. Surges R, Strzelczyk A, Scott CA, Walker MC, Sander JW. Postictal generalized electroencephalographic suppression is associated with generalized seizures. Epilepsy Behav 2011; 21: 271– 274 [DOI] [PubMed] [Google Scholar]
- 10. Poh MZ, Swenson NC, Picard RW. A wearable sensor for unobtrusive, long-term assessment of electrodermal activity. IEEE Trans Biomed Eng 2010; 57: 1243– 1252 [DOI] [PubMed] [Google Scholar]
- 11. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Stat Methodol 1995; 57: 289– 300 [Google Scholar]
- 12. Sevcencu C, Struijk JJ. Autonomic alterations and cardiac changes in epilepsy. Epilepsia 2010; 51: 725– 737 [DOI] [PubMed] [Google Scholar]
- 13. Devinsky O. Effects of seizures on autonomic and cardiovascular function. Epilepsy Curr 2004; 4: 43− 46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Surges R, Scott CA, Walker MC. Enhanced QT shortening and persistent tachycardia after generalized seizures. Neurology 2010; 74: 421– 426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Al-Aweel I, Krishnamurthy K, Hausdorff J, et al. Postictal heart rate oscillations in partial epilepsy. Neurology 1999; 53: 1590– 1590 [DOI] [PubMed] [Google Scholar]
- 16. Toth V, Hejjel L, Fogarasi A, et al. Periictal heart rate variability analysis suggests long-term postictal autonomic disturbance in epilepsy. Eur J Neurol 2010; 17: 780− 787 [DOI] [PubMed] [Google Scholar]
- 17. Venables PH. Autonomic activity. Ann NY Acad Sci 1991; 620: 191– 207 [DOI] [PubMed] [Google Scholar]
- 18. Cacioppo JT, Berntson GG, Binkley PF, Quigley KS, Uchino BN, Fieldstone A. Autonomic cardiac control. II. Noninvasive indices and basal response as revealed by autonomic blockades. Psychophysiology 1994; 31: 586– 598 [DOI] [PubMed] [Google Scholar]
- 19. Hopf HB, Skyschally A, Heusch G, Peters J. Low-frequency spectral power of heart rate variability is not a specific marker of cardiac sympathetic modulation. Anesthesiology 1995; 82: 609− 619 [DOI] [PubMed] [Google Scholar]
- 20. Boucsein W. Electrodermal Activity. New York: Plenum Press; 1992 [Google Scholar]
- 21. Simon RP, Aminoff MJ, Benowitz NL. Changes in plasma catecholamines after tonic-clonic seizures. Neurology 1984; 34: 255– 257 [DOI] [PubMed] [Google Scholar]
- 22. Hull SS, Jr, Evans AR, Vanoli E, et al. Heart rate variability before and after myocardial infarction in conscious dogs at high and low risk of sudden death. J Am Coll Cardiol 1990; 16: 978– 985 [DOI] [PubMed] [Google Scholar]
- 23. Nakagawa M, Saikawa T, Ito M. Progressive reduction of heart rate variability with eventual sudden death in two patients. Br Heart J 1994; 71: 87– 88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Martin GJ, Magid NM, Myers G, et al. Heart rate variability and sudden death secondary to coronary artery disease during ambulatory electrocardiographic monitoring. Am J Cardiol 1987; 60: 86– 89 [DOI] [PubMed] [Google Scholar]
- 25. Molgaard H, Sorensen KE, Bjerregaard P. Attenuated 24-h heart rate variability in apparently healthy subjects, subsequently suffering sudden cardiac death. Clin Auton Res 1991; 1: 233– 237 [DOI] [PubMed] [Google Scholar]
- 26. Hull SS, Jr, Vanoli E, Adamson PB, Verrier RL, Foreman RD, Schwartz PJ. Exercise training confers anticipatory protection from sudden death during acute myocardial ischemia. Circulation 1994; 89: 548– 552 [DOI] [PubMed] [Google Scholar]
- 27. Vanoli E, De Ferrari GM, Stramba-Badiale M, Hull SS, Jr, Foreman RD, Schwartz PJ. Vagal stimulation and prevention of sudden death in conscious dogs with a healed myocardial infarction. Circ Res 1991; 68: 1471– 1481 [DOI] [PubMed] [Google Scholar]
- 28. Rauscher G, DeGiorgio A, Miller P, DeGiorgio C. Sudden unexpected death in epilepsy associated with progressive deterioration in heart rate variability. Epilepsy Behav 2011; 21: 103– 105 [DOI] [PubMed] [Google Scholar]
- 29. Bird J, Dembny K, Sandeman D, Butler S. Sudden unexplained death in epilepsy: an intracranially monitored case. Epilepsia 1997; 38: S52– S56 9092961 [Google Scholar]
- 30. Lee MA. EEG video recording of sudden unexpected death in epilepsy (SUDEP). Epilepsia 1998; 39 (suppl 6): 123– 124 [Google Scholar]
- 31. McLean BN, Wimalaratna S. Sudden death in epilepsy recorded in ambulatory EEG. J Neurol Neurosurg Psychiatr 2007; 78: 1395– 1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bateman LM, Spitz M, Seyal M. Ictal hypoventilation contributes to cardiac arrhythmia and SUDEP: report on two deaths in video-EEG-monitored patients. Epilepsia 2010; 51: 916– 920 [DOI] [PubMed] [Google Scholar]
- 33. Purves SJ. Sudden death in epilepsy: single case report with video-EEG documentation. Epilepsia 1992; 33 (suppl 3): 123 [Google Scholar]
- 34. Kim AJ, Kuroda MM, Nordli DR., Jr. Abruptly attenuated terminal ictal pattern in pediatrics. J Clin Neurophysiol 2006; 23: 532 [DOI] [PubMed] [Google Scholar]
- 35. Poh MZ, Loddenkemper T, Reinsberger C, et al. Convulsive seizure detection using a wrist-worn electrodermal activity and accelerometry biosensor. Epilepsia. doi: 10.1111/j.1528-1167.2012.03444.x. Epub 2012 Mar 20. [DOI] [PubMed] [Google Scholar]
- 36. Langan Y, Nashef L, Sander JW. Sudden unexpected death in epilepsy: a series of witnessed deaths. J Neurol Neurosurg Psychiatry 2000; 68: 211 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.