Abstract
Background
Alcohol consumption is associated with increased iron stores. In sub-Saharan Africa, high dietary ionic iron and the ferroportin Q248H allele have also been implicated in iron accumulation. We examined the associations of ferroportin Q248H, alcohol and dietary iron with serum ferritin, aspartate aminotransaminase (AST) and alanine aminotransaminase (ALT) concentrations in African Americans.
Methods
Inner-city African Americans (103 men, 40 women) were recruited from the community according to reported ingestion of >4 alcoholic drinks per day or <2 per week. Typical daily heme iron, non-heme iron and alcohol were estimated using University of Hawaii’s multiethnic dietary questionnaire. Based on dietary questionnaire estimates we established categories of < versus ≥56 g alcohol per day, equivalent to 4 alcoholic drinks per day assuming 14 g alcohol per drink.
Results
Among 143 participants, 77% drank <56 g alcohol/day and 23% ≥56 g/d as estimated by the questionnaire. The prevalence of ferroportin Q248H was 23.3% with alcohol >56 g/d versus 7.5% with lower amounts (P=0.012). Among subjects with no history of HIV disease, serum ferritin concentration had positive relationships with male gender (P=0.041), alcohol consumption (P=0.021) and ALT concentration (P=0.0001) but not with dietary iron intake or ferroportin Q248H. Serum AST and ALT concentrations had significant positive associations with male gender and hepatitis C seropositivity but not with alcohol or dietary iron intake or ferroportin Q248H.
Conclusions
Our findings suggest a higher prevalence of ferroportin Q248H with greater alcohol consumption, and this higher prevalence raises the possibility that the allele might ameliorate the toxicity of alcohol. Our results suggest that alcohol but not dietary iron contributes to higher body iron stores in African Americans. Studies with larger numbers of participants are needed to further clarify the relationship of ferroportin Q248H with the toxicity of alcohol consumption.
Introduction
Iron is absorbed from the diet as elemental divalent iron or in the form of heme (Wheby et al, 1970). Heme is found primarily in such proteins as hemoglobin and myoglobin, while elemental iron is present in vegetables, cereals, and other foodstuffs. Iron absorption occurs most efficiently in the duodenum and heme iron is better absorbed than elemental iron (Cook, 1990). Heme crosses from the lumen into the enterocyte (Conrad et al, 1967), possibly via heme carrier protein 1 (Shayeghi et al, 2005), and non-heme iron uptake is mediated by divalent metal transporter 1 (Gunshin et al, 1997; Cannone-Hergaux et al, 1999). Iron is exported from the duodenal endothelial cell to the portal blood stream by ferroportin1 (Donovan et al, 2000; McKie et al, 2000; Abboud and Haile, 2000). Consumption of alcohol has been associated with increased iron stores as assessed by serum ferritin concentration in several population studies (Milman and Kirchoff, 1996, Fleming et al, 1998; Liu et al, 2003). A number of population studies conducted predominantly among Caucasians and Hispanics have shown an association between dietary heme iron, but not dietary non-heme iron, and iron stores as assessed by serum ferritin concentration (Fleming et al, 1998; Backstrand et al, 2002; Ramakrishnan et al, 2002; Liu et al, 2003). A third or more of alcoholics develop increased amounts of hepatic iron compared to controls (Fletcher et al, 1999; Suzuki et al, 2002).
In rural Africa, there is a strong association among the consumption of a traditional fermented beverage with high ionic iron concentration, increased body iron stores and liver toxicity and cirrhosis (Gordeuk et al, 1986; Gordeuk et al, 1992; Friedman et al, 1990; Moyo et al, 1997; Moyo et al, 1998). In experimental rats, dietary iron supplementation exacerbates alcohol-induced hepatocyte damage and promotes liver fibrogenesis (Tsukamoto et al, 1995). Similarly, the presence of elevated iron stores accentuates the hepatic toxicity of alcohol in the setting of HFE hemochromatosis in Caucasians (Fletcher and Powell, 2003). At the population level and based on serum ferritin concentration, African Americans have higher iron stores than Caucasian Americans (Barton et al, 2005).
The cDNA 744G>T substitution in exon 6 of the ferroportin gene (dbSNP rs11568350, www.ncbi.nlm.nih.gov), which results in the replacement of glutamine with histidine at position 248 (Q248H), is common in Africans and African Americans (prevalence of heterozygotes of 5% or more). Ferroportin is the only iron exporter in mammalian cells, and the Q248H allele may be associated with a tendency to iron loading in adults and to protection from iron deficiency in children (Gordeuk et al, 2003; Beutler et al, 2003; McNamara et al, 2005; Kasvosve et al, 2005; Rivers et al, 2007). One in vitro study indicated that the Q248H allele impairs the egress of iron when expressed in Xenopus oocytes (McGregor et al, 2005). Other studies indicated that the Q248H allele retains the ability to export iron and respond to hepcidin when expressed in HEK 293T cells (Drakesmith et al, 2005; Schimanski et al, 2005). What selection advantage this mutation may have is not known, but it is possible that altered iron trafficking could be protective of certain tissues during times of oxidant stress.
The present study was conducted to determine whether dietary iron content, alcohol consumption and ferroportin Q248H influence iron stores and hepatic function among inner city African Americans.
Materials and Methods
Participants
The research was approved by the Howard University IRB and all participants gave written informed consent. The participants were self-described African-American males and females over 18 years of age from the community who were recruited as one of the following two groups: i) self-reported average alcohol consumption of less than two drinks per week (n = 72); ii) self-reported average alcohol consumption of four or more drinks per day (n = 71).
Estimation of daily iron and alcohol intake by dietary questionnaire and categorization of patients according to alcohol intake
To quantify dietary iron content and alcohol consumption, participants filled out the University of Hawaii Multi-Ethnic Dietary Questionnaire with the help of the study research nurses. The test-retest reliability of this questionnaire has been validated (Stram et al, 2000). The questionnaire asks about average eating habits over the past year. The questionnaire was analyzed at the University of Hawaii. Estimates for average daily intake of kilocalories, alcohol, total dietary iron, dietary iron derived from meat, fish and poultry, and supplemental iron were provided. For the analyses reported in this study, the iron derived from meat, fish and poultry was classified as heme iron and the difference between total dietary iron and dietary heme iron as non-heme iron.
Laboratory tests
Peripheral blood was collected in the morning. EDTA-anticoagulated blood was used for performing complete blood count, reticulocyte count (Coulter® LH750, Beckman Coulter, Inc., Fullerton, CA) and erythrocyte sedimentation rate (ESR) (Westergren method). Serum was used to determine hepatitis B surface antigen (Diagnostic Products Corporation, Los Angeles, CA), antibody to hepatitis C (ORTHO® HCV Version 3.0 ELISA Test System, Ortho-Clinical Diagnostics, Inc., Raritan, NH), aspartate aminotransferase (AST), alanine aminotransferase (ALT), bilirubin, total protein, albumin, iron, transferrin (Unicel® DxC 600 Synchron® Clinical System, Beckman Coulter, Inc., Fullerton, CA) and ferritin (Access® 2 Beckman Coulter, Inc., Fullerton, CA). These tests were performed in the clinical laboratory of Howard University Hospital. Concentrations of C-reactive protein were determined from serum samples that had been stored at −80°C by enzyme-linked immunosorbant assay (ALPCO Diagnostics, Windham, NH, USA) (expected plasma range provided by the manufacturer of 0.068–8.2 mg/L). Transferrin saturation was calculated by dividing the serum iron in μg/dL by 1.27 X transferrin concentration in mg/L and multiplying by 100 (Gottschalk et al, 2000).
Ferroportin Q248H mutation
DNA was isolated from whole blood using the GenomicPrep Blood Isolation Kit (GE Healthcare, Little Chalfont, UK). Exon 6 of ferroportin was amplified by using a set of primers encompassing portions of the introns that flank the exon (forward primer: 5′-CAT CGC CTG TGG CTT TAT TT-3′; reverse primer: 5′-GCT CAC ATC AAG GAA GAG GG-3′). PCR reactions were performed in 25 μl volumes in standard PCR buffer containing 1.5 mM MgCl2, 200 μM dNTP, 20 nM Primers and 0.5U Taq DNA polymerase. After initial denaturation at 95°C for 5 min, a polymerase chain reaction was performed in a thermocycler (Mycycler, Bio-Rad) for 38 of cycles of heating at 95°C for 15s, annealing at 55°C 15s and extension at 72°C for 1min. Also a final cycle of 10 min at 72°C was added. Ten μl of PCR product (392 bp) was digested with PvuII enzyme (MBI Fermentas, Hanover, MD) for 2 hr at 37°C, and the resulting DNA fragments (252 bp and 140bp) were resolved on 2.5% agarose gel and detected with ethidium bromide staining.
Statistical Analysis
The study prospectively defined alcohol consumption of >4 drinks per day (>56 g per day) as heavy consumption. Linear regression was used to asses the relationships of dietary estimates of daily alcohol, heme iron and non-heme iron intake per kg with serum ferritin, AST, and ALT concentrations. Because the iron required for menstruation, child-bearing and breast feeding strongly affects the iron status of women (Bothwell et al, 1979), we prospectively planned to stratify these analyses by sex. Also, because hepatits C, liver function, BMI and age potentially influence our serum markers of interest (Prieto et al, 1975; Reissmann and Diedrich, 1956; Aungst, 1968; Meyer et al, 1984; Legget et al, 1990; Di Bisceglie et al, 1992; Fleming et al, 1992; Wrede et al, 2006), we report two betas from linear regression in each sex category, a crude beta and a beta adjusted for the effects of potential confounders for each variable as explained in the footnote of Table 2). Because history of HIV seropositivity strongly correlated with ferritin, AST, and ALT in a non-linear manner, these analyses were performed in participants who did not give a history of HIV seropositivity.
Table 2.
Effects of alcohol and heme Iron intake on serum concentrations of ferritin, AST and ALT (models exclude two cases with high calorie intake and six cases with history of HIV)
| Male | Female | ||||
|---|---|---|---|---|---|
| Independent Factor | Dependent Factor | Crude Beta (P value) | *Adjusted Beta (P value) | Crude Beta (P value) | *Adjusted Beta (P value) |
| Log Alcohol Intake (g per day) | Log Ferritin (ug/L) | 0.09 (0.004) | 0.09 (0.018)a | −0.01 (0.8) | 0.11 (0.2)a |
| Log ALT (U/L) | −0.01 (0.5) | −0.01 (0.6)b | −0.02 (0.3) | −0.03 (0.2)b | |
| Log AST (U/L) | −0.008 (0.7) | 0.002 (0.9)b | 0.002 (0.9) | −0.02 (0.095)b | |
| Log Dietary Heme Iron Intake (mg/kg per day) | Log Ferritin (ug/L) | 0.07 (0.4) | 0.10 (0.5)c | −0.53 (0.092) | 0.20 (0.7)c |
| Log ALT (U/L) | −0.02 (0.8) | 0.07 (0.4)d | −0.18 (0.027) | −0.41 (0.041)d | |
| Log AST (U/L)f | −0.08 (0.2) | −0.05 (0.6)d | −0.12 (0.049) | −0.26 (0.011)d | |
Adjusted for age, BMI, ALT, erythrocyte sedimentation rate, heme iron intake and non-heme iron intake, ferroportin Q248H
Adjusted for BMI, HCV, erythrocyte sedimentation rate, heme iron intake and non-heme iron intake, ferroportin Q248H
Adjusted for age, BMI, ALT, erythrocyte sedimentation rate, alcohol and non-heme iron intake, ferroportin Q248H
Adjusted for BMI, HCV, erythrocyte sedimentation rate, alcohol and non-heme iron intake, ferroportin Q248H
Separate models were also developed to explain ferritin, AST and ALT in the comined sample of males and females. In each case, the final model was developed with a hierarchical backward approach with a p<0.05 to keep the predictor in the model. The ferritin model initially included sex, age, BMI, alcohol intake, heme and non-heme iron intake, erythrocyte sediment rate, ferroportin Q248H and ALT concentration with terms of interaction. The AST and ALT models initially included sex, age, alcohol intake, heme and non-heme iron intake, erythrocyte sediment rate, BMI, ferroprotin Q248H, and hepatitis C seropositvity.
Serum ferritin, AST, ALT, and dietary intakes of alcohol and iron were log normal transformed for use in linear regression. Two individuals with extreme outlying values for average kilocalories ingested per day were excluded from all models.
Results
Characteristics of the study participants
The median age was 47 years and 28% of the participants were women (Table 1). The overall prevalence of the ferroportin Q248H mutation was 11%. History of HIV seropositivity was given by 4% of the participants. The prevalence of hepatitis C positivity was 28% and that of hepatitis B surface antigen 2%. Median daily estimated dietary iron was 20 mg (4 mg heme iron and 16 mg non-heme iron). Fifty percent of the subjects were recruited as drinking four or more alcoholic drinks per day, but only 23% drank this amount as estimated by dietary questionnaire, if it is assumed that one drink contains 14 g alcohol. Similarly, 50% of the participants were recruited as drinking less than two alcoholic drinks per week, but only 32% drank alcohol this rarely as estimated by the questionnaire.
Table 1.
Clinical characteristics of the 143 study participants. Results in median and interquartile range unless otherwise indicated.
| Demographics | |
| Recruitment group in no. (%) | |
| Self-reported alcohol consumption <2 drinks/week | 72 (50.3%) |
| Self-reported alcohol consumption ≥4 drinks/day | 71 (49.7%) |
| Age in years | 47 (42–54) |
| Women in no. (%) | 40 (28.0) |
| Weight (kg) | 81 (71–92) |
| Body mass index (kg/m2) | 27.8 (24.2–31.8) |
| HIV seropositive by history in no. (%) | 5 (3.5%)a |
| Results of dietary questionnaire | |
| Alcohol (g/d) | 18 (1–51)b |
| Alcohol group in no. (%) | |
| <4 g/d | 45 (31.7%) |
| >56 g/d | 32 (22.5%)b |
| Kcal/day | 3,129 (1,824–5,087)b |
| Dietary total iron (mg/d) | 20 (10–34)b |
| Dietary heme iron (mg/d) | 4 (2–6)b |
| Dietary non-heme iron (mg/d) | 16 (8–28)b |
| Supplemental iron (mg/d) | 0 (0–14) |
| Vitamin C (mg/d) | 136 (60–242)b |
| Laboratory tests | |
| Ferroportin Q248H mutation in no. (%)b | |
| Heterozygotes | 14 (10.2)c |
| Homozygotes | 1 (0.7)c |
| Hemoglobin (g/dL) | 14.1 (13.2–15.0)c |
| Mean corpuscular volume (fL) | 90 (86–93)d |
| White blood cells (10−3/μL) | 5.8 (4.4–7.5)d |
| Platelets (10−3/μL) | 252 (211–301)d |
| C-reactive protein (mg/L) | 2.2 (0.7–6.0)d |
| Erythrocyte sedimentation rate (mm/hr) | 15 (6–32)e |
| Bilirubin (mg/dL) | 0.7 (0.6–0.9)f |
| ALT (U/L) | 24 (18–37)f |
| AST (U/L) | 27 (22–37)b |
| Albumin (g/dL) | 3.9 (3.7–4.2)a |
| Protein (g/dL) | 7.3 (6.8–7.7)a |
| Anti-hepatitis C virus positive in no. (%) | 40 (28.2)b |
| Hepatitis B surface antigen positive in no. (%) | 3 (2.1)b |
| Ferritin (μg/L) | 92 (47–210)a |
| Transferrin saturation (%) | 27 (19–34)g |
N = 141;
N = 142;
N = 137;
N = 139;
N = 132;
N = 140;
N = 130
ALT = alanine aminotransferase; AST = aspartate aminotransferase
Increased prevalence of ferroportin Q248h among participants with higher alcohol consumption
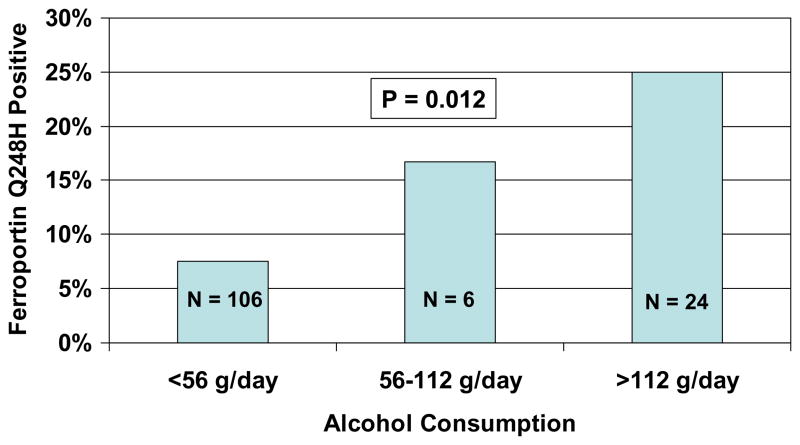
The prevalence of ferroportin Q248H increased from 7.5% in the 106 participants who consumed <56 g alcohol/day to 23.3% in the 30 participants who consumed ≥56 g alcohol/kg per day (P=0.014 by the Pearson chi square test). The group that consumed ≥56 g alcohol/day was further stratified according to consumption of 56–112 g/day (n = 6) and consumption of >112 g/day (n = 24). By the Cochran linear trend test, there was a significantly increasing prevalence of ferroportin Q248H across the three groups (P = 0.012; Figure 1).
Figure 1.
Proportions of participants with the ferroportin Q248 mutation according to alcohol consumption category.
Separate linear regression analyses in men and women
Relationship of serum ferritin concentration to alcohol consumption
Among men who did not have a history of HIV infection, the serum ferritin concentration correlated positively with the dietary questionnaire derived estimate for daily intake per kg of alcohol (Beta = 0.09; P = 0.004; Table 2). In linear regression models, beta shows the magnitude of change in the dependent factor for a unit change in a predictor. A beta of 0.09 for log alcohol and log ferritin indicates that one log increase in alcohol consumption is associated with a 0.09 increase in log ferritin concentration. The relationship between alcohol and serum ferritin persisted after adjustment for age. BMI, ALT concentration, erythrocyte sedimentation rate, heme and non-heme iron intake and ferroportin Q248H. Among women with no history of HIV seropositivity, serum ferritin concentration did not correlate significantly with estimated alcohol intake either in univariate analysis or after adjustment for potential confounders (Table 2).
Relationship of serum AST and ALT to alcohol consumption
The log AST and ALT concentrations did not correlate with alcohol intake in either men or women who did not have a history of HIV seropositivity. Controlling for the effects of hepatitis C seropositivity, erythrocyte sedimentation rate, heme and non-heme iron intake and ferroportin Q248H did not change these findings significantly (Table 2).
Relationship of serum ferritin concentration to heme Iron intake
Among men and women subjects with no history of HIV seropositivity, serum ferritin concentration did not correlate significantly with estimated dietary heme Iron intake either in univariate analysis or after adjustment for potential confounders (Table 2).
Relationship of serum AST and ALT concentrations to heme Iron intake
Among men, there was no significant correlation between heme iron intake and either AST or ALT, before or after adjustment for potential confounders. Among women there was a negative correlation between heme iron intake and both ALT and AST concentrations. These relationships remained statistically significant after controlling for potential confounders (Table 2).
Multivariate models including both men and women
Multivariate models were developed to predict the serum ferritin, AST and ALT concentrations. In each model a specific set of variables was entered into the primary model (see footnotes of Tables 3 and 4 and Statistical Methods). Nominated variables were selected from sex, age (years), BMI (kg/m2), ALT, hepatitis C seropostivity, erythrocyte sedimentation rate (mm/hr), alcohol intake (g per day), heme and non-heme iron intake (mg/kg per day) and ferroportin Q248H. Male sex, alcohol intake and ALT concentration had independent positive relationships with serum feritin concentration. The model predicted 23% of serum ferritin concentration variation in our subjects (Table 3). Male sex and hepatitis C seropositivity had independent, significant correlations with both serum ALT and AST concentrations. The models predicted 28% and 31% of the variation in ALT and AST, respectively (Table 4). We did not observe any statistically significant relationships of BMI, ferroportin Q248H, erythrocyte sedimentation rate, or dietary iron with serum ferritin, AST or ALT concentrations.
Table 3.
Beta (and P value) of predictors for log serum ferritin concentration from a multivariate linear regression model. (Model excludes two cases with high estimated caloric intake and 6 cases with history of HIV seropositivity)
| Predictor* | Beta (P value) |
|---|---|
| Sex (male) | 0.40 (0.041) |
| Log alcohol intake (g per day) | 0.06 (0.021) |
| Log ALT concentration (U/L) | 0.60 (0.0001) |
| Constant | 2.21 (0.0001) |
| Model R2 | 0.23 |
Primary variables entered into the models were: sex, age, BMI, alcohol intake, heme and non-heme iron intake, erythrocyte sedimentation rate, ferroportin Q248H and ALT
Table 4.
Beta (and P values) of predictors for log AST and log ALT by multivariate linear regression. (Models exclude two cases with high estimated caloric intake and 6 cases with history of HIV)
| Predictor* | Log ALT | Log AST |
|---|---|---|
| Sex (male) | 0.24 (0.022) | 0.34 (0.0001) |
| HCV seropositivity | 0.60 (0.0001) | 0.55 (0.0001) |
| Constant | 2.94 (0.0001) | 2.00 (0.0001) |
| Model R2 | 0.28 | 0.31 |
Primary variables entered into the models were: sex, alcohol intake, heme and non-heme iron intake, age, erythrocyte sedimentation rate, BMI, ferroportin Q248H, HCV
Discussion
The present study investigated the influence of alcohol consumption and dietary iron content on iron stores as assessed by serum ferritin concentration and on hepatic function as assessed by AST and ALT concentrations. The overall prevalence of ferroportin Q248H of 11% among inner-city African Americans from the community in the present study (Table 1) was slightly higher than the prevalence of 5–7% in African Americans that has been reported in other studies (Gordeuk et al, 2005; Rivers et al, 2007; Wrede et al, 2006). The prevalence was significantly higher in participants who consumed ≥56 g per day (23.3%) than those who consumed lesser amounts (7.5%) (P = 0.014; Figure 1). Conceivable reasons for the higher prevalence of ferroportin Q248H with greater degree of alcohol consumption are that this mutation may increase alcohol metabolism, provide a survival advantage for heavy alcohol drinkers and/or protect from toxicities that limit persistent heavy alcohol consumption.
After considering the potential confounding effects of inflammation and hepatocellular damage on serum ferritin concentration, the present study provides evidence that greater alcohol consumption is associated with higher serum ferritin concentrations and therefore increased iron stores in African-American subjects with no history of HIV disease (Tables 2 and 3). However, we did not find an association between dietary heme iron and serum ferritin concentration in the group of African Americans studied, in contrast to observations of previous studies in different population groups (Fleming et al, 1998; Backstrand et al, 2002; Ramakrishnana et al, 2002; Liu et al, 2003). Also, serum ferritin concentration did not differ significantly according to ferroportin Q248H allele status. The mechanism of possible increased iron absorption with alcohol exposure in the present study does not appear to be related to ineffective erythropoiesis, for there were no positive associations of alcohol intake with serum concentrations of LDH, haptoglobin or bilirubin. Rather, recent studies suggest that increased iron absorption associated with alcohol ingestion may be related to suppression of hepcidin production by hepatocytes (Kohgo et al, 2005; Flanagan et al, 2007; Ohtake et al, 2007). The lack of a significant association of serum ferritin concentration with alcohol consumption in the subset of African-American women in the present study size may be related to the smaller sample size. The confounding effects of menstruation and childbearing on iron stores, which are difficult to quantify historically and to account for in statistical analyses, may also be factors. The lack of a significant association of serum ferritin concentration with ferroportin Q248H may be related to the small sample size and/or to potential complex effects of the variant allele on iron metabolism.
Serum concentrations of AST and ALT had significant associations with male sex and with hepatitis C seropositivity, but not with alcohol consumption in multivariate analysis (Table 4), underscoring previous observations that a minority of individuals in the population are highly vulnerable to the hepatic toxicity of alcohol (Maddrey, 2000). The reason for the inverse associations of estimated dietary heme iron intake with AST and ALT concentrations in the subset of African-American women studied is not clear, but this observation underscores that adverse effects of dietary heme iron were not observed in this study.
Limitations to the present study include that serum ferritin concentration is an indirect measure of iron status, that dietary estimates of alcohol and iron consumption were derived from a recall questionnaire and that relatively small numbers of women and ferroportin Q248H positive participants were evaluated.
In summary, the present study points to the possibility of a previously unrecognized association of the ferroportin Q248H allele with alcohol consumption of ≥0.66 g/kg per day. It seems possible that this association may reflect some effect of ferroportin Q248H in protecting from alcohol toxicity. In addition, our findings provide further evidence that alcohol consumption is associated with greater iron stores as reflected in serum ferritin concentration in African Americans. Further research is needed to confirm and clarify these associations on the epidemiologic level. Investigations into the effects of ferroportin Q248H, alcohol and iron on cellular iron metabolism and oxidative mechanisms are also in order.
Acknowledgments
Financial Support:
Supported in part by grant nos. P20 AA014643-03 and P50 AA11199 from NIAAA, grant no. 2 R25 HL003679-08 from NHLBI, and Howard University GCRC grant no 2MOI RR10284-10 from NCRR, NIH, Bethesda, MD.
Victor R. Gordeuk, MD designed the study, performed data analyses, and drafted the paper. Sharmin F. Diaz, RN contributed to study design, conducted the study and contributed to data analysis. Gladys O. Onojobi, MD contributed to data analysis. Ishmael Kasvosve, PhD contributed to study design, data analysis and drafting the paper. Zufan Debebe, MS contributed to the laboratory, Xiomei Niu, MD, Tatyana Ammosova, PhD, Amanuel Edossa, PhD and Sergei Nekhai, PhD contributed to the laboratory methods and drafting the paper. Jeremy M. Pantin, MBBS contributed to conducting the study and analyzing the data. Shigang Xiong, MD, PhD, Hidekazu Tsukamoto, DVM, PhD and Mehdi Nouraie, MD, PhD contributed in final statistical analysis. Robert E. Taylor, MD, PhD contributed to study design, data analysis and drafting the paper.
Contributor Information
Victor R. Gordeuk, Email: vgordeuk@howard.edu.
Sharmin F. Diaz, Email: sdiaz@howard.edu.
Gladys O. Onojobi, Email: gonojobi@hotmail.com.
Ishmael Kasvosve, Email: ikasvosve@medsch.uz.ac.zw.
Zufan Debebe, Email: zufandebebe@yahoo.com.
Amanuel Edossa, Email: aedossa@hotmail.com.
Jeremy M. Pantin, Email: jpantin@gmail.com.
Shigang Xiong, Email: shigangx@usc.edu.
Sergei Nekhai, Email: snekhai@howard.edu.
Mehdi Nouraie, Email: snouraie@howard.edu.
Hidekazu Tsukamoto, Email: hidekazu.tsukamoto@keck.usc.edu.
Robert E. Taylor, Email: rtaylor@howard.edu.
References
- Abboud S, Haile DJ. A novel mammalian iron-regulated protein involved in intracellular iron metabolism. J Biol Chem. 2000;275:19906–19912. doi: 10.1074/jbc.M000713200. [DOI] [PubMed] [Google Scholar]
- Aungst CW. Ferritin in body fluids. J Lab Clin Med. 1968;71:517. [PubMed] [Google Scholar]
- Backstrand JR, Allen LH, Black AK, de Mata M, Pelto GH. Diet and iron status of nonpregnant women in rural Central Mexico. Am J Clin Nutr. 2002 Jul;76(1):156–64. doi: 10.1093/ajcn/76.1.156. [DOI] [PubMed] [Google Scholar]
- Barton JC, Acton RT, Rivers CA, Bertoli LF, Gelbart T, West C, Beutler E. Genotypic and phenotypic heterogeneity of African Americans with primary iron overload. Blood Cells Mol Dis. 2003 Nov-Dec;31(3):310–9. doi: 10.1016/s1079-9796(03)00166-9. [DOI] [PubMed] [Google Scholar]
- Barton JC, Acton RT, Dawkins FW, Adams PC, Lovato L, Leiendecker-Foster C, McLaren CE, Reboussin DM, Speechley MR, Gordeuk VR, McLaren GD, Sholinsky P, Harris EL. Initial screening transferrin saturation values, serum ferritin concentrations, and HFE genotypes in whites and blacks in the Hemochromatosis and Iron Overload Screening Study. Genet Test. 2005 Fall;9(3):231–41. doi: 10.1089/gte.2005.9.231. [DOI] [PubMed] [Google Scholar]
- Beutler E, Barton JC, Felitti VJ, Gelbart T, West C, Lee PL, Waalen J, Vulpe C. Ferroportin 1 (SCL40A1) variant associated with iron overload in African-Americans. Blood Cells Mol Dis. 2003;31:305–9. doi: 10.1016/s1079-9796(03)00165-7. [DOI] [PubMed] [Google Scholar]
- Bothwell TH, Charlton RW, Cook JD, Finch CA. Iron Metabolism in Man. Oxford: Blackwell Scientific; 1979. [Google Scholar]
- Canonne-Hergaux F, Gruenheid S, Ponka P, Gros P. Cellular and subcellular localization of the Nramp2 iron transporter in the intestinal brush border and regulation by dietary iron. Blood. 1999;93:4406–4417. [PubMed] [Google Scholar]
- Conrad ME, Benjamin BI, Williams HL, Foy AL. Human absorption of hemoglobin-iron. Gastroenterology. 1967 Jul;53(1):5–10. [PubMed] [Google Scholar]
- Cook JD. Adaptation in iron metabolism. Am J Clin Nutr. 1990 Feb;51(2):301–8. doi: 10.1093/ajcn/51.2.301. [DOI] [PubMed] [Google Scholar]
- Di Bisceglie AM, Axiotis CA, Hoofnagle JH, Bacon BR. Measurements of iron status in patients with chronic hepatitis. Gastroenterology. 1992;102:2108–13. doi: 10.1016/0016-5085(92)90339-z. [DOI] [PubMed] [Google Scholar]
- Donovan A, Brownlie A, Zhou Y, Shepard J, Pratt SJ, Moynihan J, Paw BH, Drejer A, Barut B, Zapata A, Law TC, Brugnara C, Lux SE, Pinkus GS, Pinkus JL, Kingsley PD, Palis J, Fleming MD, Andrews NC, Zon LI. Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature. 2000;403:776–781. doi: 10.1038/35001596. [DOI] [PubMed] [Google Scholar]
- Drakesmith H, Schimanski LM, Ormerod E, Merryweather-Clarke AT, Viprakasit V, Edwards JP, et al. Resistance to hepcidin is conferred by hemochromatosis-associated mutations of ferroportin. Blood. 2005;106:1092–1097. doi: 10.1182/blood-2005-02-0561. [DOI] [PubMed] [Google Scholar]
- Flanagan JM, Peng H, Beutler E. Effects of alcohol consumption on iron metabolism in mice with hemochromatosis mutations. Alcohol Clin Exp Res. 2007 Jan;31(1):138–43. doi: 10.1111/j.1530-0277.2006.00275.x. [DOI] [PubMed] [Google Scholar]
- Fleming DJ, Jacques PF, Dallal GE, Tucker KL, Wilson PW, Wood RJ. Dietary determinants of iron stores in a free-living elderly population: The Framingham Heart Study. Am J Clin Nutr. 1998 Apr;67(4):722–33. doi: 10.1093/ajcn/67.4.722. [DOI] [PubMed] [Google Scholar]
- Fleming DJ, Tucker KL, Jacques PF, Dallal GE, Wilson PW, Wood RJ. Dietary factors associated with the risk of high iron stores in the elderly Framingham Heart Study cohort. Am J Clin Nutr. 2002 Dec;76(6):1375–84. doi: 10.1093/ajcn/76.6.1375. [DOI] [PubMed] [Google Scholar]
- Fletcher LM, Halliday JW, Powell LW. Interrelationships of alcohol and iron in liver disease with particular reference to the iron-binding proteins, ferritin and transferrin. J Gastroenterol Hepatol. 1999;14:202–214. doi: 10.1046/j.1440-1746.1999.01836.x. [DOI] [PubMed] [Google Scholar]
- Fletcher LM, Powell LW. Hemochromatosis and alcoholic liver disease. Alcohol. 2003 Jun;30(2):131–6. doi: 10.1016/s0741-8329(03)00128-9. [DOI] [PubMed] [Google Scholar]
- Friedman BM, Baynes RD, Bothwell TH, Gordeuk VR, Macfarlane BJ, Lamparelli RD, Robinson EJ, Sher R, Hamberg S. Dietary iron overload in southern African rural blanks. S Afr Med J. 1990;78:301–305. [PubMed] [Google Scholar]
- Gordeuk VR, Boyd RD, Brittenham GM. Dietary iron overload persists in rural sub Sahara Africa. Lancet. 1986;1:1310–1313. doi: 10.1016/s0140-6736(86)91230-4. [DOI] [PubMed] [Google Scholar]
- Gordeuk VR, Mukiibi J, Hasstedt SJ, Samowitz W, Edwards CQ, West G, Ndambire S, Emmanual J, Nkanza N, Chapanduka Z, Randall M, Boone P, Romano P, Martell RW, Yamashita T, Effler P, Brittenham G. Iron overload in Africa: interaction between a gene and dietary iron content. N Engl J Med. 1992;326:95–100. doi: 10.1056/NEJM199201093260204. [DOI] [PubMed] [Google Scholar]
- Gordeuk VR, Caleffi A, Corradini E, Ferrara F, Jones RA, Castro O, Onyekwere O, Kittles R, Pignatti E, Montosi G, Garuti C, Gangaidzo IT, Gomo ZA, Moyo VM, Rouault TA, MacPhail P, Pietrangelo A. Iron overload in Africans and African-Americans and a common mutation in the SCL 40A1 (ferroportin 1) gene. Blood Cells Mol Dis. 2003;31:299–304. doi: 10.1016/s1079-9796(03)00164-5. [DOI] [PubMed] [Google Scholar]
- Gottschalk R, Wigand R, Dietrich C, Oremek G, Liebisch F, Hoelzer D, Kaltwasser JP. Total iron binding capacity and serum transferrin determination under the influence of several clinical conditions. Clim Chim Acta. 2000;293:127–138. doi: 10.1016/s0009-8981(99)00242-9. [DOI] [PubMed] [Google Scholar]
- Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL, Hediger MA. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388:482–488. doi: 10.1038/41343. [DOI] [PubMed] [Google Scholar]
- Kasvosve I, Gomo ZA, Nathoo KJ, Matibe P, Mudenge B, Loyevsky M, Gordeuk VR. Effect of ferroportin Q248H polymorphism on iron status in African children. Am J Clin Nutr. 2005 Nov;82:1102–6. doi: 10.1093/ajcn/82.5.1102. [DOI] [PubMed] [Google Scholar]
- Kohgo Y, Ohtake T, Ikuta K, Suzuki Y, Hosoki Y, Saito H, Kato J. Iron accumulation in alcoholic liver diseases. Alcohol Clin Exp Res. 2005 Nov;29(11 Suppl):189S–93S. doi: 10.1097/01.alc.0000189274.00479.62. Review. [DOI] [PubMed] [Google Scholar]
- Leggett BA, Brown NN, Bryant SJ, Duplock L, Powell LW, Halliday JW. Factors affecting the concentrations of ferritin in serum in a healthy Australian population. Clin Chem. 1990;36:1350. [PubMed] [Google Scholar]
- Liu JM, Hankinson SE, Stampfer MJ, Rifai N, Willett WC, Ma J. Body iron stores and their determinants in healthy postmenopausal US women. Am J Clin Nutr. 2003 Dec;78(6):1160–7. doi: 10.1093/ajcn/78.6.1160. [DOI] [PubMed] [Google Scholar]
- Maddrey WC. Alcohol-induced liver disease. Clin Liver Dis. 2000 Feb;4(1):115–31. vii. doi: 10.1016/s1089-3261(05)70099-4. Review. [DOI] [PubMed] [Google Scholar]
- McGregor JA, Shayeghi M, Vulpe CD, Anderson GJ, Pietrangelo A, Simpson RJ, McKie AT. Impaired iron transport activity of ferroportin 1 in hereditary iron overload. J Membr Biol. 2005 Jul;206(1):3–7. doi: 10.1007/s00232-005-0768-1. [DOI] [PubMed] [Google Scholar]
- McKie AT, Marciani P, Rolfs A, Brennan K, Wehr K, Barrow D, Miret S, Bomford A, Peters TJ, Farzaneh F, Hediger MA, Hentze MW, Simpson RJ. A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Molecular Cell. 2000;5:299–309. doi: 10.1016/s1097-2765(00)80425-6. [DOI] [PubMed] [Google Scholar]
- McNamara L, Gordeuk VR, MacPhail AP. Ferroportin (Q248H) mutation in African families with dietary iron overload. J Gastroenterol Hepatol. 2005 Dec;20(12):1855–8. doi: 10.1111/j.1440-1746.2005.03930.x. [DOI] [PubMed] [Google Scholar]
- Meyer TE, Kassianides C, Bothwell TH, Green A. Effects of heavy alcohol consumption on serum ferritin concentrations. S Afr Med J. 1984;66:573. [PubMed] [Google Scholar]
- Milman M, Kirchoff M. Relationship between serum ferritin, alcohol intake, and social status in 2235 Danish men and women. Ann Hematol. 1996;72:145–151. doi: 10.1007/s002770050153. [DOI] [PubMed] [Google Scholar]
- Moyo VM, Gangaidzo IT, Gomo ZAR, Khumalo H, Saungweme T, Kiire CF, Rouault T, Gordeuk VR. Traditional beer consumption and the iron status of spouse pairs from a rural community in Zimbabwe. Blood. 1997;89:2157–66. [PubMed] [Google Scholar]
- Moyo VM, Mandishona E, Hasstedt SJ, Gangaidzo IT, Gomo A, Khumalo H, Saungweme T, Kiire CF, Paterson AC, Bloom P, MacPhail AP, Rouault T, Gordeuk VR. Evidence of genetic transmission in African iron overload. Blood. 1998;91:1076–1082. [PubMed] [Google Scholar]
- Ohtake T, Saito H, Hosoki Y, Inoue M, Miyoshi S, Suzuki Y, Fujimoto Y, Kohgo Y. Hepcidin is down-regulated in alcohol loading. Alcohol Clin Exp Res. 2007 Jan;31(1 Suppl):S2–8. doi: 10.1111/j.1530-0277.2006.00279.x. [DOI] [PubMed] [Google Scholar]
- Prieto J, Barry M, Sherlock S. Serum ferritin in patients with iron overload and with acute and chronic liver disease. Gastroenterol. 1975;68:525–533. [PubMed] [Google Scholar]
- Ramakrishnan U, Frith-Terhune A, Cogswell M, Kettel Khan L. Dietary intake does not account for differences in low iron stores among Mexican American and non-Hispanic white women: Third National Health and Nutrition Examination Survey, 1988–1994. J Nutr. 2002 May;132(5):996–1001. doi: 10.1093/jn/132.5.996. [DOI] [PubMed] [Google Scholar]
- Reissmann KR, Diedrich MR. On the presence of ferritin in the peripheral blood of patients with hepatocellular disease. J Clin Invest. 1956;35:588. doi: 10.1172/JCI103312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivers CA, Barton JC, Gordeuk VR, Acton RT, Speechley MR, Snively BM, Leiendecker-Foster C, Press RD, Adams PC, McLaren GD, Dawkins FW, McLaren CE, Reboussin DM. Association of ferroportin Q248H polymorphism with elevated levels of serum ferritin in African Americans in the Hemochromatosis and Iron Overload Screening (HEIRS) Study. Blood Cells Mol Dis. 2007;38:247–252. doi: 10.1016/j.bcmd.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimanski LM, Drakesmith H, Merryweather-Clarke AT, Viprakasit V, Edwards JP, Sweetland E, Bastin JM, Cowley D, Chinthammitr Y, Robson KJ, Townsend AR. In vitro functional analysis of human ferroportin (FPN) and hemochromatosis-associated FPN mutations. Blood. 2005;105:4096–4102. doi: 10.1182/blood-2004-11-4502. [DOI] [PubMed] [Google Scholar]
- Shayeghi M, Latunde-Dada GO, Oakhill JS, Laftah AH, Takeuchi K, Halliday N, Khan Y, Warley A, McCann FE, Hider RC, Frazer DM, Anderson GJ, Vulpe CD, Simpson RJ, McKie AT. Identification of an intestinal heme transporter. Cell. 2005;22:789–801. doi: 10.1016/j.cell.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Stram DO, Hankin JH, Wilkens LR, Pike MC, Monroe KR, Park S, Henderson BE, Nomura AM, Earle ME, Nagamine FS, Kolonel LN. Calibration of the dietary questionnaire for a multiethnic cohort in Hawaii and Los Angeles. Am J Epidemiol. 2000;151:358–70. doi: 10.1093/oxfordjournals.aje.a010214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y, Saito H, Suzuki M, Hosoki Y, Sakurai S, Fujimoto AY, Kohgo Y. Up-regulation of Transferrin Receptor Expression in Hepatocytes by Habitual Alcohol Drinking Is Implicated in Hepatic Iron Overload in Alcoholic Liver Disease. Alcohol Clin Exp Res. 2002;26:26S–31S. doi: 10.1097/01.ALC.0000026830.27338.23. [DOI] [PubMed] [Google Scholar]
- Tsukamoto H, Horne W, Kamimura S, Niemela O, Parkkila S, Yla-Herttuala S, Brittenham GM. Experimental liver cirrhosis induced by alcohol and iron. J Clin Invest. 1995 Jul;96(1):620–30. doi: 10.1172/JCI118077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheby MS, Suttle GE, Ford KT., 3rd Intestinal absorption of hemoglobin iron. Gastroenterology. 1970 May;58(5):647–54. [PubMed] [Google Scholar]
- Wrede CE, Buettner R, Bollheimer LC, Scholmerich J, Palitzsch KD, Hellerbrand C. Association between serum ferritin and the insulin resistance syndrome in a representative population. Eur J Endocrinol. 2006 Feb;154(2):333–40. doi: 10.1530/eje.1.02083. [DOI] [PubMed] [Google Scholar]