Abstract
We performed a population-based study to examine the influence of healthcare-associated acquisition on pathogen distribution, antimicrobial resistance, short-and long-term mortality of community-onset Gram-negative bloodstream infections (BSI). We identified 733 unique patients with community-onset Gram-negative BSI (306 healthcare-associated and 427 community-acquired) among Olmsted County, Minnesota, residents from 1 January 1998 to 31 December 2007. Multivariate logistic regression was used to examine the association between healthcare-associated acquisition and microbiological etiology and antimicrobial resistance. Multivariate Cox proportional hazards regression was used to evaluate the influence of the site of acquisition on mortality. Healthcare-associated acquisition was predictive of Pseudomonas aeruginosa (odds ratio [OR] 3.14, 95% confidence intervals [CI]: 1.59–6.57) and the group of Enterobacter, Citrobacter, and Serratia species (OR 2.23, 95% CI: 1.21–4.21) as causative pathogens of community-onset Gram-negative BSI. Healthcare-associated acquisition was also predictive of fluoroquinolone resistance among community-onset Gram-negative bloodstream isolates (OR 2.27, 95% CI: 1.18–4.53). Healthcare-associated acquisition of BSI was independently associated with higher 28-day (hazard ratio [HR] 3.73, 95% CI: 2.13–6.93) and 1-year mortality (HR 3.60, 95% CI: 2.57–5.15). Because of differences in pathogen distribution, antimicrobial resistance, and outcomes between healthcare-associated and community-acquired Gram-negative BSI, identification of patients with healthcare-associated acquisition of BSI is essential to optimize empiric antimicrobial therapy.
Introduction
Traditionally, bloodstream infections (BSI) have been classified according to the site of acquisition as either community- or hospital-acquired (nosocomial) [1]. Owing to rapidly evolving changes within the healthcare model i.e., shortened hospital stays, increasing utilization of ambulatory care interventional services, including surgical procedures, outpatient parenteral antimicrobial therapy, and chemotherapy administration, a revision in the traditional designations of infection site of acquisition seemed necessary [2]. The universal adoption of a new classification strategy, however, has not occurred.
The clinical features of healthcare-associated BSI have been well defined for cases due to Gram-positive cocci, particularly Staphylococcus aureus, and differences in outcomes and antimicrobial resistance between community-acquired and healthcare-associated infection have been demonstrated [2, 3]. In contrast, the characteristics of healthcare-associated BSI due to Gram-negative bacilli have not been clearly defined. To our knowledge, a single investigation from a large tertiary-care center has evaluated healthcare-associated Gram-negative BSI [4]. The overwhelming majority of patients in that study had healthcare-associated BSI and only a few patients had community-acquired BSI, likely because of the referral status of the patient population in that study. Moreover, the small sample size did not allow detection of differences in outcomes or antimicrobial resistance between community-acquired and healthcare-associated Gram-negative BSI.
Therefore, we identified a large population-based cohort of patients with community-onset Gram-negative BSI in Olmsted County, Minnesota, and:
Determined the incidence rates of community-acquired and healthcare-associated Gram-negative BSI by age, sex, and calendar year
Examined the influence of healthcare-associated acquisition on pathogen distribution, fluoroquinolone and third-generation cephalosporin resistance among bloodstream isolates
Compared the 28-day and 1-year all-cause mortality rates in patients with healthcare-associated and community-acquired Gram-negative BSI and defined risk factors associated with mortality
Because the clinical distinction between nosocomial and community-onset Gram-negative BSI has been clearly established [5, 6], we did not include nosocomial BSI in this investigation.
Materials and methods
Setting
Olmsted County is located in southeastern Minnesota and has a population of 124,277 according to the 2000 census [7]. With the exception of a lower prevalence of persons who inject illegal drugs, a higher prevalence of middle-class persons, and a higher proportion of persons employed in the healthcare industry, the population characteristics of Olmsted County residents are similar to those of USA non-Hispanic whites [8, 9]. The Rochester Epidemiology Project (REP) is a unique medical records-linkage system that encompasses care delivered to residents of Olmsted County, Minnesota. The microbiology laboratories at the Mayo Medical Center and the Olmsted Medical Center are the only two laboratories in Olmsted County. These two medical centers are geographically isolated from other urban centers, as previously described [8, 10, 11], which increases the likelihood that residents obtain their healthcare at the local facilities rather than seeking healthcare at a distant location geographically.
Definitions
Gram-negative BSI was defined as the growth of any aerobic Gram-negative bacillus in a blood culture. Monomicrobial Gram-negative BSI was defined as the growth of only one species of Gram-negative bacillus in a blood culture and polymicrobial BSI was defined as the growth of more than one microorganism in a blood culture, excluding coagulase-negative staphylococci, Corynebacterium spp. and Propionibacterium spp. The growth of one of the latter three microorganisms in a blood culture in addition to Gram-negative bacilli was considered to constitute skin contamination, rather than polymicrobial BSI.
Cases were classified according to infection site of acquisition into nosocomial, healthcare-associated, and community-acquired, as previously defined [2]. Briefly, nosocomial BSI was defined as a positive blood culture obtained from patients who had been hospitalized for 48 h or longer. The remaining BSI were classified as community-onset and were further divided into healthcare-associated and community-acquired BSI. Healthcare-associated BSI was defined as BSI acquired outside the hospital or within 48 h of hospital admission in the setting of recent contact with the healthcare system (residents in a nursing home or long-term care facility, those who received intravenous therapy, including antimicrobial agents and chemotherapy, or underwent hemodialysis within 30 days of BSI, and those hospitalized for 2 or more days within 90 days of BSI). Community-acquired BSI was defined as BSI that did not meet the criteria for either nosocomial or healthcare-associated definitions.
The primary source of BSI was defined using the Centers for Disease Control and Prevention criteria [1]. AmpC β-lactamase-producing Enterobacteriaceae included Enterobacter, Citrobacter, and Serratia species [12]. Fluoroquinolone-resistant Gram-negative bloodstream isolates were defined as isolates that were resistant (minimum inhibitory concentration [MIC]≥4 μg/mL) or had intermediate in vitro susceptibility (MIC=2 μg/mL) to ciprofloxacin. Gram-negative bloodstream isolates were considered resistant to third-generation cephalosporins if they were resistant (MIC≥32 μg/mL) or had intermediate in vitro susceptibility (MIC=16 μg/mL) to ceftazidime.
Case ascertainment
All residents of Olmsted County, Minnesota, were eligible for inclusion as we used complete enumeration of the Olmsted County population from 1 January 1998 to 31 December 2007. After the institutional review boards of the Mayo Medical Center in Rochester, Minnesota, and the Olmsted Medical Center approved the study, we used the microbiology laboratory databases at both institutions to identify all patients with Gram-negative BSI during the study period. Using the REP tools, we identified residents of Olmsted County, Minnesota, for inclusion in the study and excluded those who lived outside Olmsted County at the time of BSI. We included patients with first episodes of monomicrobial BSI due to Gram-negative bacilli and excluded polymicrobial, nosocomial, and recurrent episodes of BSI to ensure that cases were independent of each other. The primary investigator (M. N.A.) reviewed the medical records of all patients to confirm the diagnosis, determine patient residency status and obtain clinical features, outcome, and in vitro antimicrobial susceptibility results.
Blood cultures were processed using standard microbiology techniques according to the Clinical and Laboratory Standards Institute (CLSI). The detailed blood culture methods used were described elsewhere [13, 14]. CLSI methods were employed to evaluate in vitro antimicrobial susceptibility results of Gram-negative isolates. The microbiology laboratories at the Mayo Medical Center in Rochester, Minnesota, and the Olmsted Medical Center are certified by the College of American Pathologists.
Statistical analysis
Descriptive statistics were used to summarize the data: medians and interquartile range (IQR) for continuous variables and counts and percentages for categorical variables. Chi-squared test was used to evaluate associations between categorical variables and the Wilcoxon rank-sum test was used to test for differences in medians across continuous variables.
The incidence rate, expressed as the number of patients with Gram-negative BSI per 100,000 person-years, was calculated assuming that the entire population of Olmsted County was at risk of BSI. The 2000 Olmsted County census figures were used to compute the age- and gender-specific person-years denominator with a projected population growth rate after 2000 of 1.9% per year. Age was categorized into five groups (0–18, 19–39, 40–59, 60–79, and ≥ 80 years) and the 10-year study period was categorized into five 2-year intervals (1998–1999, 2000–2001, 2002–2003, 2004–2005, and 2006–2007). The incidence rates of community-acquired and healthcare-associated Gram-negative BSI were directly adjusted to the USA 2000 white population [7]. The 95% confidence intervals (CI) for the incidence rate were estimated using a Poisson distribution.
Multivariate logistic regression analysis was used to identify independent predictors of P. aeruginosa, AmpC β-lactamase-producing Enterobacteriaceae, fluoroquinolone-and third-generation cephalosporin-resistant Gram-negative bacilli as causative agents of Gram-negative BSI. Variables were included in a multivariate logistic regression model if the p value was ≤ 0.10 in the univariate model. Odds ratios (OR) with 95% CI were calculated to indicate the strength of association between each variable and the causative pathogen. Logistic regression was also used to test for a linear change in antimicrobial resistance among healthcare-associated and community-acquired Gram-negative bloodstream isolates throughout the 10-year study period (divided into five 2-year intervals).
The Kaplan–Meier method was used to estimate the 28-day and 1-year all-cause mortality rates following healthcare-associated and community-acquired Gram-negative BSI. Patients were followed from the date of Gram-negative BSI until death or last healthcare encounter. Death was confirmed by reviewing medical records and Minnesota death registry database. Patients lost to follow-up were censored on the date of their last healthcare encounter. Cox proportional hazard regression was used to identify risk factors for 28-day and 1-year all-cause mortality. To identify independent risk factors for mortality, variables were included in a multivariate Cox model if the p value for a univariate association with mortality was ≤ 0.10. The proportional hazards assumption was evaluated by plotting the log integrated hazard versus time from the Kaplan–Meier method. Hazard ratios (HR) with 95% CI were presented to demonstrate the strength of association between each factor and mortality.
The following variables were considered for inclusion in the logistic regression and Cox proportional hazard regression models: age (as a continuous variable), sex (male vs female), year of diagnosis (as a continuous variable), primary source of infection (urinary vs non-urinary), and site of infection acquisition (healthcare-associated vs community-acquired). JMP (version 8.0, SAS Institute Inc, Cary, NC, USA) was used for statistical analysis. The level of significance for statistical testing was defined as p< 0.05 (two-sided) unless otherwise specified.
Results
Demographic and clinical features
Of the 733 first episodes of community-onset monomicrobial Gram-negative BSI during the 10-year study period, 306 (41.7%) were healthcare-associated and 427 (58.3%) were community-acquired. The median age of patients with healthcare-associated infection was 73 years (interquartile range [IQR]: 51–83); and 168 (54.9%) were female. Patients with community-acquired Gram-negative BSI had a median age of 66 years (IQR: 45–79) and 253 (59.3%) were female. The urinary tract was the most common primary source of infection in both community-acquired and healthcare-associated Gram-negative BSI. However, patients with community-acquired BSI were more likely than those with healthcare-associated BSI to have the primary source of infection in the urinary tract (65.8% vs 54.2%; Table 1). In contrast, patients with healthcare-associated BSI were more likely to have an unknown primary source of infection than patients with community-acquired BSI (20.3% vs 11.7%).
Table 1.
Clinical characteristics of patients with Gram-negative bloodstream infection based on site of acquisition
| Variable | Healthcare-associated N=306 |
Community-acquired N=427 |
p value* |
|---|---|---|---|
| Age: median (IQR) | 73 (51–83) | 66 (45–79) | <0.001 |
| Female sex, n (%) | 168 (54.9) | 253 (59.3) | 0.24 |
| Primary source, n (%) | <0.001 | ||
| Urinary tract | 166 (54.2) | 281 (65.8) | |
| Gastrointestinal tract | 29 (9.5) | 61 (14.3) | |
| Respiratory tract | 26 (8.5) | 22 (5.2) | |
| Skin and soft tissue | 10 (3.3) | 9 (2.1) | |
| Central venous catheter-related | 10 (3.3) | 0 (0) | |
| Other | 3 (1.0) | 4 (0.9) | |
| Unknown | 62 (20.3) | 50 (11.7) | |
| Microbiology, n (%) | <0.001 | ||
| Escherichia coli | 145 (47.4) | 271 (63.5) | |
| Klebsiella pneumoniae | 45 (14.7) | 48 (11.2) | |
| Pseudomonas aeruginosa | 28 (9.2) | 12 (2.8) | |
| Proteus mirabilis | 13 (4.2) | 11 (2.6) | |
| Enterobacter species | 20 (6.5) | 8 (1.9) | |
| Citrobacter species | 6 (2.0) | 4 (0.9) | |
| Serratia species | 5 (1.6) | 6 (1.4) | |
| Other | 44 (14.4) | 67 (15.7) | |
| Fluoroquinolone resistancea | 24/293 (8.2) | 15/406 (3.7) | 0.01 |
| Third-generation cephalosporin resistancea | 13/293 (4.4) | 11/405 (2.7) | 0.22 |
IQR: interquartile range, CI: confidence intervals
Chi-squared test was used to calculate p values for categorical variables and Wilcoxon rank-sum test for continuous variables
Among isolates with available in vitro antimicrobial susceptibility results to fluoroquinolones and third-generation cephalosporins
Epidemiology
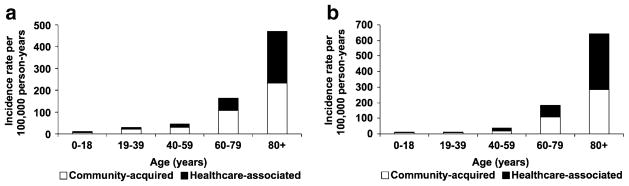
The overall age- and sex-adjusted incidence rate of community-onset Gram-negative BSI was 65.7 (95% CI: 60.9–70.5) per 100,000 person-years, including 27.7 (95% CI: 24.5–30.8) per 100,000 person-years for healthcare-associated and 38.0 (95% CI: 34.4–41.6) per 100,000 person-years for community-acquired Gram-negative BSI. The age-adjusted incidence rates of healthcare-associated and community-acquired Gram-negative BSI per 100,000 person-years were 25.8 (95% CI: 21.9–29.8) and 39.8 (95% CI: 34.9–44.8) respectively in female subjects; and 31.2 (95% CI: 25.8–36.6) and 36.9 (95% CI: 31.3–42.6) respectively in male subjects. The incidence rates of healthcare-associated and community-acquired Gram-negative BSI increased with age in both female (Fig. 1a) and male subjects (Fig. 1b). There was no apparent change in the age- and sex-adjusted incidence rate of healthcare-associated and community-acquired Gram-negative BSI throughout the 10-year study period (Fig. 2).
Fig. 1.
Incidence rate of community-onset Gram-negative bloodstream infection by age and site of acquisition in a female subjects and b male subjects, 1998–2007
Fig. 2.
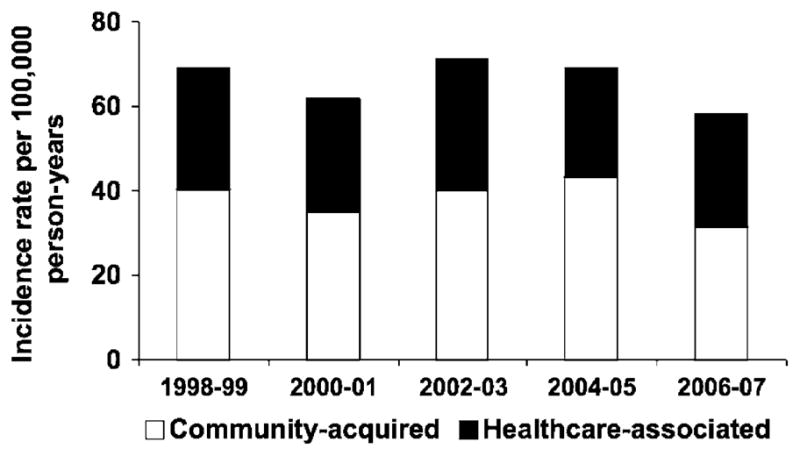
Age- and sex-adjusted incidence rate of community-onset Gram-negative bloodstream infection by calendar year and site of acquisition
Microbiology
Escherichia coli was the predominant cause of both healthcare-associated and community-acquired Gram-negative BSI; it was, however, more common among community-acquired than in healthcare-associated BSI (63.5% vs 47.4%; Table 1). In contrast, P. aeruginosa was a more common cause of Gram-negative BSI among patients with healthcare-associated BSI than those with community-acquired BSI (9.2% vs 2.8%). After adjustment for sex and primary source of infection in a multivariate logistic regression model, patients with healthcare-associated BSI were nearly three times more likely than those with community-acquired BSI to have P. aeruginosa as an etiology of Gram-negative BSI (OR 3.14, 95% CI: 1.59–6.57; Table 2).
Table 2.
Predictors of the causative pathogen and antimicrobial resistance among community-onset Gram-negative bloodstream infections
| Variable | Univariate model
|
Multivariate model
|
||
|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Pseudomonas aeruginosa | ||||
| Age (per year) | 1.00 (0.99–1.01) | 0.98 | – | – |
| Sex: male vs female | 2.97 (1.54–6.04) | 0.002 | 2.38 (1.20–4.94) | 0.02 |
| Year of diagnosis (per year) | 0.96 (0.86–1.08) | 0.54 | – | – |
| Source of infection: urinary tract vs other | 0.29 (0.14–0.56) | <0.001 | 0.38 (0.18–0.76) | 0.007 |
| Site of acquisition: HCA vs CA | 3.48 (1.78–7.22) | <0.001 | 3.14 (1.59–6.57) | 0.001 |
| AmpC-producing Enterobacteriaceae | ||||
| Age (per year) | 1.00 (0.98–1.01) | 0.59 | – | – |
| Sex: male vs female | 2.72 (1.50–5.09) | 0.001 | 2.18 (1.18–4.18) | 0.02 |
| Year of diagnosis (per year) | 1.19 (1.07–1.34) | 0.002 | 1.21 (1.08–1.36) | 0.002 |
| Source of infection: urinary tract vs other | 0.26 (0.13–0.47) | <0.001 | 0.31 (0.16–0.58) | <0.001 |
| Site of acquisition: HCA vs CA | 2.56 (1.42–4.75) | 0.002 | 2.23 (1.21–4.21) | 0.01 |
| Fluoroquinolone resistance | ||||
| Age (per year) | 1.01 (0.99–1.02) | 0.41 | – | – |
| Sex: male vs female | 1.82 (0.95–3.54) | 0.07 | 1.73 (0.90–3.40) | 0.10 |
| Year of diagnosis (per year) | 1.23 (1.08–1.40) | 0.002 | 1.22 (1.08–1.39) | 0.003 |
| Source of infection: urinary tract vs other | 1.47 (0.74–3.14) | 0.29 | – | – |
| Site of acquisition: HCA vs CA | 2.33 (1.21–4.61) | 0.01 | 2.19 (1.13–4.38) | 0.02 |
| Third-generation cephalosporin resistance | ||||
| Age (per year) | 0.98 (0.96–0.99) | 0.007 | 0.98 (0.97–1.00) | 0.02 |
| Sex: male vs female | 0.67 (0.27–1.55) | 0.37 | – | – |
| Year of diagnosis (per year) | 0.99 (0.86–1.15) | 0.92 | – | – |
| Source of infection: urinary tract vs other | 0.14 (0.05–0.35) | <0.001 | 0.15 (0.05–0.39) | <0.001 |
| Site of acquisition: HCA vs CA | 1.66 (0.73–3.84) | 0.22 | – | – |
OR: odds ratio, CI: confidence interval, HCA: healthcare-associated, CA: community-acquired
Similarly, healthcare-associated site of acquisition was also predictive of BSI due to AmpC β-lactamase-producing Enterobacteriaceae, including Enterobacter, Citrobacter, and Serratia species (OR 2.23, 95% CI: 1.21–4.21), after adjustment for sex, primary source of infection, and year of diagnosis (Table 2).
In vitro antimicrobial resistance
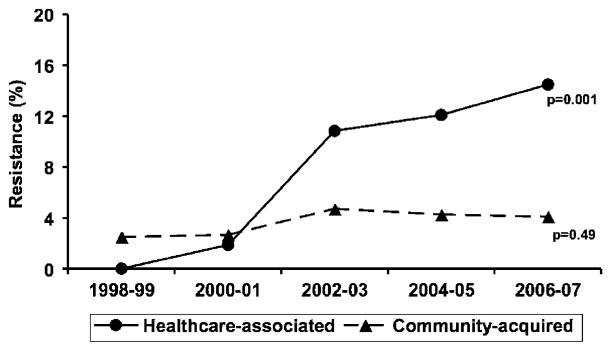
Among healthcare-associated and community-acquired Gram-negative bloodstream isolates that were tested for in vitro fluoroquinolone susceptibility, 8.2% and 3.7% respectively were resistant to fluoroquinolones (Table 1). Healthcare-associated acquisition was independently associated with fluoroquinolone resistance (OR 2.19, 95% CI: 1.13–4.38), after adjustment for sex and calendar year (Table 2). There was a linear increase in fluoroquinolone resistance among healthcare-associated Gram-negative bloodstream isolates between 1998 and 2007 (Fig. 3).
Fig. 3.
In vitro fluoroquinolone resistance rates of community-onset Gram-negative bloodstream isolates, by calendar year and site of acquisition. p value denotes a one-degree of freedom test for a linear trend using logistic regression
On the other hand, 4.4% and 2.7% of the healthcare-associated and community-acquired Gram-negative bloodstream isolates tested were resistant to third-generation cephalosporins (Table 1). There was no linear change in third-generation cephalosporin resistance during the study period; and healthcare-associated acquisition was not associated with third-generation cephalosporin resistance (OR 1.66, 95% CI: 0.73–3.84; Table 2).
Mortality
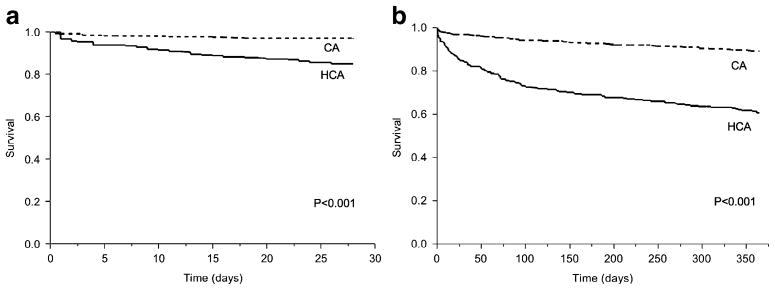
Only 5 patients (0.7%) were lost to follow-up within 28 days and 62 (8.5%) were lost to follow-up within 1 year of community-onset Gram-negative BSI. Patients with healthcare-associated Gram-negative BSI had a higher 28-day all-cause mortality than did those with community-acquired Gram-negative BSI (15.4% vs 3.5%; Fig. 4a). In a multivariate Cox proportional hazard regression model, increasing age and healthcare-associated acquisition were associated with higher 28-day all-cause mortality, whereas a urinary source of BSI was associated with lower 28-day mortality (Table 3).
Fig. 4.
Kaplan–Meier a 28-day and b 1-year survival curves of patients with community-onset Gram-negative bloodstream infection by site of acquisition, 1998–2007. CA: community-acquired, HCA: healthcare-associated. p value denotes a difference in survival using the log-rank test
Table 3.
Factors associated with 28-day and 1-year all-cause mortality in patients with community-onset Gram-negative bloodstream infection
| Variable | Univariate model
|
Multivariate model
|
||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| 28-day mortality | ||||
| Age (per year) | 1.02 (1.01–1.03) | 0.003 | 1.02 (1.01–1.03) | 0.003 |
| Sex: male vs female | 1.35 (0.82–2.23) | 0.23 | – | – |
| Year of diagnosis (per year) | 0.94 (0.86–1.03) | 0.18 | – | – |
| Source of infection: urinary tract vs other | 0.27 (0.15–0.45) | <0.001 | 0.27 (0.16–0.47) | <0.001 |
| Site of acquisition: HCA vs CA | 4.61 (2.64–8.53) | <0.001 | 3.73 (2.13–6.93) | <0.001 |
| 1–year mortality | ||||
| Age (per year) | 1.03 (1.02–1.04) | <0.001 | 1.03 (1.02–1.03) | <0.001 |
| Sex: male vs female | 1.45 (1.06–1.97) | 0.02 | 1.23 (0.90–1.68) | 0.19 |
| Year of diagnosis (per year) | 0.95 (0.90–1.00) | 0.06 | 0.93 (0.88–0.99) | 0.02 |
| Source of infection: urinary tract vs other | 0.34 (0.24–0.46) | <0.001 | 0.34 (0.24–0.47) | <0.001 |
| Site of acquisition: HCA vs CA | 4.37 (3.12–6.22) | <0.001 | 3.67 (2.61–5.24) | <0.001 |
HR: hazard ratio, CI: confidence interval, HCA: healthcare-associated, CA: community-acquired
The 1-year all-cause mortality was also higher in patients with healthcare-associated Gram-negative BSI than in patients with community-acquired Gram-negative BSI (39.5% vs 11.0%; Fig. 4b). In a multivariate Cox proportional hazard regression model, increasing age and healthcare-associated acquisition were associated with higher 1-year all-cause mortality, whereas a urinary source and a more recent diagnosis of BSI were associated with lower 1-year mortality (Table 3).
Discussion
To our knowledge, this is the first population-based study to examine the significance of healthcare-associated acquisition in patients with community-onset Gram-negative BSI. We demonstrated that healthcare-associated acquisition was predictive of the microbiological etiology of Gram-negative BSI and in vitro fluoroquinolone resistance among Gram-negative bloodstream isolates. We also demonstrated that healthcare-associated acquisition was a risk factor for increased short- and long-term mortality in patients with community-onset Gram-negative BSI.
The proportion of patients with healthcare-associated Gram-negative BSI in our survey (41.7%) was considerably lower than that described in other investigations performed at large tertiary care centers (50.9–82.5%) [2–4]. This difference was likely due to a lack of referral bias in our population-based investigation. Studies from tertiary-care centers tend to identify patients with more complex underlying medical problems that are often associated with healthcare-associated acquisition [15].
The median age of patients with healthcare-associated Gram-negative BSI in our study was higher than that in patients with community-acquired BSI, which is consistent with the results of a previous publication [4]. Moreover, the incidence rate of healthcare-associated Gram-negative BSI exceeded that of community-acquired Gram-negative BSI in elderly patients ≥ 80 years old of both genders (Fig. 1). This is likely due to the relatively higher proportion of elderly patients residing at nursing homes and long-term care facilities compared with patients in younger age groups.
The administration of inappropriate empiric antimicrobial therapy in patients with Gram-negative BSI has been associated with increased mortality [16]. Therefore, identification of patients at higher risk of BSI due to pathogens that potentially harbor antimicrobial resistance genes is essential for the selection of the initial antimicrobial regimen. Due to the inherent resistance mechanisms of P. aeruginosa, multiple previous studies have attempted to identify patients at higher risk of community-onset P. aeruginosa BSI [17, 18]. Many previously identified risk factors associated with P. aeruginosa BSI, including indwelling central venous catheters, prior chemotherapy and antimicrobial therapy, are criteria that support the designation of healthcare-associated acquisition. Our study provides further data that the classification proposed by Friedman et al. [2] allows for a simplified approach to identify patients with community-onset Gram-negative BSI who are at risk of BSI due to P. aeruginosa. Patients with healthcare-associated Gram-negative BSI should receive empiric antimicrobial therapy with antipseudomonal activity, including piperacillin-tazobactam, ticarcillin-clavulanate, ceftazidime, cefepime, carbapenems (excluding ertapenem), and aztreonam. P. aeruginosa is not the only Gram-negative bacillus that requires careful selection of empiric antimicrobial regimen. Enterobacter, Citrobacter and Serratia species have an inherent capability to produce inducible chromosomal β-lactamases, including AmpC, that confer resistance to ampicillin, ampicillin-sulbactam and first-generation cepha-losporins [12, 19]. Development of resistance has been described when patients with infections due to these micro-organisms were treated with third-generation cephalosporins [20, 21]. Therefore, identification of patients at high risk of BSI due to AmpC β-lactamase-producing Enterobacteriaceae is equally important. Our findings suggest that patients with healthcare-associated Gram-negative BSI should receive empiric antimicrobial agents that are not associated with a transient increase or induction of AmpC β-lactamase production, including cefepime and carbapenems [22, 23].
The identification of healthcare-associated acquisition as a risk factor for fluoroquinolone resistance among Gram-negative bloodstream isolates has been previously described [24, 25]. We confirmed this observation in a population-based setting with an overall lower in vitro antimicrobial resistance rate to fluoroquinolones. As previously demonstrated, studies from tertiary-care referral centers tend to over-estimate fluoroquinolone resistance rates [11]. In the current investigation, fluoroquinolone resistance rate among healthcare-associated Gram-negative bloodstream isolates approached 15% in the 2006–2007 period of the study (Fig. 3). Therefore, empiric use of fluoroquinolone mono-therapy in patients with healthcare-associated Gram-negative BSI should be discouraged.
The in vitro antimicrobial rate of resistance to third-generation cephalosporins among healthcare-associated Gram-negative bloodstream isolates in this population-based investigation was also lower than the antimicrobial resistance rates reported in tertiary-care referral centers [24, 25]. Healthcare-associated acquisition of Gram-negative BSI was not a risk factor for third-generation cephalosporin resistance in this study, which is contrary to the results of investigations performed outside the USA [24, 25].
Our findings of a higher 28-day all-cause mortality in patients with healthcare-associated Gram-negative BSI compared with those with community-acquired BSI is consistent with the results of previous large studies that included all forms of BSI [3, 24, 26]. However, investigations with smaller sample size, including one that was limited to Gram-negative BSI, did not detect a statistically significant difference in short-term mortality between patients with healthcare-associated BSI and those with community-acquired BSI [2, 4]. Studies with a large sample size have an obvious power advantage that allows detection of a difference in outcome between the two groups.
Estimating the 1-year mortality in patients with Gram-negative BSI was a unique feature of this investigation. We demonstrated that patients with community-acquired Gram-negative BSI had an excellent long-term prognosis with a 1-year all-cause mortality of 11%. In contrast, nearly 40% of patients with healthcare-associated Gram-negative BSI did not survive beyond 1 year of onset of BSI. It is of interest that the 1-year all-cause mortality in patients with Gram-negative BSI has declined over the past decade. Since there was no concomitant decline in 28-day all-cause mortality in patients with Gram-negative BSI over the same period of time, we speculate that improvements in the management of chronic underlying medical conditions, especially in elderly patients, is likely the reason for this increased long-term survival. This is likely a reflection of increased life expectancy in the general population of the USA over the past decade [27].
The primary strengths of this study were the population-based design, which decreased the possibility of referral bias affecting the results, the large sample size, and prolonged follow-up facilitated by the REP.
Our study has limitations. First, the population of Olmsted County consists mainly of middle class whites; therefore, our study results may be generalized only to communities with similar population characteristics. Second, our data were derived from one geographical area. The results of studies from multiple geographical locations might provide a more generalizable application. Finally, we did not provide data on chronic underlying medical conditions and previous exposure to antimicrobial agents.
Conclusions
Patients with healthcare-associated Gram-negative BSI have substantially increased short- and long-term mortality rates compared with those with community-acquired Gram-negative BSI. These differences emphasize the importance of identification of patients with healthcare-associated acquisition among those with community-onset Gram-negative BSI to optimize empiric antimicrobial therapy based on a predicted causative pathogen and antimicrobial resistance rates. Empiric antimicrobial therapy in patients with healthcare-associated Gram-negative BSI should include antimicrobial agents with antipseudomonal activity and without the ability to induce AmpC β-lactamase production. The use of fluoroquinolone monotherapy for the empiric treatment of healthcare-associated Gram-negative BSI should be discouraged because of increasing fluoroquinolone resistance among healthcare-associated Gram-negative bloodstream isolates.
Acknowledgments
The authors thank Emily Vetter and Mary Ann Butler for providing us with vital data from the microbiology laboratory databases at the Mayo Clinic, Rochester and the Olmsted Medical Center. The authors thank Susan Schrage, Susan Stotz, R.N., and all the staff at the Rochester Epidemiology Project for their administrative help and support.
Funding The study received funding from the Small Grants Program and the Baddour Family Fund at the Mayo Clinic, Rochester, MN, USA. The funding source had no role in study design.
This work was made possible by research grant R01-AG034676 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (National Institutes of Health, US Public Health Service).
Footnotes
Potential conflicts of interest MNA, JEE, and LMB: No conflict.
MNA has full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Contributor Information
M. N. Al-Hasan, Email: majdi.alhasan@uky.edu, Department of Medicine, Division of Infectious Diseases, University of Kentucky Medical Center, 800 Rose Street, Room MN 672, Lexington, KY 40536, USA
J. E. Eckel-Passow, Division of Biomedical Statistics and Informatics, Department of Health Sciences Research, Mayo Clinic, Rochester, MN, USA
L. M. Baddour, Department of Medicine, Division of Infectious Diseases, Mayo Clinic, Rochester, MN, USA
References
- 1.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988;16:128–140. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 2.Friedman ND, Kaye KS, Stout JE, McGarry SA, Trivette SL, Briggs JP, et al. Health care-associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med. 2002;137:791–797. doi: 10.7326/0003-4819-137-10-200211190-00007. [DOI] [PubMed] [Google Scholar]
- 3.Shorr AF, Tabak YP, Killian AD, Gupta V, Liu LZ, Kollef MH. Healthcare-associated bloodstream infection: a distinct entity? Insights from a large U.S. database. Crit Care Med. 2006;34:2588–2595. doi: 10.1097/01.CCM.0000239121.09533.09. [DOI] [PubMed] [Google Scholar]
- 4.Marschall J, Fraser VJ, Doherty J, Warren DK. Between community and hospital: healthcare-associated Gram-negative bacteremia among hospitalized patients. Infect Control Hosp Epidemiol. 2009;30:1050–1056. doi: 10.1086/606165. [DOI] [PubMed] [Google Scholar]
- 5.Olesen B, Kolmos HJ, Orskov F, Orskov I. A comparative study of nosocomial and community-acquired strains of Escherichia coli causing bacteraemia in a Danish University Hospital. J Hosp Infect. 1995;31:295–304. doi: 10.1016/0195-6701(95)90208-2. [DOI] [PubMed] [Google Scholar]
- 6.Kang CI, Kim SH, Bang JW, Kim HB, Kim NJ, Kim EC, et al. Community-acquired versus nosocomial Klebsiella pneumoniae bacteremia: clinical features, treatment outcomes, and clinical implication of antimicrobial resistance. J Korean Med Sci. 2006;21:816–822. doi: 10.3346/jkms.2006.21.5.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.US Census Bureau. [Accessed 21 April 2008];Olmsted County QuickFacts. http://quickfacts.census.gov.
- 8.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 9.Steckelberg JM, Melton LJ, 3rd, Ilstrup DM, Rouse MS, Wilson WR. Influence of referral bias on the apparent clinical spectrum of infective endocarditis. Am J Med. 1990;88:582–588. doi: 10.1016/0002-9343(90)90521-e. [DOI] [PubMed] [Google Scholar]
- 10.Tleyjeh IM, Steckelberg JM, Murad HS, Anavekar NS, Ghomrawi HM, Mirzoyev Z, et al. Temporal trends in infective endocarditis: a population-based study in Olmsted County, Minnesota. JAMA. 2005;293:3022–3028. doi: 10.1001/jama.293.24.3022. [DOI] [PubMed] [Google Scholar]
- 11.Al-Hasan MN, Wilson JW, Lahr BD, Eckel-Passow JE, Baddour LM. Incidence of Pseudomonas aeruginosa bacteremia: a population-based study. Am J Med. 2008;121:702–708. doi: 10.1016/j.amjmed.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pfaller MA, Jones RN, Marshall SA, Coffman SL, Hollis RJ, Edmond MB, et al. Inducible AmpC beta-lactamase producing gram-negative bacilli from blood stream infections: frequency, antimicrobial susceptibility, and molecular epidemiology in a national surveillance program (SCOPE) Diagn Microbiol Infect Dis. 1997;28:211–219. doi: 10.1016/s0732-8893(97)00064-3. [DOI] [PubMed] [Google Scholar]
- 13.Uslan DZ, Crane SJ, Steckelberg JM, Cockerill FR, 3rd, St Sauver JL, Wilson WR, et al. Age- and sex-associated trends in bloodstream infection: a population-based study in Olmsted County, Minnesota. Arch Intern Med. 2007;167:834–839. doi: 10.1001/archinte.167.8.834. [DOI] [PubMed] [Google Scholar]
- 14.Al-Hasan MN, Eckel-Passow JE, Baddour LM. Recurrent gram-negative bloodstream infection: a 10-year population-based cohort study. J Infect. 2010;61:28–33. doi: 10.1016/j.jinf.2010.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Hasan MN, Eckel-Passow JE, Baddour LM. Influence of referral bias on the clinical characteristics of patients with Gram-negative bloodstream infection. Epidemiol Infect. 2011 doi: 10.1017/s09502688100001x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang CI, Kim SH, Park WB, Lee KD, Kim HB, Kim EC, et al. Bloodstream infections caused by antibiotic-resistant gram-negative bacilli: risk factors for mortality and impact of inappropriate initial antimicrobial therapy on outcome. Antimicrob Agents Chemother. 2005;49:760–766. doi: 10.1128/AAC.49.2.760-766.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schechner V, Nobre V, Kaye KS, Leshno M, Giladi M, Rohner P, et al. Gram-negative bacteremia upon hospital admission: when should Pseudomonas aeruginosa be suspected? Clin Infect Dis. 2009;48:580–586. doi: 10.1086/596709. [DOI] [PubMed] [Google Scholar]
- 18.Cheong HS, Kang CI, Wi YM, Kim ES, Lee JS, Ko KS, et al. Clinical significance and predictors of community-onset Pseudomonas aeruginosa bacteremia. Am J Med. 2008;121:709–714. doi: 10.1016/j.amjmed.2008.03.034. [DOI] [PubMed] [Google Scholar]
- 19.Jacoby GA. AmpC beta-lactamases. Clin Microbiol Rev. 2009;22:161–182. doi: 10.1128/CMR.00036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chow JW, Fine MJ, Shlaes DM, Quinn JP, Hooper DC, Johnson MP, et al. Enterobacter bacteremia: clinical features and emergence of antibiotic resistance during therapy. Ann Intern Med. 1991;115:585–590. doi: 10.7326/0003-4819-115-8-585. [DOI] [PubMed] [Google Scholar]
- 21.Kaye KS, Cosgrove S, Harris A, Eliopoulos GM, Carmeli Y. Risk factors for emergence of resistance to broad-spectrum cephalosporins among Enterobacter spp. Antimicrob Agents Chemother. 2001;45:2628–2630. doi: 10.1128/AAC.45.9.2628-2630.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang K, Guglielmo BJ. Diagnosis and treatment of extended-spectrum and AmpC beta-lactamase-producing organisms. Ann Pharmacother. 2007;41:1427–1435. doi: 10.1345/aph.1K213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodriguez-Bano J, de Cueto M, Retamar P, Galvez-Acebal J. Current management of bloodstream infections. Expert Rev Anti Infect Ther. 2010;8:815–829. doi: 10.1586/eri.10.49. [DOI] [PubMed] [Google Scholar]
- 24.Sun HY, Chen SY, Chang SC, Pan SC, Su CP, Chen YC. Community-onset Escherichia coli and Klebsiella pneumoniae bacteremia: influence of health care exposure on antimicrobial susceptibility. Diagn Microbiol Infect Dis. 2006;55:135–141. doi: 10.1016/j.diagmicrobio.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Siegman-Igra Y, Fourer B, Orni-Wasserlauf R, Golan Y, Noy A, Schwartz D, et al. Reappraisal of community-acquired bacteremia: a proposal of a new classification for the spectrum of acquisition of bacteremia. Clin Infect Dis. 2002;34:1431–1439. doi: 10.1086/339809. [DOI] [PubMed] [Google Scholar]
- 26.Kollef MH, Zilberberg MD, Shorr AF, et al. Epidemiology, microbiology and outcomes of healthcare-associated and community-acquired bacteremia: a multicenter cohort study. J Infect. 2011;62:130–135. doi: 10.1016/j.jinf.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 27.Arias E. United States life tables, 2006. Natl Vital Stat Rep. 2010;58:1–40. [PubMed] [Google Scholar]