Abstract
The challenge in developing an anti-cocaine vaccine is that cocaine is a small molecule, invisible to the immune system. Leveraging the knowledge that adenovirus (Ad) capsid proteins are highly immunogenic in humans, we hypothesized that linking a cocaine hapten to Ad capsid proteins would elicit high-affinity, high-titer antibodies against cocaine, sufficient to sequester systemically administered cocaine and prevent access to the brain, thus suppressing cocaine-induced behaviors. Based on these concepts, we developed dAd5GNE, a disrupted E1−E3− serotype 5 Ad with GNE, a stable cocaine analog, covalently linked to the Ad capsid proteins. In pre-clinical studies, dAd5GNE evoked persistent, high titer, high affinity IgG anti-cocaine antibodies, and was highly effective in blocking cocaine-induced hyperactivity and cocaine self-administration behavior in rats. Future studies will be designed to expand the efficacy studies, carry out relevant toxicology studies, and test dAd5GNE in human cocaine addicts.
Keywords: Cocaine, addiction, adenovirus, vaccine, anti-cocaine antibody, passive immunity
INTRODUCTION
Cocaine addiction is a major social and medical problem for which there is no effective therapy [1]. Because addiction is a chronic relapsing illness, characterized by cycles of compulsive drug use and abstinence, and reinstatement of such cycles of abuse, vaccination against cocaine could be a lifetime therapeutic. The challenge in developing an anti-cocaine vaccine is that cocaine is a small molecule, invisible to the immune system [2]. Previous attempts to generate anti-cocaine antibodies by linking cocaine as a hapten to a protein carrier have had limited success, likely because the protein carriers have not been sufficiently immunogenic to evoke high affinity, high titer antibodies sufficient to block cocaine from reaching its receptors in the brain [3–7].
We have developed a novel strategy for the development of immunity to cocaine, leveraging the knowledge that adenovirus (Ad) capsid proteins are highly immunogenic in humans [8, 9]. We hypothesized that covalently linking a cocaine analog to Ad capsid proteins would elicit high-affinity, high-titer antibodies against cocaine sufficient to sequester systemically administered drug and prevent access to the brain, thus suppressing cocaine-induced behavior. We hypothesized that we could circumvent any risk of using an infectious virus by disrupting the Ad, resulting in a vaccine comprised of capsid proteins that retain the immunologic adjuvant properties of an infectious Ad, with the capacity to stimulate the immune system to generate high-titer, anti-drug antibodies [10]. Based on these concepts, we developed dAd5GNE, using a cocaine hapten termed GNE, a stable cocaine analog that was covalently linked to the capsid proteins of a disrupted E1−E3− serotype 5 Ad [11]. This review summarizes the development and assessment of dAd5GNE in murine and rat models challenged with cocaine.
BACKGROUND
Cocaine abuse is an intractable societal problem, with an estimate of 2 million regular users in the US [1] and >500,000 annual visits to the emergency room due to problems arising from chronic cocaine abuse [12]. Despite a myriad of drugs and therapeutics tested over the last 3 decades, there are no FDA-approved therapies for cocaine addiction [13].
Based on the concept that high titers of drug-specific antibodies would bind to cocaine in blood and this antibody-cocaine complex could not cross the blood brain barrier, an anti-cocaine vaccine would prevent administered drug from reaching its cognate receptors in the brain. Over the last decade, investigators have tested cocaine analog hapten-carrier protein-based vaccines using bovine serum albumin (BSA), keyhole limpet hemocyanin (KLH), and cholera toxin [3, 14, 15]. Only TA-CD (succinyl norcocaine coupled to a recombinant cholera toxin B subunit), has been tested in clinical trials (phase I [16], phase II [17]). Publically available data indicates only 38% of treated subjects achieved high serum antibody titers, with only 58% of those subjects reducing their cocaine usage by half [6].
We put forth a strategy to develop a successful anti-cocaine vaccine, using a hapten termed GNE, a stable cocaine analog [11], tethered to the capsid proteins of a disrupted serotype 5 adenovirus (Ad5), a virus that is highly immunogenic in humans. Because GNE has greater inherent chemical-stability at the 2’ position than other cocaine haptens, we anticipated that this structure, when linked to highly immunogenic Ad capsid proteins, would evoke persistent, high affinity, high titer anti-cocaine antibodies. This derives from our extensive experience with Ad5 gene transfer vectors which evoke high titer anti-Ad5 capsid protein antibodies in humans, including healthy subjects [18–20]. Another important aspect of this novel vaccine is the recognition that disruption of Ad before covalently linking GNE to the capsid proteins elicits higher anti-cocaine antibody titers than does an intact Ad5-based vaccine. The resulting dAd5GNE vaccine meets the challenge for an anticocaine vaccine: it is highly immunogenic in experimental animals due to combinations of the ligand chemistry, the conjugation process and, most importantly, the adjuvant properties of the Ad capsid proteins.
DRUG DESCRIPTION AND CHARACTERIZATION
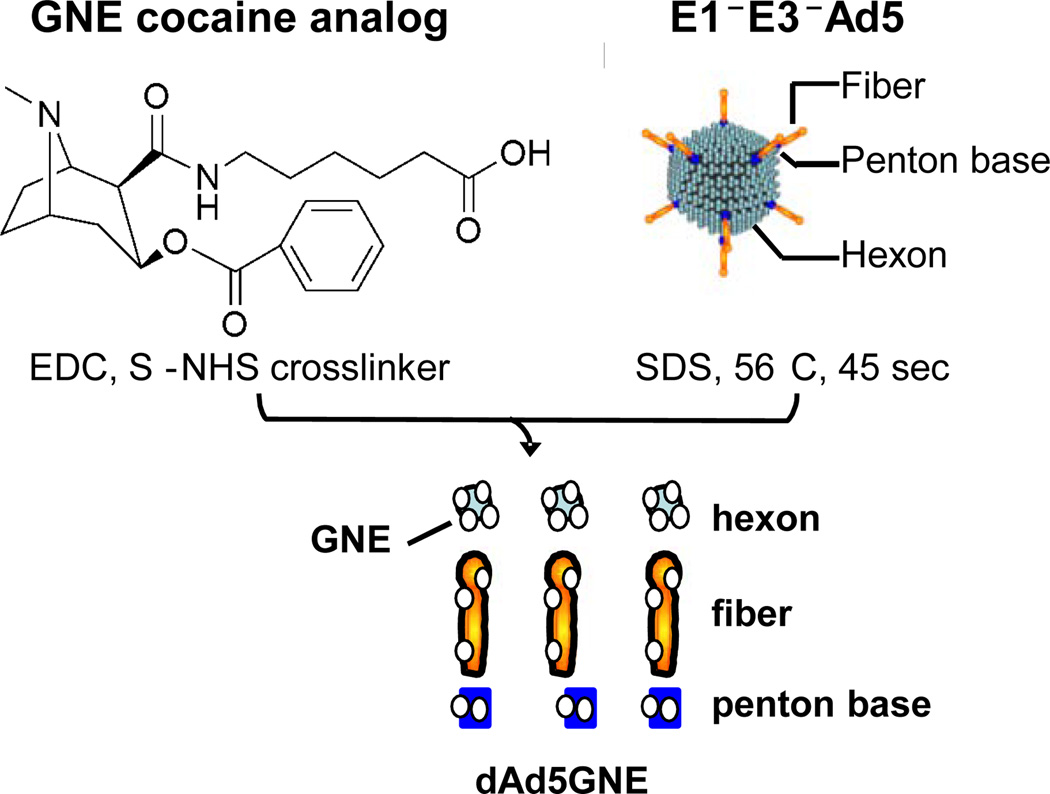
The dAd5GNE vaccine consists of a cocaine analog, GNE (6-(2R,3S)-3-(benzoyloxy)-8-methyl-8-azabicyclo [3.2.1] octane-2-carboxoamido-hexanoic acid), chemically linked to disrupted Ad5LacZ, an E1−E3−replication deficient serotype 5 Ad, 1st generation gene transfer vector with the bacterial LacZ (β-galactosidase) gene in the deleted adenovirus E1 region. The purpose of the LacZ gene is to provide a marker to insure that the final product is not infectious [10]. The vector is denatured by exposure to detergent (sodium dodecyl sulfate) at 56°C and then the amines on the capsid proteins are chemically joined to GNE with the bifunctional agent EDC (1-ethyl-3-[3-dimethylaminopropyl] carbodiimide hydrochloride) (Fig. 1). We have demonstrated that the product cannot infect cells. The vaccine is dialyzed to remove excess reagents and formulated immediately before administration with Adjuplex, a proprietary adjuvant from Advanced BioAdjuvants, LLC (Omaha, NB), which is oil, detergent, and preservative free and made without animal or microbial derived ingredients.
Fig. (1).
Production of dAd5GNE. A clinical grade, serotype E1−E3− adenovirus(Ad5) gene transfer vector coding for β-galactosidase is disrupted and coupled to the cocaine analog GNE with the EDC (1-ethyl-3-[3-dimethylaminopropyl] carbodiimide hydrochloride) cross-linking agent. Just before use, the dialyzed dAd5GNE is mixed with Adjuplex adjuvant. Used with permission from Hicks et al. [10].
EFFICACY STUDIES WITH dAd5GNE IN MICE
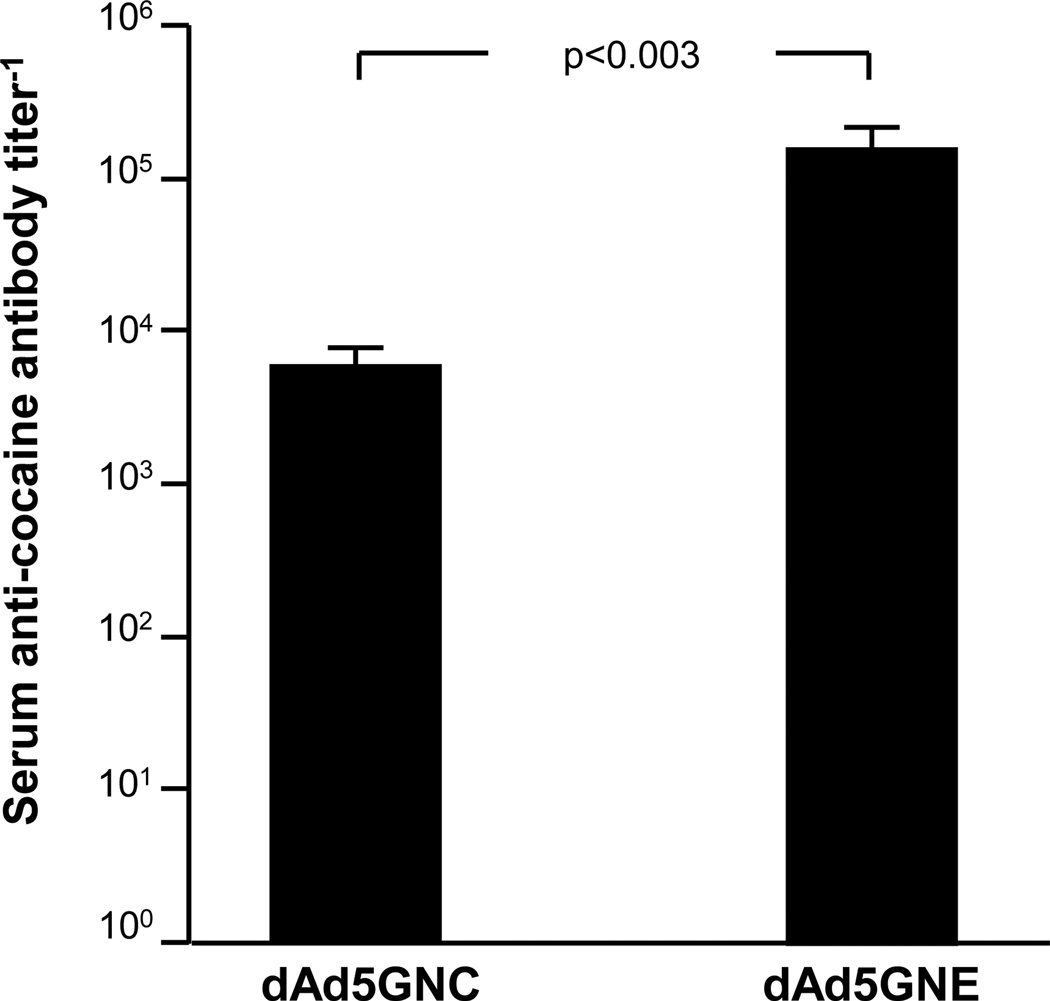
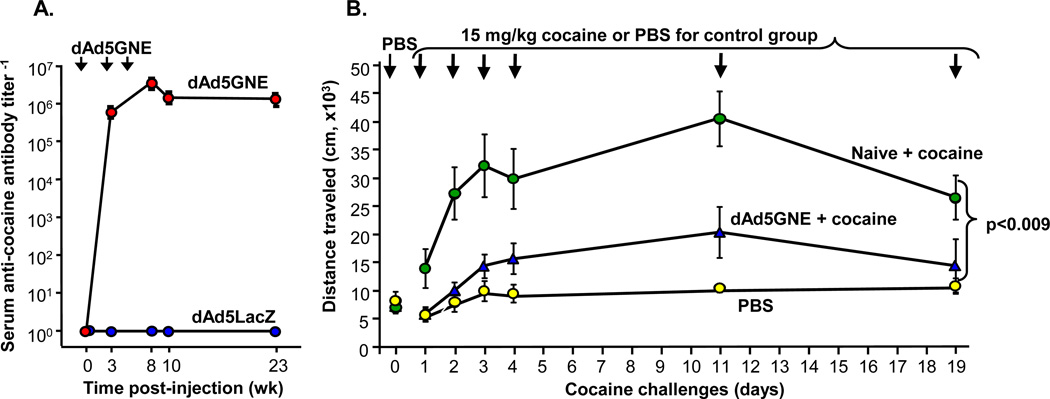
Our 1st generation anti-cocaine Ad vaccine program was initiated using GNC, a 1st generation cocaine hapten, that was attached to denatured Ad5 to create dAd5GNC [10]. Immunization of mice with dAd5GNC evoked a high-titer humoral response to cocaine with a dominant IgG1 subtype and abundant titers of IgG2a and IgG2b. We also examined GNE, a cocaine hapten that was designed to impart better chemical stability embedded within the naturally labile cocaine ester periphery when presented to the immune system. Head-to-head comparison of dAd5GNC vs dAd5GNE demonstrated that the dAd5GNE vaccine evoked higher titers in mice (Fig. 2). Also, dAd5GNE was equipotent in evoking anti-cocaine antibodies in naive mice as in mice previously immunized with Ad5 (not shown). This finding is important, as sero-epidemiologic studies have shown a high prevalence of anti- Ad5 capsid antibodies in human populations [21]. Based on this data, dAd5GNE was further evaluated for efficacy in mice and rats. When the dAd5GNE vaccine was administered to BALB/c mice, high levels of anti-cocaine titers were elicited (>106 titer−1 by 8 wk; (Fig. 3A). Importantly, vaccination with dAd5GNE suppressed repetitive intraperitoneal cocaine-induced hyper-locomotion in mice (Fig. 3B).
Fig. (2).
Superiority of dAd5GNE over dAd5GNC. Balb/C mice received intramuscular injection of 4 µg of dAd5GNC or dAd5GNE vaccine. After 3 wk the anti-cocaine serum titer was assessed by ELISA against GNC conjugated to bovine serum albumin (BSA). The data represent geometric mean and standard deviation for n=5/group.
Fig. (3).
dAd5GNE efficacy in mice. A. Anti-cocaine IgG antibody titers over time. BALB/c mice (n=20) were vaccinated intramuscularly with 4 µg dAd5GNE + Adjuplex at 0, 3 and 6 wk. Antibody titers were assessed by ELISA at 0, 3, 8, 10 and 23 wk. The control was unconjugated dAd5LacZ. B. Cocaine-induced locomotor activity was assessed upon 6 cocaine exposures over a 3 wk period. All test groups were initially assayed with PBS to establish baseline activity (shown by non-connected symbols at day 0). Mice were sensitized to cocaine (15 mg/kg, i.p.) during the 1st wk of the trial (days 1–4), and then challenged weekly (n=10/group).
EFFICACY STUDIES WITH dAd5GNE IN RATS
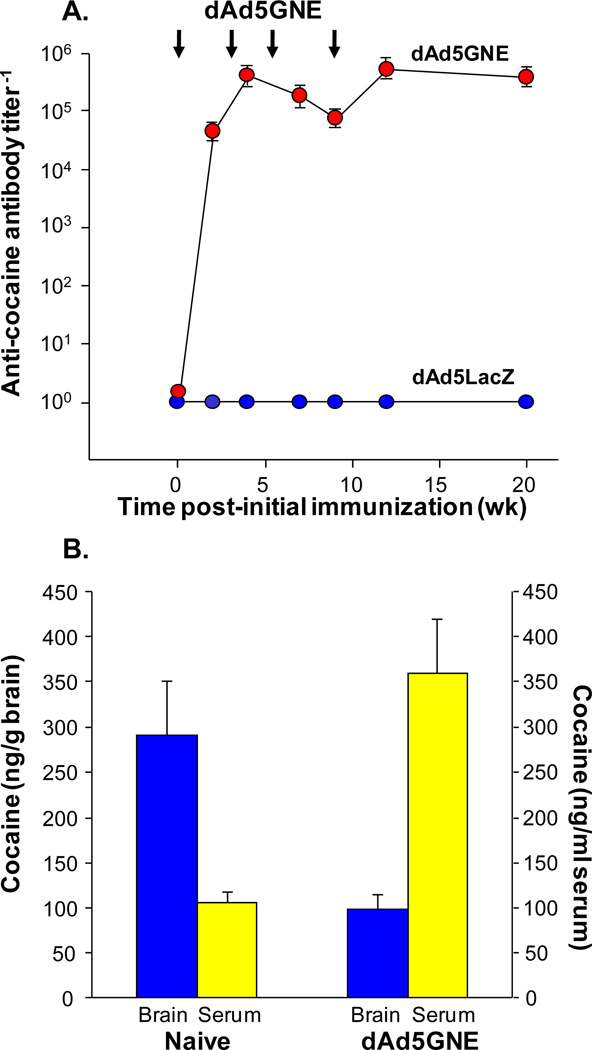
As in mice, the dAd5GNE evoked high anti-cocaine titers in rats which persisted for >20 wk, the longest time point evaluated (Fig. 4A). Control rats injected with non-conjugated, denatured Ad5 vector had no detectable anti-cocaine antibody titers [11]. Pharmacokinetic studies in rats, measuring the partition at 2 min post intravenous radioactive cocaine challenge between brain and blood in naive vs vaccinated rats, demonstrated significant blockade of drug access to the CNS (Fig. 4B). The antibody dissociation coefficient (Kd), evaluated by competitive binding assay, was 4 nM.
Fig. (4).
Efficacy of dAd5GNE in rats. A. Sustained anti-cocaine immunity evoked by dAd5GNE. Total anti-cocaine IgG antibody titers in male Wistar rats vaccinated intramuscularly with 10 µg dAd5GNE plus Adjuplex. Control animals were similarly vaccinated with unconjugated denatured dAd5LacZ. (The Ad5 vector used in the dAd5GNE vaccine.) The vaccine was administered to rats (n=12) at 0, 3, 5, and 10 wk, and the titers (by ELISA) assessed up to 20 wk. Plotted are geometric means and standard deviation. B. Partition of cocaine between brain and blood in naive and dAd5GNE-vaccinated rats (n=5). Levels of cocaine in brain (ng/g brain) and serum (ng/ml serum) of naive and dAd5GNE-vaccinated rats 2 min following [3H]-cocaine challenge (25 µg). The study was at 16 wk post-vaccination. Significance by one-way paired two sample t-test: Brain t = 3.0, p<0.009; serum t = 4.1, p<0.002. Used with permission from Wee et al. [11].
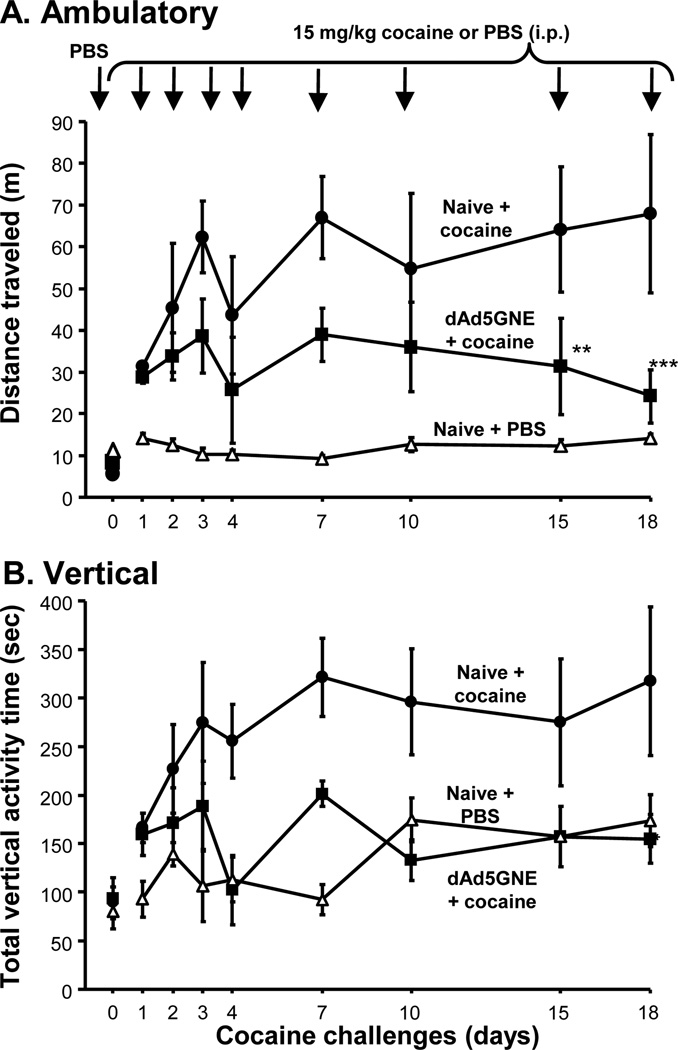
The anti-cocaine titers elicited by dAd5GNE vaccination suppressed the cocaine-induced hyper-locomotion expected from repeated cocaine administrations at 15 mg/kg; a challenge that establishes drug serum levels in the rat comparable to that of the typical human doses.
Rats were sensitized by daily intraperitoneal injections of 15 mg/kg cocaine during the 1st wk, and subsequently challenged with cocaine and evaluated for drug-induced hyperactivity twice weekly for 3 wk [11]. In response to cocaine challenge, naive rats traveled over a greater distance (>2-fold) than dAd5GNE vaccinated rats (p<0.003; Fig. 5A). In addition, naive rats responding to cocaine challenge had a dramatic elevation of time spent exercising vertical activity, whereas dAd5GNE vaccinated rats showed vertical activity similar to rats that received phosphate-buffered saline (PBS) instead of cocaine (p>0.4) and the dAd5GNE vaccinated rats were significantly different from the naive rats receiving cocaine that had more vertical activity (p<0.05; Fig. 5B).
Fig. (5).
Effect of dAd5GNE on suppressing cocaine-induced locomotor activity in rats. Cocaine-induced locomotor activity was assessed upon 8 cocaine challenges over 3 wk. dAd5GNE + Adjuplex vaccinated (■) and naive + cocaine (●) rats (n=8/group) were sensitized to cocaine (15 mg/kg, i.p.) during the 1st wk of the trial and then challenged biweekly with cocaine during wk 2 and 3. Naive rats with PBS challenge were used as a control (Δ). A. Total distance traveled inside an infra-red beam monitored open field apparatus (AccuScan, 40 × 40 cm chambers) in 30 min immediately post cocaine or PBS challenges. All test groups were initially assayed with PBS to establish baseline activity (shown by non-connected symbols at day 0). B. Rat hyperactivity monitored by amount of time spent displaying vertical activity (breaking z-axis beams). Total vertical activity time over 30 min post PBS or cocaine (15 mg/kg) challenges plotted for each trial (n=8/group). Used with permission from Wee et al. [11].
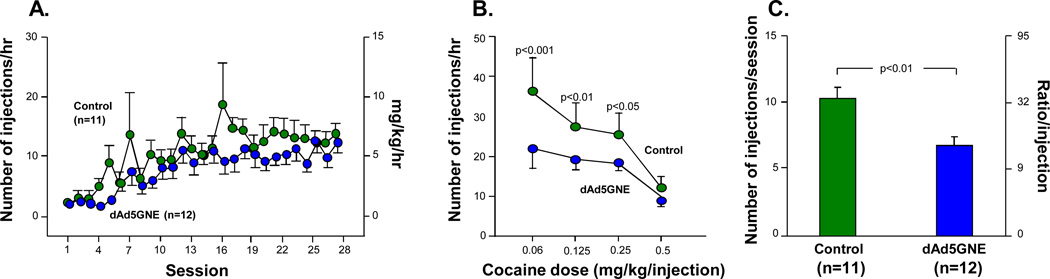
Further assessment of vaccine efficacy using cocaine self-administration behavior in rats reflects the neuropharmacological effects known to motivate drug seeking. All drugs of abuse that are intravenously self-administered by rats have high abuse potential. When rats were allowed to self-administer 0.5 mg/kg/injection of cocaine, vaccinated and control rats gradually increased the number of responses for cocaine [11]. There was no difference in acquisition of cocaine self-administration across sessions or between the groups (Fig. 6A), p>0.1; vaccination, p>0.1; self-administration session, p<0.01; control and vaccine groups, p>0.1]. Both groups of rats significantly increased their responses as the unit dose of cocaine for self-administration decreased (Fig. 6B). The control rats tended to take more cocaine than the vaccinated rats, but there was no significant difference between the groups (vaccination × cocaine dose interaction, p>0.1; vaccination, p>0.1; cocaine dose, p<0.001). However, under a progressive ratio schedule, whereby the work (lever presses) to obtain each successive cocaine injection is increased, the vaccinated rats showed a significant decrease in the number of lever presses emitted compared with the control rats (Fig. 6C; p<0.01). To demonstrate that these differences were not mediated by an indeterminate variable, but were due to the drug-vaccine interaction, similar studies with methamphetamine or food pellets showed no difference between vaccine and control rats (not shown, methamphetamine, p>0.1; food, p>0.1).
Fig. (6).
Effect of dAd5GNE vaccine on cocaine reward and drug seeking in rats. dAd5GNE + Adjuplex (10 µg) vaccinated and control (dAd5LacZ) rats (n=12) were fitted with intravenous catheters 1 wk post final vaccination. After recovery, they were allowed to self-administer 0.5 mg/kg/injection of cocaine in daily 1 hr sessions. After 20 sessions, cocaine dose-response function and responding were determined, using both fixed and progressive ratio schedules. Lastly the rats were tested for food self-administration under a progress ratio schedule. A. Acquisition of cocaine self-administration under a fixed ratio schedule. Shown are the number of cocaine injections (left) and cocaine intake (mg/kg) (right). B. Cocaine dose-response function. Shown are the number of injections per hour at each dose of cocaine. P values compared data with responding for 0.5 mg/kg/injection of cocaine. C. Cocaine self-administration under a progressive ratio schedule. Shown are the number of injections per session (left) and ratio requirement per injection (right). Used with permission from Wee et al. [11].
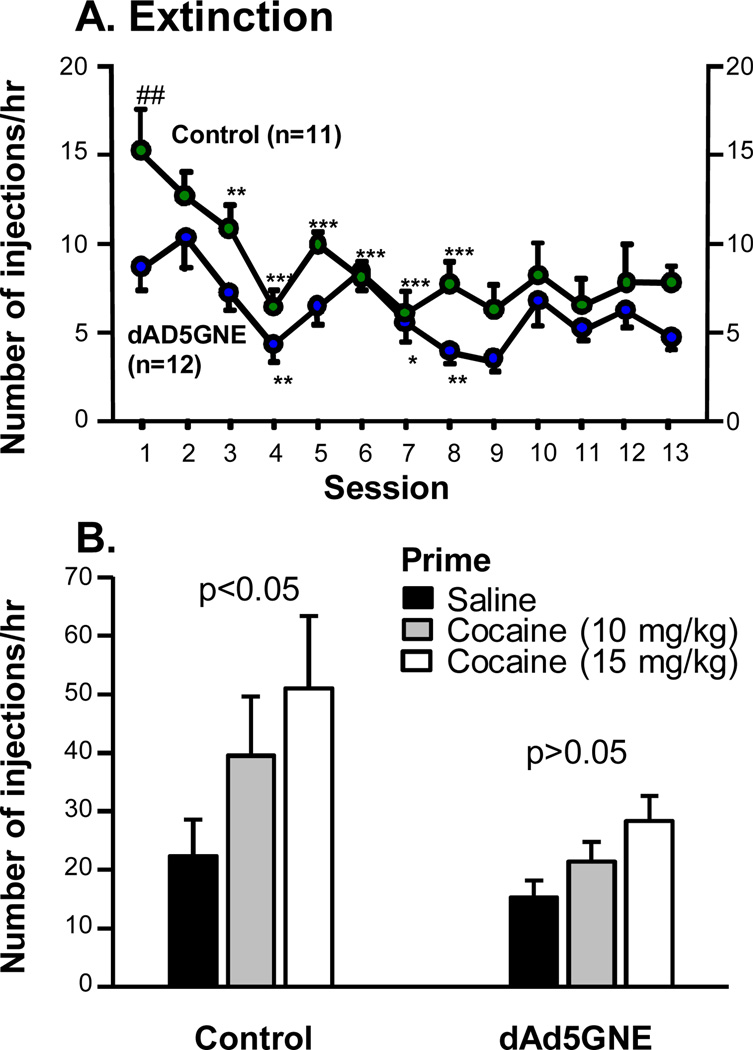
When cocaine was replaced with saline, the control rats showed a significantly higher level of work (lever presses) on the previously cocaine-associated lever (extinction burst) than the vaccinated rats during the 1st session, but both groups significantly decreased responding in 8 sessions (Fig. 7A), vaccination × extinction session interaction, p<0.05; vaccination, p<0.05; extinction session, p<0.001. Importantly, control rats primed with cocaine significantly reinstated responding on the previously cocaine-associated lever, whereas the vaccinated rats did not (Fig. 7B), vaccination × primed drug interaction, p>0.05; vaccination, p>0.05; primed drug, p=0.01. The fact that the acquisition of cocaine self-administration is not significantly blunted in the vaccinated rats combined with the robust blockade of progressive ratio responding could be interpreted as suggesting that the efficacy of the vaccine is less when more cocaine is available. However, this is not likely the case in that almost the same mg/kg/time (e.g., 5 vs 5.1 mg/kg/hour) of cocaine is actually self-administered in each situation. Most of the responding under the progressive ratio schedule occurs during the first hour and the actual mg/kg delivered over a session is not changed by the unit dose over time. Thus, we believe that the blockade of the reinforcing actions of cocaine with our vaccine is equivalent to data obtained in the preclinical studies with other manipulations known to block the reinforcing actions of cocaine [22].
Fig. (7).
Effect of dAd5GNE on extinction and reinstatement of responding for cocaine. A. The extinction session in which cocaine was replaced with saline. Extinction *p<0.05, **p<0.01, ***p<0.001 and compared with extinction session 1. ##p<0.01 compared with the dAd5GNE group in the same session. B. For the reinstatement test, rats were injected i.p. with saline or cocaine (10, 15 mg/kg) and immediately placed in an operant chamber. During the reinstatement session, response on the lever previously associated with cocaine delivery resulted in saline delivery with cue light. Reinstatement p<0.01 compared with saline. Used with permission from Wee et al. [11].
DISCUSSION
These preclinical data establish the dAd5GNE vaccine as highly effective in the generation of high anti-cocaine antibody titers which can sequester intravenously administered cocaine, at weight adjusted doses equivalent to the typical human dose, in the blood of mice and rats. Cocaine induced hyper-locomotor behaviors in mice and rats are blocked by the vaccine and the vaccine dramatically blocks the effort that rats will expend to obtain cocaine reward, facilitates extinction of cocaine reward, and blocks reinstatement of cocaine seeking in an animal model of relapse to cocaine taking.
A key element in treating drug addiction is to prevent relapse to drug use after prolonged drug abstinence. Stressors, drug-associated stimuli, and brief exposure to the drug itself can trigger drug craving and relapse to drug use in humans [23–25]. In an animal model of drug-induced craving and relapse, animals that undergo extinction of drug self-administration reinstate responding when they are primed with a previously self-administered drug or other drugs of the same class [26]. When saline was initially substituted for cocaine in the dAd5GNE vaccine study, the control rats showed an extinction-like burst in responding, whereas the vaccinated rats did not. Interestingly, the results of the control group are similar to extinction-like bursts observed in rats with high cocaine intake, while the dAd5GNE experimental group shows similarity in lack of extinction-like bursts observed in rats with low cocaine intake [27]. Additionally, when primed with cocaine, control rats reinstated responding on the previously cocaine-associated lever, whereas vaccinated rats did not, consistent with previous results [4]. Thus, the data suggest that the vaccine prevented cocaine from reaching the brain to prime cocaine-seeking mechanisms in rats, and if extrapolated to cocaine addiction in humans, upon exposure (priming) to cocaine, vaccinated humans might likely show a similar reduction in cocaine-seeking behavior.
Consistent with the robust effects of the vaccine in blocking the motivation to seek cocaine in the present study, we did not observe a "compensatory-like" increase in cocaine self-administration in vaccinated rats as has been observed with low antibody titers from other studies [28], and low doses of dopamine antagonists [29]. Such increases in cocaine self-administration due to lowering the dose of cocaine are thought to reflect shifts to the right of an inverted U- shaped dose effect function relating unit dose of cocaine to intake. However, in our study there was no such increase in baseline responding, suggesting that the high, stable antibody titers at the time of exposure to cocaine self administration may reflect a more complete antagonism and a downward shift, rather than a rightward shift in the dose-intake function for cocaine self-administration in the vaccinated rats.
FUTURE STUDIES WITH dAd5GNE
Future plans include the execution of additional preclinical efficacy studies to identify adjuvant potency, optimal vaccination regimen, minimum effective dose, and vaccine efficacy in the context of multiple cocaine challenges. This optimization would be followed by standard toxicology studies in mice, rats and nonhuman primates to demonstrate a suitable starting human dose and establish a product safety profile. Should these studies support the concept that the dAd5GNE is a safe and effective vaccine in humans, our plans are to carry out a phase I placebo controlled, double blinded, dose escalation assessment of the dAd5GNE vaccine in cocaine addicts who have chosen to discontinue cocaine use. In addition to safety parameters, the primary efficacy parameter will be anti-cocaine serum titers and urine cocaine metabolites; the secondary efficacy parameters will be self-reporting of cocaine usage and the extent of the subjective effect or “high” if they do use cocaine.
ACKNOWLEDGMENTS
We thank N Mohamed and R Hamid for help in preparing the manuscript. These studies were supported, in part, by DA025305, DA028847 and DA004398. MJH is supported, in part, by T32 HL094284; KDJ, in part, by R01 DA008590. The authors thank the National Institute on Drug Abuse (NIDA) drug supply program for the cocaine and cocaine metabolites used in this study; and Advanced BioAdjuvants, LLC of Omaha, NE for supplying AduplexTM.
ABBREVIATIONS
- Ad
Adenovirus
- GNC
A 1st generation cocaine hapten
- GNE
(6-(2R,3S)-3-(benzoyloxy)-8-methyl-8-azabicyclo [3.2.1] octane-2-carboxoamido-hexanoic acid), a cocaine hapten
- PBS
Phosphate-buffered saline
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts to disclose.
REFERENCES
- 1.Summary of National Findings. Rockville, MD: 2010. Substance Abuse and Mental Health Services Administration, Anonymous, Results from the 2009 National Survey on Drug Use and Health: Volume I. (Office of Applied Studies, NSDUH Series H-38A, HHS Publication No. SMA 10-4586Findings. [Google Scholar]
- 2.Kinsey BM, Jackson DC, Orson FM. Anti-drug vaccines to treat substance abuse. Immunol. Cell Biol. 2009;87:309–314. doi: 10.1038/icb.2009.17. [DOI] [PubMed] [Google Scholar]
- 3.Carrera MR, Ashley JA, Parsons LH, Wirsching P, Koob GF, Janda KD. Suppression of psychoactive effects of cocaine by active immunization 11. Nature. 1995;378:727–730. doi: 10.1038/378727a0. [DOI] [PubMed] [Google Scholar]
- 4.Carrera MR, Ashley JA, Zhou B, Wirsching P, Koob GF, Janda KD. Cocaine vaccines: antibody protection against relapse in a rat model. Proc. Natl. Acad. Sci. USA. 2000;97:6202–6206. doi: 10.1073/pnas.97.11.6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carrera MR, Ashley JA, Wirsching P, Koob GF, Janda KD. A second-generation vaccine protects against the psychoactive effects of cocaine. Proc. Natl. Acad. Sci. 2001;98:1988–1992. doi: 10.1073/pnas.041610998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martell BA, Orson FM, Poling J, Mitchell E, Rossen RD, Gardner T, Kosten TR. Cocaine vaccine for the treatment of cocaine dependence in methadone-maintained patients: a randomized, double-blind, placebo-controlled efficacy trial 22. Arch. Gen. Psychiatry. 2009;66:1116–1123. doi: 10.1001/archgenpsychiatry.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haney M, Gunderson EW, Jiang H, Collins ED, Foltin RW. Cocaine-specific antibodies blunt the subjective effects of smoked cocaine in humans. Biol. Psychiatry. 2010;67:59–65. doi: 10.1016/j.biopsych.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chirmule N, Propert K, Magosin S, Qian Y, Qian R, Wilson J. Immune responses to adenovirus and adeno-associated virus in humans 16. Gene Ther. 1999;6:1574–1583. doi: 10.1038/sj.gt.3300994. [DOI] [PubMed] [Google Scholar]
- 9.Harvey BG, Hackett NR, El-Sawy T, Rosengart TK, Hirschowitz EA, Lieberman MD, Lesser ML, Crystal RG. Variability of human systemic humoral immune responses to adenovirus gene transfer vectors administered to different organs. J. Virol. 1999;73:6729–6742. doi: 10.1128/jvi.73.8.6729-6742.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hicks MJ, De BP, Rosenberg JB, Davidson JT, Moreno AY, Janda KD, Wee S, Koob GF, Hackett NR, Kaminsky SM, Worgall S, Toth M, Mezey JG, Crystal RG. Cocaine analog coupled to disrupted adenovirus: a vaccine strategy to evoke high-titer immunity against addictive drugs. Mol. Ther. 2011;19:612–619. doi: 10.1038/mt.2010.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wee S, Hicks MJ, De BP, Rosenberg JB, Moreno AY, Janda KD, Kaminsky SM, Crystal RG, Koob GF. Novel cocaine vaccine linked to a disrupted adenovirus gene transfer vector blocks cocaine psychostimulant and reinforcing effects. Neuropsychopharmacology. 2011 doi: 10.1038/npp.2011.200. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drug Abuse Warning Network. National Estimates of Drug-Related Emergency Department Visits: 2009. [accessed December 31, 2011]; http://www.samhsa.gov/data/2k11/DAWN/2k9DAWNED/HTML/DAWN2k9ED.htm.
- 13.Orson FM, Kinsey BM, Singh RA, Wu Y, Kosten TR. Vaccines for cocaine abuse. Hum. Vaccin. 2009;I:194–199. doi: 10.4161/hv.5.4.7457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bagasra O, Forman LJ, Howeedy A, Whittle P. A potential vaccine for cocaine abuse prophylaxis. Immunopharmacology. 1992;23:173–179. doi: 10.1016/0162-3109(92)90023-6. [DOI] [PubMed] [Google Scholar]
- 15.Fox BS, Kantak KM, Edwards MA, Black KM, Bollinger BK, Botka AJ, French TL, Thompson TL, Schad VC, Greenstein JL, Gefter ML, Exley MA, Swain PA, Briner TJ. Efficacy of a therapeutic cocaine vaccine in rodent models. Nat. Med. 1996;2:1129–1132. doi: 10.1038/nm1096-1129. [DOI] [PubMed] [Google Scholar]
- 16.Kosten TR, Rosen M, Bond J, Settles M, Roberts JS, Shields J, Jack L, Fox B. Human therapeutic cocaine vaccine: safety and immunogenicity. Vaccine. 2002;20:1196–1204. doi: 10.1016/s0264-410x(01)00425-x. [DOI] [PubMed] [Google Scholar]
- 17.Martell BA, Mitchell E, Poling J, Gonsai K, Kosten TR. Vaccine pharmacotherapy for the treatment of cocaine dependence. Biol. Psychiatry. 2005;58:158–164. doi: 10.1016/j.biopsych.2005.04.032. [DOI] [PubMed] [Google Scholar]
- 18.Hackett NR, Kaminsky SM, Sondhi D, Crystal RG. Antivector and antitransgene host responses in gene therapy. Curr. Opin. Mol. Ther. 2000;2:376–382. [PubMed] [Google Scholar]
- 19.Worgall S, Busch A, Rivara M, Bonnyay D, Leopold PL, Merritt R, Hackett NR, Rovelink PW, Bruder JT, Wickham TJ, Kovesdi I, Crystal RG. Modification to the capsid of the adenovirus vector that enhances dendritic cell infection and transgene-specific cellular immune responses. J. Virol. 2004;78:2572–2580. doi: 10.1128/JVI.78.5.2572-2580.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Worgall S, Krause A, Rivara M, Hee KK, Vintayen EV, Hackett NR, Roelvink PW, Bruder JT, Wickham TJ, Kovesdi I, Crystal RG. Protection against P. aeruginosa with an adenovirus vector containing an OprF epitope in the capsid. J. Clin. Invest. 2005;115:1281–1289. doi: 10.1172/JCI23135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foy HM, Grayston JT. Adenoviruses. In: Evans AS, editor. Viral infections of humans: epidemiology and control. New York: Plenum, Plenum; 1976. pp. 53–69. [Google Scholar]
- 22.Koob G, Moal ML, editors. Neurobiology of Addiction. San Diego, CA: Academic Press; 2005. [Google Scholar]
- 23.Carrera MR, Ashley JA, Zhou B, Wirsching P, Koob GF, Janda KD. Cocaine vaccines: antibody protection against relapse in a rat model. Proc. Natl. Acad. Sci. USA. 2000;97:6202–6206. doi: 10.1073/pnas.97.11.6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology. 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yahyavi-Firouz-Abadi N, See RE. Anti-relapse medications: preclinical models for drug addiction treatment. Pharmacol. Ther. 2009;124:235–247. doi: 10.1016/j.pharmthera.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stewart J. Pathways to relapse: the neurobiology of drug- and stress-induced relapse to drug-taking. J. Psychiatry Neurosci. 2000;25:125–136. [PMC free article] [PubMed] [Google Scholar]
- 27.Edwards S, Whisler KN, Fuller DC, Orsulak PJ, Self DW. Addiction-related alterations in D1 and D2 dopamine receptor behavioral responses following chronic cocaine self-administration. Neuropsychopharmacology. 2007;32:354–366. doi: 10.1038/sj.npp.1301062. [DOI] [PubMed] [Google Scholar]
- 28.Kantak KM, Collins SL, Bond J, Fox BS. Time course of changes in cocaine self-administration behavior in rats during immunization with the cocaine vaccine IPC-1010. Psychopharmacology. 2001;153:334–340. doi: 10.1007/s002130000555. [DOI] [PubMed] [Google Scholar]
- 29.Caine SB, Koob GF. Effects of dopamine D-1 and D-2 antagonists on cocaine self-administration under different schedules of reinforcement in the rat. J. Pharmacol. Exp. Ther. 1994;270:209–218. [PubMed] [Google Scholar]