A high burden of chronic lung disease (CLD) was found among 116 consecutive adolescents with vertically acquired human immunodeficiency virus in Zimbabwe. The main cause of HIV-associated CLD appears to be obliterative bronchiolitis, which has not previously been recognized among this patient group.
Abstract
Background. Long-term survivors of vertically acquired human immunodeficiency virus (HIV) infection are reaching adolescence in large numbers in Africa and are at high risk of delayed diagnosis and chronic complications of untreated HIV infection. Chronic respiratory symptoms are more common than would be anticipated based on the HIV literature.
Methods. Consecutive adolescents with presumed vertically acquired HIV attending 2 HIV care clinics in Harare, Zimbabwe, were recruited and assessed with clinical history and examination, CD4 count, pulmonary function tests, Doppler echocardiography, and chest radiography (CXR). Those with suspected nontuberculous chronic lung disease (CLD) were scanned using high-resolution computed tomography (HRCT).
Results. Of 116 participants (43% male; mean age, 14 ± 2.6 years, mean age at HIV diagnosis, 12 years), 69% were receiving antiretroviral therapy. Chronic cough and reduced exercise tolerance were reported by 66% and 21% of participants, respectively; 41% reported multiple respiratory tract infections in the previous year, and 10% were clubbed. More than 40% had hypoxemia at rest (13%) or on exercise (29%), with pulmonary hypertension (mean pulmonary artery pressure >25 mm Hg) in 7%. Forced expiratory volume in 1 second (FEV1) was <80% predicted in 45%, and 47% had subtle CXR abnormalities. The predominant HRCT pattern was decreased attenuation as part of a mosaic attenuation pattern (31 of 56 [55%]), consistent with small airway disease and associated with bronchiectasis (Spearman correlation coefficient (r2 = 0.8) and reduced FEV1 (r2 = −0.26).
Conclusions. Long-term survivors of vertically acquired HIV in Africa are at high risk of a previously undescribed small airway disease, with >40% of unselected adolescent clinic attendees meeting criteria for severe hypoxic CLD. This condition is not obvious at rest. Etiology, prognosis, and response to treatment are currently unknown.
More than 90% of the estimated 2 million global pediatric human immunodeficiency virus (HIV) infections occur in sub-Saharan Africa, predominantly due to mother-to-child transmission [1]. Although untreated HIV infection in infants is associated with a high risk of rapid disease progression [2], up to one-third of HIV-infected infants are “slow progressors,” with an estimated median survival of a decade or more [3–5]. Two percent to 3% of all 10-year-olds living in Southern Africa may be HIV-infected long-term survivors, the majority still undiagnosed [5, 6]. These estimated burdens reflect high regional HIV prevalence rates in pregnant women during the 1990s and lack of interventions to prevent mother-to-child transmission at that time.
Clinicians in sub-Saharan Africa have become familiar with the clinical manifestations of untreated slow progressors, who typically present in later childhood with pronounced stunting, severe immunosuppression, or chronic complications of untreated HIV [6]. The best-documented regional epidemic is in Zimbabwe, where 43% of 25 000 children attending HIV care clinics in 2008 were aged ≥10 years [7]. Recently diagnosed or still undiagnosed vertically acquired HIV is now the most common cause of admission to the hospital and the most common cause of in-hospital deaths among adolescents in the region [8]. Undocumented epidemics of similar magnitude are thought to affect neighboring countries.
The natural history of HIV infection in older children is less well defined than for infants or adults. Presentation with chronic cough is common and often leads to presumptive treatment for unconfirmed tuberculosis. Respiratory illness, frequently with a history suggestive of acute-on-chronic lung disease, was the most common cause of death among HIV-infected adolescents admitted to a hospital in Harare [8]. Among younger children, the most common cause of chronic lung disease (CLD) is lymphocytic interstitial pneumonitis (LIP), found in 30%–40% of HIV-infected untreated children [9]. LIP is associated with a slower progression of HIV infection and responds well to antiretroviral therapy (ART) [9, 10]. The cause of HIV-related CLD in older children and adolescents has not previously been investigated.
The aim of this study was to investigate the clinical features and morphologic characteristics of CLD in older children with vertically acquired HIV infection attending HIV care outpatient services in 2 public-sector hospitals in Harare, Zimbabwe.
METHODS
Study Participants
Consecutive patients aged 10–19 years attending 2 HIV care outpatient clinics at Parirenyatwa Hospital and Harare Central Hospital (Harare, Zimbabwe) were enrolled (maximum 8 per day for logistic ease). Exclusion criteria were horizontally acquired HIV infection, pregnancy, being too ill to participate (requiring immediate hospitalization), living outside Harare, and having pulmonary Kaposi sarcoma, recently diagnosed smear-positive tuberculosis, or acute respiratory tract infection.
Clinical Data Collection
Interview data recorded the reasons for HIV testing, duration of ART, respiratory symptoms, and exercise tolerance (New York Heart Association [NYHA] scale [11]). Standard respiratory examination and anthropometric assessment, including height, weight, and Tanner staging, were performed. All participants underwent CD4 count measurement (CyFlow counter, Partec GmbH), spirometry, Doppler echocardiography, and chest radiography (CXR); high-resolution computed tomography (HRCT) was performed if CLD was suspected. The World Health Organization (WHO) Adult Classification was used to stage HIV infection [12]. HIV infection was presumed to be vertically acquired if the following case definition was met: maternal and/or sibling death or known maternal HIV infection, plus no history of sexual debut or blood transfusion, and at least 2 of the following: frequent infections during early childhood, stunting, or pubertal delay [6].
Pulmonary Function Tests
Oxygen saturation (O2 sat) and respiratory rate were measured at rest and after exercise (a 200-m brisk walk). Participants with a respiratory rate >25 breaths/minute or O2 sat <92% at rest were not exercised.
Spirometry was performed using a Microloop spirometer (Mk8, MicroMedical). Participants were actively encouraged during the maneuver verbally and with pictorial incentives to blow out maximally. A nose clip was used; 3 reproducible, technically adequate measurements were taken in the standing position, and the maximal values were recorded. At least 30 seconds were allowed between each maneuver.
Laboratory Investigations
Participants with cough of any duration were asked to provide 2 sputum specimens. Bacterial pathogens were identified using standard techniques [13]. Concentrated decontaminated sputum specimens were examined using Auramine and Ziehl-Neelsen staining and cultured on Lowenstein-Jensen media, with positive mycobacterial cultures confirmed by genotyping using the Hain assay (Hain LifeScience GmbH).
Radiologic Data
Participants with clinically suspected CLD or CXR abnormality (assessed locally) were invited to undergo HRCT scanning (Siemens Emotion 6). Those with suspected active mycobacterial disease were excluded. In separate sessions, CXR and HRCT scans were scored independently by 2 thoracic radiologists blinded to clinical data. CXR abnormalities were quantified using a previously developed scoring system [14]. HRCT abnormalities were categorized on the basis of patterns reflecting either airway pathology or parenchymal disease using standard definitions (see Supplementary Data) [15].
Statistical Analysis
The z scores for height-for-age and weight-for-age were calculated using British Growth Reference Curves [16]. Scores of ≤2 were considered to represent stunting and wasting, respectively. Predicted spirometric indices were calculated using age-, sex-, and height-adjusted standards in healthy Malawian schoolchildren [17]. Quantitative interrelationships were examined using Spearman rank correlation coefficient: HRCT lobar scores were summed to provide global HRCT scores for specific variables. Interobserver variation for HRCT and CXR abnormalities with a prevalence of ≥15% was examined using the kappa (κ) coefficient of agreement.
Clinical Case Definitions
Acute Respiratory Tract Infection
Acute onset of respiratory tract infection (<2 weeks) was defined as the presence of ≥2 of the following: fever, cough, purulent sputum, and clinical response to antibiotics (amoxicillin and/or erythromycin).
Tuberculosis
Definite tuberculosis was defined as a positive, genotype-confirmed culture for Mycobacterium tuberculosis; probable active mycobacterial disease was defined as 2 sputum smears positive for acid-fast bacilli on Ziehl-Neelsen staining without culture confirmation.
Clinically Suspected CLD
CLD was defined as at two or more of chronic cough (present most days for 3 months of the year in the past 2 years), recurrent respiratory tract infections (> antibiotic courses in the last year) and moderate to severe limitation in physical activity caused by breathlessness (NYHA class 2–4), and/or an existing diagnosis and/or signs of cor pulmonale (finger clubbing, raised jugular venous pressure), or hypoxia (O2 sat ≥92%) at rest, or desaturation (O2 sat ≥5%) on exercise.
Ethical Considerations
Written informed consent was obtained from all participants and from guardians of participants aged <16 years. Ethical approval was obtained from the Medical Research Council of Zimbabwe, the London School of Hygiene and Tropical Medicine Ethics Committee, and the Biomedical Research and Training Institute Institutional Review Board.
RESULTS
Demographic and Clinical Characteristics
Five individuals were excluded (1 with a recent diagnosis of tuberculosis, 3 who did not obtain parental consent, and 1 with likely horizontally acquired HIV infection). Characteristics of the 116 participants (mean age, 14 ± 2.6 years, 43% male) are summarized in Table 1. None were cigarette smokers or had smoke exposure from indoor open fires. HIV was diagnosed a median of 23 months (interquartile range [IQR], 10–45) before recruitment, and 99 patients (85%) had WHO stage 3/4 disease. Reasons for HIV testing were presumed tuberculosis in 20 (17%), repeated chest infections or coughing in 23 (20%), other illnesses in 60 (52%), and following HIV diagnosis in a parent or parental death in 13 (11%). Eighty (69%) participants were taking ART for a median duration of 20 months (IQR, 5–40). Overall, median CD4 count was 384 cells/µL (IQR, 180–584) and did not differ between those who were receiving ART (median CD4 count, 402 cells/µL) and those who were not (median CD4 count, 351 cells/µL) (P < .58).
Table 1.
Baseline Demographic and Clinical Characteristics of Study Participants (N = 116)
| Baseline Characteristic | No. (%) |
|---|---|
| Age, years | |
| ≤12 | 34 (29) |
| 13–15 | 39 (34) |
| 16–18 | 43 (37) |
| Male | 50 (43) |
| Orphanhood | |
| Both parents alive | 9 (8) |
| Single orphan | 51 (44) |
| Double orphan | 56 (48) |
| Educational level | |
| None | 3 (2) |
| Primary | 52 (45) |
| Secondary | 61 (53) |
| Age at HIV diagnosis, y, median (IQR) | 12 (10–15) |
| Taking co-trimoxazole prophylaxis | 111 (96) |
| Taking ART | 80 (69) |
| Type of ART | |
| 2 NRTI + 1 NNRTI | 72 (62) |
| 2 NRTI + PI | 6 (5) |
| Height-for-age z score, median (IQR) | −1.96 (−2.9 to −1.3) |
| Weight-for-age z score, median (IQR) | −1.74 (−2.9 to −0.79) |
| BMI z score, median (IQR) | −0.69 (−1.7 to 0.1) |
| Pubertal delay (Tanner stage 1/2 in ≥14 y)a | 21 (18) |
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; HIV, human immunodeficiency virus; IQR, interquartile range; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
a Data missing for 2 participants.
Eighty-two (71%) participants had a prior diagnosis of CLD made by a physician, and 42 (36%) had been treated for suspected tuberculosis at least once. Disabling respiratory insufficiency was common (Table 2): 24 (21%) reported reduced exercise tolerance (NYHA class >2), 41 (35%) were dyspneic at rest and/or hypoxic at rest, and 21 (29%) had a ≥5% fall in O2 sat following exercise. Abnormal lung function (forced expiratory volume in 1 second [FEV1] < 80% predicted) was noted in 52 (45%) participants. Of ART-naive participants with CD4 counts >350 cells/µL, 8 of 18 (44%) had clinically defined CLD and 5 of 18 (28%) had an abnormal HRCT scan. There was no association between FEV1 and duration of ART or current CD4 count (P = .65 and P = .31, respectively). On Doppler echocardiography, 16 patients (14%) had raised mean pulmonary artery pressure >20 mm Hg and 7% had pulmonary hypertension (mean pulmonary artery pressure >25 mm Hg) [18].
Table 2.
Respiratory Symptoms and Signs in Study Participants
| Characteristic | Patients, No. (%) |
|---|---|
| Clinical history | |
| Previously treated for tuberculosisa | 42 (36) |
| History of other cardiorespiratory conditions | |
| Treatment for asthma | 11 (9) |
| Treatment for PCP | 7 (6) |
| Dilated cardiomyopathy | 1 (1) |
| Hospitalized for LRTI in the past year | 19 (16) |
| >2 courses of antibiotics for LRTI in the past year | 48 (41) |
| Recurrentb cough ± purulent sputum | 77 (66) |
| NYHA classc | |
| 1 | 72 (62) |
| 2 | 20 (17) |
| 3 | 22 (19) |
| 4 | 2 (2) |
| Symptoms (current/recent) | |
| Cough | 84 (72) |
| Sputum | 54 (47) |
| Exertional chest tightness | 49 (42) |
| Clinical assessment | |
| Clubbing | 12 (10) |
| Bibasal crackles | 37 (32) |
| Wheeze on auscultation | 2 (2) |
| Tachycardia (HR >100), at rest | 35 (30) |
| Respiratory rate >25/min at rest | 33 (28) |
| Resting O2 sat <92% at rest | 15 (13) |
| Drop of ≥5% O2 sat on exercise testing (n = 72)d | 21 (29) |
| Pulmonary arterial pressure (n = 110)e | |
| <20 mm Hg | 94 (86) |
| 20–25 mm Hg | 8 (7) |
| >25 mm Hg | 8 (7) |
| FEV1, % predicted | |
| 80–100 | 64 (55) |
| 50–79 | 40 (35) |
| <50 | 12 (10) |
| PEFR, % predicted | |
| 80–100 | 83 (72) |
| 50–79 | 28 (24) |
| <50 | 5 (4) |
Abbreviations: FEV1, forced expiratory volume in 1 second; HR, heart rate; LRTI, lower respiratory tract infection; NYHA, New York Heart Association; PCP, pneumocystis pneumonia; PEFR, peak expiratory flow rate.
a Three participants treated twice.
b Most days in at least 3 mo in the year in past 2 years.
c NYHA functional classification:1 = no symptoms and no limitation in physical activity; 2 = mild symptoms and slight limitation during physical activity; 3 = marked limitation of activity due to symptoms; 4 = severe limitations in activity, symptoms at rest.
d Three not mobile; 26 had respiratory rate >25; 7 had O2 sat <92% and RR>25; 8 had O2 sat <92%.
e Measured on Doppler echocardiography.
Microbiologic Findings
Sputum, obtained from 54 (46%) participants, was culture positive for bacteria/fungi in 18 (33%): Haemophilus influenzae (n = 6), Staphylococcus aureus (n = 5), Moraxella catarrhalis (n = 5), Pseudomonas aeruginosa (n = 3), Streptococcus pneumoniae (n = 2), Klebsiella pneumoniae (n = 1), Listeria monocytogenes (n = 1), and Candida albicans (n = 3). Mycobacterial cultures were positive in 12 patients: M. tuberculosis was grown from 8 participants (smear positive = 7); additionally, 31 (27%) participants had organisms seen on sputum microscopy (staining on both auramine-O and Ziehl-Neelsen stains, but negative culture results from specimens taken at recruitment). Of these, Mycobacterium gordonae was cultured from 4 (3%) participants, and 18 (16%) had confirmation of positive microscopy but negative cultures when repeat sputum specimens were taken before commencement of antituberculous treatment and processed in a second independent mycobacterial culture laboratory. Unprocessed sputum specimens were sent to the second laboratory because of the unexpectedly high prevalence of smear positivity and concern about possible laboratory artifact (morphology was reported to be unusual for M. tuberculosis) or a nosocomial epidemic of M. tuberculosis.
Radiologic Findings
Abnormalities on chest radiography were reported in 55 participants (47%), the most common being ring and tramline opacities. Features of posttuberculous lung disease (such as volume loss and fibrosis) were not prominent, despite the high proportion of participants who had been treated for tuberculosis.
One hundred participants (86%) met study case definitions for suspected CLD (see the “Methods” section), of whom 56 (56%) had HRCT performed: the investigation was withheld in 23 because of positive sputum microscopy infection control hazard, and an additional 21 participants were referred but did not attend, including 1 who had died. The median interval between CXR and HRCT in the remaining 56 participants was 2 months. Interobserver agreement was good for presence of specific HRCT abnormalities (bronchiectasis, κ = 0.74; bronchial wall thickening, κ = 0.70; small airway (centrilobular) plugging, κ = 0.66; consolidation, κ = 0.56; decreased attenuation, κ = 0.75; noncavitating nodules, κ = 0.57).
CXR and HRCT Abnormalities in Participants Meeting Case Definition for CLD
Radiologic findings in the 56 participants who received HRCT scans are summarized in Table 3. Of note, the prevalence of CXR abnormalities was not significantly different between participants meeting case definitions for CLD who received HRCT scans and those who met case definitions but did not undergo HRCT (data not shown). Decreased attenuation consistent with small airway disease was the most common and most extensive HRCT abnormality, followed by (and associated with) large airway abnormalities (bronchial wall thickening, small and large airway plugging, and bronchiectasis; Figure 1, Table 3). Our protocol obtained HRCT images at full inspiration (having not anticipated the high prevalence of small airway disease), but pronounced decreased attenuation was observed in 4 HRCT scans serendipitously performed in the expiratory phase. Ground-glass opacification and nodules were seen in a minority of cases, and features suggesting interstitial lung disease were also rare.
Table 3.
Prevalence of Abnormalities on Chest Radiography and High-Resolution Computed Tomography in 56 Patients Undergoing Full Imaging Evaluation
| Radiologic Abnormality | Prevalence of Abnormality, No. (%) | With >5% Extent of Abnormality, No. (%) |
|---|---|---|
| CXR | ||
| Rings/tramline opacities | 18 (32) | 18 (32) |
| Consolidation | 7 (12) | 6 (11) |
| Volume loss | 7 (12) | NA |
| Paucity of vascular markings | 5 (9) | NA |
| Noncavitating nodules | 4 (7) | 4 (7) |
| Ground-glass opacification | 2 (4) | 2 (4) |
| Hyperexpansion | 2 (4) | NA |
| Reticular pattern | 0 (0) | 0 (0) |
| HRCT | ||
| Airway abnormality | ||
| Decreased attenuation | 31 (55) | 27 (48) |
| Bronchiectasis | 24 (43) | NA |
| Bronchial wall thickening | 20 (36) | NA |
| Small airway plugging | 13 (23) | NA |
| Large airway plugging | 6 (11) | NA |
| Parenchymal abnormality | ||
| Consolidation | 18 (32) | 4 (7) |
| Noncavitating nodules | 11 (20) | 2 (4) |
| Reticular pattern | 1 (2) | 0 (0) |
| Emphysema | 8 (14) | 1 (2) |
| Cysts | 6 (11) | 3 (5) |
| Ground-glass opacification | 6 (11) | 1a (2) |
| Cavitation | 3 (5) | 0 (0) |
Abbreviation: CXR, chest radiography; HRCT, high-resolution computed tomography; NA, not applicable.
a 90% extent.
Figure 1.
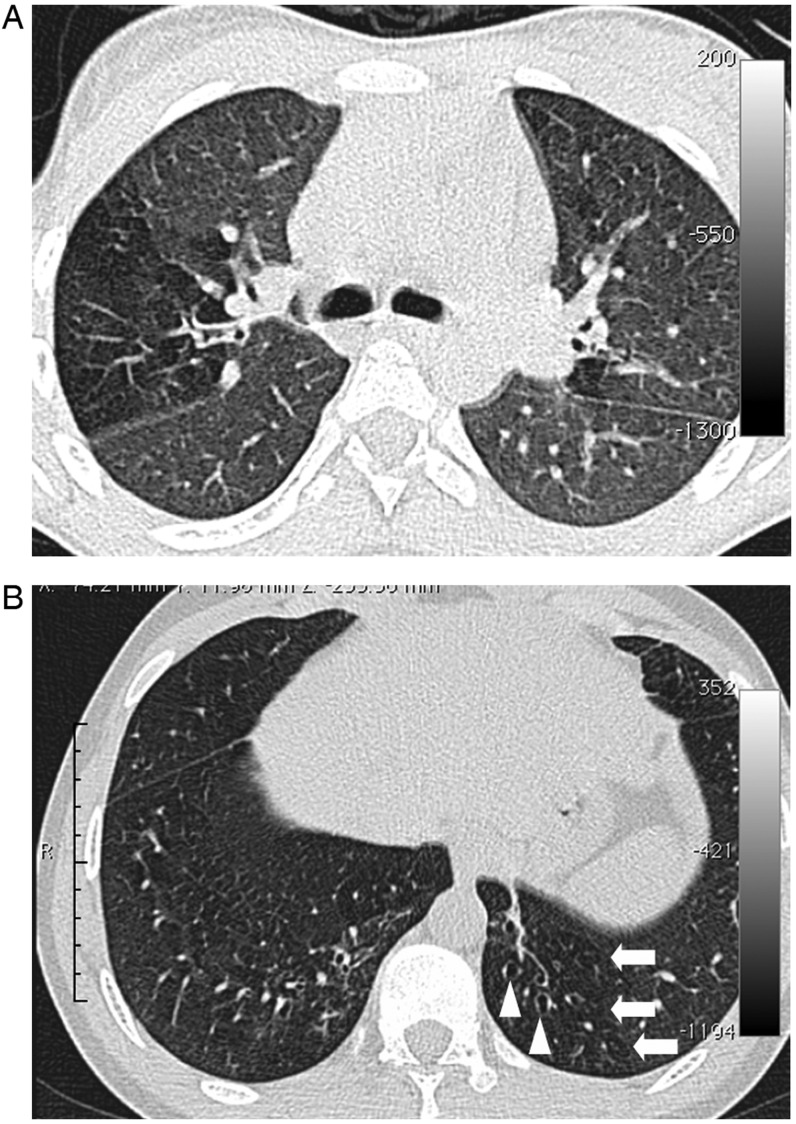
Lung high-resolution computed tomography findings in participants. A, Image section at the level of the carina in a 15-year-old female. There is a clear zone of decreased attenuation in the right upper lobe (and, to a lesser extent, the left lung). In regions of decreased attenuation there is reduction in the caliber of pulmonary vessels; there was no bronchiectasis in this patient. B, Image section in a 19-year-old male through the lower zones demonstrating focal areas of decreased attenuation in both lungs (arrows) and bronchiectasis in the left lower lobe (arrowheads).
On HRCT, the extent of decreased attenuation was strongly correlated with extent of bronchiectasis (Spearman correlation coefficient, rs = 0.80; P < .001) and with other features indicating large airway pathology (severity of bronchial wall dilatation (rs = 0.80; P < .001) and bronchial wall thickening (rs = 0.73; P < .001). The association between extent of decreased attenuation and a composite score of the extent of small and large airway plugging on HRCT (“secretion score”) was weaker but statistically significant (rs = 0.48; P < .001). The extent of bronchiectasis was also significantly associated with secretion score (rs = 0.60; P < .001). Ring/tramline opacities on CXR were associated with secretion score (P = .006) but not with any other HRCT abnormalities, including decreased attenuation, bronchiectasis, bronchial wall thickening, or bronchial dilatation. Consolidation on CXR was also significantly correlated with HRCT secretion score (P = .005).
Association Between HRCT Patterns and FEV1
The extent of decreased attenuation was inversely correlated with FEV1 (rs = −0.26; P = .06), as was consolidation (rs = −0.3; P = .05). No other HRCT features, including bronchiectasis, correlated with FEV1.
DISCUSSION
The major finding of this study was the high burden of severe respiratory insufficiency among HIV-infected older children receiving HIV care after delayed diagnosis of presumed vertically acquired HIV infection. Two-thirds had chronic cough; recurrent lower respiratory tract infections and active mycobacterial disease were common, including persistent culture-negative smear positivity potentially reflecting nontuberculous mycobacterial disease. Pulmonary function tests were abnormal in 45% of participants, and a similar proportion had resting or exertional hypoxia. Pulmonary arterial hypertension was present in 7%. HRCT suggested small airway disease as the predominant underlying pathology, a previously undescribed finding with major implications for the health and long-term survival of perinatally HIV-infected adolescents.
We had anticipated a high prevalence of LIP in our cohort because it is the most common cause of HIV-associated CLD in younger children and because slow HIV progression and LIP are strongly correlated [9, 10, 19]. However, there was a striking absence of features (cysts and ground-glass opacification) to support active LIP or subsequent fibrosis [20]. This may reflect the natural history of LIP for self-resolution and prompt response to ART [21]. Instead, the most prevalent abnormality on HRCT was patchy (mosaic) decreased attenuation, for which the differential diagnosis is small airway disease, chronic pulmonary thromboembolic lung disease, or infiltrative lung disease. Of these, small airway disease is by far the most likely because of the significant association with (1) FEV1 (an index of airflow limitation) and (2) large airway abnormalities such as bronchiectasis.
The most common small airway disease is asthma, but the relatively stable clinical condition of hypoxic participants (attending a routine outpatient clinic and not in acute distress when recruited) argues against this diagnosis, as does the low prevalence of wheezing. Moreover, we have since confirmed a similarly high prevalence of hypoxic lung disease and shown lack of responsiveness to inhaled bronchodilators (excluding asthma) among older children and adolescents receiving HIV care in Blantyre, Malawi [22]. This leaves obliterative bronchiolitis as the most common cause of CLD in this patient group [23, 24].
Obliterative bronchiolitis is a life-threatening condition that responds poorly to treatment. It can progress to hypoxic respiratory failure and cor pulmonale or it can develop acutely and then remain at a relatively stable level of disability (eg, when due to adenovirus infection). In young children it may impair subsequent lung development. Obliterative bronchiolitis can result from a number of insults, including drug reactions; inhalation of allergens or toxins; and infection with a number of pathogens including adenovirus, mycoplasma, respiratory syncytial virus, or influenza, It is also an integral feature of the pathology of cystic fibrosis and chronic rejection following lung transplantation, with some evidence for genetic predisposition to severe forms [25]. It is thought to result from epithelial injury in the small airways, which undergo repair with fibrosis, leading to narrowing and airflow limitation [26]. Obliterative bronchiolitis has not been recognized in European or American cohorts of HIV-infected children, suggesting either geographic variability (eg, infantile adenoviral obliterative bronchiolitis has a Southern hemisphere predominance) or that ART during early childhood is preventive [27].
Obliterative bronchiolitis is prone to misdiagnosis and diagnostic delay because patients often appear well at rest with only subtle clinical and plain radiologic abnormalities [28]. In our study, the main HRCT finding had no CXR correlate, except in the subgroup with concurrent bronchiectasis. Decreased attenuation on HRCT was reported from lobes without overt bronchiectasis, implying that small airway disease precedes development of bronchiectasis [29], a phenomenon well recognized in obliterative bronchiolitis due to cystic fibrosis and adenovirus infection [25, 30].
HIV has not previously been recognized as predisposing to childhood obliterative bronchiolitis , but untreated HIV-infected children frequently experience multiple bacterial and viral respiratory tract infections over many years [6]. We postulate that these infections, together with HIV-induced impairment of innate immune responses [31, 32], predispose to chronic lower respiratory tract inflammation that in turn increases susceptibility to further respiratory tract infections. Small airway disease may then result from cumulative inflammatory damage incurred during unusually frequent or severe viral/bacterial infections (similar to HIV-negative children with adenovirus, measles, or Bordetella infection). Alternatively, HIV-infected children may be predisposed to an inflammatory obliterative bronchiolitis that does not require a secondary pathogen other than HIV itself. In either event, host pulmonary inflammatory responses may be modulated by HIV and potentially by genetically determined immunologic responses. Persistent airway inflammation facilitates airway remodeling and development of bronchiectasis [33–36].
Previous studies of HIV-related CLD in this age group are limited to CXR findings [9, 37, 38], which are insensitive and subject to observer error and inconsistent use of terminology [39]. The strengths of this study are its prospective design, unselected recruitment, exclusion of acutely unwell patients, use of HRCT, and low interobserver variability in CXR and HRCT scoring. Limitations include the cross-sectional design, incomplete uptake of HRCT in participants meeting definitions for chronic lung disease, and lack of histologic confirmation. Although histology provides the “gold standard” for diagnosis of obliterative bronchiolitis, open or thorascopic lung biopsy or postmortem examination is required because of the patchy and peripheral nature of the disease process [40]. Increasingly, HRCT without biopsy is considered to be sufficiently characteristic to be used for initial diagnosis, and the chronic symptoms, structural damage, mosaic pattern of decreased attenuation, and strong association with FEV1 provide strong evidence for obliterative bronchiolitis in our patients [41]. Finally, although we were careful to avoid further selection bias, our recruitment was limited to adolescents known to be receiving HIV care services, whereas the majority of African adolescent survivors of perinatal HIV infection are still undiagnosed [7].
Our study showed a substantial burden of CLD in vertically HIV-infected adolescents, even at high CD4 counts and following several years of ART. Other than a single case report in an HIV-infected 11-year-old, this is the first description of small airway disease as a cause of HIV-associated CLD [42]. As HIV epidemics mature, increasing numbers of previously undiagnosed HIV-infected older children are being identified [6]. Our findings suggest that HIV-infected children with chronic respiratory symptoms or evidence of airflow obstruction should be investigated for possible obliterative bronchiolitis as well as for mycobacterial disease. Earlier diagnosis of HIV infection may be crucial to preventing the development of CLD and suggests that international recommendations (which currently do not support immediate initiation of ART in children diagnosed over the age of 2 years) need to take the high risk of CLD and potential for prevention with early ART initiation into account. The lack of association between CD4 count or duration of ART and pulmonary function infers that once established, lung damage may be irreversible. Possible intervention strategies include aggressive management of intercurrent infection and use of prophylactic antibiotics, which may delay progression of airway disease and reduce morbidity, and, potentially, corticosteroids [43]. Further investigation of the natural history, histopathology, geographic distribution, pathogenesis, and management of this newly described and highly prevalent HIV-associated CLD is needed.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://www.oxfordjournals.org/our_journals/cid/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Prudence Jarrett and the staff at Parirenyatwa Hospital and Harare Central Hospital for their help with recruitment; the Diagnostic Imaging Centre, Harare, for performing the chest radiographs and the HRCT scans; and Professor James Hakim and Professor Jonathan Matenga for performing the Doppler echocardiograms.
Financial support. The study was funded by the Wellcome Trust. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.AIDS epidemic update. Geneva, Switzerland: UNAIDS and WHO; 2009. Joint United Nations Programme on HIV/AIDS. [PubMed] [Google Scholar]
- 2.Newell ML, Coovadia H, Cortina-Borja M, Rollins N, Gaillard P, Dabis F. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet. 2004;364:1236–43. doi: 10.1016/S0140-6736(04)17140-7. [DOI] [PubMed] [Google Scholar]
- 3.Marston M, Zaba B, Salomon JA, Brahmbhatt H, Bagenda D. Estimating the net effect of HIV on child mortality in African populations affected by generalized HIV epidemics. J Acquir Immune Defic Syndr. 2005;38:219–27. doi: 10.1097/00126334-200502010-00015. [DOI] [PubMed] [Google Scholar]
- 4.Stover J, Walker N, Grassly NC, Marston M. Projecting the demographic impact of AIDS and the number of people in need of treatment: updates to the Spectrum projection package. Sex Transm Infect. 2006;82(Suppl 3):iii45–50. doi: 10.1136/sti.2006.020172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrand RA, Corbett EL, Wood R, et al. AIDS among older children and adolescents in Southern Africa: projecting the time course and magnitude of the epidemic. AIDS. 2009;23:2039–46. doi: 10.1097/QAD.0b013e32833016ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrand RA, Munaiwa L, Matsekete J, et al. Undiagnosed HIV infection among adolescents seeking primary health care in Zimbabwe. Clin Infect Dis. 2010;51:844–51. doi: 10.1086/656361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrand R, Lowe S, Whande B, et al. Survey of children accessing HIV services in a high prevalence setting: time for adolescents to count? Bull World Health Organ. 2010;88:428–34. doi: 10.2471/BLT.09.066126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrand RA, Bandason T, Musvaire P, et al. Causes of acute hospitalization in adolescence: burden and spectrum of HIV-related morbidity in a country with an early-onset and severe HIV epidemic: a prospective survey. PLoS Med. 2010;7:e1000178. doi: 10.1371/journal.pmed.1000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeena PM, Coovadia HM, Thula SA, Blythe D, Buckels NJ, Chetty R. Persistent and chronic lung disease in HIV-1 infected and uninfected African children. AIDS. 1998;12:1185–93. doi: 10.1097/00002030-199810000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Sharland M, Gibb DM, Holland F. Respiratory morbidity from lymphocytic interstitial pneumonitis (LIP) in vertically acquired HIV infection. Arch Dis Child. 1997;76:334–6. doi: 10.1136/adc.76.4.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sitbon O, Lascoux-Combe C, Delfraissy JF, et al. Prevalence of HIV-related pulmonary arterial hypertension in the current antiretroviral therapy era. Am J Respir Crit Care Med. 2008;177:108–13. doi: 10.1164/rccm.200704-541OC. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization. Geneva, Switzerland: WHO; 2005. Interim WHO clinical staging of HIV/AIDS and HIV/AIDS case definitions for surveillance–African Region. Report No.: WHO/HIV/2005.02. [Google Scholar]
- 13.Munyati SS, Dhoba T, Makanza ED, et al. Chronic cough in primary health care attendees, Harare, Zimbabwe: diagnosis and impact of HIV infection. Clin Infect Dis. 2005;40:1818–27. doi: 10.1086/429912. [DOI] [PubMed] [Google Scholar]
- 14.Desai SR, Copley SJ, Barker RD, et al. Chest radiography patterns in 75 adolescents with vertically-acquired human immunodeficiency virus (HIV) infection. Clin Radiol. 2011;66:257–63. doi: 10.1016/j.crad.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hansell DM, Bankier AA, MacMahon H, McLoud TC, Muller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246:697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 16.Cole TJ. Growth monitoring with the British 1990 growth reference. Arch Dis Child. 1997;76:47–9. doi: 10.1136/adc.76.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zverev Y, Gondwe M. Ventilatory capacity indices in Malawian children. East Afr Med J. 2001;78:14–8. doi: 10.4314/eamj.v78i1.9105. [DOI] [PubMed] [Google Scholar]
- 18.Galie N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT) Eur Heart J. 2009;30:2493–537. doi: 10.1093/eurheartj/ehp297. [DOI] [PubMed] [Google Scholar]
- 19.Zar HJ. Chronic lung disease in human immunodeficiency virus (HIV) infected children. Pediatr Pulmonol. 2008;43:1–10. doi: 10.1002/ppul.20676. [DOI] [PubMed] [Google Scholar]
- 20.Johkoh T, Muller NL, Pickford HA, et al. Lymphocytic interstitial pneumonia: thin-section CT findings in 22 patients. Radiology. 1999;212:567–72. doi: 10.1148/radiology.212.2.r99au05567. [DOI] [PubMed] [Google Scholar]
- 21.Dufour V, Wislez M, Bergot E, Mayaud C, Cadranel J. Improvement of symptomatic human immunodeficiency virus-related lymphoid interstitial pneumonia in patients receiving highly active antiretroviral therapy. Clin Infect Dis. 2003;36:e127–30. doi: 10.1086/374665. [DOI] [PubMed] [Google Scholar]
- 22.Rylance J, Mwalukomo T, Rylance S, et al. Seattle, WA: 2012. Lung function and bronchodilator response in perinatally HIV-infected Malawian adolescents. Program and abstracts of the 19th Conference on Retroviruses and Opportunistic Infections. [Google Scholar]
- 23.Worthy SA, Muller NL, Hartman TE, Swensen SJ, Padley SP, Hansell DM. Mosaic attenuation pattern on thin-section CT scans of the lung: differentiation among infiltrative lung, airway, and vascular diseases as a cause. Radiology. 1997;205:465–70. doi: 10.1148/radiology.205.2.9356630. [DOI] [PubMed] [Google Scholar]
- 24.Garg K, Lynch DA, Newell JD, King TE., Jr Proliferative and constrictive bronchiolitis: classification and radiologic features. AJR Am J Roentgenol. 1994;162:803–8. doi: 10.2214/ajr.162.4.8140994. [DOI] [PubMed] [Google Scholar]
- 25.Tiddens HA, Koopman LP, Lambert RK, et al. Cartilaginous airway wall dimensions and airway resistance in cystic fibrosis lungs. Eur Respir J. 2000;15:735–42. doi: 10.1034/j.1399-3003.2000.15d18.x. [DOI] [PubMed] [Google Scholar]
- 26.Kraft M, Mortenson RL, Colby TV, Newman L, Waldron JA, Jr, King TE., Jr Cryptogenic constrictive bronchiolitis. A clinicopathologic study. Am Rev Respir Dis. 1993;148(4 Pt 1):1093–101. doi: 10.1164/ajrccm/148.4_Pt_1.1093. [DOI] [PubMed] [Google Scholar]
- 27.Colom AJ, Teper AM, Vollmer WM, Diette GB. Risk factors for the development of bronchiolitis obliterans in children with bronchiolitis. Thorax. 2006;61:503–6. doi: 10.1136/thx.2005.044909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Breatnach E, Kerr I. The radiology of cryptogenic obliterative bronchiolitis. Clin Radiol. 1982;33:657–61. doi: 10.1016/s0009-9260(82)80395-4. [DOI] [PubMed] [Google Scholar]
- 29.Hansell DM, Wells AU, Rubens MB, Cole PJ. Bronchiectasis: functional significance of areas of decreased attenuation at expiratory CT. Radiology. 1994;193:369–74. doi: 10.1148/radiology.193.2.7972745. [DOI] [PubMed] [Google Scholar]
- 30.Becroft DM. Bronchiolitis obliterans, bronchiectasis, and other sequelae of adenovirus type 21 infection in young children. J Clin Pathol. 1971;24:72–82. doi: 10.1136/jcp.24.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Noursadeghi M, Katz DR, Miller RF. HIV-1 infection of mononuclear phagocytic cells: the case for bacterial innate immune deficiency in AIDS. Lancet Infect Dis. 2006;6:794–804. doi: 10.1016/S1473-3099(06)70656-9. [DOI] [PubMed] [Google Scholar]
- 32.Tsang J, Chain BM, Miller RF, et al. HIV-1 infection of macrophages is dependent on evasion of innate immune cellular activation. AIDS. 2009;23:2255–63. doi: 10.1097/QAD.0b013e328331a4ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tiddens H, Silverman M, Bush A. The role of inflammation in airway disease: remodeling. Am J Respir Crit Care Med. 2000;162(2 Pt 2):S7–10. doi: 10.1164/ajrccm.162.supplement_1.maic-2. [DOI] [PubMed] [Google Scholar]
- 34.Bush A, Tiddens H, Silverman M. Clinical implications of inflammation in young children. Am J Respir Crit Care Med. 2000;162(2 Pt 2):S11–4. doi: 10.1164/ajrccm.162.supplement_1.maic-3. [DOI] [PubMed] [Google Scholar]
- 35.Rao S, Grigg J. New insights into pulmonary inflammation in cystic fibrosis. Arch Dis Child. 2006;91:786–8. doi: 10.1136/adc.2004.069419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elizur A, Cannon CL, Ferkol TW. Airway inflammation in cystic fibrosis. Chest. 2008;133:489–95. doi: 10.1378/chest.07-1631. [DOI] [PubMed] [Google Scholar]
- 37.Norton KI, Kattan M, Rao JS, et al. Chronic radiographic lung changes in children with vertically transmitted HIV-1 infection. AJR Am J Roentgenol. 2001;176:1553–8. doi: 10.2214/ajr.176.6.1761553. [DOI] [PubMed] [Google Scholar]
- 38.Sheikh S, Madiraju K, Steiner P, Rao M. Bronchiectasis in pediatric AIDS. Chest. 1997;112:1202–7. doi: 10.1378/chest.112.5.1202. [DOI] [PubMed] [Google Scholar]
- 39.Cleveland RH, Schluchter M, Wood BP, et al. Chest radiographic data acquisition and quality assurance in multicenter studies. Pediatr Radiol. 1997;27:880–7. doi: 10.1007/s002470050262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parambil JG, Yi ES, Ryu JH. Obstructive bronchiolar disease identified by CT in the non-transplant population: analysis of 29 consecutive cases. Respirology. 2009;14:443–8. doi: 10.1111/j.1440-1843.2008.01445.x. [DOI] [PubMed] [Google Scholar]
- 41.Devakonda A, Raoof S, Sung A, Travis WD, Naidich D. Bronchiolar disorders: a clinical-radiological diagnostic algorithm. Chest. 2010;137:938–51. doi: 10.1378/chest.09-0800. [DOI] [PubMed] [Google Scholar]
- 42.Mauskar A, Shanbag P, Dadge D. Bronchiolitis obliterans in a child with HIV infection. Indian J Pediatr. 2011;78:112–14. doi: 10.1007/s12098-010-0231-x. [DOI] [PubMed] [Google Scholar]
- 43.Ratjen F, Doring G. Cystic fibrosis. Lancet. 2003;361:681–9. doi: 10.1016/S0140-6736(03)12567-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.