A single vaccination with MVA-NP+M1 boosts T-cell responses to conserved influenza antigens in humans. Protection against influenza disease and virus shedding was demonstrated in an influenza virus challenge study.
Abstract
Background. The novel influenza vaccine MVA-NP+M1 is designed to boost cross-reactive T-cell responses to internal antigens of the influenza A virus that are conserved across all subtypes, providing protection against both influenza disease and virus shedding against all influenza A viruses. Following a phase 1 clinical study that demonstrated vaccine safety and immunogenicity, a phase 2a vaccination and influenza challenge study has been conducted in healthy adult volunteers.
Methods. Volunteers with no measurable serum antibodies to influenza A/Wisconsin/67/2005 received either a single vaccination with MVA-NP+M1 or no vaccination. T-cell responses to the vaccine antigens were measured at enrollment and again prior to virus challenge. All volunteers underwent intranasal administration of influenza A/Wisconsin/67/2005 while in a quarantine unit and were monitored for symptoms of influenza disease and virus shedding.
Results. Volunteers had a significantly increased T-cell response to the vaccine antigens following a single dose of the vaccine, with an increase in cytolytic effector molecules. Intranasal influenza challenge was undertaken without safety issues. Two of 11 vaccinees and 5 of 11 control subjects developed laboratory-confirmed influenza (symptoms plus virus shedding). Symptoms of influenza were less pronounced in the vaccinees and there was a significant reduction in the number of days of virus shedding in those vaccinees who developed influenza (mean, 1.09 days in controls, 0.45 days in vaccinees, P = .036).
Conclusions. This study provides the first demonstration of clinical efficacy of a T-cell–based influenza vaccine and indicates that further clinical development should be undertaken.
Clinical Trials Registration. NCT00993083.
A recent meta-analysis of influenza vaccine efficacy and effectiveness [1] concluded that protection against virologically confirmed influenza is at best moderate, and in some seasons is greatly reduced or completely absent. Even in the most favorable situation when the vaccine is exceptionally well matched to the circulating virus, as was the case for pandemic H1N1 vaccines, median effectiveness in adults <65 years was 69%. The size of the influenza vaccine market was US$2.8 billion in 2008–2009 in 7 major markets (United States, Japan, France, Germany, Italy, Spain, and United Kingdom) [2]. An increasingly greater proportion of the population is vaccinated, with vaccination for all individuals aged >6 months recommended in some countries, but vaccines with considerably improved and more consistent effectiveness are required in order to bring about a greater reduction in influenza-related morbidity and mortality.
Trivalent inactivated vaccines are used as influenza vaccines in most circumstances, with live attenuated influenza vaccines sometimes used in children. Although cytotoxic T-cell–mediated immunity against influenza is an important component of naturally acquired immunity [3, 4], the trivalent inactivated vaccine does not stimulate this response, and live attenuated influenza vaccine has been found to prime a T-cell–mediated response in young children but not to boost it in adults who have already acquired T-cell responses to influenza antigens following natural exposure to the virus [5]. Because the main targets of T-cell recognition are internal antigens of the influenza virus that are well conserved between influenza A virus subtypes, [6] T-cell–mediated immunity should provide much broader protection than antibodies specific for the highly polymorphic external glycoproteins of the virus.
We have previously reported on the use of a novel influenza vaccine, to boost these cross-reactive T-cell responses in adult volunteers, in a phase 1 study that demonstrated the safety and immunogenicity of the vaccine [7]. MVA-NP+M1 is a modified vaccinia virus Ankara (MVA) vector (replication-deficient) expressing the conserved internal influenza antigens nucleoprotein (NP) and matrix protein 1 (M1). T-cell responses to these antigens are known to be induced by influenza infection [6]. We now describe a phase 2a vaccination and influenza challenge study, the first study to test the efficacy of an influenza vaccine designed to boost T-cell responses without inducing antihemagglutinin antibodies. The study confirmed vaccine safety and immunogenicity and provides preliminary evidence of vaccine efficacy, with a 60% reduction of laboratory-confirmed influenza in vaccinated subjects.
MATERIALS AND METHODS
Vaccine Design and Manufacture
MVA-NP+M1 design and manufacture are described in [7].
Study Population
Volunteers were recruited and enrolled following written informed consent under a protocol approved by the UK Medicines and Healthcare Products Regulatory Agency and the Oxfordshire NHS Research Ethics Committee. Recruitment took place at the Centre for Clinical Vaccinology and Tropical Medicine, Oxford and the Wellcome Trust Clinical Research Facility, Southampton. Volunteers were aged 18–45 years and were initially screened by hemagglutination inhibition (HI) assay against the virus to be used in the challenge phase of the study to ensure susceptibility to challenge. Those with a titer ≤1:10 were eligible for further screening. Enrolled volunteers were seronegative for human immunodeficiency virus (HIV), hepatitis B virus, and hepatitis C virus and had not received seasonal influenza vaccination for at least 1 year prior to enrollment. Results of routine hematological and biochemical tests on enrolled volunteers were all within normal limits.
Vaccination and Follow-up Regimen
Following receipt of study information, volunteers attended a screening visit to assess their suitability for the study. Two volunteers were screened, vaccinated, and underwent influenza challenge ahead of the main cohort. Subsequent eligible volunteers were enrolled first into the vaccination group and subsequently into the control challenge group. The volunteers taking part in the main efficacy cohort were screened for enrollment between 3 August 2009 and 9 September 2009. Influenza transmission rates during this period were low in the areas in which volunteers were recruited, and no volunteer had experienced an influenza-like illness prior to screening. Vaccinated volunteers received a single intramuscular injection of 1.5 × 108 plaque-forming units (PFUs) of MVA-NP+M1 (dose volume, 1154 μL) 28 days prior to entry to the quarantine unit. Volunteers were reviewed on day 2 after vaccination to assess adverse events and on day 21 for exploratory immunology blood sampling.
Ex Vivo Interferon γ Enzyme-Linked Immunosorbent Spot Assay
The ex vivo interferon γ (IFN-γ) enzyme-linked immunosorbent spot assay (ELISpot) was performed as previously described [7]. Fifteen- to 20-mer peptides overlapping by 10 amino acid residues, spanning the whole of the NP + M1 insert in pools of 10 peptides, were used to stimulate peripheral blood mononuclear cells (PBMCs) at a concentration of 10 μg/mL. Fifty microliters of PBMCs (2 × 105 cells) and 50 μL of the peptides was tested in triplicate. R10 was used as a negative control, and phytohemagglutinin at a final concentration of 10 μg/mL was used as a positive control. Following an 18–20-hour incubation at 37°C, the ELISpot plates were developed, dried, and read with an AID ELISpot reader (AID Diagnostika). The results are expressed as spot-forming units (SFUs) per million PBMCs after background subtraction.
Flow cytometry, quarantine, and challenge procedures are described in the Supplementary Data.
RESULTS
Vaccine Safety and Immunogenicity
A total of 15 volunteers (11 for the main study, 2 for a pilot challenge study, and 2 volunteers who were vaccinated but then excluded from the influenza challenge for either increase in HI titer to the challenge virus or evidence of recent mild respiratory tract infection) were administered 1.5 × 108 PFU MVA-NP+M1 intramuscularly. The study timeline is shown in Supplementary Figure 1A. Supplementary Figure 1B shows the numbers of volunteers recruited for the vaccine and control groups, with demographic information given in Supplementary Table 1. The safety profile was comparable to other MVA-vectored vaccines, with the majority of adverse events being mild in severity. No severe systemic adverse events were reported.
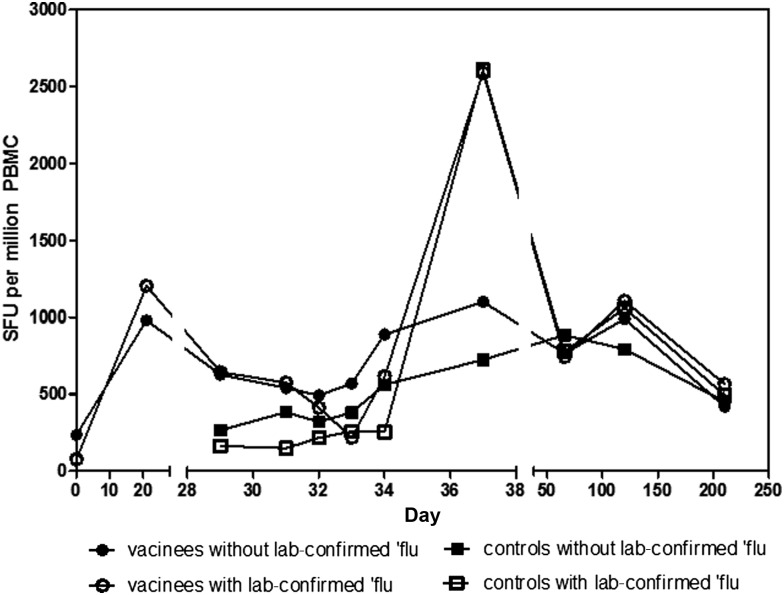
T-cell responses to the influenza antigens NP and M1 were measured in all volunteers at screening and again on day of vaccination and 21 days later in the vaccinees and in all volunteers on the day prior to challenge, as well as 8 occasions after influenza challenge (Figure 1). As previously observed [7], there was a clearly detectable response in all volunteers at the time of screening, with a median response of 258 SFUs per million PBMCs in the group who went on to receive the vaccine and 300 SFUs per million PBMCs in the controls. The level of response was stable prior to vaccination, significantly boosted to 980 SFUs per million PBMCs 21 days after vaccination (P < .001 vs day 0) and then declined to 627 SFUs per million PBMCs 8 days later (the day prior to influenza challenge, P < .05 vs day 0). The response in the control group remained stable prior to influenza challenge, with a median of 215 SFUs per million PBMCs measured on the day prior to influenza challenge (day 29). Although there was no significant difference in the responses between the 2 groups at screening, responses to NP and M1 at day 29 were significantly higher in the vaccinees compared to controls (P < .05) (Figure 1).
Figure 1.
Ex vivo interferon γ enzyme-linked immunosorbent spot assay responses to nucleoprotein (NP) and matrix protein 1 (M1). The graph represents the summed response to NP and M1 antigens in vaccinees (circles) and controls (squares) at the relevant time points; lines represent the median per group and open symbols represent subjects who developed laboratory-confirmed influenza. Control subjects were not assayed at day 0 or day 21. Vaccination took place on day 0 and influenza challenge on day 30. Data were analyzed with a Kruskal-Wallis 1-way analysis of variance with selected pairs of data analyzed with a Dunn positive test. No significant difference between the median response in the vaccinated and control group was observed at time of screening (day 0 for vaccinees, day 29 for controls). A significant increase in the response was observed in vaccinees between days 0 and 21 and days 0 and 29 (P < .001, P < .05, respectively). A significant difference between vaccinees and controls was observed at day 29 (P < .05). Abbreviations: PBMC, peripheral blood mononuclear cell; SFU, spot-forming units.
The response to all influenza antigens in addition to those included in the vaccine was also measured on day 29 using overlapping peptides for each antigen (Supplementary Figure 2). The only statistically significant difference between responses in vaccinees and controls was the magnitude of the response to the vaccine antigens, with the response to NP predominating in most vaccinees.
T-Cell Phenotype of the Immunodominant Response to M158–66 in Vaccinated and Control Volunteers
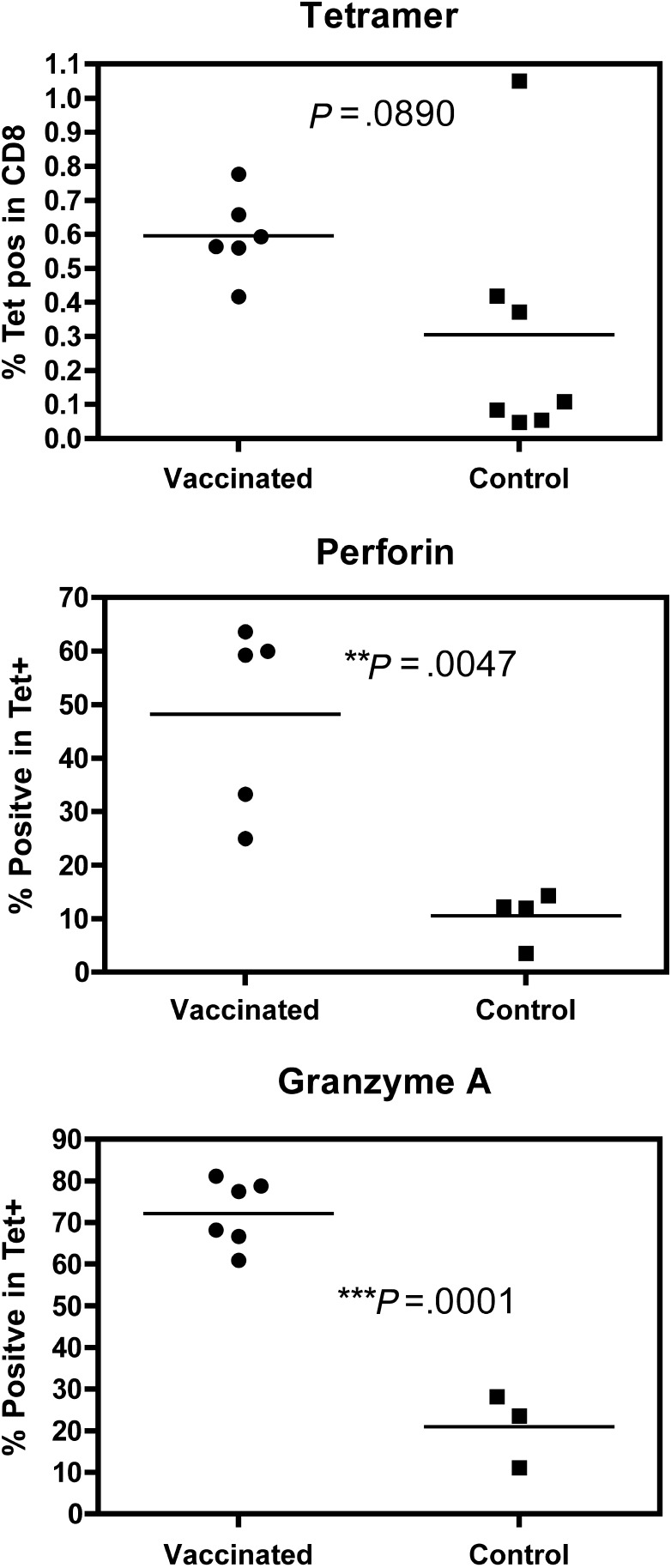
Six of the vaccinees and 7 of the controls were positive for human leukocyte antigen A*0201 and therefore likely to have preexisting T-cell responses to the known A2-restricted immunodominant epitope M158–66. A tetramer for this epitope was used to measure phenotypic markers in PBMCs from these volunteers. A significant difference between vaccinated and control donors on the day prior to influenza challenge was observed in the expression of the cytotoxic markers perforin (D48 epitope [8]) and granzyme A (Figure 2), indicating that the antigen-specific CD8+ T cells in the vaccinees were more highly activated than T cells from the control donors.
Figure 2.
Responses to M158–66 in human leukocyte antigen A2–positive volunteers. Whole blood drawn 1 day prior to virus challenge was labeled for tetramer (A*0201/GILGFVFTL) followed by perforin or granzyme A staining. Values shown are the percentage of CD8+ T cells or Tet+ cells; individuals are shown as a single point with lines representing the median per group. Open symbols represent samples from volunteers who subsequently developed laboratory-confirmed influenza. For each marker the data were analyzed with an unpaired t test; P values are shown for statistically significant differences between vaccinees and controls.
Influenza Challenge Outcome
The safety of the influenza challenge protocol in healthy volunteers is well established, but as this was the first study to our knowledge in which T-cell responses to influenza antigens were boosted by vaccination prior to influenza challenge of human volunteers by intranasal administration, we conducted a pilot safety study of 2 vaccinated volunteers to make an initial assessment of the safety of the protocol prior to the main study. These 2 volunteers underwent the same screening, vaccination, quarantine, and challenge protocol as for the main study, including twice-daily symptom questionnaires and once-daily physician-directed examination to assess their response to influenza challenge following MVA-NP+M1 vaccination. The majority of symptoms recorded were mild, with some evidence of upper respiratory tract infection. Rhinorrhea was the commonest symptom, but no cough or other symptoms of lower respiratory tract infection or severe illness were observed. Following safety review, permission was granted to proceed with influenza challenge for the main study of 11 vaccinees and 11 control subjects.
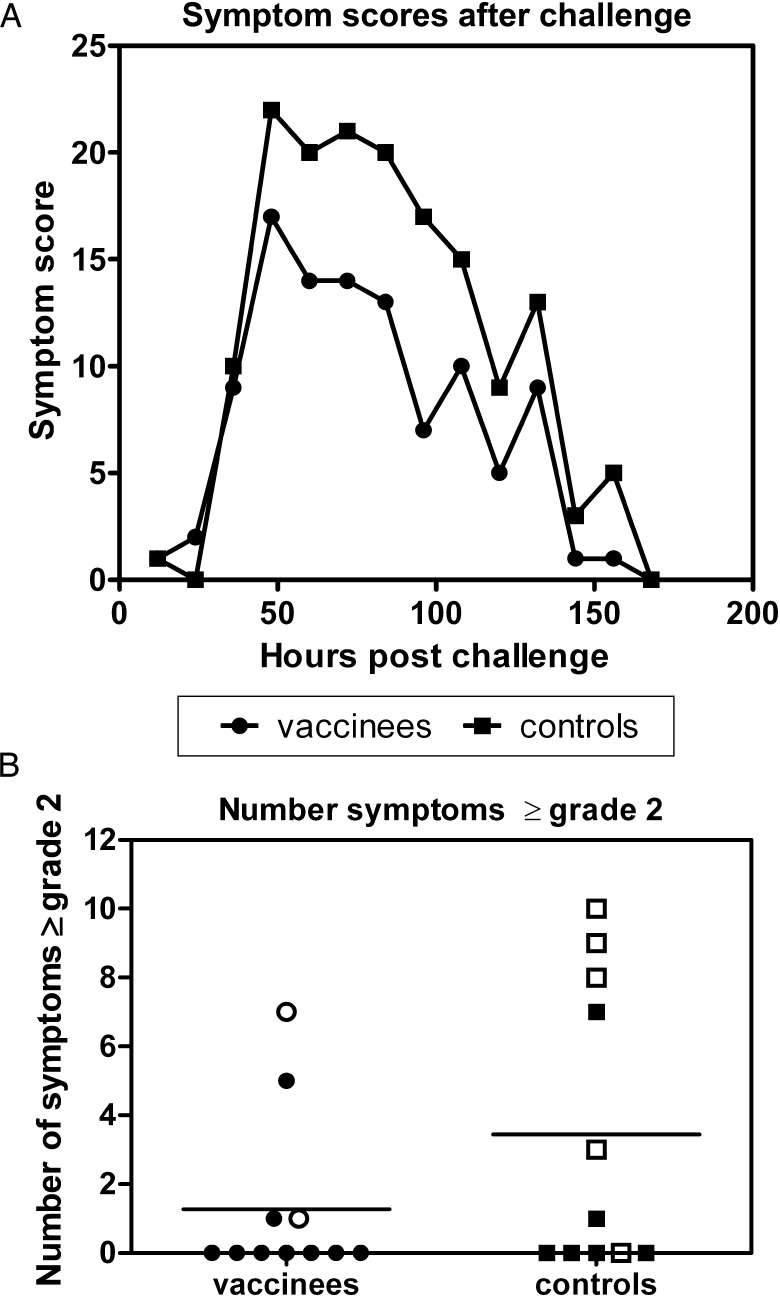
The primary outcome of the challenge study was the number of subjects in each group diagnosed with laboratory-confirmed influenza, defined as mild or moderate/severe symptoms of influenza infection plus laboratory detection of influenza virus in any of the daily nasal washes conducted following influenza challenge (Table 1). In total, 2 vaccinees and 5 controls developed laboratory-confirmed influenza. Of these, 1 vaccinee and 4 controls experienced moderate to severe symptoms in addition to virus shedding. Comparing the vaccinated and control groups as a whole, symptoms were fewer in vaccinees at all time points following influenza challenge (Figure 3A), with symptoms peaking on the second and third days. Vaccinees as a group experienced a significant reduction in the number of days of virus shedding in the presence of laboratory-confirmed influenza (5 of 55 days in vaccinees and 12 of 55 days in controls; P = .036).
Table 1.
Clinical Outcome of Challenge
| Virus (Log10 TCID50) Shed on
Day After Challenge |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Vol No. | Total Symptom Score | Symptom Severity | 1 | 2 | 3 | 4 | 5 | Lab-Confirmed Influenza | HI Postchallenge |
| A: Vaccinees | |||||||||
| 58 | 26 | Mild | N | <10 | |||||
| 76 | 3 | None | N | 20 | |||||
| 79 | 0 | None | N | 40 | |||||
| 80 | 0 | None | 1.75 | 3.00 | N | <10 | |||
| 19 | 0 | None | N | 80 | |||||
| 32 | 12 | Mild | N | <10 | |||||
| 37 | 27 | Mild | N | 160 | |||||
| 39 | 29 | Mod/sev | 3.25 | 4.25 | Y | >640 | |||
| 41 | 3 | None | N | 320 | |||||
| 64 | 12 | Mild | 2.5 | 2.96 | 3.75 | Y | 226 | ||
| 70 | 0 | None | N | >640 | |||||
| B: Controls | |||||||||
| 72 | 29 | Mod/sev | N | 160 | |||||
| 81 | 20 | Mild | 3.25 | 2.00 | Y | <10 | |||
| 84 | 29 | Mod/sev | 2.75 | 3.25 | Y | 40 | |||
| 95 | 4 | Mild | 3.5 | Y | <10 | ||||
| 100 | 35 | Mod/sev | 3.5 | 5.5 | 1.75 | Y | 160 | ||
| 87 | 38 | Mod/sev | 3.00 | 3.25 | 2.5 | 1.75 | Y | 80 | |
| 86 | 0 | None | N | 320 | |||||
| 93 | 1 | None | N | 320 | |||||
| 96 | 4 | Mild | N | 20 | |||||
| 108 | 0 | None | N | 20 | |||||
| 109 | 8 | Mild | N | 80 | |||||
Laboratory-confirmed influenza is defined as mild or moderate to severe symptoms of influenza infection plus shedding of influenza virus on at least 1 day after challenge. Standardized nasal washes and virus assays were performed each day for 5 days on each subject, with no missing data points, but only positive results are shown in the table. The severity of the symptoms is defined by the symptom + examination score, with mild flu having a score of 4–28, moderate to severe is ≥29. HI titers were all <10 at screening and on entry to the quarantine unit. The figures given above are HI titers at study day 66 (26 days after influenza challenge) with the exception of volunteer 093 (study day 120, 90 days after influenza challenge).
Abbreviations: HI, hemagglutination titer; TCID50, median tissue culture infective dose.
Figure 3.
Total of symptom scores at each time point following challenge (A) or total grade 2 and 3 symptom and examination scores (B) for vaccinees (circles) and controls (squares), with the group mean indicated by a line. Open symbols denote subjects who developed laboratory-confirmed influenza after challenge.
Investigation of Immune Responses Associated With Protective Outcome
T-cell responses to all influenza antigens in PBMCs on the day prior to influenza challenge by IFN-γ ELISpot assay were measured (Supplementary Figure 2). Following influenza challenge, there was no correlation between the total symptom score for each subject and the T-cell response to the vaccine antigens NP and M1, to all internal antigens (NP, M1, M2, NS1, NS2, PB1, PB2, PA) or to all influenza antigens (internal plus HA, NA) on the day prior to challenge. ELISpot assays were repeated on days 1, 2, 3, 4, and 7 after challenge (study days 31–37), measuring responses to NP and M1 only. There were minor fluctuations in the number of PBMCs secreting IFN-γ in response to NP and M1 between days 29 and 34, with a pronounced increase on day 37 in those volunteers who developed laboratory-confirmed influenza (Figure 1). There was a significant positive correlation (P = .0008) between the total symptom score for each volunteer and the fold increase in ELISpot response from day 29 to day 37 (Supplementary Figure 3) when both control subjects and vaccinees are assessed.
Blood samples were also taken for ELISpot assay at follow-up visits on days 66, 120, and 210. The median response to NP and M1 declined between day 37 and day 66, marginally increased at day 120 (not significant) and decreased at day 210 in both vaccinated and control groups (Figure 1).
HI Titers After Challenge
HI titers to the challenge virus were repeated 36 days following influenza challenge (Table 1). There was no correlation between symptoms and virus shedding and rise in HI titer.
DISCUSSION
In this phase 2a study, we have demonstrated the safety of MVA-NP+M1 at a dose of 1.5 × 108 PFU given as a single intramuscular injection. The majority of adverse events were mild in severity, with no serious systemic adverse events and no rigors experienced by any of the 15 subjects who were vaccinated, indicating a satisfactory safety profile at this dose. This dose is now being tested in an additional phase 1 study of subjects aged >50 years.
In the phase 1 study, the T-cell response was measured by ex vivo IFN-γ ELISpot assay at the peak of response 7 days after vaccination, and at 21 days. Median responses were 2793 and 2088 SFUs per million PBMCs at 7 and 21 days in the high-dose (2.5 × 108 PFU) group when fresh PBMCs were used in the assay. In the phase 2a study reported here, employing an intermediate dose of MVA-NP+M1 and using fresh PBMCs, the median response of the vaccinees 21 days after vaccination was 980, falling to 627 on the day prior to influenza challenge. Although it is not unexpected that the response measured by this assay is reduced when the vaccine dose is reduced, the small numbers of volunteers in both studies do not allow an accurate determination of the magnitude of this reduction.
Following influenza virus challenge, only 5 of 11 control subjects developed laboratory-confirmed influenza, defined as symptoms of influenza disease plus virus shedding. This figure is lower than expected for challenge studies of this type, although it has previously been shown that approximately one-third of individuals undergoing influenza challenge are protected despite not having detectable antibodies against the challenge virus [3] and it is a known feature of this challenge model that not all control subjects will develop influenza. In this study only 2 vaccinated volunteers developed laboratory-confirmed influenza, the total number of symptoms recorded was lower in the vaccinated group at all time points following challenge, the number of grade 2 and 3 symptoms recorded was lower, and virus shedding was significantly reduced, supporting a protective effect of the vaccine against both disease severity and virus shedding.
It was notable that there was no consistent rise in HI titer following influenza challenge, even among volunteers who developed laboratory-confirmed influenza.
Having demonstrated a significant increase in the number of T cells producing IFN-γ in response to NP and M1 following vaccination, and with fewer vaccinated volunteers developing influenza than control subjects, we attempted to confirm the association of vaccine-induced T-cell responses with this protective outcome. In a large study of 2172 children in the Philippines and Thailand, it was found that the majority of infants and young children with >100 SFUs per million PBMCs in an IFN-γ ELISpot assay utilizing whole influenza virus as antigen were protected against clinical influenza [9]. In our own small-scale study of adults, who would have had multiple prior exposures to influenza prior to vaccination resulting in memory populations of influenza-specific T and B cells, we were not able to define a correlate of protection based on responses detected in PBMCs using the IFN-γ ELISpot assay prior to challenge. Following influenza challenge, only minor fluctuations in the IFN-γ ELISpot were detected for a period of 4 days, increasing by the seventh day in subjects who developed influenza disease, whereas virus shedding was detected on the second and third day. This suggests that changes in responses measured in circulating PBMCs are occurring only after respiratory tract symptoms, and cannot be used to predict protection or susceptibility. However an anamnestic mucosal T-cell response predictive of protection cannot be excluded. For future studies, a systems biology approach should be taken to understanding multifactorial mechanisms of protection that may be missed when only a small number of measures of immune system status are used.
This study provides evidence that intranasal challenge with influenza virus appears safe in individuals with elevated T-cell responses after MVA-NP+N1 immunization. The absence of any lower respiratory symptoms or signs, together with normal oxygen saturations and spirometry after influenza challenge, makes immunopathology highly unlikely . This supports previous work in several nonhuman species (particularly mice and ferrets [10]) and pigs [11], indicating the apparent safety of intranasal influenza virus challenge after immunization with T-cell–inducing vaccines.
This first efficacy study of a vaccine designed to boost T-cell responses to conserved influenza antigens has demonstrated the safety of this vaccination approach. Vaccinees were exposed to influenza virus at a time when anti-influenza T-cell responses had been increased by vaccination with no ill effects and no evidence of lower respiratory tract infection or inflammation. It also elucidated the efficacy of the vaccine in boosting the T-cell response to the vaccine antigens and in reducing laboratory-confirmed influenza in the vaccinees compared with control subjects. This reduction equates to 60% vaccine efficacy, which is a similar level to that shown for inactivated influenza vaccines when the circulating virus and the strain used in the vaccine are well matched [12], although further studies using a larger sample size will be required to reach a more precise and robust estimate of vaccine efficacy.
The majority of studies on T-cell–mediated protection against influenza have been conducted in the mouse model. A small number of studies in other species have indicated that T-cell responses to conserved influenza antigens can protect against disease and virus shedding [13–16], but this is the first clinical efficacy study of a vaccine designed to protect in this way. The results of this first clinical study are encouraging and provide initial evidence that this approach will be successful. Further studies are indicated to characterize safety and efficacy in larger numbers of individuals and to assess vaccine immunogenicity in both older and younger age groups.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://www.oxfordjournals.org/our_journals/cid/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We are grateful for the assistance of Hazel Poyntz, and Jenner Institute and Retroscreen Virology Ltd clinical project managers and clinical trial nurses in completing this study, and to Simon Draper for review of the manuscript. The quarantine phase of the study was conducted by Retroscreen Virology Ltd at the phase 1 quarantine unit.
Financial support. This work was supported by the Wellcome Trust (grant number 081865) and the Isis Innovation University Challenge Seed Fund with additional support from the UK National Institute for Health Research Oxford Biomedical Research Centre. T. K. B., A. J. S., and T. L. are supported by the Oxford Martin School. C. J. A. D. is supported by the Wellcome Trust. S. G. and A. V. S. H. are Jenner Institute Investigators.
Potential conflicts of interest. A. V. S. H. and S. G. are named as inventors on patent applications relating to induction of T-cell responses by vaccination. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:36–44. doi: 10.1016/S1473-3099(11)70295-X. [DOI] [PubMed] [Google Scholar]
- 2.Kresse H, Rovini H. Influenza vaccine market dynamics. Nat Rev Drug Discov. 2009;8:841–2. doi: 10.1038/nrd3026. [DOI] [PubMed] [Google Scholar]
- 3.McMichael AJ, Gotch F, Cullen P, Askonas B, Webster RG. The human cytotoxic T cell response to influenza A vaccination. Clin Exp Immunol. 1981;43:276–84. [PMC free article] [PubMed] [Google Scholar]
- 4.Epstein SL. Prior H1N1 influenza infection and susceptibility of Cleveland Family Study participants during the H2N2 pandemic of 1957: an experiment of nature. J Infect Dis. 2006;193:49–53. doi: 10.1086/498980. [DOI] [PubMed] [Google Scholar]
- 5.He XS, Holmes TH, Zhang C, et al. Cellular immune responses in children and adults receiving inactivated or live attenuated influenza vaccines. J Virol. 2006;80:11756–66. doi: 10.1128/JVI.01460-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee LY, Ha do LA, Simmons C, et al. Memory T cells established by seasonal human influenza A infection cross-react with avian influenza A (H5N1) in healthy individuals. J Clin Invest. 2008;118:3478–90. doi: 10.1172/JCI32460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berthoud TK, Hamill M, Lillie PJ, et al. Potent CD8+ T-cell immunogenicity in humans of a novel heterosubtypic influenza A vaccine, MVA-NP+M1. Clin Infect Dis. 2011;52:1–7. doi: 10.1093/cid/ciq015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Makedonas G, Banerjee PP, Pandey R, et al. Rapid up-regulation and granule-independent transport of perforin to the immunological synapse define a novel mechanism of antigen-specific CD8+ T cell cytotoxic activity. J Immunol. 2009;182:5560–9. doi: 10.4049/jimmunol.0803945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forrest BD, Pride MW, Dunning AJ, et al. Correlation of cellular immune responses with protection against culture-confirmed influenza virus in young children. Clin Vaccine Immunol. 2008;15:1042–53. doi: 10.1128/CVI.00397-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laddy DJ, Yan J, Kutzler M, et al. Heterosubtypic protection against pathogenic human and avian influenza viruses via in vivo electroporation of synthetic consensus DNA antigens. PLoS One. 2008;3:e2517. doi: 10.1371/journal.pone.0002517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wesley RD, Tang M, Lager KM. Protection of weaned pigs by vaccination with human adenovirus 5 recombinant viruses expressing the hemagglutinin and the nucleoprotein of H3N2 swine influenza virus. Vaccine. 2004;22:3427–34. doi: 10.1016/j.vaccine.2004.02.040. [DOI] [PubMed] [Google Scholar]
- 12.CDC. Interim within-season estimate of the effectiveness of trivalent inactivated influenza vaccine–Marshfield, Wisconsin, 2007–08 influenza season. MMWR Morb Mortal Wkly Rep. 2008;57:393–8. [PubMed] [Google Scholar]
- 13.Breathnach CC, Clark HJ, Clark RC, Olsen CW, Townsend HG, Lunn DP. Immunization with recombinant modified vaccinia Ankara (rMVA) constructs encoding the HA or NP gene protects ponies from equine influenza virus challenge. Vaccine. 2005;24:1180–90. doi: 10.1016/j.vaccine.2005.08.091. [DOI] [PubMed] [Google Scholar]
- 14.Donnelly JJ, Friedman A, Martinez D, et al. Preclinical efficacy of a prototype DNA vaccine: enhanced protection against antigenic drift in influenza virus. Nat Med. 1995;1:583–7. doi: 10.1038/nm0695-583. [DOI] [PubMed] [Google Scholar]
- 15.Epstein SL, Tumpey TM, Misplon JA, et al. DNA vaccine expressing conserved influenza virus proteins protective against H5N1 challenge infection in mice. Emerg Infect Dis. 2002;8:796–801. doi: 10.3201/eid0808.010476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laddy DJ, Yan J, Khan AS, et al. Electroporation of synthetic DNA antigens offers protection in nonhuman primates challenged with highly pathogenic avian influenza virus. J Virol. 2009;83:4624–30. doi: 10.1128/JVI.02335-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.