Abstract
Retinoic acid (RA) induces embryonic stem cell differentiation. The effects of RA are mediated by retinoic acid receptors (RARs) that promote epigenetic changes controlling gene transcription. We show here that RARγ, in the absence of the ligand RA, is required for deposition of the histone variant H2A.Z and the Polycomb protein Suz12 at RA target genes, and that both RARγ and Suz12 exist in a multi-protein complex in the absence of ligand. Addition of RA causes removal of H2A.Z and Suz12 from RARγ target genes when the genes are transcriptionally activated.
Keywords: Polycomb group, epigenetic, gene expression, retinoic acid
INTRODUCTION
Embryonic stem (ES) cells are pluripotent cells that have the potential to differentiate into many types of cells (Kashyap et al., 2009). Retinoic acid (RA), the most potent natural form of vitamin A, can induce differentiation of ES cells into different cell types (Eiges and Benvenisty, 2002; Rohwedel et al., 1999; Schuldiner et al., 2000). The biological effects of retinoids are mainly mediated by two classes of nuclear receptors: RARs and RXRs, encoded by six distinct genes: RARα, RARβ, RARγ, RXRα, RXRβ and RXRγ (Mangelsdorf, 1994). Of particular interest is that RARγ exhibits similar patterns of expression in mouse and human undifferentiated ES cells and F9 stem cells and during early embryoid body formation (Jeong and Mangelsdorf, 2009; Su and Gudas, 2008).
RA promotes epigenetic changes. RA treatment of ES cells decreases H3k27me3 and increases H3k4me3 marks (Campos and Reinberg, 2009). Moreover, RA displaces Suz12 from some of RA response elements (RAREs) in F9 cells (Gillespie and Gudas, 2007; Lee et al., 2007b). Suz12, a member of Polycomb Repressive Complex-2 (PRC2) involved in H3K27me3 methylation and in maintaining ES cell self-renewal, is a powerful repressor of homeobox (Hox) gene expression (Lee et al., 2006b). Hox gene clusters are expressed during ES cell differentiation and transcriptionally activated by RA (Gillespie and Gudas, 2007; Langston and Gudas, 1994; Mainguy et al., 2003; Tarchini and Duboule, 2006).
The histone H2A variant H2A.Z is of particular interest because it is essential in multicellular organisms (Faast et al., 2001; Ridgway et al., 2004). In mammalian cells, H2A.Z loci have been found predominantly at sites occupied by RNA Polymerase II, along with enhancer regions (Barski et al., 2007). In undifferentiated ES cells H2A.Z is enriched at a large set of silent, developmental genes in a manner that is highly similar to that of the PRC2 protein Suz12 (Boyer et al., 2006; Creyghton et al., 2008). Moreover, H2A.Z expression is required for proper ES cell differentiation (Creyghton et al., 2008).
We show here that H2A.Z is highly enriched in undifferentiated ES cells at RARγ target genes, which correlates with Suz12 occupancy and the presence of the H3k27me3 mark at these genes. Importantly, these genes are not expressed in undifferentiated ES cells. We also demonstrate that upon RA treatment gene transcription is activated and is accompanied by a decrease in H2A.Z occupancy and removal of Suz12. Furthermore, we show that RARγ is important for H2A.Z and Suz12 recruitment in ES cells cultured without RA. Our studies also demonstrate that RARγ is present with Suz12 in a multi-protein complex in undifferentiated cells; RA treatment disrupts the interaction of RARγ with Suz12. Together, our findings establish a critical role for RARγ in H2A.Z and Suz12 recruitment to RA target genes in ES cells in the absence of RA and in the removal of both H2A.Z and Suz12 proteins upon RA treatment.
MATERIALS AND METHODS
Cell Culture
Mouse ES cells and all-trans-retinoic acid (Sigma) was used as described previously (Chen et al., 2003). The RARγ knockout (KO) ES cells were generated as described (Kashyap et al, Submitted 2010).
Antibodies
Anti-HA (#ab9110), anti-H3k27me3 (#ab6002), and anti-H2A.Z (#ab4174) antibodies were purchased from Abcam. Anti-Suz12 (#3737) antibody was obtained from Cell Signaling Technology. Anti-Ash2l (A300-107A) was obtained from Bethyl Labs. Secondary anti-rabbit IgG (#sc-2027) antibody was obtained from Santa Cruz Biotechnology. Anti-RARγ was generated as described (Gillespie and Gudas, 2007).
RNA analysis
Cells were harvested in Trizol (Invitrogen), and total cellular RNA was extracted according to the manufacturer’s protocol and as described (Kashyap et al., 2009).
Chromatin immunoprecipitation
ChIP and two-step ChIP (HA-ChIP) were carried out as described previously (Kashyap et al., 2009).
Semi-quantitative and Real Time PCR Analysis
Semi-quantitative PCR was carried out using commercial Taq polymerase (Quanta). PCR reactions were run on a Bio-Rad iCycler as described previously (Kashyap et al., 2009).
Immunoprecipitation (IP)
Whole cell lysates were prepared from COS, CCE and RARγ KO cells in 50mM Tris-HCl, 0.1M NaCl, 10mM EDTA, 1% Nonidet P-40 (pH 7.4) containing a protease inhibitor mixture (Roche Diagnostics). Whole cell lysate fractions were IP using 500μg or 1mg of total protein using Trueblot anti-Rabbit Ig IP beads (eBioscience San Diego, CA) according to the manufacturer’s instructions. Proteins were separated by SDS-PAGE gels, and transferred to Immobilon-P membranes (Millipore).
Western Blot Analysis
WT CCE, RARγ KO and COS cells were lysed in 50mM Tris-HCl, 0.1M NaCl, 10mM EDTA, 1% Nonidet P-40 (pH 7.4) containing a protease inhibitor mixture (Roche Diagnostics). The samples were boiled and 20–40μg of protein sample was separated on an SDS-PAGE gel and transferred to a nitrocellulose membrane.
RESULTS AND DISCUSSION
RARγ and Suz12 are present in the same multi-protein complex in vivo in murine ES cells; this interaction is inhibited by RA
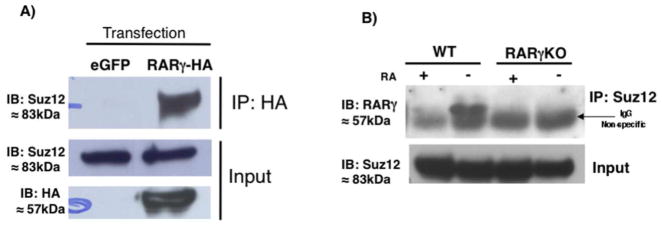
RARs interact with some histone modifying complexes (Blanco et al., 1998; Dietze et al., 2003; Dilworth et al., 2000; Flajollet et al., 2006; Fujiki et al., 2009; Lee et al., 2006a). Suz12, a PRC2 component, is recruited to some RARγ target genes in F9 cells (Gillespie and Gudas, 2007). For these reasons we tested the possibility that Suz12 and RARγ interact. First, we overexpressed an RARγ-HA (hemagglutinin) tagged fusion protein in COS cells, and then immunoprecipitated (IP) RARγ using an HA antibody. In HA-IP extracts we detected Suz12, indicating that Suz12 and RARγ can interact in vivo (Fig 1A). Since we were interested in understanding the interacting partners of RARγ in ES cells, we also performed co-IP assays in murine wildtype (WT) ES cells. When an anti-Suz12 antibody (Ab) was used to IP the cell extracts, we found substantial levels of RARγ in the IP material in untreated ES cells (Fig 1B). The signal was specific for RARγ, we did not detect any signal in the ES RARγ Knock-out (KO) cells (Fig 1B).
Fig. 1.
RARγ interacts with Suz12 in vivo. (A) Immunoprecipitation (IP) analysis of the interaction of RARγ with Suz12. COS cells transfected with eGFP or RARγ-HA expression vector. Cell extracts were IP using an anti-HA Ab and immunoblotted (IB) with an anti-Suz12 Ab (top panel). Whole-cell extracts were probed with anti-Suz12 to confirm equivalent Suz12 input before IP (middle panel) or anti-HA to check RARγ-HA expression (bottom panel). (B) ES cell extracts were IPd using Suz12 Ab and IBd with an anti-RARγ Ab (top panel). Whole-cell extracts were probed with the anti-Suz12 Ab to confirm equivalent Suz12 levels before IP (bottom panel). This experiment was performed three times.
Because Suz12 in F9 cells is removed from some RA target genes upon RA treatment we studied the effects of RA on the interaction of RARγ and Suz12 in ES cells. We observed that upon RA treatment the interaction between RARγ and Suz12 was impaired (Fig 1B). Others have suggested that Suz12 protein levels decrease after RA exposure (Lee et al., 2007a); however, we did not observe changes in the total Suz12 protein level after 24h of RA (input levels, Fig 1B). Taken together, these results show for the first time that RARγ interacts with a PRC2 component only in undifferentiated ES cells. We did not observe any interaction between RARγ and Suz12 in the presence of RA, suggesting that the ligand changes the multi-protein complex structure. We cannot discount the possibility that RARγ can interact with other histone modifying complexes such as MLL. Although we were not able to detect an interaction between RARγ and Ash2L, a common component of MLLs, in ES cells, we did detect Ash2L in HA IP extracts when we overexpressed both Ash2L and RARγ in COS cells (Supplementary Fig S1).
RARγ binds to the promoter regions of RARβ2, Cyp26a1, and C/EBPα; genes transcriptionally activated by RA in ES cells
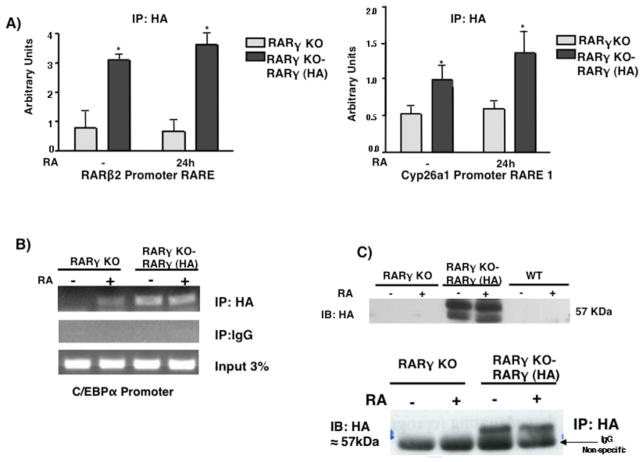
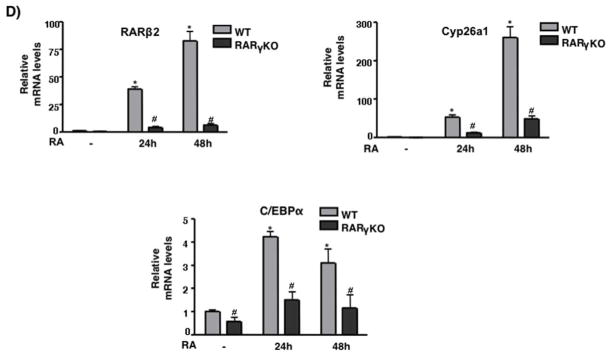
We previously showed that RARγ binds to RAREs in the promoters of RARβ2 and Cyp26a1 in F9 cells (Gillespie and Gudas, 2007). To determine whether RARγ binds in these regions in ES cells we replaced RARγ with RARγ-HA (see previous section) in a RARγ-KO ES cell line (Fig 2B). We performed chromatin-immunoprecipitation (ChIP) using an HA Ab and observed that RARγ-HA binds to the RARβ2 and Cyp26a1 RAREs (Fig 2A). We found that RARγ-HA was bound even in the absence of RA, consistent with the model for the actions of RARs (Chambon, 1996). Upon RA treatment of the RARγ(KO)-RARγ-HA restored ES cells, RARγ-HA binding did not show a statistical difference versus binding seen in the untreated cells. We also measured binding of RARγ-HA at the C/EBPα gene because researchers had previously shown by ChIP-on-ChIP that RARγ was bound at its promoter region after 2h of RA (Delacroix et al., 2010). Our results indicate that RARγ-HA is bound to the promoter region of C/EBPα even in untreated ES cells. Thus, RA did not increase RARγ-HA binding at the C/EBPα promoter, as occurred at the RARβ2 and Cyp26a1 promoters. We also observed that RARβ2, Cyp26a1, and C/EBPα transcript levels were greatly increased upon RA treatment of WT ES cells. However, in the RARγ KO cells RA induction of these genes was severely reduced (Fig 2C).
Fig. 2.
RARγ binding on RAR target genes in ES cells. ES RARγKO or RARγ-HA restoration cells were cultured +/− 1μM RA for 24h. (A) ChIP was performed using HA Ab or IgG and bound DNA was quantitated by real-time PCR. The data are presented as fold enrichment (mean ± S.E.) at RARβ2 or Cyp26a1 promoter. Error bars indicate standard error of three biological replicates corrected by GAPDH DNA bound for normalization. Statistically significant differences (p<0.05), asterisk. (B) Semi-quanitative PCR of RARγ-HA binding at the C/EBPα promoter. (C) Whole-cell extracts from RARγKO, RARγ-HA restoration, or WT ES cells were probed with the anti-HA Ab to confirm RARγ-HA expression. Representative Western blotting of three different biological replicates (top panel). IP analysis of RARγ-HA (bottom panel). ES cell extracts were IP’d using an Ab directed against HA and IB’d with an anti-HA Ab. (D) WT and RARγKO ES cells were cultured −/+ 1μM RA for 24h. Induction of the RA target gene mRNAs was measured by quantitative RT-PCR. Error bars, standard error of three biological replicates. Data in E were normalized to 36B4 mRNA. Statistically significant differences (p<0.05), asterisk (RA vs -) and # (WT vs RARγKO).
Suz12 levels are high at RARγ target genes in WT cells, and the RARγ Knock-out ES cell line shows reduced Suz12 levels
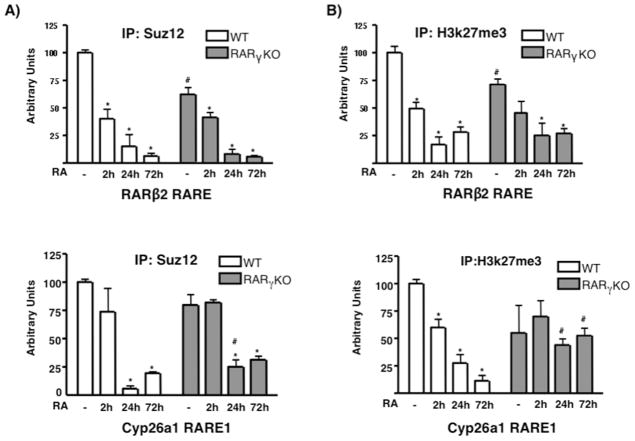
We found that RARγ and Suz12 are present together in a multi-protein complex in ES cells. We then studied Suz12 recruitment at the regulatory regions of the RARγ target genes RARβ2 and Cyp26a1. Using ChIP, we showed that Suz12 was highly recruited to the RARβ2 and Cyp26a1 promoters that contain RAREs in WT ES cells (Fig 3A). Furthermore, the H3k27me3 mark (associated with PRC2 activity) was present at these promoter regions in WT ES cells (Fig 3B). We observed that a 2h RA treatment caused a reduction of 50% in Suz12 and 25% in the H3k27me3 mark; these changes were even greater at 24 and 72h after RA addition, when both the Suz12 and H3k27me3 marks were completely removed, becoming equal to IgG levels at the RARγ target genes in WT ES cells (Fig 3B).
Fig. 3.
Suz12 and H3k27me3 proteins are present at the RARβ2 and Cyp26a1 RAREs in WT ES cells. WT and RARγKO ES cells were treated +/− 1μM RA for 2, 24 or 72h. ChIP was performed using anti-Suz12 (A) or anti-H3k27me3 Ab (B), and bound DNA was quantitated by real-time PCR. Each experiment was repeated at least three times. Data are show as % of input DNA before IP (mean ± S.E.) and normalized by GAPDH DNA bound. Error bars, standard error of three biological replicates. Statistically significant differences (p<0.05), asterisk (RA vs -) and # (WT vs RARγKO).
We then determined the effect of RARγ by comparing Suz12 and H3k27me3 occupancy in the ES WT and RARγ KO cells (Fig 3A, B). We observed that both Suz12 levels and the H3k27me3 mark were reduced under basal conditions in the ES RARγ KO cells, especially at the RARβ2 promoter, where the reduction was between 25–50%. These results indicate that RARγ plays an important role in the correct deposition of these chromatin components even in the absence of RA.
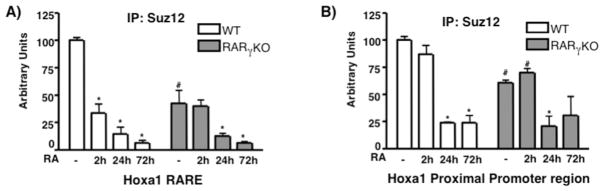
We then studied Suz12 occupancy on the Hoxa1 gene cluster, which is a Suz12 - RARγ target (Gillespie and Gudas, 2007; Kashyap et. al, submitted). We analyzed Suz12 recruitment at the proximal promoter (PP) and at a RARE element located ~2kb downstream of the gene (Langston and Gudas, 1992). Suz12 occupancy was high at both regions, and upon RA treatment Suz12 was removed (Fig 4). However, when we compared the Suz12 occupancy in WT and RARγ KO ES cells, we found that Suz12 levels were diminished in the RARγ KO cell line (Fig 4). Interestingly, we found that in the RARγ KO cells Suz12 was less abundant at the Hoxa1 RARE than at the PP region, indicating the importance of RARγ for Suz12 binding at the RARE. Based on the ChIP data and the results from the previous section, we propose that in basal conditions RARγ and PRC2 proteins are present in the same multi-protein complex at the regulatory regions of RARγ target genes in ES cells, when the genes are not transcriptionally activated. In the presence of RA, the RARγ and PRC2 interaction is disrupted and the PRC2 proteins are removed from the regulatory regions, leading to transcriptional activation.
Fig. 4.
Suz12 is present at the 3′ downstream Hoxa1 RARE and at the proximal promoter region in WT ES cells. WT and RARγKO ES cells were treated +/− 1μM RA for 2, 24 or 72h. ChIP was performed using an anti-Suz12 Ab and bound DNA was quantitated by real time PCR at the RARE (A) or Hoxa1 PP region (B). Each experiment was repeated at least three times. Data are presented as %s of input DNA before IP (mean ± S.E.) and normalized by GAPDH DNA bound. Error bars, standard error of three biological replicates. Statistically significant differences (p<0.05), asterisk (RA vs -) and # (WT vs RARγ KO). 352×264mm (72 × 72 DPI)
H2A.Z occupancy is high at RAREs and H2A.Z is removed upon RA treatment
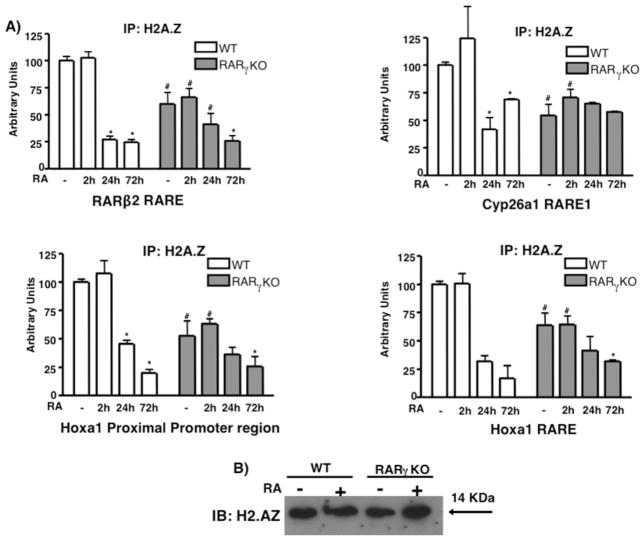
H2A.Z occupancy displays a high overlap with Suz12 occupancy in undifferentiated ES cells (Creyghton et al., 2008). Furthermore, H2A.Z is linked to signaling by ERα (Gevry et al., 2009), a receptor with structural similarity to the RARs. For these reasons, we determined if some RARγ target genes displayed H2A.Z recruitment. ChIP using an H2A.Z Ab detected high levels of H2A.Z at the RARβ2, Cyp26a1, and Hoxa1 RAREs, as well as at the Hoxa1 PP in ES WT cells, while RA dramatically reduced H2A.Z levels in those regions (Fig 5A). We performed ChIP at different time points and observed that H2A.Z was removed slowly, as opposed to Suz12 (see Fig 3A, 4). At 2 and 3h (Fig 5A, and data not shown) after RA addition, H2A.Z levels remained similar or were even higher than those in untreated WT cells. However, after a 24h RA treatment H2A.Z levels were greatly reduced, and after 72h H2A.Z levels were comparable to the IgG signal. These data indicate a different dynamic of H2A.Z removal from RA target genes as compared to Suz12. Because we observed major differences in Suz12 recruitment in the ES RARγ KO cells, we compared H2A.Z occupancy in RARγ KO cells with H2A.Z occupancy in WT. RARγ KO ES cells displayed a 50% reduction in H2A.Z levels compared to WT ES cells (Fig 5A) at the RARβ2, Cyp26a1, and Hoxa1 PP/RARE regions. Furthermore, the effect of RA on H2A.Z removal in RARγ KO cells was severely impaired in comparison to the WT. We observed that 24h after RA WT ES cells showed a 75% reduction of H2A.Z. However, H2A.Z levels in RARγ KO ES cells after 24h of RA treatment were similar to the levels in untreated RARγ KO ES cells. We considered whether RA treatment or the loss of RARγ affected H2A.Z protein levels. We performed Western blotting in WT and RARγ KO ES cells and observed that H2A.Z protein levels did not change upon RA treatment of both ES cell lines (Fig 5C).
Fig. 5.
H2A.Z is present at RARγ target genes and its level decreases upon RA treatment of ES cells. ES WT and RARγKO cells were treated +/− 1μM RA for 2, 24 or 72h. ChIP was performed using an anti-H2A.Z Ab (A) and bound DNA was quantitated by real time PCR. Each experiment was repeated at least three times. Data are presented as %s of input DNA before IP (mean ± S.E.) and normalized to bound GAPDH DNA. Error bars, standard error of three biological replicates. Statistically significant differences (p<0.05), asterisk (RA vs -) and # (WT vs RARγKO). (B) Representative of three immunoblots showing H2A.Z protein levels in WT and in RARγKO cells.
Taken together, we show that H2A.Z recruitment is affected by the retinoid signaling pathway in ES cells. We show that H2A.Z is present at RARγ target genes and is removed from these target genes upon RA addition, when these genes are transcriptionally activated in ES cells; this is a different result from that reported for H2A.Z and estrogen receptor α (ERα) targets (Gevry et al, 2009). Although we have described an interaction between RARγ and Suz12, we failed to detect H2A.Z using an HA Ab to IP RARγ-HA from the RARγ (KO)/RARγ-HA cell extracts (data not shown). It should be noted that the removal of H2A.Z displayed a different time course than that for Suz12 removal. RA promoted Suz12 removal as early as 2h. However, H2A.Z levels remained equal to the basal levels at 2h after RA addition. It was not until 24h after RA addition that we observed a ~75 % reduction of H2A.Z occupancy, indicating a different mechanism of removal. Moreover, we found that H2A.Z removal in the RARγ KO cell line after RA was severely impaired (Fig 5A), in contrast to Suz12, which eventually was removed from RA target genes even in the absence of RARγ (Fig 3, 4).
Our data demonstrate that RARγ is necessary for the correct deposition of H2A.Z and PRC2 on RA target genes. This is the first time that H2A.Z recruitment has been linked to RARs and the importance of a specific RAR isoform for the deposition of both Suz12 and H2A.Z in ES cells has been demonstrated. Furthermore, RA greatly reduces H2A.Z occupancy on RA target genes when they are transcriptionally activated.
Acknowledgments
We thank Dr. Kristian Laursen for the RARγ-HA expression vector, Dr. Pierre Chambon for the RARγ KO mice, Tamara Weissman for editorial assistance, and Vasundhra Kashyap for critically reading this manuscript. This research was supported by NIH RO1 CA043796 to Dr. Lorraine J. Gudas.
LITERATURE CITED
- Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, Wei G, Chepelev I, Zhao K. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129(4):823–837. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Blanco JC, Minucci S, Lu J, Yang XJ, Walker KK, Chen H, Evans RM, Nakatani Y, Ozato K. The histone acetylase PCAF is a nuclear receptor coactivator. Genes Dev. 1998;12(11):1638–1651. doi: 10.1101/gad.12.11.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer LA, Plath K, Zeitlinger J, Brambrink T, Medeiros LA, Lee TI, Levine SS, Wernig M, Tajonar A, Ray MK, Bell GW, Otte AP, Vidal M, Gifford DK, Young RA, Jaenisch R. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature. 2006;441(7091):349–353. doi: 10.1038/nature04733. [DOI] [PubMed] [Google Scholar]
- Campos EI, Reinberg D. Histones: annotating chromatin. Annu Rev Genet. 2009;43:559–599. doi: 10.1146/annurev.genet.032608.103928. [DOI] [PubMed] [Google Scholar]
- Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996;10(9):940–954. [PubMed] [Google Scholar]
- Chen AC, Yu K, Lane MA, Gudas LJ. Homozygous deletion of the CRABPI gene in AB1 embryonic stem cells results in increased CRABPII gene expression and decreased intracellular retinoic acid concentration. Arch Biochem Biophys. 2003;411(2):159–173. doi: 10.1016/s0003-9861(02)00732-4. [DOI] [PubMed] [Google Scholar]
- Creyghton MP, Markoulaki S, Levine SS, Hanna J, Lodato MA, Sha K, Young RA, Jaenisch R, Boyer LA. H2AZ is enriched at polycomb complex target genes in ES cells and is necessary for lineage commitment. Cell. 2008;135(4):649–661. doi: 10.1016/j.cell.2008.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delacroix L, Moutier E, Altobelli G, Legras S, Poch O, Choukrallah MA, Bertin I, Jost B, Davidson I. Cell-pecific interaction of retinoic acid receptors with target genes in mouse embryonic fibroblasts and embryonic stem cells. Mol Cell Biol. 2010;30(1):231–244. doi: 10.1128/MCB.00756-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietze EC, Troch MM, Bowie ML, Yee L, Bean GR, Seewaldt VL. CBP/p300 induction is required for retinoic acid sensitivity in human mammary cells. Biochem Biophys Res Commun. 2003;302(4):841–848. doi: 10.1016/s0006-291x(03)00266-3. [DOI] [PubMed] [Google Scholar]
- Dilworth FJ, Fromental-Ramain C, Yamamoto K, Chambon P. ATP-driven chromatin remodeling activity and histone acetyltransferases act sequentially during transactivation by RAR/RXR In vitro. Mol Cell. 2000;6(5):1049–1058. doi: 10.1016/s1097-2765(00)00103-9. [DOI] [PubMed] [Google Scholar]
- Eiges R, Benvenisty N. A molecular view on pluripotent stem cells. FEBS Lett. 2002;529(1):135–141. doi: 10.1016/s0014-5793(02)03191-5. [DOI] [PubMed] [Google Scholar]
- Faast R, Thonglairoam V, Schulz TC, Beall J, Wells JR, Taylor H, Matthaei K, Rathjen PD, Tremethick DJ, Lyons I. Histone variant H2A.Z is required for early mammalian development. Curr Biol. 2001;11(15):1183–1187. doi: 10.1016/s0960-9822(01)00329-3. [DOI] [PubMed] [Google Scholar]
- Flajollet S, Lefebvre B, Rachez C, Lefebvre P. Distinct roles of the steroid receptor coactivator 1 and of MED1 in retinoid-induced transcription and cellular differentiation. J Biol Chem. 2006;281(29):20338–20348. doi: 10.1074/jbc.M603023200. [DOI] [PubMed] [Google Scholar]
- Fujiki R, Chikanishi T, Hashiba W, Ito H, Takada I, Roeder RG, Kitagawa H, Kato S. GlcNAcylation of a histone methyltransferase in retinoic-acid-induced granulopoiesis. Nature. 2009;459(7245):455–459. doi: 10.1038/nature07954. [DOI] [PubMed] [Google Scholar]
- Gevry N, Hardy S, Jacques PE, Laflamme L, Svotelis A, Robert F, Gaudreau L. Histone H2A.Z is essential for estrogen receptor signaling. Genes Dev. 2009;23(13):1522–1533. doi: 10.1101/gad.1787109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie RF, Gudas LJ. Retinoid regulated association of transcriptional co-regulators and the polycomb group protein SUZ12 with the retinoic acid response elements of Hoxa1, RARbeta(2), and Cyp26A1 in F9 embryonal carcinoma cells. J Mol Biol. 2007;372(2):298–316. doi: 10.1016/j.jmb.2007.06.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong Y, Mangelsdorf DJ. Nuclear receptor regulation of stemness and stem cell differentiation. Exp Mol Med. 2009;41(8):525–537. doi: 10.3858/emm.2009.41.8.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashyap V, Rezende NC, Scotland KB, Shaffer SM, Persson JL, Gudas LJ, Mongan NP. Regulation of stem cell pluripotency and differentiation involves a mutual regulatory circuit of the NANOG, OCT4, and SOX2 pluripotency transcription factors with polycomb repressive complexes and stem cell microRNAs. Stem Cells Dev. 2009;18(7):1093–1108. doi: 10.1089/scd.2009.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langston AW, Gudas LJ. Identification of a retinoic acid responsive enhancer 3′ of the murine homeobox gene Hox-1.6. Mech Dev. 1992;38(3):217–227. doi: 10.1016/0925-4773(92)90055-o. [DOI] [PubMed] [Google Scholar]
- Langston AW, Gudas LJ. Retinoic acid and homeobox gene regulation. Curr Opin Genet Dev. 1994;4(4):550–555. doi: 10.1016/0959-437x(94)90071-a. [DOI] [PubMed] [Google Scholar]
- Lee ER, Murdoch FE, Fritsch MK. High histone acetylation and decreased polycomb repressive complex 2 member levels regulate gene specific transcriptional changes during early embryonic stem cell differentiation induced by retinoic acid. Stem Cells. 2007a;25(9):2191–2199. doi: 10.1634/stemcells.2007-0203. [DOI] [PubMed] [Google Scholar]
- Lee MG, Villa R, Trojer P, Norman J, Yan KP, Reinberg D, Di Croce L, Shiekhattar R. Demethylation of H3K27 regulates polycomb recruitment and H2A ubiquitination. Science. 2007b;318(5849):447–450. doi: 10.1126/science.1149042. [DOI] [PubMed] [Google Scholar]
- Lee S, Lee DK, Dou Y, Lee J, Lee B, Kwak E, Kong YY, Lee SK, Roeder RG, Lee JW. Coactivator as a target gene specificity determinant for histone H3 lysine 4 methyltransferases. Proc Natl Acad Sci U S A. 2006a;103(42):15392–15397. doi: 10.1073/pnas.0607313103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TI, Jenner RG, Boyer LA, Guenther MG, Levine SS, Kumar RM, Chevalier B, Johnstone SE, Cole MF, Isono K, Koseki H, Fuchikami T, Abe K, Murray HL, Zucker JP, Yuan B, Bell GW, Herbolsheimer E, Hannett NM, Sun K, Odom DT, Otte AP, Volkert TL, Bartel DP, Melton DA, Gifford DK, Jaenisch R, Young RA. Control of developmental regulators by Polycomb in human embryonic stem cells. Cell. 2006b;125(2):301–313. doi: 10.1016/j.cell.2006.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainguy G, In der Rieden PM, Berezikov E, Woltering JM, Plasterk RH, Durston AJ. A position-dependent organisation of retinoid response elements is conserved in the vertebrate Hox clusters. Trends Genet. 2003;19(9):476–479. doi: 10.1016/S0168-9525(03)00202-6. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf DJ. Vitamin A receptors. Nutr Rev. 1994;52(2 Pt 2):S32–44. doi: 10.1111/j.1753-4887.1994.tb01385.x. [DOI] [PubMed] [Google Scholar]
- Ridgway P, Brown KD, Rangasamy D, Svensson U, Tremethick DJ. Unique residues on the H2A.Z containing nucleosome surface are important for Xenopus laevis development. J Biol Chem. 2004;279(42):43815–43820. doi: 10.1074/jbc.M408409200. [DOI] [PubMed] [Google Scholar]
- Rohwedel J, Guan K, Wobus AM. Induction of cellular differentiation by retinoic acid in vitro. Cells Tissues Organs. 1999;165(3–4):190–202. doi: 10.1159/000016699. [DOI] [PubMed] [Google Scholar]
- Schuldiner M, Yanuka O, Itskovitz-Eldor J, Melton DA, Benvenisty N. Effects of eight growth factors on the differentiation of cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A. 2000;97(21):11307–11312. doi: 10.1073/pnas.97.21.11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su D, Gudas LJ. Gene expression profiling elucidates a specific role for RARgamma in the retinoic acid-induced differentiation of F9 teratocarcinoma stem cells. Biochem Pharmacol. 2008;75(5):1129–1160. doi: 10.1016/j.bcp.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarchini B, Duboule D. Control of Hoxd genes’ collinearity during early limb development. Dev Cell. 2006;10(1):93–103. doi: 10.1016/j.devcel.2005.11.014. [DOI] [PubMed] [Google Scholar]