Abstract
Stem cells have been identified in organs with both low and high cell turnover rates. They are characterized by the expression of key marker genes for undifferentiated cells, the ability to self-renew, and the ability to regenerate tissue after cell loss. Several recent reports present evidence for the presence of pituitary stem cells. Here we offer a critical review of the field and suggest additional studies that could resolve points of debate. Recent reports have relied on different markers, including SOX2, nestin, GFRa2, and SCA1, to identify pituitary stem cells and progenitors. Future studies will be needed to resolve the relationships between cells expressing these markers. Members of the Sox family of transcription factors are likely involved in the earliest steps of pituitary stem cell proliferation and the earliest transitions to differentiation. The transcription factor PROP1 and the NOTCH signaling pathway may regulate the transition to differentiation. Identification of the stem cell niche is an important step in understanding organ development. The niche may be the marginal zone around the lumen of Rathke's pouch, between the anterior and intermediate lobes of mouse pituitary, because cells in this region apparently give birth to all six pituitary hormone cell lineages. Stem cells have been shown to play a role in recurrent malignancies in some tissues, and their role in pituitary hyperplasia, pituitary adenomas, and tumors is an important area for future investigation. From a therapeutic viewpoint, the ability to cultivate and grow stem cells in a pituitary predifferentiation state might also be helpful for the long-term treatment of pituitary deficiencies.
Introduction
Pituitary Growth and Regeneration
-
Potential Pituitary Stem Cell Populations
The SOX2+ cell population
The GFRa2+ cell population
The “side population” cells
The nestin+ cell population
The folliculostellate cells
Prospective for future analyses
Pituitary Stem Cells: from Differentiation to Pathogenesis
Pituitary Stem Cells as Potential Therapeutic Tools
Conclusion
I. Introduction
Stem cells exist in various adult organs, including heart, brain, lungs, gonads, and many others. In all these organs, stem cells are characterized by two main criteria (1):
The ability to self-renew. Stem cells undergo symmetric division to produce two stem cells. Cells can also divide asymmetrically to give birth to one stem cell and one committed progenitor cell. The latter gives rise to a daughter cell that proliferates and forms a population of expanding transit-amplifying cells before final differentiation.
The ability to regenerate tissue after cell loss by completing the differentiation programs for multiple cell fates.
Yoshimura et al. (2) described the first purported progenitor cells in the rat pituitary gland in 1969. Transplantation of anterior pituitary chromophobe cells into the hypothalamus of hypophysectomized rats led to viable, differentiated pituitary cells. Chromophobe cells were defined as nonsecreting cells, in contrast to acidophils, which secrete GH and/or prolactin, and basophils secreting LH, FSH, TSH, and to a lesser extent ACTH. The distinction between different cell types was not clear in this study, precluding any firm conclusions. Later studies suggested that the chromophobes were progenitors within the pituitary gland that could be induced to differentiate further in response to hypothalamic-releasing factors (3).
Initial indirect evidence for pituitary multipotent progenitors was based on the responsiveness of the pituitary to physiological or pathological conditions. This response could employ a variety of mechanisms including pituitary stem cells, proliferation of committed progenitors, transdifferentiation of another type of secreting cell, or expansion of limited potential precursors (4). The pituitary may regenerate after tissue loss either from surgery or from immune diseases such as hypophysitis (5). During pregnancy and lactation, there is an increased demand for prolactin production, which was thought to be accomplished by an increase in lactotroph cell number but may occur primarily through hypertrophy (6). The capacity for plasticity and regeneration requires more study.
Transgenic mouse studies provide clear evidence for regeneration of GH cells after massive cell loss. Mice carrying the herpes virus thymidine kinase gene under the control of GH promoter exhibited ablation of more than 95% of the somatotroph cells after administration of an anti-herpes drug; a few GH cells regenerated in 3 wk after drug withdrawal, and more were evident at 6 wk, although the recovery was not complete (7). Acquired or congenital hypothyroidism can result in massive expansion of the thyrotrope cell population and thyrotroph hyperplasia (8). Finally, the number of gonadotropes increases substantially during puberty (9). The type of progenitors that are recruited to fuel these population expansions is not known. The low cell turnover rate in the pituitary gland has been used to argue against the existence of pituitary stem cells, but stem cells clearly exist in other organs with a low turnover rate like heart and lungs (1).
Indirect evidence for pituitary regeneration and plasticity likely suggest the existence of pituitary stem cells. However, the nature of pituitary stem cells remains a matter of debate because the key criteria remain to be proven. The variety of markers and approaches used to identify pituitary progenitors and stem cells makes it difficult to compare results and integrate the findings without further analyses. In this review we summarize the literature and evaluate it critically, proposing experiments that could be carried out to clarify the nature of pituitary progenitors and stem cells in rodents. In addition, we will discuss the potential for human pituitary stem cells to contribute to malignancies and to be harnessed for human therapeutic use.
II. Pituitary Growth and Regeneration
Fate-mapping studies demonstrate that the vertebrate pituitary gland originates from the most anterior aspect of the anterior neural ridge (10). The cells that become the hypothalamus are located in the midline just posterior to the cells that become the anterior lobe, which contains specialized cells that produce polypeptide hormones that regulate many body processes. These cells include somatotrophs that produce GH, lactotrophs producing prolactin, gonadotrophs that secrete both LH and FSH, thyrotrophs producing TSH, and corticotrophs that express proopiomelanocortin (POMC) and cleave it to produce ACTH. In early development, the oral ectoderm is closely juxtaposed with the neural ectoderm. In response to signals from the neural ectoderm, the oral ectoderm invaginates to produce Rathke's pouch, which later develops into the anterior lobe and intermediate lobe in the rodent. The mature intermediate lobe contains melanotrophs that express POMC, which is cleaved to produce MSH and endorphin. The neural ectoderm evaginates to produce the pituitary stalk or infundibulum and develops into the posterior lobe of the pituitary gland, which differentiates and contains the terminals for the neurons that secrete oxytocin and vasopressin. All of the hormone-producing cell types differentiate by birth in the rodent, although the sizes of the various cell populations change after birth as the gland grows in response to physiological needs.
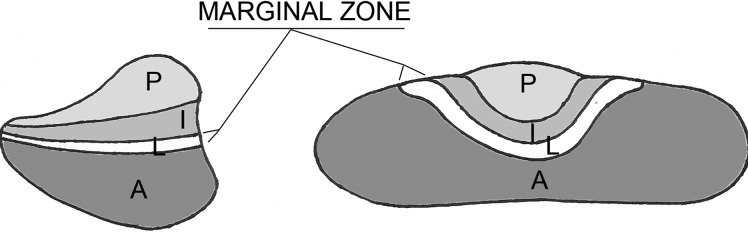
Growth and proliferation levels in Rathke's pouch suggest two major time points for stem cell or progenitor cell activity: embryogenesis and early postnatal days. During mouse embryogenesis, between embryonic day (e) 11.5 and e18.5, cells in the pituitary evolve from primarily proliferating to mostly differentiating cells. At e13.5, there is a clear division in cell-cycle state between the dorsal side of the anterior lobe, which contains proliferating cells, and the ventral side where the first differentiated cells appear (11). At e14.5, proliferating cells are highly enriched around the lumen of Rathke's pouch. This multilayer heterogeneous zone is described as the marginal zone or the niche for putative pituitary stem cells (Fig. 1).
Fig. 1.
Schematic representation of the marginal zone, a niche for presumed pituitary stem cells. P, Posterior lobe; I, intermediate lobe; A, anterior lobe; L, lumen.
Several pieces of evidence suggest a niche role for the marginal zone (reviewed in Ref. 12). Marginal zone cells display primitive morphological features that are suggestive of stem cell properties, such as the absence of secretory granules, poorly developed endoplasmic reticulum, and abundance of free ribosomes and polysomes. Transplantation of rat hemipituitaries under the kidney capsule leads to mitosis of marginal zone cells and apoptosis of endocrine cells. After castration, LH+ cells undergo hypertrophy, and new LH+ cells are observed beneath the marginal epithelium. This observation implies that at least a part of the endocrine cells are coming from marginal zone cells, or that marginal zone cells are supportive of newly formed cells. Evidence that stem/progenitor cells originate in the marginal zone also comes from recent reports based on potential pituitary stem cell markers that will be detailed later in this review (13–17).
From embryogenesis to adulthood, the number of proliferating cells decreases progressively, whereas the number of differentiated cells increases (11). Although all hormone-producing cell types are formed during embryonic development, the pituitary cell constitution at birth is far from finalized, and the rodent anterior pituitary gland still grows and matures substantially during the first postnatal weeks (18). Interestingly, terminally differentiated cells can reenter the cell cycle a few days after birth (19), thereby increasing the population size for each cell type so that the neonate can function independently (14, 16, 19). There is no direct evidence that the proliferating cells migrate rapidly from the lumen to the developing anterior lobe after birth; progenitors scattered throughout the gland could also be a source of renewal. The presence of a somatotroph network throughout the pituitary implies that newly born somatotrophs should actively or passively move away from the lumen toward the somatotroph network (20). The signals that lead to the formation of cell type-specific networks in the pituitary remain unknown. In addition, endothelial progenitors could be supportive cells for either differentiation of stem cells or guidance through these hormone-specific networks.
As suggested by the work of Levy (21), a likely mechanism to account for the large increase in cell number is a population of transient amplifying cells that proliferate outside of the marginal zone, or alternatively, reentry of differentiated cells into the cell cycle. Several studies report continuous proliferation of stem cells that give birth to transit-amplifying cells in basal conditions in adult pituitary. The expansion and differentiation of this compartment is such that the whole pituitary could be renewed in 5–8 wk, theoretically. In the absence of a stimulus corresponding to physiological needs for growth of differentiated pituitary cells, most of the transient-amplifying cells undergo apoptosis. In acute stress conditions or after injury, transit-amplifying cells give birth to nascent null cells (meaning hormone negative) and then to mature null cells differentiating into specific endocrine cells. This whole process takes about 3 wk, suggesting that early stress-induced modifications of pituitary composition could be due to preexisting mature null cells, probably scattered through the gland. These rare cells could be expressing PROP1, a paired homeodomain transcription factor, when they are quiescent, or PROP1 could be expressed as a triggering signal to promote differentiation. Another hypothesis could be proliferation of differentiated cells (21, 22). In rats, however, the expansion of a specific type of differentiated pituitary cell seems to involve nascent or mature null cells rather than differentiated cells. In animals with combined adrenalectomy and gonadectomy, the majority of dividing cells in the anterior pituitary are not positive for either ACTH or LH during the peak period of mitotic response (23). A population of undifferentiated cells may be responding to the cues for increased ACTH and/or gonadotropin production; otherwise, cells may transdifferentiate, or differentiated cells may undergo mitosis. It is possible that some combination of these mechanisms is used to respond to the physiological need for increased hormone production. There is no direct evidence for cell migration from the marginal zone or from mini-niches within the gland in acute stress conditions.
Characterization of migration and differentiation steps in response to injury and or stress is complicated by the variety of protocols in use and possible species differences. Rats spontaneously develop very aggressive pituitary carcinomas from hyperplastic pituitary zones, which is rarely observed in humans. This suggests that the vulnerable steps regulating proliferation and differentiation are different in each species. Most human studies used very different protocols in patients of various ages: regeneration studies after partial hypophysectomy, electrocoagulation for metastases, or postmortem analyses gave different results, probably due to different amounts of resected tissue or different degrees of inhibitory phenomena like local inflammation, or different ages of the patients/animals leading to different capacities of regeneration (24, 25). Of note, some of these experiments described an expansion of chromophobe cells, but without any further detail allowing a more precise definition of these cells.
The precise signals that trigger expansion of a specific cell population are unknown but likely involve feedback loops, growth factors, signaling pathways, and transcription factors. Some components of this regulatory process may be similar to those observed during pituitary development to promote gland growth, but there certainly could be pathways unique to postnatal maintenance. The roles of hypothalamic signals are evident, given the failure of specific cell types to expand after birth in mice with genetic defects in hypothalamic-releasing factor production and/or response. Presumably, the receptors for hypothalamic-releasing hormones are expressed in committed cells that are hormone positive, and therefore, proliferation stimulated by releasing hormones would be limited to hormone-producing cells. Hypothalamic signals could be involved in the migration of differentiated cells to their network and in the process of proliferation or transdifferentiation of differentiated cells. The latter theory is supported by evidence that receptors for hypothalamic-releasing hormones are not strictly restricted to individual cell types (22).
To conclude, the possibility of pituitary stem cells is very likely. The mechanism(s) that regulate progenitor proliferation and differentiation during development and after birth are not completely understood. We recently completed a study that birth-dated hormone-producing cells derived from Rathke's pouch with an analysis of their locations in the gland at birth; the cells are not located dorsal-ventral or rostral-caudal based on their birth date, which supports the idea of migration and organization into homotypic networks (26).
Over the past 5 yr, innovative approaches have been used to define new populations of multipotent pituitary progenitor cells, based on potential pituitary stem cell markers. It will be interesting to apply this new knowledge of markers for stem cells and progenitors to better understand how the pituitary gland responds to physiological challenges and to understand the relative importance of hypertrophy, transdifferentiation, and new cell generation in the response.
III. Potential Pituitary Stem Cell Populations
Recent studies have reported potential populations of stem cells in the pituitary. Each study was focused on a different marker: SOX2, GFRa2, SCA1, nestin, S100β (Table 1). Each of these markers is expressed in stem cells of other organs, and the population of pituitary cells positive for these markers was characterized.
Table 1.
Summary of markers potentially involved in pituitary stem cells, progenitors, and differentiation
| Gene name | Protein encoded | Main characteristic |
|---|---|---|
| Transcription factors | ||
| Sox2 | SRY-related HMG box transcription factor | Expressed in pituitary marginal zone, stem cell marker in several tissues |
| Sox9 | SRY-related HMG box transcription factor | Might play a role in the transition between pituitary stem cells/progenitors and transit-amplifying cells |
| Prop1 | Paired homeodomain transcription factor | Expressed in pituitary marginal zone prior to emergence of most differentiated cell types |
| Tpit (Tbx19) | Pituitary T box transcription factor | Promotes corticotroph differentiation |
| Oct4 (Pou5f1) | POU homeodomain transcription factor | Expressed in several tissue stem cells |
| Pou1f1 (Pit1) | POU homeodomain transcription factor | Involved in differentiation of somatolactotroph and thyrotroph lineages |
| Nanog | Homeobox transcription factor | Involved in maintaining stem cell pluripotency |
| Hes1 | Basic helix loop helix transcription factor | Notch downstream target, repressor of cell cycle inhibitors. Expressed in S/G2/M/G1, not in G0 |
| Cell cycle regulators | ||
| Bmi1 | BMI1 polycomb ring finger oncogene | Regulates cell cycle inhibitor genes |
| Cyclin D1 (Ccnd1) | G1/S specific cyclin D1 | Involved in G1/S cell cycle transition |
| Cyclin D2 (Ccnd2) | G1/S specific cyclin D2 | Involved in G1/S cell cycle transition |
| Cyclin E (Ccne1) | Cyclin E | Allows progression to S phase |
| Cdk4 | Cyclin-dependent kinase 4 | Involved in cell cycle G1 phase progression |
| Ki67 | Antigen Ki-67 | Nuclear protein associated with cell proliferation. Marks all steps except G0 |
| Intermediate filament proteins | ||
| Nestin | Type VI intermediate filament protein | Expressed in pituitary marginal zone |
| Gfap | Glial fibrillary acidic protein (intermediate filament protein) | Expressed in folliculostellate cells |
| Cytokeratin 8 (Krt8) | Keratin-containing intermediate filament protein | Expressed in pituitary marginal zone in adulthood |
| Adhesion and cell surface proteins, receptors, and others | ||
| E-cadherin (Cdh1) | Calcium-dependent adhesion molecule (type 1 transmembrane protein) | Might be involved in transition from proliferation to differentiation |
| Epcam (CD326) | Epithelial cell adhesion molecule | Pan-epithelial differentiation antigen expressed in carcinomas |
| CD90 | Thy1 or CD90 (cell surface protein) | Marker of a variety of stem cells |
| Gfra2 | GDNF receptor α 2 | Stem cell marker in testis and ovary. Expressed in pituitary marginal zone |
| S100β | S100 calcium binding protein B | Expressed in folliculostellate cells |
| Sca1(Ataxin1) | Stem cell antigen 1 | Expressed in the side population cells and pericytes |
| c-Kit (CD117) | Cytokine receptor CD117 | Expressed in hematopoietic stem cells |
| Notch1 | Notch 1 (transmembrane receptor) | Involved in progenitor differentiation in CNS |
| Rb | Retinoblastoma | Tumor suppressor gene. Haploinsufficiency increases risk of retinoblastoma in humans and intermediate lobe adenomas in mice |
| AIP | Aryl hydrocarbon receptor interacting protein | Involved in familial pituitary adenomas |
A. The SOX2+ cell population
SOX2 is a member of the family of high-mobility group (HMG) box transcription factors. It is required for the maintenance of several stem cell populations in humans during central nervous system development (27). The best evidence for pituitary stem cells comes from a study by Fauquier et al. (14). They discovered a SOX2+ cell population located around the lumen in the marginal zone between the anterior and the intermediate lobes and scattered in small clusters inside the pituitary gland of mice (Fig. 2, a and b). The lumen is created when oral ectoderm cells that face the oral cavity invaginate to produce Rathke's pouch (14). These SOX2+ cells are initially present throughout Rathke's pouch (e11.5), and they become progressively restricted to the marginal zone around both sides of the lumen. SOX2+ cells express E-cadherin, which is a nonspecific marker of marginal zone cells. Some SOX2+ cells are scattered throughout the gland. It is not clear whether these scattered SOX2+ cells have transitioned to a niche-independent state or whether supporting cells, such as folliculostellate cells, serve as mini-niches.
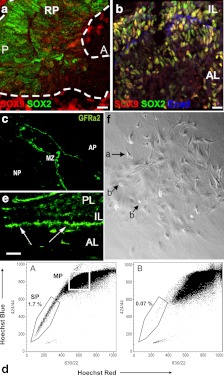
Fig. 2.
Markers of putative pituitary stem cells. a and b, SOX9 is expressed in the embryonic pituitary gland and defines two populations of SOX2+ cells. At e12.5 (a), the majority of SOX2+ cells (green) are SOX9 negative (red) (RP, Rathke's pouch; A, anterior; P, posterior) (14). At e18.5 (b) and in adults, the majority of SOX2+ cells are SOX9+ cells (yellow staining); these cells are probably transit-amplifying cells (14). Some cells are still SOX2+, SOX9− (green) (AL, Anterior lobe; IL, intermediate lobe). E-cadherin (blue) is a marker of marginal cells and folliculostellate cells (14). c, The marginal zone of human adult pituitary contains a niche of progenitors expressing GFRa2 (15) (AP, Anterior pituitary; MZ, marginal zone; NP, neuropituitary). d, The adult mouse anterior pituitary contains a side population divided into SCA1high- and non-SCA1high - expressing cells. The SCA1high group are presumably endothelial progenitors, and non-SCA1high are presumably pituitary stem cells. Dual wave length FACS analysis reveals the presence of typical side population cells (1.7% of total living cells) in the adult anterior pituitary that are Hoechst low (d, A) and can be blocked by verapamil (d, B) (3) (SP, Side population; MP, main population). e, Nestin-GFP cells in mouse pituitary at birth. The cells are located almost exclusively in the perilumenal area of the pituitary (16) (AL, Anterior lobe; IL, intermediate lobe, PL, posterior lobe). f, Colony-forming pituitary cells in low-density culture colonies at d 8. Phase contrast low-power view showing stellate-shaped cells with long cytoplasmic processes (arrow a) and round refractile cells (arrow b). These cells express S100β and GFAP, which are folliculostellate markers (17). Panels a and b are reproduced from reference 14, with permission. © 2008, National Academy of Sciences. Panel c is reproduced from reference 15. Panel d is reproduced from reference 3, with permission. © 2005, The Endocrine Society. Panel e is reproduced from reference 16, with permission. © 2008, The National Academy of Sciences. Panel f is reproduced from reference 17, with permission. © 2005, Elsevier.
In adult mice, SOX2+ cells represent 3–5% of the whole population of the anterior lobe, which is consistent with the theoretical limited number of stem cells in adult organs. For instance, less than 1% of cells of mature adult organs (pancreas, skin, mammary gland) are able to form spheres that evolve into differentiated cells (28–30).
There is a stepwise progression of stem cell marker expression during embryogenesis: cells only express SOX2 at e12.5, whereas they express SOX2 and SOX9 at e18.5. During embryogenesis the SOX2+, SOX9− cells are highly proliferating, which is consistent with a role of progenitors involved in pituitary organogenesis. The location of these cells in the developing pituitary is consistent with studies of cell cycle markers that indicate a regional separation of cells transitioning from proliferation to differentiation (11, 31). Interestingly, in adult pituitaries, the majority of SOX2+ cells also express SOX9, whereas only rare cells are SOX2+, SOX9−. Although the role of adult SOX2+ cells remains to be determined, it is plausible that these cells are a reserve of quiescent, multipotent cells for organ maintenance. These SOX2+ cells are indeed slowly dividing, a feature observed for some stem cells. The SOX2+, SOX9+ cells are more rapidly dividing, which is consistent with the idea that they represent transit-amplifying cells. Shortly after birth the level of proliferation of SOX2+, SOX9+ cells is increased, in agreement with the wave of cell proliferation necessary to accommodate pituitary growth in the first week of life (18). We hypothesize that the progression from SOX2+, SOX9− cells to SOX2+, SOX9+ cells observed during embryogenesis also occurs in adulthood. About half of the pituitary SOX2+ cells also express epithelial markers like E-cadherin, suggesting that an epithelial to mesenchymal transition might be necessary before these cells become rapidly dividing, differentiating cells.
The role of SOX9+ cells in the pituitary is unclear. SOX9 has opposite roles in the development of the pancreas and intestinal epithelium, suggesting tissue-specific differences in function. Seymour et al. (32) demonstrated that SOX9 is necessary in a mitotically active, Notch-responsive subset of progenitors during pancreas development. SOX9 expression maintains pancreatic progenitors by stimulating their proliferation, survival, and persistence in an undifferentiated state. Because SOX2 expression was not evaluated in the pancreas, it is difficult to draw an analogy to pituitary SOX2+, SOX9+ cells. In contrast to the role of SOX9 in the pancreas, SOX9 seems to promote differentiation in the intestinal epithelium because its inactivation leads to increased proliferation of progenitors and differentiation into Paneth cells (33, 34). These apparently different roles of SOX9 might be correlated with different levels of expression in the progenitors or stem cells. In support of this idea, Sox9 transgenes with low expression support proliferative capacity, whereas high SOX9 expression suppresses proliferation and induces differentiation (35). In the embryonic pituitary, SOX9 is probably a marker of pituitary progenitor/transit-amplifying cells, representing one step after initial SOX2+ stem cells. SOX2+, SOX9− and SOX2+, SOX9+ cells might be called upon in case of tissue loss or in response to physiological demands.
The methodology of sphere formation follows the progression of single cells into a heterogeneous population of differentiated cells. Pituispheres cultured from dispersed adult anterior pituitaries exhibited the ability to self-renew and differentiate into hormone-producing cells (see Fig. 4a). The pituispheres displayed a pattern of marker expression evolving from SOX2, SCA1, and E-cadherin positivity in the first few days to cells expressing SOX2, SOX9, and then S100β after 6–7 d (14). SCA1 expression was not detectable at the later time point, which is consistent with another study focused on a side population of cells identified by cell sorting (13). The delayed expression of S100β in pituispheres favors a transient state of S100β expression during progenitor cell differentiation. Pituispheres derived from adult pituitaries were able to form secondary spheres, demonstrating the ability to self-renew. In different cell culture conditions (mainly with Matrigel), these pituispheres differentiated into the five anterior pituitary cell lineages, demonstrating their multipotent status. Some of the cells in the pituispheres exhibited coexpression of SOX2 and hormones, a feature not observed in vivo. The reason for this discrepancy is not clear. It will be important to assess the potential of purified cell types to form pituispheres and self-renew, i.e., fluorescence-activated cell sorting (FACS) purified SOX2+, SOX9− and SOX2+, SOX9+ cells. This will establish the potential of each progenitor for self-renewal and differentiation.
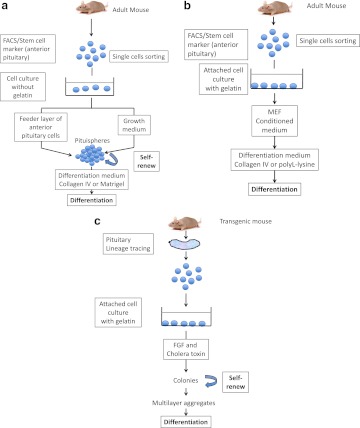
Fig. 4.
Different ways to isolate and differentiate pituitary stem cells. a, Cells of the anterior lobe are dissociated into single cells and sorted by FACS based on specific characteristics. Side population cells display verapamil-sensitive Hoechst dye efflux capacity and/or a stem cell marker such as SCA1 and GFRa2. Cells can be seeded in dishes with medium enriched in growth factors (DMEM/Ham's F12 supplemented with growth factors) (13–15). Adult anterior pituitary cells can also be used as a feeder layer (13). After 6–8 d, pituispheres appear (16). These can be handpicked individually, dispersed into single cells, and cultured to evaluate their self-renewal capacity (generation of secondary spheres). To induce differentiation, growth medium can be replaced by a specific differentiation medium. Cells are coated on growth-factor reduced Matrigel (13, 14). 4b, Anterior pituitary cells are dissociated into single cells and sorted by FACS based on a stem cell marker (AMCA) (15). Cells can be cultured on gelatin with mouse embryonic fibroblasts (MEF). To induce differentiation, growth medium can be replaced by a specific differentiation medium. Cells are coated on Collagen IV or poly-L-lysine culture slides. c, Lineage tracing involves isolation of nestin-expressing cells based on a GFP transgene (16). Cells are cultured on gelatin. After 6- to 8-d culture in fibroblast growth factor 2 (FGF2) medium and cholera toxin, colonies can be visualized. They can be handpicked individually, dispersed into single cells, and cultured to evaluate their self-renewal capacity. After multilayer aggregates formation, differentiated cells can be observed.
The pituispheres derived from adult pituitary cells could be passaged for only two generations, which falls short of expectations for stem cells, but it implies that the adult pituitary does contain progenitors. The classical definition of stem cells requires at least five passages as spheres (36). The limitation in passage capacity might be due to the fact that these SOX2+ cells are already progenitors rather than stem cells. Alternatively, the apparent limitation could be due to technical issues, such as the lack of appropriate culture conditions. Further studies may uncover growth factors or contacts with neighboring cells that simulate the niche and permit additional passages.
To summarize, Fauquier et al. (14) have proven that SOX2+ cells have characteristics of progenitors and stem cells because they normally do not express pituitary hormone markers, are capable of at least limited self-renewal, and are able to differentiate into the five anterior pituitary cell types. SOX2 expression is detectable during embryogenesis and at adulthood, consistent with the idea that the adult pituitary gland has progenitors or stem cells that can regenerate all the cell types in the organ, and that the process for adult cell replenishment may be similar to the process that occurs in development. Although it is exciting that SOX2+ cells gave birth to all five anterior pituitary cell lineages, it will be important to assess the capacity for these cells to expand and regenerate a functional organ after tissue loss. An interesting experiment would be to graft wild-type SOX2+ stem cells into Prop1 mutant mice and evaluate their ability to correct the hypoplasia and hypopituitarism that characterize these mice (19). A similar kind of experiment conducted in the early 1960s showed that transplanting normal pituitary cells in dwarf mice rescued the phenotype, but the essential cell types were not identified (37).
B. The GFRa2+ cell population
Garcia-Lavandeira et al. (15) recently used the glial cell line-derived neurotrophic factor (GDNF) receptor α2 (GFRa2) as a marker for pituitary stem cells. This receptor is expressed in putative stem cells of the testis and ovaries (38). It belongs to a family of glycosyl-phosphatidyl receptors that modulate signaling pathways induced by their ligands, the main one being GDNF. In developing neurons, GDNF was originally characterized as a growth factor promoting the survival of ventral midbrain dopaminergic neurons. Later, it was found to have a potent survival effect on motor neurons and other neuronal subpopulations in the central and peripheral nervous systems. In addition to its role as a survival factor, GDNF is also essential for proliferation, migration, and differentiation of neuronal cells (39).
A population of GFRa2+ cells exists in adulthood that are restricted to a single layer in the marginal zone of the mouse pituitary gland (Fig. 2c). In adults, the population of GFRa2+ cells represented less than 1% of the whole pituitary cell population. Interestingly, more than 90% of these cells expressed SOX2 and SOX9, which would suggest that these cells are the progenitors/transit-amplifying cells described by Fauquier et al. (14). Other points of concordance are the expression of the epithelial marker E-cadherin in the majority of cells and S100β in about 50% of the cells. Interestingly, these GFRa2+ cells expressed PROP1, a pituitary transcription factor important for the POU1F1 lineage, comprised of somatolactotroph and thyrotroph cell types (40). This might imply that the GFRa2+ cells are in a predifferentiated state. Given the critical role of PROP1 during pituitary organogenesis, one might expect pituitary stem cells to progress through a PROP1+ stage to produce new hormone-producing cells. PROP1 is also required for normal E-cadherin expression, which is present in all GFRa2+ cells (41). Finally, GFRa2+ cells were able to form primary and secondary pituispheres and to differentiate into the five pituitary cell lineages (see Fig. 4b). It is significant that a comparison of GFRa2+ cells with GFRa2− cells clearly showed that the latter were not able to form pituispheres, suggesting that GFRa2− cells are not multipotent. Only a limited number of passages of GFRa2+ cells were possible, similar to the observations by Fauquier et al. (14) for SOX2+ cells. With special medium conditions, spheres derived from GFRa2+ cells are capable of differentiation into tubulin-β III-expressing cells, a marker characteristic of neurons (15). Thus, these GFRa2+ cells are clearly multipotent progenitors, but they may not be truly “stem cells.”
The precise role of GFRa2 in these cells remains to be determined. GFRa2 is not detectable when the cells express pituitary hormone markers, suggesting that it has a transient role during the differentiation process. It will be particularly interesting to establish the expression profile of this receptor during embryonic development to determine whether GFRa2 is only present in adult stem cells or whether it also plays a role in pituitary development during embryogenesis. To confirm the idea that GFRa2 characterizes progenitors rather than stem cells, the size of the pituispheres should be evaluated. Pituispheres are larger if they are derived from stem cells rather than progenitors with more limited potential.
To summarize, the study by Garcia-Lavandeira et al. (15) made very important contributions by demonstrating that GFRa2+ cells have the characteristics of progenitor/stem cells because they: 1) expressed no pituitary hormone markers; 2) were capable of at least limited self-renewal (they form pituispheres when cultured with specific media); and 3) are able to differentiate into the five pituitary cell types. As with the study of Fauquier et al. (14), it will be important to explore the ability of GFRa2+ cells to drive additional self-renewal and to regenerate tissue.
C. The “side population” cells
FACS has been used to identify a side population enriched in stem cells in several tissues (42). The initial description of these cells in bone marrow was based on verapamil-sensitive dye efflux capacity (42). Vankelecom and colleagues (3, 13) were the first to apply this technology to the pituitary gland and proceeded to identify cells in mouse, rat, and chicken with a “side-population” phenotype (Fig. 2d). These cells clonally replicate as nonadherent spheres and express candidate stem cell markers (3). The side population isolated from the anterior pituitary of 3- to 8-wk-old mice represents 1.7% of the whole anterior pituitary cell population. This side population is composed of cells expressing SCA1 at a high level (SCA1high; about 60% of the side population cells) and at a lower level (non-SCA1high; less than 40% of the cells). SCA1, a putative stem cell marker (43), has been observed as a transient marker in pituispheres derived from SOX2+ cells (14). In contrast, the main population of pituitary cells, which is mostly differentiated cells, contains only 5% of so-called SCA1low cells and 2.5% of SCA1high cells (the majority of cells being SCA1 negative). It would be valuable to have a more precise definition and/or additional markers to describe the SCA1high, non-SCA1high, and SCA1low cells.
Vankelecom and colleagues (3, 13) compared SCA1high and non-SCA1high side population cells in adult mice. The SCA1high cells express several stem cell markers, including OCT4 and Nanog mRNA, and they express higher levels of nestin, BMI1, Notch1, and HES1 compared with the main population (other anterior pituitary cells). Some of these SCA1high cells also expressed S100β. This suggests that the SCA1high cell population might contain folliculostellate cells. Microarray analysis revealed prominent expression of angiogenesis-related genes in the SCA1high cells. Because SCA1 is involved in endothelial cell development (44), these SCA1high cells may be endothelial progenitors rather than pituitary progenitors. In contrast, SOX2 and SOX9 were expressed in about 50% of non-SCA1high cells and absent from the SCA1high cells. OCT4, Bmi-1, and nestin displayed similar levels of expression between SCA1high and non-SCA1high populations, suggesting that these markers are probably not specific to pituitary stem cells or that SCA1 level does not perfectly define the stem cell population. Most of the transcription factors involved in pituitary development (LHX3, LHX4, PITX2, ISL1, PITX1, and OTX2) (40) were expressed at increased levels (2.5- to 25-fold more) in non-SCA1high cells relative to SCA1high cells. Interestingly, the non-SCA1high cells also expressed PROP1 and HESX1. These transcription factors are normally expressed at different developmental times and are essential for normal pituitary organogenesis (40). Thus, the non-SCA1high population is likely to be heterogeneous, possibly including both stem cells and progenitors at various steps of differentiation.
Vankelecom and colleagues (3, 13) derived pituispheres from non-SCA1high cells using a standard culture medium supplemented with growth factors (see Fig. 4a). Neither the SCA1high cells nor the main population of sorted cells were able to produce pituispheres. In 6-d-old spheres, the majority of the cells expressed SOX2 and nestin. Because 50% of non-SCA1high cells express SOX2, the cells that formed pituispheres may have been cells initially expressing SOX2. The presence of SOX2 expression at d 6 is concordant with other studies (14, 15). Again, it was difficult to maintain an ability to self renew during extended serial passaging. Second-generation spheres cultured in the appropriate medium gave birth to the five pituitary cell lineages and ceased expression of SOX2, as expected. The authors agree with Fauquier et al. (14) that the SOX2+ cells are around the lumen in the presumptive stem cell niche and in clusters scattered over the anterior pituitary. The relationship of SOX2+ cells to non-SCA1high cells is not clear. A unique marker for non-SCA1high cells is necessary for identifying the location of these cells in the gland. Coexpression of SOX2 and pituitary hormone markers was observed in some adult pituitary cells. This is intriguing because other studies did not detect SOX2 in fully differentiated cells (14, 15). The authors hypothesize that SOX2 might be localized to the cytoplasm during the differentiation steps and localized to the nucleus during the stem cell state. Further studies are needed to clarify this issue.
To summarize, Vankelecom and colleagues (3, 13) have made an important contribution by using cell sorting to purify cell populations and examine gene expression and pluripotency. They have shown that the non-SCA1high side population cells express no pituitary hormone markers, are capable of at least limited self-renewal because they form pituispheres when cultured in specific media, and are able to differentiate into the five anterior pituitary cell types. Future studies will need to address the potential of non-SCA1high side population cells to self-renew more extensively and to expand to regenerate tissue. Gene expression profiling of this cell population has been useful for comparing the side population with other studies using a variety of markers, yet discrepancies in SOX2 expression need to be resolved. All studies of pituitary progenitors could benefit by implementing FACS to analyze gene expression in cell populations purified using other markers and to assess the potential for pituisphere formation.
D. The nestin+ cell population
Nestin is an intermediate filament protein mostly expressed in nerve cells, where it is implicated in the radial growth of axons (45). Nestin is a marker of stem cells in several types of tissues, including adult and embryonic neural stem cells (45). Gleiberman et al. (16) used transgenic mice expressing green fluorescent protein (GFP) driven by regulatory elements of the nestin gene to characterize a potential progenitor population in the pituitary (Fig. 2e). They also used a nestin-cre transgene and cre-responsive GFP reporter strain to carry out lineage tracing of nestin cells. This approach is different from the previous ones in which the cell population of interest was isolated from the pituitary using an endogenous marker and cultured to form pituispheres. The transgenic marker approach carries the inherent risk of ectopic expression of the transgene, and conclusions from this study are diminished because the transgene was not proven to recapitulate endogenous nestin gene expression. Indeed, there are examples of various nestin-cre transgenes that do not mimic endogenous nestin gene expression (46, 47). Despite this caveat, the authors did prove that a set of genetically marked cells in Rathke's pouch can differentiate into all hormone-producing cell types.
Nestin-GFP-expressing cells were first detected at e11.5 in the dorsal part of Rathke's pouch; they represented about 2% of the whole cells of the pouch. In adulthood, cells in this region or “niche” express SOX2 and epithelial markers cytokeratin 8 and EpCAM. The coexpression of SOX2 and nestin during development remains to be demonstrated, and a recent report presents convincing evidence that the pituitary gland, indeed, does not express endogenous nestin during development (47). The GFP-expressing cells also expressed LHX3, a LIM domain transcription factor involved in the early steps of pituitary development (40). To trace the lineage of these cells, a nestin-cre line was crossed with the ROSA26-loxP-stop-loxP-GFP reporter line. Progeny carrying both the cre reporter and the cre-expressing transgenes reveal which differentiated cells are derived from the cre-expressing progenitors. The cre-expressing precursor cells undergo cre-mediated recombination of the reporter gene in the genomic DNA, which permanently marks the precursors and the differentiated cells derived from them with GFP expression. The number of GFP-expressing cells represented a maximum of 20% of the whole population in adulthood (5 months of age), whereas it only represented 2% of the anterior pituitary cells immediately after birth. A small fraction of the GFP+ cells expressed POU1F1 (PIT1), a pituitary transcription factor essential for establishing the fate of somatolactotroph and thyrotroph lineages (40). POU1F1 expression in GFP+ cells is consistent with them having achieved a differentiated state. Interestingly, early postnatal days were the only period (in comparison with later postnatal weeks) during which these cells stained for Ki67, a marker of proliferating cells. Ward et al. (19) had previously shown that POU1F1 cells had a very high proliferation rate in the neonatal period.
In adulthood, nestin-GFP-positive cells were observed mainly in the marginal zone (Fig. 1), consistent with the localization of pituitary progenitor cells in other studies (14, 16). GFP expression from the cre-reporter was detected in the five types of terminally differentiated pituitary cells at adulthood, suggesting that nestin-cre-expressing progenitors were able to differentiate into each kind of pituitary cell. This approach, however, was not based on pituisphere formation (see Fig. 4c). Gleiberman et al. (16) suggest that the nestin-GFP+ cells might be pituitary stem cells in adulthood, in contrast to other stem cells that might be used during embryogenesis; the number of GFP+ cells indeed remains unchanged during embryogenesis and increases only after birth. The lack of characterization of the nestin-GFP and nestin-cre transgenes relative to endogenous nestin expression weakens the conclusions that can be drawn from this study. Nestin could be mainly expressed during embryogenesis in endothelial progenitors, as suggested by Chen et al. (13), and in pituitary progenitors after birth. A precise evaluation of nestin expression coupled to cell markers during embryogenesis and adulthood has recently been carried out (47), contradicting the conclusions drawn by Gleiberman and colleagues. In the study by Rizzoti and colleagues (47), nestin expression was not observed in Rathke's pouch before 18.5 d post coitum, and nestin-cre activity was not detected until at least 16.5 d post coitum. In the adult, very few cells showed transgenic activity. Fauquier et al. (14) reported nestin expression in pituispheres after SOX2, suggesting that nestin was probably playing a role in stem/progenitor cell development. However, results of Rizzoti and colleagues (47) suggest that nestin+ cells are unlikely to be pituitary stem cells.
Interestingly, GFRa2+ cells are all negative for nestin (15), whereas the non-SCA1high side population is positive for nestin. Both populations are expected to be SOX2+, SOX9+. This suggests four possibilities: 1) a technical difficulty underlies some of the results (15); 2) the pituitary contains distinct populations of multipotent progenitor cells with unique sets of expressed genes; 3) there is only one population of progenitors that expresses different markers at each time point; or 4) the nestin-GFP transgene has ectopic expression in a set of cells within Rathke's pouch that happens to mark the stem cell niche (13). The work of Rizzoti and colleagues (47) strongly supports the latter possibility. Because nestin+ cells were mainly dividing after birth, the authors' final hypothesis was that these cells were probably quiescent progenitors necessary for the initial wave of pituitary growth after birth, and pituitary maintenance function at adulthood, different from the ones involved during embryonic development (pituitary organ formation).
The concept of nestin as a pituitary stem cell marker is intriguing, but this idea requires more rigorous investigation. The nestin expression profile in the developing rat pituitary gland is dynamic (48). Nestin expression increased progressively after birth, between postnatal day (P) 5 and P12 and decreased precipitously at P21, a time when much of the pituitary organ growth has been achieved. Levels of nestin expression remained significant, however, in adulthood and in 2-yr-old rats, particularly in zones that might correspond to hyperplastic aberrant nodular growth. Two different morphological nestin+ cell populations were observed: first, a filamentous cell type, close to the lumen; and second, cells close to blood vessels, likely involved in vascular development. The increased expression of nestin in hyperplastic nodules relative to normal tissue could be linked to increased vascularization of the adenoma. The nestin-GFP+ population described by Gleiberman et al. (16) could include both kinds of nestin+ cells. A portion of these would be stem cells expressing SOX2 and LHX3, whereas other nestin+ cells would be supportive cells involved in vascular development and would be negative for SOX2 and LHX3 expression. These data are consistent with a study that reported nestin-expressing pituitary cells to be very heterogeneous, including a subpopulation frequently associated with endothelial cells (49). This hypothesis is also in agreement with the study reported by Chen et al. (13), showing that nestin was equally present in the non-SCA1high cells, which are presumably pituitary progenitors, and the SCA1high cells of the side population that are likely endothelial progenitors.
To summarize, Gleiberman et al. (16) have used a lineage tracing to determine which hormone cell types arise from transgene marked cells. The transgene-expressing cells also express key markers of the undifferentiated state, and they seem to give birth to all five pituitary lineages. In addition, transgene-expressing cells are proliferative during the postnatal growth period, when the demand for new progenitor cells is high. Future studies testing the capability of transgene-marked cells to form pituispheres and differentiate into each cell type would provide a valuable confirmation of the ability of these cells to self-renew and exhibit pluripotency. Another important corollary experiment is to test the ability of transgene-negative cells to form colonies.
E. The folliculostellate cells
Initial work suggested that folliculostellate cells could be pituitary stem cells (12, 50, 51). Folliculostellate cells are non-hormone-producing, agranular cells, in contrast to pituitary polypeptide hormone-secreting cells, which are filled with secretory granules. The folliculostellate cells are located in the parenchymal tissue of the anterior pituitary lobe, mainly around the lumen of large follicles scattered throughout the lobe, and they constitute about 5–10% of the whole population of cells (12, 50, 51). They have a stellate morphology comprised of long cytoplasmic projections between the glandular cells. Folliculostellate cells have several roles in the pituitary, including acting as scavenger cells with phagocytic activity and as supportive cells through both mechanical structure and by production of cytokines and growth factors. They also facilitate interactions between the endocrine and immune systems, between pituitary cells via their own functional network, and as supportive cells for the GH network (52).
Their main markers are S100β protein and GFAP (glial fibrillary acidic protein). Interestingly, S100β is detectable only after birth, first in the marginal zone (51). Using S100β as a folliculostellate marker is ambiguous because not all folliculostellate cells express this marker. Moreover, S100β may not be specific to the folliculostellate cells (51). Indirect evidence suggests the possibility of retrodifferentiation of endocrine cells into folliculostellate cells (53) or expansion of the folliculostellate compartment in parallel with gonadotrophs after castration in rats (54). It will be valuable to have more precise markers for folliculostellate cells and to perform more direct experiments testing their ability to self-renew (pituisphere approach).
Lepore et al. (17) described a pituitary cell population able to form colonies in adult mice. The pituitary colony-forming cells represent 0.2% of the cells in the whole anterior pituitary (Fig. 2f). These cells are contained in a subpopulation of pituitary cells that import fluorescent β-Ala-Lys-N ε-AMCA (Y-amino-4-methylcoumarin-3-acetic acid). They are stellate, with long cytoplasmic processes. All of them express S100β and GFAP, whereas only 40% express SCA1 and angiotensin-converting enzyme, and a few express GH. The expression of both S100β and GFAP is consistent with the idea that these colony-forming cells are folliculostellate cells. No other theoretical pituitary stem cell marker has been evaluated. The ability to self-renew and to differentiate into the five pituitary lineages has to be shown to confirm the progenitor or stem cell status of these cells (17, 55).
A recent study reported ongoing work on transgenic mice expressing GFP under a cell-specific promoter of the S100β protein (56). This approach, if not compromised by ectopic expression of the transgene, might allow for cell sorting, expression profiling, and development of reliable markers as well as determination of the potential of these cells to differentiate into pituitary hormone-secreting cells.
F. Prospective for future analyses
Future studies will facilitate a direct comparison of the stem cell populations identified in these four reports by comparing each of the markers during similar periods of observation. A suggested schema of pituitary progenitor cell marker expression during progression to differentiated cells is shown in Fig. 3. None of the studies demonstrated more than two rounds of pituisphere self-renewal. The difference in outcome observed with Matrigel compared with classical growth factors is interesting, and it suggests the possibility that a specific three-dimensional structure is necessary to promote the renewal and differentiation of the cells. Thus, another important advance will be to identify conditions that permit five consecutive generations of pituisphere formation. This will firmly establish the existence of stem cells, instead of multipotent progenitors, which is the current state of the art. Culture conditions leading to differentiated cells are summarized in Fig. 4.
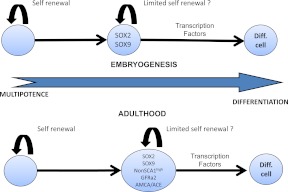
Fig. 3.
Stem cells during embryogenesis and adulthood. Pituitary stem cells differentiate during embryogenesis and form the pituitary lineages (14). These cells evolve from SOX2 to SOX9 positivity. No other marker has been evaluated during embryogenesis. Nestin does not seem to play a role at this time point because nestin+ cells begin to proliferate after birth (16). In adults, rare cells express only SOX2. The majority of adult “progenitors” are SOX2+, SOX9+ (14). It is likely that the same cells express GFRa2 (15), AMCA, and angiotensin-converting enzyme (ACE) (55) and have a little or no SCA1 expression. These cells are probably transit-amplifying cells and are capable of limited self-renewal. Nestin may be expressed in a subset of these cells, although it is not a specific marker of pituitary cells (16). Final differentiation requires spatiotemporal regulation by several transcription factors and signaling pathways (review in Ref. 40). Diff. cell, Differentiated cell.
We hypothesize that there are two critical roles of stem cells: one in establishing the pituitary gland during development, and the other involved in maintenance of the mature pituitary gland in response to physiological challenges and normal cell turnover. The hypothesis of two different populations of stem cells, one involved in embryogenesis and one involved in maintenance function after birth, remains highly controversial. Given the evidence for ectopic nestin transgene expression, there is no compelling evidence for different stem cell populations. The fundamental question of whether adult cell renewal follows the same or a different pathway than initial embryonic differentiation remains to be resolved.
An important next step in the analysis of pituitary stem cells is to expand the data on embryonic marker profiles, including coexpression studies with attention to spatial and temporal location of each cell type. This would expand on the critical studies by Fauquier et al. (14), which is the only group that examined embryonic time points revealing the progression from SOX2+ to SOX2+, SOX9+. Examination of GFRa2, SCA1, and other markers during embryogenesis will be invaluable for establishing the steps in normal pituitary development. A brief summary of the different markers used to characterize pituitary progenitors and differentiated cells is given in Table 1.
IV. Pituitary Stem Cells: from Differentiation to Pathogenesis
In the pituitary, Notch might play a major role in proliferation and fate selection. HES1, a downstream target of Notch, is necessary for suppression of differentiation: loss of HES1 results in a cell fate switch such that intermediate lobe cells differentiate as GH-producing somatotropes instead of POMC-expressing melanotropes (57). This suggests that intermediate lobe cells, normally fated to become melanotropes, can differentiate into different pituitary cell lineages depending on transcription factor interactions (58). Interestingly, Chen et al. (59) have shown that the Notch signaling system is active in the side population cells of the postnatal pituitary. Activation of Notch signaling increased the number of side population cells, whereas down-regulation of Notch reduced the proportion of side population cells. The impact of this study was limited by its reliance on the entire side population cells, which is heterogeneous, containing both the subpopulation of SCA1high cells, which were reported as endothelial progenitors by the same group (13), and non-SCA1high cells, which are the potential pituitary stem cells. Further study is needed to determine the role of Notch signaling in the subset of non-SCA1high side population cells. The Notch signaling pathway might also interact with SOX9 in the proliferation and differentiation steps of progenitors, as demonstrated in pancreas development. SOX9 controls the maintenance of pluripotent pancreatic progenitors by stimulating their proliferation and survival. Interestingly, SOX9-deficient progenitors have reduced expression of the Notch target HES1, suggesting possible interactions between SOX9 and the Notch signaling pathway in stem cell maintenance or fate selection (32). Moreover, there is a feedback regulatory loop between PROP1, which is involved in the transition from proliferating progenitors to quiescent differentiating cells, and Notch2; PROP1 activates expression of Notch2, and Notch signaling feeds back to maintain and/or enhance PROP1 expression (31, 41, 60, 61). In Prop1df/df mice, proliferating cells are retained in the perilumenal zone, and there are many fewer proliferating progenitors in the anterior lobe after birth. This suggests that PROP1 may be necessary for migration of progenitors into the anterior lobe.
Transcription factors from the LIM family, including ISL1, LHX3, and LHX4, are likely to be involved in the early steps of pituitary stem cell and/or progenitor differentiation because they are known to be essential for expansion of the pituitary primordium and differentiation of hormone-producing cells during embryogenesis (40, 62). There are contradictory reports on coexpression of these transcription factors and SOX2 or SOX9 (14–16). The hypoplasia in Lhx3 and Lhx4 mutants results from reduced proliferation and increased cell death (62–64). Lhx3 and Lhx4 have overlapping functions consistent with dosage-sensitive effects, and part of the Lhx4 mutant phenotype could be attributable to a delay in Lhx3 activation (62). Chen et al. (59) have reported that withdrawal of LIF, which is necessary to maintain stem cells in an undifferentiated state, results in extinguishing LHX4 and ISL1 (another LIM domain transcription factor) expression. This suggests that LIM domain transcription factors are involved in proliferation rather than differentiation of progenitors and/or stem cells. Surprisingly, however, pituitary hyperplasia has also been observed in some human patients with loss of function mutations of LIM domain transcription factors (65, 66). PROP1, a paired-like homeodomain transcription factor, is probably involved later in the pathway, promoting transition from proliferation to differentiation, thereby generating precursor cells capable of becoming the hormone-producing cells of the anterior lobe (19). Interestingly, a recent report suggested that PROP1 might interact with the transcription factor SOX2 to promote POU1F1-dependant lineage differentiation (somatolactotroph and thyrotroph) (67).
The roles of stem cells in the pathology of pituitary hypoplasia and hyperplasia are less clear. We have some clues about the transitions between proliferation and differentiation from patients with hypopituitarism. A good example is the pituitary morphology modification induced by PROP1 mutations in combined pituitary hormone deficiencies (CPHD). Some patients present with pituitary hypoplasia, whereas others frequently present a transient pituitary hyperplasia, leading to a secondary hypoplasia (40, 68, 69). Based on analyses of Prop1 mutant mice, Ward et al. (19) implied that the pituitary hyperplasia in humans might be due to migratory and/or cell adhesion defects of progenitors, which generates dysmorphology and apparent overgrowth that is eliminated through apoptosis. Proliferating cells are retained in the perilumenal zone of the Prop1df/df pituitaries, instead of colonizing the anterior lobe at e12.5–e14.5. This suggests that cells are unable to differentiate and that the ultimate hypoplasia of Prop1 mutant pituitaries might not be due to a failure of early progenitors to proliferate, but rather to a defect in transitioning to differentiation and in seeding the anterior lobe with cells able to reenter the cell cycle at later stages (19). This idea could also explain the progressive nature of the hormone deficiency in human patients.
Cell cycle regulation of stem cells is probably under the control of several cyclin-dependent kinases and kinase inhibitors. Landmark studies by Drouin and colleagues (11) have demonstrated that two members of the Cip/Kip families of cell cycle inhibitors, p27 and p57, play a major role in the p57-dependent cell cycle exit and progression to differentiation during embryogenesis. Cyclins D1, D2, and E are important actors in the different steps of the cell cycle (11). Notch and HES1 also play roles in the control of cell cycle exit (31, 41). An important area of future research is investigation of the regulation of the cell cycle, including exit for differentiation and reentry for expansion of cell populations.
The hypothesis that hypoplasia can result from defects in the transition from proliferation to differentiation is supported by studies in CDK4-deficient mice. CDK4 is necessary for stem cells to enter into a transit-amplifying state leading to differentiated cells (70). Mice deficient in CDK4 have hypoplastic pituitaries with a dramatic reduction in all hormone secretory cells in the anterior pituitary during postnatal life (15). Interestingly, the number of progenitors, based on the marker GFRa2, was increased in the pituitaries of these mice. This suggests that CDK4-deficient progenitors are able to proliferate but are unable to undergo differentiation into hormone-producing cells. This situation is reversed by reexpression of CDK4 (15). The reason these CDK4-deficient progenitors do not enter the differentiated phase is unknown.
Are stem or progenitor cells involved in pituitary tumorigenesis? Few studies have addressed this issue. Characteristics of pituitary adenomas are unusual compared with other solid tumors. Pituitary adenomas are usually benign, slow growing (25% of pituitary adenomas are discovered on autopsy procedures), do not lead to metastases, and can respond to physiological signals (for instance, dopamine agonists can regulate prolactinomas, and somatostatin analogs can control somatotropinomas) (22). Some pituitary adenomas can resolve spontaneously after several years of quiescence; some secretion profiles can be cyclic, with hypersecretion preceding a long period of normal secretion. Another intriguing point is that not all microadenomas grow into macroadenomas. This suggests that the classical model of tumorigenesis and the recent studies about cancer stem cells might not be fully applicable to pituitary adenomas (71). Factors underlying pituitary tumorigenesis likely include both intrinsic pituicyte alterations and altered availability of regulatory factors including hypothalamic hormones, peripheral hormones, and paracrine growth factors.
Pituitary adenomas could originate from either differentiated cells or progenitors/stem cells. The fact that pituitary tumors can have unusual secretion profiles compared with the classical one observed in normal pituitary cells could favor a stem cell/progenitor origin rather than a predifferentiated cell (22). However, results of X-inactivation studies and loss-of-heterozygosity analysis favor the expansion of a single cell (72). Occasionally, multifocal polyclonal pituitary adenomas associated with hyperprolactinemia may arise due to either extrinsic changes in hypothalamic factors or to pituitary stalk compression, which could block lactotroph inhibition by dopamine. Pituitary tumors could also be originating from several differentiated cells, clonally skewed, leading to a predominant type of secretion. The origin of pituitary tumors is of importance because it might be a clue to determine the triggering signal. One might hypothesize that hypothalamic factors could likely be involved in tumorigenesis from differentiated cells, whereas microenvironment and growth factors could promote tumorigenesis from null mature cells, leading to hormonally nonfunctioning “null cell” adenomas (21, 22).
Markers of stem/progenitor cells have been identified in pituitary adenomas. Gleiberman et al. (16) observed nestin-GFP+ cells in pituitary tumors of nestin-GFP, retinoblastoma, Rb+/−, mice. Rb heterozygotes that carry one functional allele of retinoblastoma Rb1 gene develop tumors in the intermediate lobe of the pituitary (16). These cells express SOX2 and LHX3, but no pituitary hormones. These cells might be an undifferentiated cell compartment connected to the initiation and growth of pituitary tumors (16). On the other hand, these nestin-GFP+ cells might be indicative of modified vasculature induced by the tumor. Interestingly, adult and aged (2-yr-old) rats have at least a 2-fold increase in nestin expression in apparent hyperplastic nodules. These nodules also had enriched vasculature, and it is thus not clear whether the nestin cells were stem cells involved in proliferation or supportive cells involved in vasculature development. Moreover, the precise nature of these masses (Glial-like or adenomatous-like) has not been determined (48). Interestingly, benign pituitary adenomas contain cells capable of producing pituispheres that express markers of stem cells in other tissues, namely OCT4, CD90, and nestin. No SOX2, SOX9, SCA1, or GFRa2 staining was performed, however (73). AIP, encoding aryl hydrocarbon interacting protein, is correlated with increased risk of familial pituitary adenomas (74–76), and members of the AIP complex are expressed during mouse pituitary development (77, 78). This suggests the possibility that an early developmental mechanism for growth regulation of progenitors may be involved in adenoma formation.
The role of transcription factors involved in pituitary development is also difficult to determine in tumorigenesis. POU1F1 is a transcription factor involved in somatolactotroph and thyrotroph differentiation. However, despite the fact that POU1F1 transcripts are usually increased in somatotroph adenomas, pituitary tumorigenesis is not associated with altered POU1F1 expression.
Pituitary hyperplasia has been observed in patients with mutations of PROP1, LHX3, and LHX4 (40). However, only rare patients with hyperplasia or mutations in these transcription factors presented a secondary pituitary adenoma. In contrast with murine models where hyperplasia usually precedes pituitary adenoma, pituitary hyperplasia in humans does not seem to be a prerequisite for tumorigenesis. Resected tissue from transsphenoidal surgery is usually normal around the tumor. Estrogen promotes lactotroph hyperplasia, activates the pituitary tumor transforming gene, and stimulates growth factor release, all of which are implicated in pituitary tumorigenesis (79). However, only rare cases of prolactinoma formation have been reported in patients receiving high estrogen doses (80). Human pituitary hyperplasia associated with rare hypothalamic or ectopic GHRH- or CRH-secreting carcinoid tumors are rarely associated with adenoma development.
Clearly, more basic studies on pituitary progenitors are needed as a foundation for exploring the role of progenitors in adenoma development, progression and recurrence.
V. Pituitary Stem Cells as Potential Therapeutic Tools
The presence of pituitary stem cells that can give rise to all pituitary hormone cell types implies that these critical endocrine cells can be replaced after loss or damage. These stem cells could thus be of major interest in the treatment of congenital (CPHD) or acquired hypopituitarism (induced by surgery, radiotherapy, or traumatic injury) (81, 82).
CPHD is characterized by multiple pituitary hormone deficiencies, including somatotroph, thyrotroph, lactotroph, corticotroph, and/or gonadotroph deficiencies. The condition occurs between 1 in 3000 and 1 in 4000 births. It is important to diagnose and treat CPHD to avoid morbidity and mortality and to maintain a high quality of life (83). CPHD can be due to mutations of several genes encoding pituitary transcription factors involved in pituitary ontogenesis, leading predominantly to GH deficiency combined with variable loss of other hormones (40). Management requires an appropriate replacement of deficient hormones and strict follow-up because delayed deficiencies commonly appear for some individuals with PROP1 deficiency (68, 69). Substitutive treatment remains challenging for all hormones; pituitary hormones are indeed not substituted, and peripheral hormones, although efficient to decrease morbidity and allow a normal daily life, do not ideally mimic the physiological secretions of each endocrine organ. Moreover, these treatments have other drawbacks including potential side effects, high expense, and sometimes daily injection (for instance for GH substitution). GH treatment in adulthood remains a matter of debate because contradictory data have been published on beneficial effects in terms of bone, metabolism, and quality of life, among other physiological measures (84).
If pituitary stem cells are able to self-renew and give rise to a population of expanding transit-amplifying cells before final differentiation leading to all five pituitary lineages, then these cells would be able to replace deficient pituitary cells and contribute to organ regeneration. A few studies have reported the ability to differentiate embryonic stem cells in vitro to obtain pituitary hormone-secreting cells. These studies were based on embryonic stem cells, which are derived from the inner cell mass of blastocyst embryos (85). Embryonic stem cells have been isolated from several species including mice and humans. They have unlimited self-renewal ability and are pluripotent, capable of generating differentiated cells from ectoderm, mesoderm, and endoderm tissues (1, 85). Embryonic stem cells can differentiate into a wide variety of cell types, although predictability of differentiation and efficiency remain largely unsolved problems.
Embryonic stem cells and neural stem cells are nonpituitary cells that each have the capacity to differentiate into pituitary hormone-producing cells in culture. U et al. (86) cultured neural stem cells derived from the fetal rat brain in the presence of medium conditioned by the GH3 pituitary tumor cell line and reported transdifferentiation into GH- and prolactin-expressing cells. Although this suggests that factors secreted from somatotrophs and/or lactotrophs may be sufficient to induce transdifferentiation of neural stem cells, these studies were limited by the lack of genetically marked cells, which is necessary to prove that the rat neural stem cells were not contaminated with rat GH3 cells. In a follow-up study, U et al. (56) grafted GFP-expressing fetal rat central nervous system stem cells into adult rodent pituitary glands. The authors reported for the first time that about 10% of implanted cells eventually expressed POU1F1, and secondarily GH, prolactin, and (unexpectedly) FSHβ. In contrast, expression of TSHβ and ACTH was rarely observed. These cells survived for at least 4 wk, acquiring the morphology of original pituitary cells. This result suggests that undifferentiated cells can differentiate into a specific cell type, provided they are in an appropriate environment. Exposure to the pituitary host cells was essential because the same cells grafted in the hippocampus did not express any pituitary markers. This experiment is of major importance for potential therapeutic approaches in the future. There are, however, several caveats that must be explored. The main concern is that the recipient rats had a normal pituitary. If remaining normal pituitary tissue is essential, the application to CPHD would be significantly limited. Replication of this approach in various animal models of pituitary deficiency is important. In most of the plasticity studies, genetically marked cells from one organ of an adult mouse apparently give rise to cell type characteristics of other organs after transplantation. A critical aspect of the observation of adult stem cell plasticity is that in order for plasticity to occur, cell injury is necessary. This suggests that microenvironmental exposure to the products of injured cells may play a key role in determining the differentiated expression of stem cells after a graft (87). More research is necessary to determine the molecular mechanism of cell injury and the molecular components of the host pituitary cells that are important.
Mouse embryonic stem cells have been differentiated in vitro to produce pituitary hormone secreting cells by two different laboratories. Mouse embryonic stem cells from the 129/Sv strain were cultured to form embryoid bodies from which LHβ- and FSHβ-producing cells were identified (88). No POU1F1 mRNA was detected in the cultures, indicating the failure of these embryonic stem cells to generate POU1F1 lineages. Another study produced embryoid bodies from mouse D3 embryonic stem cells and detected differentiation of the POU1F1 lineages (89). D3 embryonic stem cells are also derived from a 129/Sv line, but due to the genetic variation among the 129 substrains of mice, we do not know how related these two strains of embryonic stem cell are (90). In the absence or presence of GH3-conditioned media, these embryoid bodies produced GH, prolactin, and occasionally TSHβ, albeit inefficiently. PROP1 and POU1F1 expression was detected after 9 d in culture and pituitary hormone markers after d 15. This suggests that embryonic stem cell differentiation might progress with a time course that mimics normal pituitary development. Although some embryonic stem cells are able to concentrate hormone proteins from the culture medium (91, 92), the demonstration that the embryoid bodies activated PROP1 expression and contain hormone transcripts makes this scenario very unlikely. Further studies are necessary to determine the critical differences in the culturing conditions that produce these unique pituitary lineages and to enhance the efficiency and predictability of the process. Additional studies of transdifferentiation are also warranted because this may be a more feasible method for controlled production of new hormone-producing cells (93).
A challenge for any type of therapeutic transplantation is to not simply produce hormone but to do it in a fashion that is capable of physiological rescue without risk of harmful side effects. Somatotroph cells are interconnected via a functional network in the pituitary; this network likely coordinates the levels of GH secretion (pulsatility for instance) (20). Grafted GH-secreting cells might be required to interconnect to this network to become physiologically functional. There is some risk that grafted GH cells might not replicate the full differentiation program, rendering them unresponsive to appropriate feedback regulation, which could translate into insufficient hormone production or excessive, unregulated GH secretion, hyperplasia, and/or development of somatotroph adenomas. The length of survival of these cells and their immune tolerance is a major confounding factor. Because the use of immunosuppressive agents is not desirable, a method to obtain immune-compatible adult stem cells will be needed for stem cells to be considered as an alternate treatment to pituitary deficiencies in the coming years. Adult stem cells isolated from patients could overcome the problem of immunological rejection and the impracticality of embryonic stem cell use (94). Lepore et al. (17, 55) transplanted enriched populations of pituitary colony-forming cells into an in vivo microchamber in SCID mice (95). Donor cells survived in chambers and underwent division. After 6 wk, GH cells were detected in grafts, suggesting that pituitary colony-forming cells have the capacity to divide and differentiate into somatotroph cells in vivo. At least two points remain to be addressed. Why would stem cells only differentiate into GH cells and not the other types of cells? How would these cells be regulated properly if grafted into the groin region, far from any hypothalamic or pituitary stimuli? At this time, it is difficult to extrapolate from these results to a cure for human hypopituitarism.
The pathophysiological mechanisms of pituitary hypo- and hyperplasia are not completely understood, leaving crucial questions unanswered. Do patients with pituitary hyperplasia and hypopituitarism have excess pituitary progenitors that are blocked in differentiation? If the signals necessary for progenitor differentiation are lacking, grafts of undifferentiated stem cells would probably produce more undifferentiated cells and ineffective treatment. It is clear that much more needs to be known about the signals necessary for differentiation before therapies will be feasible.
Patients with pituitary hypoplasia may lack pituitary progenitors and respond to a stem cell graft. Pituitary progenitor cells may yield more differentiated pituitary cells than progenitors collected from other tissue types. Garcia-Lavandeira et al. (15) showed that GFRa2+ cells are able to differentiate into neurons or pituitary hormone cell types. The majority of pituitary progenitors may be predetermined to give birth to differentiated pituitary cells. Central nervous system (CNS) tissue may also have undergone some changes in programming that make it challenging to be converted to pituitary cells. This may explain the small fraction of CNS cells that differentiate into pituitary cells (10% ratio of pituitary differentiation of CNS stem cells) (15). This suggests that CNS tissue may have undergone changes in programming that make it challenging to convert them to pituitary cells.
Replacing deficient cells by predetermined stem cells has already been performed efficiently with bone marrow transplantation. During the treatment of hematological malignancies, a patient's cancerous cells are first destroyed by chemo/radiotherapy and replaced with hematopoietic stem cells transplanted from a human leukocyte antigen-matched donor (96). Another option is to collect hematopoietic stem cells before the treatment and reinfuse them into the patients after the course of the aggressive chemotherapy, or to reconstitute immune cells as a treatment for autoimmune disorders (97). In this latter case, this has to be performed early during disease development to avoid a destruction of stem cell contingent. Future work on pituitary deficiency treatment might evaluate the possibility of collecting adult pituitary stem cells from the patient (or from an immunecompatible patient) and injecting them as a substitutive treatment. A solution for the immunocompatibility issue might be the use of induced pluripotent stem (iPS) cells; pituitary stem cells could theoretically be obtained from adult somatic cells by inducing a “forced” expression of the genes of pituitary transcription factors. For instance, Takahashi et al. (98) used SOX2 to induce mouse embryonic and adult fibroblasts to become pluripotent stem cells. As a proof of concept, iPS cells generated from autologous skin have been used to treat sickle cell anemia in mouse (99). The main advantage would be to use a somatic cell from the patient, which would ensure a perfect immune compatibility. The iPS cells could be induced to form pituitary progenitor cells or differentiated hormone-secreting cells, which could then be used as a therapeutic replacement. Although iPS cells have been induced to form dopaminergic neurons (100), there have been no reports of pituitary cell types being differentiated from iPS cells. Alternatively, iPS cells could be injected directly into the pituitary. However, IPS cells could behave more aggressively than normal pituitary cells and give rise to uncontrolled pituitary carcinomas. The technical challenge of grafting the cells stereotactically is also daunting. It is clear that further studies are needed before cell substitution or replacement therapies could be contemplated as an alternative to hormone replacement.
VI. Conclusion
There are several lines of evidence suggesting the presence of stem cells in the pituitary gland. These cells might play a major role during embryogenesis because they give birth to all five pituitary lineages. The number of multipotent progenitors in adulthood appears decreased. Their role in organ maintenance is not clear. Future studies will need to optimize the culturing conditions necessary to differentiate pituitary stem cells into the specific cell types. Knowledge of the factors necessary for pituitary organogenesis will be useful for understanding of the mechanisms of differentiation and pituitary adenoma development. These studies will lay the foundation for exploring the use of pituitary stem cells as therapeutic tools for pituitary deficiencies.
Acknowledgments
We thank Dr. Deborah Gumucio for critical reading of the manuscript.
Financial support came from Novo Nordisk, Societe Francaise d'endocrinologie, Novartis, Ipsen, Association pour le Developpement de la Recherche Medicale, and the Center for Genetics in Health and Medicine, University of Michigan (all for F.C.), and the National Institutes of Health (R37HD30428, R01HD34283, to S.A.C.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CNS
- Central nervous system
- CPHD
- combined pituitary hormone deficiencies
- e
- embryonic day
- FACS
- fluorescence-activated cell sorting
- GDNF
- glial cell line-derived neurotrophic factor
- GFAP
- glial fibrillary acidic protein
- GFP
- green fluorescent protein
- GFRa2
- GDNF receptor α2
- HMG
- high-mobility group
- iPS
- induced pluripotent stem
- P
- postnatal day
- POMC
- proopiomelanocortin.
References
- 1. Murry CE, Keller G. 2008. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell 132:661–680 [DOI] [PubMed] [Google Scholar]
- 2. Yoshimura F, Harumiya K, Ishikawa H, Otsuka Y. 1969. Differentiation of isolated chromophobes into acidophils or basophils when transplanted into the hypophysiotrophic area of hypothalamus. Endocrinol Jpn 16:531–540 [DOI] [PubMed] [Google Scholar]
- 3. Chen J, Hersmus N, Van Duppen V, Caesens P, Denef C, Vankelecom H. 2005. The adult pituitary contains a cell population displaying stem/progenitor cell and early embryonic characteristics. Endocrinology 146:3985–3998 [DOI] [PubMed] [Google Scholar]
- 4. Vidal S, Horvath E, Kovacs K, Cohen SM, Lloyd RV, Scheithauer BW. 2000. Transdifferentiation of somatotrophs to thyrotrophs in the pituitary of patients with protracted primary hypothyroidism. Virchows Arch 436:43–51 [DOI] [PubMed] [Google Scholar]
- 5. Thodou E, Asa SL, Kontogeorgos G, Kovacs K, Horvath E, Ezzat S. 1995. Clinical case seminar: lymphocytic hypophysitis: clinicopathological findings. J Clin Endocrinol Metab 80:2302–2311 [DOI] [PubMed] [Google Scholar]
- 6. Castrique E, Fernandez-Fuente M, Le Tissier P, Herman A, Levy A. 2010. Use of a prolactin-Cre/ROSA-YFP transgenic mouse provides no evidence for lactotroph transdifferentiation after weaning, or increase in lactotroph/somatotroph proportion in lactation. J Endocrinol 205:49–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Borrelli E, Heyman RA, Arias C, Sawchenko PE, Evans RM. 1989. Transgenic mice with inducible dwarfism. Nature 339:538–541 [DOI] [PubMed] [Google Scholar]
- 8. Alkhani AM, Cusimano M, Kovacs K, Bilbao JM, Horvath E, Singer W. 1999. Cytology of pituitary thyrotroph hyperplasia in protracted primary hypothyroidism. Pituitary 1:291–295 [DOI] [PubMed] [Google Scholar]
- 9. Ebling FJ. 2005. The neuroendocrine timing of puberty. Reproduction 129:675–683 [DOI] [PubMed] [Google Scholar]
- 10. Kawamura K, Kouki T, Kawahara G, Kikuyama S. 2002. Hypophyseal development in vertebrates from amphibians to mammals. Gen Comp Endocrinol 126:130–135 [DOI] [PubMed] [Google Scholar]
- 11. Bilodeau S, Roussel-Gervais A, Drouin J. 2009. Distinct developmental roles of cell cycle inhibitors p57Kip2 and p27Kip1 distinguish pituitary progenitor cell cycle exit from cell cycle reentry of differentiated cells. Mol Cell Biol 29:1895–1908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vankelecom H. 2007. Stem cells in the postnatal pituitary? Neuroendocrinology 85:110–130 [DOI] [PubMed] [Google Scholar]
- 13. Chen J, Gremeaux L, Fu Q, Liekens D, Van Laere S, Vankelecom H. 2009. Pituitary progenitor cells tracked down by side population dissection. Stem Cells 27:1182–1195 [DOI] [PubMed] [Google Scholar]
- 14. Fauquier T, Rizzoti K, Dattani M, Lovell-Badge R, Robinson IC. 2008. SOX2-expressing progenitor cells generate all of the major cell types in the adult mouse pituitary gland. Proc Natl Acad Sci USA 105:2907–2912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Garcia-Lavandeira M, Quereda V, Flores I, Saez C, Diaz-Rodriguez E, Japon MA, Ryan AK, Blasco MA, Dieguez C, Malumbres M, Alvarez CV. 2009. A GRFa2/Prop1/stem (GPS) cell niche in the pituitary. PloS One 4:e4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gleiberman AS, Michurina T, Encinas JM, Roig JL, Krasnov P, Balordi F, Fishell G, Rosenfeld MG, Enikolopov G. 2008. Genetic approaches identify adult pituitary stem cells. Proc Natl Acad Sci USA 105:6332–6337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lepore DA, Roeszler K, Wagner J, Ross SA, Bauer K, Thomas PQ. 2005. Identification and enrichment of colony-forming cells from the adult murine pituitary. Exp Cell Res 308:166–176 [DOI] [PubMed] [Google Scholar]
- 18. Carbajo-Pérez E, Watanabe YG. 1990. Cellular proliferation in the anterior pituitary of the rat during the postnatal period. Cell Tissue Res 261:333–338 [DOI] [PubMed] [Google Scholar]
- 19. Ward RD, Raetzman LT, Suh H, Stone BM, Nasonkin IO, Camper SA. 2005. Role of PROP1 in pituitary gland growth. Mol Endocrinol 19:698–710 [DOI] [PubMed] [Google Scholar]
- 20. Bonnefont X, Lacampagne A, Sanchez-Hormigo A, Fino E, Creff A, Mathieu MN, Smallwood S, Carmignac D, Fontanaud P, Travo P, Alonso G, Courtois-Coutry N, Pincus SM, Robinson IC, Mollard P. 2005. Revealing the large-scale network organization of growth hormone-secreting cells. Proc Natl Acad Sci USA 102:16880–16885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Levy A. 2008. Molecular and trophic mechanisms of tumorigenesis. Endocrinol Metab Clin North Am 37:23–50, vii [DOI] [PubMed] [Google Scholar]
- 22. Melmed S. 2003. Mechanisms for pituitary tumorigenesis: the plastic pituitary. J Clin Invest 112:1603–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nolan LA, Levy A. 2006. A population of non-luteinising hormone/non-adrenocorticotrophic hormone-positive cells in the male rat anterior pituitary responds mitotically to both gonadectomy and adrenalectomy. J Neuroendocrinol 18:655–661 [DOI] [PubMed] [Google Scholar]
- 24. Landolt AM. 1973. Regeneration of the human pituitary. J Neurosurg 39:35–41 [DOI] [PubMed] [Google Scholar]
- 25. Saeger W, Warnecke H. 1980. Ultrastructural examination of the regeneration of the rat adenohypophysis after partial hypophysectomy. Virchows Arch A Pathol Anat Histol 387:279–288 [DOI] [PubMed] [Google Scholar]
- 26. Davis SW, Mortensen AH, Camper SA. 2011. Birthdating studies reshape models for pituitary gland cell specification. Dev Biol 352:215–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Episkopou V. 2005. SOX2 functions in adult neural stem cells. Trends Neurosci 28:219–221 [DOI] [PubMed] [Google Scholar]
- 28. Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, Wicha MS. 2003. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev 17:1253–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Seaberg RM, Smukler SR, Kieffer TJ, Enikolopov G, Asghar Z, Wheeler MB, Korbutt G, van der Kooy D. 2004. Clonal identification of multipotent precursors from adult mouse pancreas that generate neural and pancreatic lineages. Nat Biotechnol 22:1115–1124 [DOI] [PubMed] [Google Scholar]
- 30. Fernandes KJ, McKenzie IA, Mill P, Smith KM, Akhavan M, Barnabé-Heider F, Biernaskie J, Junek A, Kobayashi NR, Toma JG, Kaplan DR, Labosky PA, Rafuse V, Hui CC, Miller FD. 2004. A dermal niche for multipotent adult skin-derived precursor cells. Nat Cell Biol 6:1082–1093 [DOI] [PubMed] [Google Scholar]
- 31. Monahan P, Rybak S, Raetzman LT. 2009. The Notch target gene Hes1 regulates cell cycle inhibitor expression in the developing pituitary. Endocrinology 150:4386–4394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Seymour PA, Freude KK, Tran MN, Mayes EE, Jensen J, Kist R, Scherer G, Sander M. 2007. SOX9 is required for maintenance of the pancreatic progenitor cell pool. Proc Natl Acad Sci USA 104:1865–1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bastide P, Darido C, Pannequin J, Kist R, Robine S, Marty-Double C, Bibeau F, Scherer G, Joubert D, Hollande F, Blache P, Jay P. 2007. Sox9 regulates cell proliferation and is required for Paneth cell differentiation in the intestinal epithelium. J Cell Biol 178:635–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mori-Akiyama Y, van den Born M, van Es JH, Hamilton SR, Adams HP, Zhang J, Clevers H, deCrombrugghe B. 2007. SOX9 is required for the differentiation of Paneth cells in the intestinal epithelium. Gastroenterology 133:539–546 [DOI] [PubMed] [Google Scholar]
- 35. Formeister EJ, Sionas AL, Lorance DK, Barkley CL, Lee GH, Magness ST. 2009. Distinct SOX9 levels differentially mark stem/progenitor populations and enteroendocrine cells of the small intestine epithelium. Am J Physiol Gastrointest Liver Physiol 296:G1108–G1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vankelecom H, Gremeaux L. 2010. Stem cells in the pituitary gland: a burgeoning field. Gen Comp Endocrinol 166:478–488 [DOI] [PubMed] [Google Scholar]
- 37. Carsner RL, Rennels EG. 1960. Primary site of gene action in anterior pituitary dwarf mice. Science 131:829. [DOI] [PubMed] [Google Scholar]
- 38. Hofmann MC. 2008. Gdnf signaling pathways within the mammalian spermatogonial stem cell niche. Mol Cell Endocrinol 288:95–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Paratcha G, Ledda F. 2008. GDNF and GFRα: a versatile molecular complex for developing neurons. Trends Neurosci 31:384–391 [DOI] [PubMed] [Google Scholar]
- 40. Kelberman D, Rizzoti K, Lovell-Badge R, Robinson IC, Dattani MT. 2009. Genetic regulation of pituitary gland development in human and mouse. Endocr Rev 30:790–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Himes AD, Raetzman LT. 2009. Premature differentiation and aberrant movement of pituitary cells lacking both Hes1 and Prop1. Dev Biol 325:151–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Challen GA, Little MH. 2006. A side order of stem cells: the SP phenotype. Stem Cells 24:3–12 [DOI] [PubMed] [Google Scholar]
- 43. Asakura A, Seale P, Girgis-Gabardo A, Rudnicki MA. 2002. Myogenic specification of side population cells in skeletal muscle. J Cell Biol 159:123–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cherqui S, Kurian SM, Schussler O, Hewel JA, Yates JR, 3rd, Salomon DR. 2006. Isolation and angiogenesis by endothelial progenitors in the fetal liver. Stem Cells 24:44–54 [DOI] [PubMed] [Google Scholar]
- 45. Gilyarov AV. 2008. Nestin in central nervous system cells. Neurosci Behav Physiol 38:165–169 [DOI] [PubMed] [Google Scholar]
- 46. Sclafani AM, Skidmore JM, Ramaprakash H, Trumpp A, Gage PJ, Martin DM. 2006. Nestin-Cre mediated deletion of Pitx2 in the mouse. Genesis 44:336–344 [DOI] [PubMed] [Google Scholar]
- 47. Galichet C, Lovell-Badge R, Rizzoti K. 2010. Nestin-Cre mice are affected by hypopituitarism, which is not due to significant activity of the transgene in the pituitary gland. PloS One 5:e11443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gautron L, De-Smedt V, Layé S. 2009. Age-related changes in Nestin immunoreactivity in the rat pituitary gland. Neuroendocrinology 90:19–30 [DOI] [PubMed] [Google Scholar]
- 49. Krylyshkina O, Chen J, Mebis L, Denef C, Vankelecom H. 2005. Nestin-immunoreactive cells in rat pituitary are neither hormonal nor typical folliculo-stellate cells. Endocrinology 146:2376–2387 [DOI] [PubMed] [Google Scholar]
- 50. Vankelecom H. 2007. Non-hormonal cell types in the pituitary candidating for stem cell. Semin Cell Dev Biol 18:559–570 [DOI] [PubMed] [Google Scholar]
- 51. Devnath S, Inoue K. 2008. An insight to pituitary folliculo-stellate cells. J Neuroendocrinol 20:687–691 [DOI] [PubMed] [Google Scholar]
- 52. Fauquier T, Guérineau NC, McKinney RA, Bauer K, Mollard P. 2001. Folliculostellate cell network: a route for long-distance communication in the anterior pituitary. Proc Natl Acad Sci USA 98:8891–8896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Horvath E, Kovacs K. 2002. Folliculo-stellate cells of the human pituitary: a type of adult stem cell? Ultrastruct Pathol 26:219–228 [DOI] [PubMed] [Google Scholar]
- 54. Shirasawa N, Kihara H, Yamaguchi S, Yoshimura F. 1983. Pituitary folliculo-stellate cells immunostained with S-100 protein antiserum in postnatal, castrated and thyroidectomized rats. Cell Tissue Res 231:235–249 [DOI] [PubMed] [Google Scholar]
- 55. Lepore DA, Jokubaitis VJ, Simmons PJ, Roeszler KN, Rossi R, Bauer K, Thomas PQ. 2006. A role for angiotensin-converting enzyme in the characterization, enrichment, and proliferation potential of adult murine pituitary colony-forming cells. Stem Cells 24:2382–2390 [DOI] [PubMed] [Google Scholar]
- 56. U HS, Wu B, Wilkes N, Ho A, Saljooque F. 2007. Brain stem cells adopt a pituitary fate after implantation into the adult rodent pituitary gland. Neuroendocrinology 86:58–68 [DOI] [PubMed] [Google Scholar]
- 57. Raetzman LT, Cai JX, Camper SA. 2007. Hes1 is required for pituitary growth and melanotrope specification. Dev Biol 304:455–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pulichino AM, Vallette-Kasic S, Tsai JP, Couture C, Gauthier Y, Drouin J. 2003. Tpit determines alternate fates during pituitary cell differentiation. Genes Dev 17:738–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chen J, Crabbe A, Van Duppen V, Vankelecom H. 2006. The notch signaling system is present in the postnatal pituitary: marked expression and regulatory activity in the newly discovered side population. Mol Endocrinol 20:3293–3307 [DOI] [PubMed] [Google Scholar]
- 60. Zhu X, Zhang J, Tollkuhn J, Ohsawa R, Bresnick EH, Guillemot F, Kageyama R, Rosenfeld MG. 2006. Sustained Notch signaling in progenitors is required for sequential emergence of distinct cell lineages during organogenesis. Genes Dev 20:2739–2753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kita A, Imayoshi I, Hojo M, Kitagawa M, Kokubu H, Ohsawa R, Ohtsuka T, Kageyama R, Hashimoto N. 2007. Hes1 and Hes5 control the progenitor pool, intermediate lobe specification, and posterior lobe formation in the pituitary development. Mol Endocrinol 21:1458–1466 [DOI] [PubMed] [Google Scholar]
- 62. Raetzman LT, Ward R, Camper SA. 2002. Lhx4 and Prop1 are required for cell survival and expansion of the pituitary primordia. Development 129:4229–4239 [DOI] [PubMed] [Google Scholar]
- 63. Ellsworth BS, Butts DL, Camper SA. 2008. Mechanisms underlying pituitary hypoplasia and failed cell specification in Lhx3-deficient mice. Dev Biol 313:118–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sheng HZ, Moriyama K, Yamashita T, Li H, Potter SS, Mahon KA, Westphal H. 1997. Multistep control of pituitary organogenesis. Science 278:1809–1812 [DOI] [PubMed] [Google Scholar]
- 65. Castinetti F, Saveanu A, Reynaud R, Quentien MH, Buffin A, Brauner R, Kaffel N, Albarel F, Guedj AM, El Kholy M, Amin M, Enjalbert A, Barlier A, Brue T. 2008. A novel dysfunctional LHX4 mutation with high phenotypical variability in patients with hypopituitarism. J Clin Endocrinol Metab 93:2790–2799 [DOI] [PubMed] [Google Scholar]
- 66. Netchine I, Sobrier ML, Krude H, Schnabel D, Maghnie M, Marcos E, Duriez B, Cacheux V, Moers A, Goossens M, Grüters A, Amselem S. 2000. Mutations in LHX3 result in a new syndrome revealed by combined pituitary hormone deficiency. Nat Genet 25:182–186 [DOI] [PubMed] [Google Scholar]
- 67. Yoshida S, Kato T, Susa T, Cai LY, Nakayama M, Kato Y. 2009. PROP1 coexists with SOX2 and induces PIT1-commitment cells. Biochem Biophys Res Commun 385:11–15 [DOI] [PubMed] [Google Scholar]
- 68. Reynaud R, Barlier A, Vallette-Kasic S, Saveanu A, Guillet MP, Simonin G, Enjalbert A, Valensi P, Brue T. 2005. An uncommon phenotype with familial central hypogonadism caused by a novel PROP1 gene mutant truncated in the transactivation domain. J Clin Endocrinol Metab 90:4880–4887 [DOI] [PubMed] [Google Scholar]
- 69. Reynaud R, Chadli-Chaieb M, Vallette-Kasic S, Barlier A, Sarles J, Pellegrini-Bouiller I, Enjalbert A, Chaieb L, Brue T. 2004. A familial form of congenital hypopituitarism due to a PROP1 mutation in a large kindred: phenotypic and in vitro functional studies. J Clin Endocrinol Metab 89:5779–5786 [DOI] [PubMed] [Google Scholar]
- 70. Kuwahara R, Kofman AV, Landis CS, Swenson ES, Barendswaard E, Theise ND. 2008. The hepatic stem cell niche: identification by label-retaining cell assay. Hepatology 47:1994–2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sergeant G, Vankelecom H, Gremeaux L, Topal B. 2009. Role of cancer stem cells in pancreatic ductal adenocarcinoma. Nat Rev Clin Oncol 6:580–586 [DOI] [PubMed] [Google Scholar]
- 72. Levy A. 2001. Monoclonality of endocrine tumours: What does it mean? Trends Endocrinol Metab 12:301–307 [DOI] [PubMed] [Google Scholar]
- 73. Xu Q, Yuan X, Tunici P, Liu G, Fan X, Xu M, Hu J, Hwang JY, Farkas DL, Black KL, Yu JS. 2009. Isolation of tumour stem-like cells from benign tumours. Br J Cancer 101:303–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Vierimaa O, Georgitsi M, Lehtonen R, Vahteristo P, Kokko A, Raitila A, Tuppurainen K, Ebeling TM, Salmela PI, Paschke R, Gündogdu S, De Menis E, Mäkinen MJ, Launonen V, Karhu A, Aaltonen LA. 2006. Pituitary adenoma predisposition caused by germline mutations in the AIP gene. Science 312:1228–1230 [DOI] [PubMed] [Google Scholar]
- 75. Barlier A, Vanbellinghen JF, Daly AF, Silvy M, Jaffrain-Rea ML, Trouillas J, Tamagno G, Cazabat L, Bours V, Brue T, Enjalbert A, Beckers A. 2007. Mutations in the aryl hydrocarbon receptor interacting protein gene are not highly prevalent among subjects with sporadic pituitary adenomas. J Clin Endocrinol Metab 92:1952–1955 [DOI] [PubMed] [Google Scholar]
- 76. Daly AF, Vanbellinghen JF, Khoo SK, Jaffrain-Rea ML, Naves LA, Guitelman MA, Murat A, Emy P, Gimenez-Roqueplo AP, Tamburrano G, Raverot G, Barlier A, De Herder W, Penfornis A, Ciccarelli E, Estour B, Lecomte P, Gatta B, Chabre O, Sabaté MI, Bertagna X, Garcia Basavilbaso N, Stalldecker G, Colao A, Ferolla P, Wémeau JL, Caron P, Sadoul JL, Oneto A, Archambeaud F, Calender A, Sinilnikova O, Montañana CF, Cavagnini F, Hana V, Solano A, Delettieres D, Luccio-Camelo DC, Basso A, Rohmer V, Brue T, Bours V, Teh BT, Beckers A. 2007. Aryl hydrocarbon receptor-interacting protein gene mutations in familial isolated pituitary adenomas: analysis in 73 families. J Clin Endocrinol Metab 92:1891–1896 [DOI] [PubMed] [Google Scholar]
- 77. Brinkmeier ML, Davis SW, Carninci P, MacDonald JW, Kawai J, Ghosh D, Hayashizaki Y, Lyons RH, Camper SA. 2009. Discovery of transcriptional regulators and signaling pathways in the developing pituitary gland by bioinformatic and genomic approaches. Genomics 93:449–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Davis SW, Potok MA, Brinkmeier ML, Carninci P, Lyons RH, MacDonald JW, Fleming MT, Mortensen AH, Egashira N, Ghosh D, Steel KP, Osamura RY, Hayashizaki Y, Camper SA. 2009. Genetics, gene expression and bioinformatics of the pituitary gland. Horm Res 71(Suppl 2):101–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Heaney AP, Horwitz GA, Wang Z, Singson R, Melmed S. 1999. Early involvement of estrogen-induced pituitary tumor transforming gene and fibroblast growth factor expression in prolactinoma pathogenesis. Nat Med 5:1317–1321 [DOI] [PubMed] [Google Scholar]
- 80. Nolan LA, Levy A. 2009. The trophic effects of oestrogen on male rat anterior pituitary lactotrophs. J Neuroendocrinol 21:457–464 [DOI] [PubMed] [Google Scholar]
- 81. Castinetti F, Régis J, Dufour H, Brue T. 2010. Role of stereotactic radiosurgery in the management of pituitary adenomas. Nat Rev Endocrinol 6:214–223 [DOI] [PubMed] [Google Scholar]
- 82. Castinetti F, Morange I, Dubois N, Albarel F, Conte-Devolx B, Dufour H, Brue T. 2009. Does first-line surgery still have its place in the treatment of acromegaly? Ann Endocrinol (Paris) 70:107–112 [DOI] [PubMed] [Google Scholar]
- 83. Davis SW, Castinetti F, Carvalho LR, Ellsworth BS, Potok MA, Lyons RH, Brinkmeier ML, Raetzman LT, Carninci P, Mortensen AH, Hayashizaki Y, Arnhold IJ, Mendonça BB, Brue T, Camper SA. 2010. Molecular mechanisms of pituitary organogenesis: in search of novel regulatory genes. Mol Cell Endocrinol 323:4–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Molitch ME, Clemmons DR, Malozowski S, Merriam GR, Shalet SM, Vance ML, Stephens PA. 2006. Evaluation and treatment of adult growth hormone deficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 91:1621–1634 [DOI] [PubMed] [Google Scholar]
- 85. Trounson A. 2006. The production and directed differentiation of human embryonic stem cells. Endocr Rev 27:208–219 [DOI] [PubMed] [Google Scholar]
- U HS, Alilain W, Saljooque F. 2002. Fetal brain progenitor cells transdifferentiate to fates outside the nervous system. Mol Endocrinol 16:2645–2656 [DOI] [PubMed] [Google Scholar]
- 87. Smith AG. 2001. Embryo-derived stem cells: of mice and men. Annu Rev Cell Dev Biol 17:435–462 [DOI] [PubMed] [Google Scholar]
- 88. Zhao X, Teng R, Asanuma K, Okouchi Y, Johkura K, Ogiwara N, Sasaki K. 2005. Differentiation of mouse embryonic stem cells into gonadotrope-like cells in vitro. J Soc Gynecol Investig 12:257–262 [DOI] [PubMed] [Google Scholar]
- 89. Wagner J, Lepore D, Thomas P. 2007. Differentiation of mouse embryonic stem cells into growth hormone and prolactin expressing cells in vitro. Mol Cell Endocrinol 273:68–74 [DOI] [PubMed] [Google Scholar]
- 90. Simpson EM, Linder CC, Sargent EE, Davisson MT, Mobraaten LE, Sharp JJ. 1997. Genetic variation among 129 substrains and its importance for targeted mutagenesis in mice. Nat Genet 16:19–27 [DOI] [PubMed] [Google Scholar]
- 91. Lumelsky N, Blondel O, Laeng P, Velasco I, Ravin R, McKay R. 2001. Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets. Science 292:1389–1394 [DOI] [PubMed] [Google Scholar]
- 92. Rajagopal J, Anderson WJ, Kume S, Martinez OI, Melton DA. 2003. Insulin staining of ES cell progeny from insulin uptake. Science 299:363. [DOI] [PubMed] [Google Scholar]
- 93. Collombat P, Xu X, Ravassard P, Sosa-Pineda B, Dussaud S, Billestrup N, Madsen OD, Serup P, Heimberg H, Mansouri A. 2009. The ectopic expression of Pax4 in the mouse pancreas converts progenitor cells into α and subsequently β cells. Cell 138:449–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Hombach-Klonisch S, Panigrahi S, Rashedi I, Seifert A, Alberti E, Pocar P, Kurpisz M, Schulze-Osthoff K, Mackiewicz A, Los M. 2008. Adult stem cells and their trans-differentiation potential—perspectives and therapeutic applications. J Mol Med 86:1301–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Lepore DA, Thomas GP, Knight KR, Hussey AJ, Callahan T, Wagner J, Morrison WA, Thomas PQ. 2007. Survival and differentiation of pituitary colony-forming cells in vivo. Stem Cells 25:1730–1736 [DOI] [PubMed] [Google Scholar]
- 96. Gratwohl A, Baldomero H. 2009. Trends of hematopoietic stem cell transplantation in the third millennium. Curr Opin Hematol 16:420–426 [DOI] [PubMed] [Google Scholar]
- 97. Snowden JA, Martin-Rendon E, Watt SM. 2009. Clinical stem cell therapies for severe autoimmune diseases. Transfus Med 19:223–234 [DOI] [PubMed] [Google Scholar]
- 98. Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. 2007. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131:861–872 [DOI] [PubMed] [Google Scholar]
- 99. Hanna J, Wernig M, Markoulaki S, Sun CW, Meissner A, Cassady JP, Beard C, Brambrink T, Wu LC, Townes TM, Jaenisch R. 2007. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science 318:1920–1923 [DOI] [PubMed] [Google Scholar]
- 100. Swistowski A, Peng J, Liu Q, Mali P, Rao MS, Cheng L, Zeng X. 2010. Efficient generation of functional dopaminergic neurons from human induced pluripotent stem cells under defined conditions. Stem Cells 28:1893–1904 [DOI] [PMC free article] [PubMed] [Google Scholar]