Abstract
We evaluated human risk for infection with Babesia microti at a site in eastern Switzerland where several B. microti–infected nymphal Ixodes ricinus ticks had been found. DNA from pooled nymphal ticks amplified by polymerase chain reaction was highly homologous to published B. microti sequences. More ticks carried babesial infection in the lower portion of the rectangular 0.7-ha grid than in the upper (11% vs. 0.8%). In addition, we measured seroprevalence of immunoglobulin (Ig) G antibodies against B. microti antigen in nearby residents. Serum from 1.5% of the 396 human residents of the region reacted to B. microti antigen (>1:64), as determined by indirect immunofluorescence assay (IgG). These observations constitute the first report demonstrating B. microti in a human-biting vector, associated with evidence of human exposure to this agent in a European site.
Keywords: Babesia microti, Ixodes ricinus, human babesiosis, Europe
A malaria-like syndrome due to Babesia microti infection has been recognized in parts of the northeastern United States for more than three decades (1,2). This protozoon pathogen was first isolated more than half a century earlier from a Portuguese vole (3); the pathogen has since been detected in small mammals and ticks throughout Eurasia (3,4).
Despite its broad geographic distribution, B. microti has not been implicated as a cause of human illness in Europe. A host-specific, rodent-feeding tick, Ixodes trianguliceps, is widely regarded as the main enzootic vector on that continent. I. ricinus, the most common human-biting tick of Europe, transmits the Lyme borreliosis spirochete, tick-borne encephalitis virus, the agent of human granulocytic ehrlichiosis, and B. divergens, but I. ricinus was believed to be infected only occasionally with B. microti (5). This vector-pathogen association may account for the absence of human disease due to B. microti (3,6). However, subadult I. ricinus ticks feed abundantly on the reservoirs of B. microti, such as voles and mice, and appear to be competent vectors for B. microti (7). In fact, recent studies indicate that Swiss residents may have concurrent infection with the Lyme disease spirochete and B. microti (8) and that the human population of certain parts of Germany is exposed to B. microti (9).
Human exposure to B. microti may occur more often in Europe than has been recognized. Accordingly, we assessed the potential of zoonotic transmission in eastern Switzerland, where other I. ricinus–transmitted infections are present. In particular, we determined how frequently B. microti parasites infect I. ricinus ticks locally, how infection in ticks is spatially distributed in space, and how frequently the sera of nearby residents react to B. microti antigen.
Methods
Tick Collection and B. microti Detection in Ticks
To assess local prevalence of B. microti in host-seeking nymphal I. ricinus ticks, we developed a tick-sampling procedure with high spatial resolution (Figure 1). The roughly rectangular, 0.7-ha field site, Ruetiwis (9° 38´ E, 46° 59´ N), is located on a steep southwesterly slope near Seewis in the lower Praettigau Valley of eastern Switzerland at an approximate mean altitude of 850 m above sea level. The site is characterized by abandoned pastures that are partly overgrown by young stands of deciduous and coniferous trees and bushes, as well as by mature mixed forest. All ticks were collected by flagging in July 1997.
Figure 1.
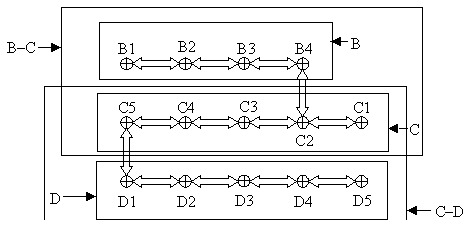
Schematic drawing of the sampling scheme. Distance between sampling points is 30 m. The letters and solid arrows denote the sections used for prevalence estimation. Sampling lines that connect points not belonging to a section are not included in that section (e.g., line B4–C2 is not part of section B).
To ensure the quality of tick-derived DNA, pools of 2–11 nymphal ticks were transferred to 0.5-mL microcentrifuge tubes containing 50 µL guanidium thiocyanate solution and stored at room temperature until further processing. Before homogenization with a glass pestle, the solution containing the ticks was incubated at 60°C for 2 h. DNA was extracted from the tick homogenate by the phenol-chloroform method. The resulting DNA pellet was suspended in 30 µL DNAse-free H20.
To determine whether B. microti–specific DNA was present, the extracted DNA was subjected to polymerase chain reaction (PCR) with the primer pair Bab-1 (5´-ttagtataagcttttatacagc-3´) and Bab-4 (5´-ataggtcagaaacttgaatgataca-3´) (10), which targets a 250-bp fragment of the 18s rRNA gene of B. microti. After denaturation for 2 min at 94°C, 40 cycles were performed, with 45 sec at 94°C, 45 sec at 55°C, and 45 sec at 72°C, followed by a 7-min final extension. Amplification products were separated on 2% agarose gel in Tris-borate-EDTA buffer, stained with ethidium bromide, and visualized under UV light. To differentiate the sequence of interest from a frequently observed, slightly smaller fragment, purified PCR products were digested with XhoI (Life Technologies, Invitrogen Corp., Gaithersburg, MD). The resulting two fragments specific for B. microti migrate in one band on the 2% agarose gel. The corresponding sequence of B. divergens lacks the restriction site.
To verify the taxonomic status of the Babesia spp. detected in these ticks, we conducted a phylogenetic analysis on a representative sample from which DNA had been amplified (Bab-1/Bab-4). For this purpose we used the primer pair PIRO A and PIRO B, which also targets 18s rDNA but is less specific for B. microti; the resulting sequence is longer (400 bp) than the Bab-1/Bab-4 amplicon (11). After PCR amplification, the respective band was excised from the agarose gel, purified with spin columns (Qiagen Inc., Valencia, CA), and sent to the University of Maine sequencing facility for sequence analysis. The resulting sequence was aligned against other Babesia spp. listed in GenBank by using Clustal X and consecutive adjustment visually. Phylogenetic analysis was performed by both maximum parsimony (Swofford D. Phylogenetic analysis using parsimony, PAUP*4b61; Sinauer Associates, Inc., Sunderland, MA) and neighbor-joining analyses (12) with Toxoplasma gondii (GenBank accession no. X68523) as outgroup. Robustness of the nodes was assessed by bootstrap analysis with 500 bootstrap replicates.
Data Analysis
To accurately estimate the local prevalence of B. microti infection in I. ricinus ticks, we developed a maximum likelihood method for point estimation as well as a method for calculating confidence intervals. Briefly, the maximum likelihood estimate (MLE) of the prevalence p is based on the likelihood
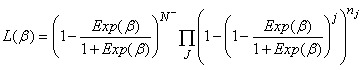 , ,
|
where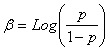 , N– is the number of ticks in negative pools, J is the set of pool sizes with at least one positive pool, and nj is the number of positive pools of size j. Test-inversion bootstrap confidence intervals (13) were calculated for the prevalence estimate. The method will be described in detail elsewhere (Foppa, unpub. data). , N– is the number of ticks in negative pools, J is the set of pool sizes with at least one positive pool, and nj is the number of positive pools of size j. Test-inversion bootstrap confidence intervals (13) were calculated for the prevalence estimate. The method will be described in detail elsewhere (Foppa, unpub. data).
|
|
To determine whether infected ticks were clustered in the study site, we tested the null hypothesis β1 = β2 = 0 by the model |
Serologic Survey
To determine whether humans may be exposed to B. microti in the study area, we recruited 400 blood donors living within 10 km of the field site for a serologic survey of tick-borne zoonoses. Volunteers were recruited for this cross-sectional seroprevalence study during blood drives from December 1997 to May 1998 in towns within a 10-km radius of the study site. This protocol was approved by the Human Subjects Committee of the Harvard School of Public Health (protocol number 9712THEE). The sera of participants who gave their written informed consent were tested by indirect immunofluorescence assay (IFA) as described (14). Antigen slides were prepared from erythrocytes of B. microti–infected hamsters (the GI strain, originally derived from a Nantucket Island patient). Sera were first screened by IFA at 1:64 dilution. A panel of sera included all samples reactive in the screening test for which enough serum was available, including samples with borderline reactivity and representative controls. This panel was coded and blindly retested for B. microti IFA at the University of Connecticut laboratories, which specialize in Babesia serology. An IFA titer >1:64 was considered reactive. All reactive sera were titrated to endpoint.
Results
Tick Survey
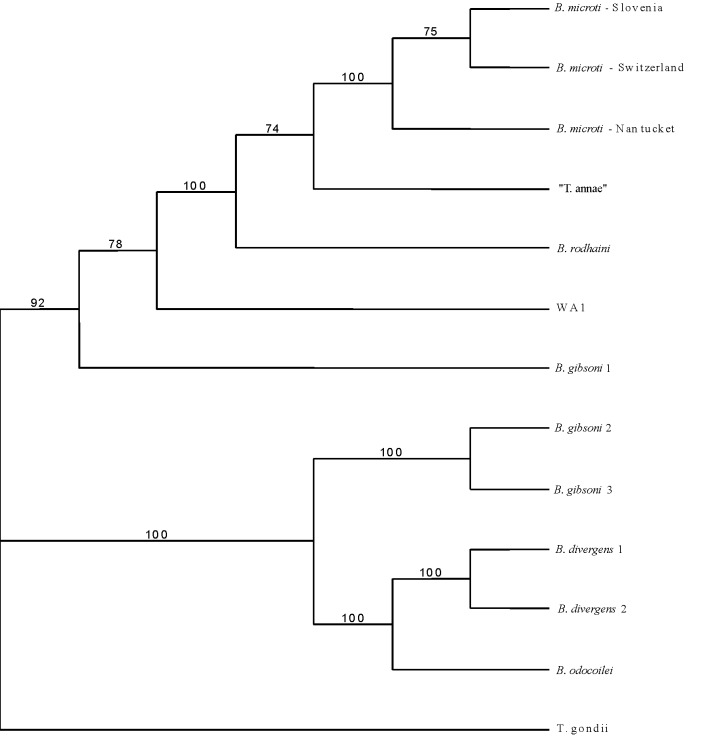
To verify the identity of the amplified DNA, a phylogenetic analysis was performed. The sequence amplified from our I. ricinus ticks, which was deposited in GenBank (accession no. AF494286), differed by l bp from the North American B. microti sequence (GenBank accession no. AF231348) and was identical with that of B. microti from Slovenia (GenBank accession no. AF373332). Accordingly, our sequence clearly clustered with the European and the American strain of B. microti (Figure 2), with concordant results from both maximum parsimony and neighbor-joining analyses. Therefore, the piroplasms detected in I. ricinus ticks from Switzerland must be considered B. microti.
Figure 2.
Maximum parsimony bootstrap consensus tree of 18S rDNA. GenBank accession nos.: Babesia microti-Slovenia AF373332; B. microti-Switzerland AF494286; B. microti- Nantucket AF231348; "Toxoplasma annae" AF188001; B. rodhaini AB049999; WA1 AF158700; B. gibsoni 1 AF158702; B. divergens 1 U07885; B. divergens 2 U16370; B. odocoilei U16369; B. gibsoni 2 AF175300; B. gibsoni 3 AF175301; and Toxoplasma gondii X68523.
We then determined the prevalence of B. microti infection in nymphal I. ricinus ticks from the study site, on the basis of PCR amplification of B. microti in DNA tick pools. Overall, we analyzed 408 ticks in 64 pools. We detected B. microti–specific DNA in 14 pools (Table 1). Thus, B. microti infection appears to be common in human-biting ticks at this central European study site.
Table 1. Babesia microti infection in nymphal Ixodes ricinus ticks as determined by polymerase chain reaction, eastern Switzerland.
| Sampling point or line | Pool 1 | Pool 2 | Pool 3 | Pool 4 | Pool 5 | No. of pools | No. of ticks |
|---|---|---|---|---|---|---|---|
| B1 | 11 | 11 | 12 | — | — | 3 | 34 |
| B1–2 | 10 | 10 | 10 | — | — | 3 | 30 |
| B2 | 4 | 3 | — | — | — | 2 | 7 |
| B2–3 | 3 | 3 | 3 | — | — | 3 | 9 |
| B3 | 3 | — | — | — | — | 1 | 3 |
| B3–4 | 8a | 8 | — | — | — | 2 | 16 |
| B4 | 1 | — | — | — | — | 1 | 1 |
| B4–C2 | 5 | 5 | 4 | 5 | — | 4 | 19 |
| C1 | 1 | — | — | — | — | 1 | 1 |
| C1–2 | 9 | 9 | 9 | 9 | — | 4 | 36 |
| C2 | 7 | 7 | 5 | — | — | 3 | 19 |
| C2–3 | 5 | 5 | 5 | 5 | 5 | 5 | 25 |
| C3 | 10 | — | — | — | — | 1 | 10 |
| C3–4 | 4 | — | — | — | — | 1 | 4 |
| C4 | 4 | — | — | — | — | 1 | 4 |
| C4–5 | 7 | 6 | — | — | — | 2 | 13 |
| C5 | 8 | 7 | 6 | — | — | 3 | 21 |
| C5–D1 | 8 | 7 | 7 | 8 | — | 4 | 30 |
| D1 | 5 | 5 | 6 | — | — | 3 | 16 |
| D1–2 | 8 | 7 | — | — | — | 2 | 15 |
| D2 | 6 | 6 | — | — | — | 2 | 12 |
| D2–3 | 5 | 5 | 5 | 6 | 6 | 5 | 27 |
| D3 | 4 | — | — | — | — | 1 | 4 |
| D3–4 | 6 | 6 | 5 | — | — | 3 | 17 |
| D4 | 11 | 11 | 10 | — | — | 3 | 32 |
| D4–5 | 3 | — | — | — | — | 1 | 3 |
| D5 | 0 | — | — | — | — | 0 | 0 |
| Total | 64 | 408 |
aPools in bold are positive; dashes represent the absence of further pools.
To determine whether the prevalence of B. microti is distributed homogeneously within the study site, prevalence of infection in ticks was estimated for selected segments of the sampling grid. The overall prevalence of B. microti infection in ticks, as estimated by MLE, was close to 4%. More detailed spatial analysis, however, indicates that the distribution of babesial infection at the site is heterogeneous (Table 2). In the lower portion of the site (section D), >10% of all ticks were infected, while in the upper portion (B–C), prevalence was <1%. Prevalence of infection in ticks was similar in sections B and C (p=0.161), but greater in section D than in sections B and C (p=0.003). Thus, B. microti transmission is focal at this site.
Table 2. Global and local prevalence estimates of Babesia microti infection in nymphal Ixodes ricinus ticks as determined by polymerase chain reaction, eastern Switzerland.
| Point estimate (%) | 95% confidence interval | |
|---|---|---|
| MLEa, overall | 3.6 | 0.2 to 9.06 |
| MLE, section B | 1.04 | 0.10 to 9.01 |
| MLE, section C | 0.77 | 0.08 to 8.47 |
| MLE, section D | 10.99 | 6.17 to 17.71 |
| MLE, sections B,C | 0.82 | 0.61 to 2.65 |
| MLE, sections C,D | 5.25 | 2.96 to 10.43 |
aMLE (maximum likelihood estimate) of the point estimates are shown with 95% bootstrap confidence limits.
Serologic Survey
To determine how frequently humans are exposed to B. microti infection in the region around the study site, sera of local residents were screened by IFA for the presence of antibodies to B. microti antigen. Most sera (80%) were collected from December through March, when I. ricinus is not active. Five (1.5%) of 396 human sera were immunoglobulin (Ig) G-reactive against B. microti antigen at a titer ³1:64. All the samples positive on initial testing and one with a borderline result were confirmed on retesting to be reactive at a titer >1:64, while none of the samples with a negative screening result reacted (Table 3). B. microti–specific IgM seroreactivity was not found in any of the sera, a finding compatible with the time of the year when most of the sera had been collected. These results indicate that residents of our central European study site are exposed to bites of ticks infected with B. microti.
Table 3. Reactivity of sera tested against Babesia microti as determined by indirect immunofluorescent assay.
| Titer | No. of sera (%) |
|---|---|
| <1:64 | 391 (98.7) |
| 1:64 | 1 (0.3) |
| 1:128 | 1 (0.3) |
| 1:256 | 1 (0.3) |
| 1:512 | 2 (0.5) |
| Total | 396 (100.0) |
Discussion
B. microti–infected nymphal I. ricinus ticks are present at Swiss study site, and human residents of the area appear to be exposed to this agent. However, the presence of B. microti in I. ricinus ticks has been reported only once before (5).
A phylogenetic analysis clearly demonstrated that the piroplasms found in our study site belong to B. microti, rather than to other Babesia or Theileria species (Figure 2). The detection of B. microti DNA in host-seeking nymphal I. ricinus ticks may, however, simply reflect “spill-over” from enzootic transmission by the accepted maintenance vector for B. microti in Eurasia, the tick I. trianguliceps (15). This tick, which does not bite humans, infests small rodents that also are abundantly parasitized by I. ricinus (3,16). The prevalence of infection in ticks may be underestimated as only one genome copy is present per parasite in unfed ticks before sporogony. Therefore, low parasite loads may escape detection, thus increasing the specificity of the assay.
In our study, maximizing specificity was desirable because infection of I. ricinus with B. microti was a priori assumed to be rare, and underestimation of prevalence therefore is conservative. Regardless, the proportion of ticks that appear to be infected by B. microti is similar to that in coastal New England (S. Telford, unpub. data). Recently, Duh et al. reported a similarly high prevalence of B. microti infection in nymphal I. ricinus ticks collected in Slovenia (7 of 69 ticks tested by PCR) (17). In combination with our findings, this report suggests that B. microti infection in I. ricinus ticks is far more common than traditionally thought. In addition, vector competence of I. ricinus for B. microti has been demonstrated experimentally (7,18). The frequent infection of I. ricinus with this piroplasm therefore implies zoonotic relevance of this vector-pathogen association in Switzerland and possibly in other parts of Europe.
B. microti transmission is clustered in the study site. Similar to tick-borne encephalitis virus, B. microti seems to be maintained in small focal areas. The risk of human infection therefore is spatially highly variable and may be conditional on tick density. Preliminary analysis of tick infection data from the study site over a 3-year period (Foppa, unpub. data) suggests that B. microti is locally maintained, especially in the lower portion of that site, while the exact location of the maximum risk changes over the years.
Residents of this site in eastern Switzerland appear to be exposed to bites by ticks infected with B. microti. The serologic result is unlikely to reflect low specificity of the assay, as previous evaluation of this IFA has demonstrated high specificity (19,20). As part of this evaluation, we tested 50 sera from residents of Iceland, where ticks capable of transmitting B. microti are absent; none of the sera reacted with B. microti antigen (19). We recently repeated testing of these sera and obtained similar results. This finding suggests a satisfactory positive predictive value of the serologic test even in settings of low prevalence.
In the northeastern United States, B. microti seroprevalence has varied in endemic regions, from 3.7% in Red Cross blood donors on Cape Cod, Massachusetts (21), to 2.5% and 9.5% in Connecticut residents who were seronegative and seroreactive, respectively, to Borrelia burgdorferi (22). Our findings of high local prevalence of B. microti infection in I. ricinus ticks may seem counterintuitive given the lower seroprevalence in residents of our study area. The findings may, however, reflect a high degree of spatial clustering of transmission with a low average risk (18). Alternatively, the low seroprevalence may be the result of low test sensitivity resulting from antigenic differences between North American B. microti strains, which were used for the serologic testing, and the European strains to which our study population had been exposed.
At least locally, the potential for zoonotic transmission of B. microti by I. ricinus is considerable, which explains serologic evidence in human beings of exposure to this agent in parts of Europe. The lack of recognized human pathology associated with European strains of B. microti, despite exposure to infectious tick bites, may be a consequence of a lower virulence of European strains than those of North American. Disease episodes due to B. microti, on the other hand, may be overlooked because of the relative nonspecificity of signs and symptoms and the presumption that this agent rarely infects I. ricinus.
Acknowledgments
We thank Alexandra Weld for technical assistance.
This study was partly supported by grants from the National Institutes of Health (AI 42402, 37993, 39002). The serologic survey was partly supported by a grant from Immuno AG Switzerland.
Biography
Dr. Foppa is assistant professor of epidemiology at the Norman J. Arnold School of Public Health, University of South Carolina, Columbia. His main interests include quantitative methods in infectious disease epidemiology and dynamics of vector-borne zoonoses.
Footnotes
Suggested citation: Foppa IM, Krause PJ, Spielman A, Goethert H, Gern L, Brand B, et al. Entomologic and Serologic Evidence of Zoonotic Transmission of Babesia microti, Eastern Switzerland. Emerg Infect Dis. [serial on the Internet]. 2002 Jul [date cited]. http://dx.doi.org/10.3201/eid0807.010459
References
- 1.Spielman A. Human babesiosis on Nantucket Island: transmission by nymphal Ixodes ticks. Am J Trop Med Hyg. 1976;25:784–7. [DOI] [PubMed] [Google Scholar]
- 2.Spielman A. The emergence of Lyme disease and human babesiosis in a changing environment. Ann N Y Acad Sci. 1994;740:146–56. 10.1111/j.1749-6632.1994.tb19865.x [DOI] [PubMed] [Google Scholar]
- 3.Sebek Z, Rosicky B, Sixl W. The occurrence of babesiasis affecting small terrestrial mammals and the importance of this zoonosis in Europe. Folia Parasitol (Praha). 1977;24:221–8. [PubMed] [Google Scholar]
- 4.Telford SR, Gorenflot A, Brasseur P, Spielman A. Babesial infections in humans and wildlife. In: Kreier JP, editor. Parasitic protozoa. Vol. 5. New York: Academic Press; 1993. p. 1–47. [Google Scholar]
- 5.Walter G. Isolierung von Babesia microti (Franca 1912) aus freilebenden Nymphen von Ixodes ricinus (Linnaeus 1758). Acta Trop. 1981;38:187–8. [PubMed] [Google Scholar]
- 6.Hussein HS. Ixodes trianguliceps: seasonal abundance and role in the epidemiology of Babesia microti infection in north-western England. Ann Trop Med Parasitol. 1980;74:531–9. [DOI] [PubMed] [Google Scholar]
- 7.Gray J, von Stedingk LV, Gurtelschmid M, Granstrom M. Transmission studies of Babesia microti in Ixodes ricinus ticks and gerbils. J Clin Microbiol. 2002;40:1259–63. 10.1128/JCM.40.4.1259-1263.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehr-Scherrer L. Babesia-Infektionen nun auch in der Schweiz? Bulletin of the Swiss Federal Office of Public Health 1999;978–9.
- 9.Hunfeld KP, Allwinn R, Peters S, Kraiczy P, Brade V. Serologic evidence for tick-borne pathogens other than Borrelia burgdorferi (TOBB) in Lyme borreliosis patients from midwestern Germany. Wien Klin Wochenschr. 1998;110:901–8. [PubMed] [Google Scholar]
- 10.Persing DH, Mathiesen D, Marshall WF, Telford SR, Spielman A, Thomford JW, et al. Detection of Babesia microti by polymerase chain reaction. J Clin Microbiol. 1992;30:2097–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Armstrong PM, Katavolos P, Caporale DA, Smith RP, Spielman A, Telford SR. Diversity of Babesia infecting deer ticks (Ixodes dammini). Am J Trop Med Hyg. 1998;58:739–42. [DOI] [PubMed] [Google Scholar]
- 12.Kumar S, Tamura K, Jakobsen IB, Nei M. MEGA2: molecular evolutionary genetics analysis software. Bioinformatics. 2001;17:1244–5. 10.1093/bioinformatics/17.12.1244 [DOI] [PubMed] [Google Scholar]
- 13.Carpenter J, Bithell J. Bootstrap confidence intervals: when, which, what? A practical guide for medical statisticians. Stat Med. 2000;19:1141–64. [DOI] [PubMed] [Google Scholar]
- 14.Krause PJ, Telford SR, Pollack RJ, Ryan R, Brassard P, Zemel L, et al. A. Babesiosis: an underdiagnosed disease of children. Pediatrics. 1992;89:1045–8. [PubMed] [Google Scholar]
- 15.Randolph SE. Quantifying parameters in the transmission of Babesia microti by the tick Ixodes trianguliceps amongst voles (Clethrionomys glareolus). Parasitology. 1995;110:287–95. 10.1017/S0031182000080872 [DOI] [PubMed] [Google Scholar]
- 16.Gern L, Aeschlimann A. Etude seroepidemiologique de 2 foyers a babesie de micromammiferes en Suisse. Schweiz Arch Tierheilkd. 1986;128:587–600. [PubMed] [Google Scholar]
- 17.Duh D, Petrovec M, Avsic-Zupanc T. Diversity of Babesia infecting European sheep ticks (Ixodes ricinus). J Clin Microbiol. 2001;39:3395–7. 10.1128/JCM.39.9.3395-3397.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Telford SR, Urioste SS, Spielman A. Clustering of host-seeking nymphal deer ticks (Ixodes dammini) infected by Lyme disease spirochetes (Borrelia burgdorferi). Am J Trop Med Hyg. 1992;47:55–60. [DOI] [PubMed] [Google Scholar]
- 19.Krause PJ, Telford SR, Ryan R, Conrad PA, Wilson M, Thomford JW, et al. Diagnosis of babesiosis: evaluation of a serologic test for the detection of Babesia microti antibody. J Infect Dis. 1994;169:923–6. [DOI] [PubMed] [Google Scholar]
- 20.Krause PJ, Ryan R, Telford S, Persing D, Spielman A. Efficacy of immunoglobulin M serodiagnostic test for rapid diagnosis of acute babesiosis. J Clin Microbiol. 1996;34:2014–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Popovsky MA, Lindberg LE, Syrek AL, Page PL. Prevalence of Babesia antibody in a selected blood donor population. Transfusion. 1988;28:59–61. 10.1046/j.1537-2995.1988.28188127955.x [DOI] [PubMed] [Google Scholar]
- 22.Krause PJ, Telford SR, Ryan R, Hurta AB, Kwasnik I, Luger S, et al. Geographical and temporal distribution of babesial infection in Connecticut. J Clin Microbiol. 1991;29:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]