ABSTRACT
Despite advances in diagnosis and therapy, esophageal cancer remains a highly lethal disease. The incidence of esophageal adenocarcinoma (EAC) has risen faster than that of any other cancer in the western world, and Barrett's esophagus (BE) may be a significant contributing factor. In-depth knowledge of biology of cancer progression and cancer could lead to the identification of biomarkers that are the hallmark of BE's progression. By integrating validated biomarkers of progression into clinical practice, there is a possibility of identifying high-risk patient population for targeted surveillance, and such biomarkers may serve as novel therapeutic targets for chemoprevention and therapy. Clinical management of BE has improved considerably due to the improvements in endoscopic resection and ablation techniques. We discuss the current status of biology and therapeutic approaches to BE.
Esophageal cancer (EC) is an aggressive neoplasm and a major cause of cancer-related deaths worldwide.1 A total of 16,470 new cases and 14,530 deaths were projected to occur in the United States in 2009.2 Despite advances in diagnosis, 50% of patients present with advanced disease.3 Five-year relative survival rates are low (14%), and the improvement in comparison with 20 years earlier (10%) is not substantial.4,5 Moreover, systemic nature and the intrinsic resistance to therapy are hallmarks of EC.
The incidence of esophageal adenocarcinoma (EAC) is rising faster than that of any other cancer in the western world.6 Barrett's esophagus (BE) is a known premalignant condition of EAC and is characterized by the replacement of squamous stratified epithelium with a columnar metaplasia in distal esophagus.7,8 Whether the presence of intestinal-type differentiation is a requirement for its definition is still a matter of debate. The American Gastroenterological Association Chicago workshop required the presence of intestinal metaplasia, but meanwhile the British Society of Gastroenterology does not require it to diagnose BE.9,10
In a Swedish study, the prevalence of BE in general population was approximately 1.6%, but in a US study it was 5.5%.11,12 This may be due to the prevalence of obesity in US adults. Furthermore, 5–15% of patients with gastroesophageal reflux disease (GERD) are expected to have a diagnosis of BE.13 The risk of EAC in patients with BE is low, 0.15–0.5% per year, but the lifetime risk is 10- to 125-fold higher than the general population.14,15 Progression of BE to EAC is a stepwise process beginning with metaplasia and progressing to low-grade dysplasia (LGD) to high-grade dysplasia (HGD) and finally to EAC. However, the risks from LGD remain controversial with regard to its frequency to progress to HGD or EAC.16–18 This unpredictability could be the result of sampling error, inter- or intraobserver variability, or instability of the dysplastic lesion. HGD's natural history is better defined and understood with a progression to EAC of almost 6.6% per year.19 It is important to underline that a multifocal or nodular HGD designation raises the risk of EAC progression even higher.20
Since BE tissue is relatively easily accessible, BE can serve as a model to study molecular alterations associated with its progression to EAC.
RISK FACTORS
BE is correlated with GERD.21 White, elderly men have an increased risk of BE diagnosis.22 Hiatal hernia and central obesity (rather than diffuse obesity) as well as the presence of acid and bile in the refluxate have also been considered as strong risk factors for development of BE.23,24 In contrast, smoking and alcohol appear to be low-risk factors.25 Contradictory results have been published regarding the damage that bile without acid could cause to the esophageal tissue.26,27
PATHOGENESIS OF METAPLASIA
Histogenesis Stem Cell Theory
When squamous mucosa is damaged in the presence of extrinsic factors such as cytokines, gastric acid, and bile-stem cells could be involved in BE pathogenesis. Their location in the esophagus is not yet identified. Three main theories exist for BE histogenesis: (1) Stem cells are in the papillae in squamous mucosa (de novo metaplasia therapy). An analogous model is identified and described in vaginal mucinosis.28 (2) Stem cells exist in the neck regions of glands of esophageal ducts (duct cell metaplasia theory). A similar model (ulcer-associated cell lineage) occurs next to ulceration in the gastrointestinal tract.29 (3) Stem cells are located at the gastroesophageal junction (GEJ) (transition zone metaplasia theory). This model is identified in cervical metaplasia.30 One hypothesis suggests that the bone marrow stem cells could seed to the area of epithelial injury and subsequently differentiate to columnar cells. This hypothesis is supported by a case report and in experiments done in an animal model.31,32
Stem cell theory has become more popular in recent days for cancer development. It is stated that the inflammatory milieu in the esophagus exposed to chronic GERD would result in animation of stem cells for repair. This repeated call for repair that demands proliferation would lead to mistakes in the DNA of stem cells. These mistakes (mutations) are sometimes not repaired, leading to clone formation. Several clones are generated over time, and some clones will survive the stressful environment to form cancer.
Familial Barrett's Esophagus (FBE)
Over the past few decades, accumulated results suggest that BE may be a multifactorial condition where genetic predisposition may result in susceptibility that leads to inadequate detoxification of enzymes and cell-cycle regulators.33–35 Currently little is known regarding the genes that lead to genetic predisposition. Moreover, previous reports on familial aggregation of BE and its associated cancers that is termed FBE are interesting and shed light on this issue.36–38 However, familial clustering may indicate either genetic predisposition or common exposure to environmental agents. Chak et al reported that FBE can occur in 7% of patients with BE, EAC, and adenocarcinoma of GEJ.39 In FBE, 20% of the relatives have BE compared to 10% in sporadic BE.40 Despite the fact that no genes responsible are yet identified, several reports suggest that FBE is a complex genetic disease inherited in an autosomal dominant fashion.41,42
Epigenetic Molecular Alterations
On the other hand, the epigenetic mechanisms associated with the development of BE are better studied. Caudal homeobox gene 1 (CDX 1) and 2 (CDX 2) are involved and are critical points in this process.43 Nuclear factor kappa b (NF-Kb), fibroblast growth factor (FGF), bone morphogenetic protein 4 (Bmp-4), and hedgehog and wnt pathways are related with CDX gene regulation.44–48 Furthermore, p63 suppression that is a determinant of squamous phenotype may also be an important factor.49
NEOPLASTIC PROGRESSION
Linear models of multistage carcinogenesis may not be ideal to define progression of BE to EAC. One of their limitations is that they cannot sufficiently deal with genetic heterogeneity. It should be emphasized that in the study by Smith et al a linear model of colorectal carcinogenesis applied only to 6.6% of cases.50
Interestingly, normal stem cells and certain cancer cells share properties. They both have the ability to self-renew, to differentiate, to migrate, to interact with stroma (niche), and to activate antiapoptotic pathways.51 Because of this evidence and because stem cells are subject to mutations when repeatedly called to repair under highly chronically inflammatory conditions, a cancer stem cell hypothesis could be central to unraveling the biomarkers of progression from BE to EAC. This hypothesis postulates that tumors may originate from adult tissue stem cells or their immediate progenitor cells.52 Cancer stem cells would then organize themselves as an organ (cancer mass). These cells can self-renew and give rise to differentiating progeny. A deregulated self-renewal process due to several mutations on genes that regulate this process could be important to neoplastic progression. Hedgehog, Wnt, Bmi-1, and Notch pathways are implicated to self-renewal process, and their deregulation could lead to tumorigenesis.53–56 Various studies conducted in leukemia, breast cancer, and brain cancers appear consistent with this model of carcinogenesis.57–59 For normal human esophageal epithelial cells, a candidate stem/progenitor cell fraction is characterized by the expression of the low-affinity p75 neurotrophin receptor (p75NTR).60 Further studies on esophageal squamous cell cancer specimens and cell lines have demonstrated that p75NTR expression was related with cancer stem cells characteristics, such as self-renewal and chemotherapy resistance.61,62
During the 1970s, Nowell presented his clonal evolution hypothesis.63 The evolutionary theory describes that during progression, clonal expansions occurred. These are intermittent selective sweeps of clones with advantageous mutations. Various mutations could happen in a cell without defining a distinct change (neutral mutations). Only when they occur before or after an advantageous mutation (hitchhiked mutation) will they be selected and dominate the neoplasm (fixation).
In BE it appears that loss of heterozygosity (LOH), promoter hypermethylation, or sequence mutations of p16 are the initial and main events that drive and select the sweep of clones necessary for clonal expansion through the tumor.64 This hypothesis was developed upon the observation that the identical p16 mutations or LOH patterns are identified in large areas of BE lesions, thus suggesting that p16 inactivation is associated with selective sweeps.65 Subsequent inactivation of p53 by LOH or mutation drives aneuploidy and cancer progression.66
Genomic Instability
In epithelial tumors during neoplastic progression tumor genome acquires extensive chromosomal changes. The degree of genetic instability increases during the multiple displacement amplication sequence.67 These alterations are also extensive and multifocal, indicating the field cancerization effect. Genomic instability mainly consists of genome-wide copy losses, gains, and LOH. Whole-genome single-nucleotide polymorphism (SNP) and comparative genomic hybridization (CGH) -based technologies have been used for biomarker discovery in premalignant lesions that are not well characterized yet, such as BE.68 Li et al analyzed 42 samples of patients in different stages from BE to EAC using a SNP platform.69 They found that chromosomal instability was increased between early- and late-stage BE (P < .001). SNP alterations highly correlated with DNA aneuploidy and were suitable to identify EAC patients. Lai et al studied copy number alterations (CNAs) in six patients using a high-resolution CGH platform.70 They found that genomic instability increases in severity during neoplastic progression. In addition, Paulson and coworkers using a CGH array examined 98 patients with BE or EAC.71 They showed that CNAs were more frequent in late stages of carcinogenesis and highly correlated with DNA content aneuploidy. Tissues with CNAs involving >70 Mbp indicated patients at increased risk of progression to DNA changes or EAC (HR = 4.9, 95% confidence interval [CT], 1.6–14.8, P = .0047).
Recent studies on genetic diversity and BE heterogeneity presented intriguing results that need further validation. Genetic clonal diversity's impact was described by Maley et al72 Using three measures of clonal diversity, i.e., number of clones per lesion, divergence between samples, and Shannon index of diversity, this group analyzed 268 patient samples with BE with a median follow-up of 4.4 years. It was shown that clonal diversity predicts further progression to adenocarcinoma development and is a greater risk factor rather than a clonal expansion. It seems that diversity drives transformation in a multilineage rather than a sequential events and there may be barriers that prevent frequent selective sweeps.
Leedham et al genetically studied individual crypts.73 Their results proposed that BE heterogeneity arises from multiple independent clones that probably expand during adenocarcinoma development but not from a single founder mutation sweeping through an entire area to fixation.
The marked heterogeneity observed in BE lesions underline the suggestion that multiple molecular pathways are involved and interact through tumor development. To that direction, a panel of prediction factors could be more useful than single factors alone. Galipeau et al showed that a combination of p53 and p16 LOH, aneuploidy, and tetraploidy provided better risk prediction.74 Unfortunately, this panel required a combination of techniques that are difficult to implement in the clinic. Advanced modern technology such as SNP-based technologies and high-resolution comparative genomic hybridization arrays seem to be promising options that could be clinically useful.
Table 1 lists the altered genes in BE and EAC.
Table 1.
Altered genes/pathways in BE and EAC (these genes may not represent all genes that may be involved)
| BE | EAC |
|---|---|
| CDX1, CDX2 | |
| NF-κB targets | NF-κB targets |
| Genes related to hedgehog signaling | Genes related to hedgehog signaling |
| Genes in the mTOR pathway | Genes in the mTOR pathway |
| Notch pathway genes | Notch pathway genes |
| BMP4 | — |
| Rb pathway genes | — |
| p53 | p53 |
| Ras pathway | Ras pathway |
| VEGF-related genes | VEGF-related genes |
| Telomerase-related pathway | Telomerase-related pathway |
| Cell-cycle-related pathway genes | Cell-cycle-related pathway genes |
| STAT-3 | STAT-3 |
| Sox 9 | Sox 9 |
| MMPs | MMPs |
| Wnt signaling | Wnt signaling |
| — | Rho |
| c-MET pathway | c-MET pathway |
CURRENT CLINICAL MANAGEMENT
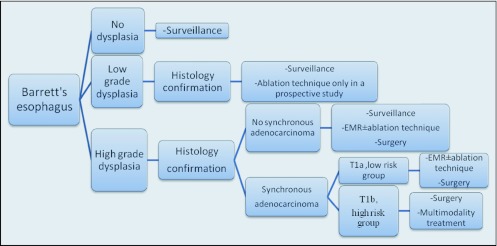
The appropriate management of BE patients depends on the presence of dysplasia and the type of dysplasia that occurs (Figure 1). Because of the small proportion of BE patients that progress to EAC, the value of surveillance programs is a matter of debate. However, in a high-risk group of patients surveillance programs have significant impact.75 BE is so prevalent that it is crucial to identify a high-risk population for targeted sufrveillance.
Figure 1.
Therapeutic recommendations for various categories of Barrett's esophagus.
Histologic designation should be made by an experienced gastrointestinal (GI) pathologist. For LGD, esophago-gastro-duodeno scopy (EGD) should be repeated after six months and then annually until two serial EGDs are negative for dysplasia. In the case of HGD, histology needs confirmation by a second GI pathologist. The risk of concomitant early EAC also should be investigated. The options offered at this stage (BE/HGD) are aggressive surveillance through endoscopy and biopsies every three months as well as ablation therapy followed by surveillance or esophagectomy.75
Esophagectomy
A high concordance that reaches 30–50% of the cases with HGD is observed between HGD and occult EC.76,77 Because of this, esophagectomy was traditionally the standard treatment for BE with HGD. However, with increasing sophistication and favorable results from endoscopic approaches, surgery is not always recommended as an immediate first option. Esophagectomy in patients with EAC without muscularis mucosa involvement confers 5-year survival rates >80%.78 Despite advances in surgical techniques, esophagectomy can result in significant mortality, ranging from 1% to 10% depending on the volume of the center.79 Furthermore, morbidity rates can range from 30% to 50%.80 Although minimally invasive approaches of esophagectomy have been found to be safe with better perioperative outcomes, randomized trials are needed to evaluate them in comparison with open procedures.81
Ablation Techniques
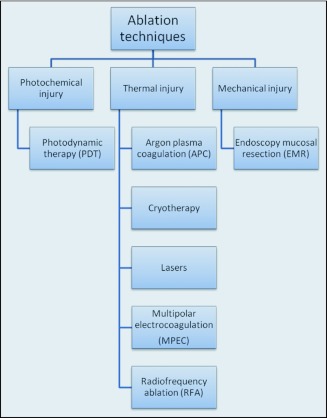
Mucosal adenocarcinomas result in a low rate of lymph node involvement (<2%).82 This observation provides the basis that less invasive local approaches, such as ablation techniques, could be performed as curative strategies in this group of patients with expectations of equivalent effectiveness but with a significant decrease in mortality and morbidity rates (Figure 2).
Figure 2.
Types of ablative approaches available.
Prior to the ablative techniques, esophageal endoscopic ultrasound and computed tomography scanning should be performed to evaluate the size, length, depth of lesion, lymph node enlargement, and distal metastases.
Lasers
A light amplification by stimulated emission of radiation (LASER) beam is directed against the lesion and destroys it. There are various types of lasers: argon, neodymium:yttrium-aluminum-gannet (Nd:YAG), potassium titanyl phosphate (KTP), and KTP:YAG with different wavelength emissions. Gossner and colleagues studied 10 patients (LGD = 4, HGD = 4, early EC=2) using a (Nd:YAG) KTP laser system.83 After a mean follow-up of 10.6 months a complete response was observed in all. In two patients Barrett' s submucosa was identified. Weston et al also presented the results of 14 patients with BE and HGD/IMC treated with a Nd:YAG contact laser.84 They reported successful elimination of HGD and or cancer in all patients. Eleven of 14 achieved complete histological ablation of Barrett's tissue, and no buried columnar epithelial tissue was observed. Odynophagia and early dysphagia were reported in 30.6% and 16.3% of the patients, respectively.
Photodynamic Therapy (PDT)
The basis for PDT is the administration of a photosensitizer [porfimer sodium (iv), 5-aminolevulinic acid (per os)] that has properties to bind the neoplastic area through an unknown mechanism. After an exposure to intense laser light, vascular thrombosis and cell necrosis is caused. Overholt and colleagues studied 100 patients (LGD = 14, HGD = 73, IMC = 13) with a mean follow-up of 19 months.85 Elimination of BE and HGD was observed in 43% and 88% of the patients, respectively. Progression or failure was found in 21 patients. Complications observed were stricture (34%) and subsquamous Barrett's esophagus (6%). An international randomized phase III trial was also conducted by Overholt et al86 that studied 208 patients to compare PDT using porfimer sodium (POR) plus omeprazole (n = 138) vs. omeprazole only (n = 70). There was a significant difference (P < .0,001) in favor of PORPDT compared with omeprazole [39% (27/70)] in complete eradication of HGD at any time during the follow-up period. The occurrence of EAC in the PORPDT group was 13% and significantly lower compared with the omeprazole group, being 28% (P < .006).
Multipolar Electrocoagulation (MPEC)
Two or more electrodes of MPEC probe allow the delivery of thermal energy to the desired area and destroy tissue. In a multicenter trial, 58 patients were studied, and after a follow-up of six months, 78% of the patients had a complete response. Residual BE was identified in 4 of 58 patients. One patient developed stricture and the most common side effect was chest pain (19/58).87 Kovacs et al studied 27 patients with BE treated with MPEC and lansoprazole with an intention to reverse histology.88 Twenty-two patients had successful reversal, and the most common side effect was dysphagia (41%).
Argon Plasma Coagulation (APC)
Through the flow of ionized argon gas, a high-frequency monopolar current is directed to the neoplastic tissues. Attwood et al studied 29 patients with HGD with a mean follow-up of 37 months.89 The median number of treatments was two, and complete regression was observed in 25 out of 29 patients (86%). Recurrence was identified in four out of 25 patients (16%). Ackroyd and coresearchers randomized patients with BE to intervention with APC (n = 20) vs. surveillance (n = 20).90 After a five-year follow-up 14/20 patients treated with APC achieved >95% BE regression vs. 5/20 in the surveillance arm. No patients in the intervention group progressed to HGD. On the contrary, 2/20 in the surveillance group progressed. Two patients treated with APC developed a stricture but could be managed endoscopically.
Radiofrequency Ablation (RFA)
This technique requires the application of a balloon with circular electrodes delivering radio-frequency energy in circumferential way (HALO360). In addition, for focal lesions, a plate device can be used (HALO90). Roorda et al studied 13 patients (6 with BE, 4 with LGD, and 3 with HGD).91 After a mean follow-up of 12 months, eradication of BE was observed in 6 patients (46%) and eradication of dysplasia in 5 out of 7 (71%). Fleischer et al presented their data on 61 patients with intramucosal carcinoma.92 A complete remission was observed in 98% of patients after a median follow-up of 30 months. In both studies no complications were reported. Shaheen and co-researchers reported on a randomized, multicenter prospective trial comparing RFA with a sham procedure in BE with dysplasia.93 One hundered and twenty-seven patients (LGD = 64, HGD = 63) with a 12-month follow-up were studied. Complete eradication of LGD and HGD occurred in 90.5% and 81% in the ablation group (P < .001). On the other hand, complete elimination of LGD and HGD occurred in 22.7% and 19% in the control group (P < .001). A clear superiority for RFA was observed. Furthermore, this superiority was also significant for eradication of BE with 77.4% (RFA group) as compared with 2.3% of those in the control group (P < .001). Patients in the ablation group had fewer cancers (1.2% vs. 9.3%, P = .045) and less disease progression (3.6% vs. 16.3%, P = .03). Six percent of patients treated with RFA developed a stricture, and one had gastrointestinal bleeding.
Endoscopic Mucosal Resection (EMR)
In EMR, local, endoscopic resection is performed after the injection of fluid to separate the mucosal and muscle layers. Ell et al reported their experience with EMR in BE patient with dysplasia or IMC.94 They studied 64 BE patients with HGD or IMC. Thirty-five patients belonged to the low-risk group and 29 to the high-risk group according to histological grade, lesion size, and macroscopic appearance. BE eradication was observed in 97% and 59% in low- and high-risk groups, respectively, after a mean follow-up of 12 months. Recurrence or metachronous lesions incidence was 13.6% and 17.1%, respectively.
Larghi et al also reported their results with this technique in 24 BE patients with HGD or IMC after a mean follow-up of 28 months.95 Complete eradication was observed in 87.5% of the patients (21 out of 24). Complications were observed in five patients (two with bleeding and three with stricture). Persistence or de novo BE, developing underneath the newly formed squamous mucosa (subsquamous), was identified in two patients (8%).
Cryo-Spray Ablation (CSA)
Through the application of liquid nitrogen gas or CO2, cold temperatures (−196°C, −70° respectively) generated can freeze the tissues, and ischemic necrosis can occur. Furthermore, cryo-ablation induces apoptosis and immune stimulation. A prospective trial evaluating safety and efficacy of CSA in patients with BE and HGD or IMCA by Dumot and colleagues was reported in 30 patients with a median follow-up of 12 months. At the last follow-up, responses persisted in 68% for HGD and 80% for IMCA.96 Greenwald et al presented results of parallel prospective treatment studies at four tertiary care medical centers.97 Seventy-seven patients (BE = 7, BE with HGD = 45, BE with IMCA = 13, EC = 10, and severe squamous dysplasia = 2) were treated. Out of 23 patients completing therapy, in 17 patients with HGD, there was a complete response in 94% and complete elimination of BE in 53%. In all four patients with IMC, a complete response was noted for cancer and 75% of BE eradication. In all three patients with esophageal cancer (inoperable or refused surgery, ineligible or refused radiation or systemic therapy) a complete response was observed for cancer and 67% of BE elimination. One major complication occurred during their study, consisting of a gastric perforation caused by gastric distention due to nitrogen gas. The most common side effect in procedures was chest pain (17.6%) and dysphagia (13.3%).
Optimal management strategy of BE remains in flux, and large prospective trials are needed. In three retrospective studies comparing esophagectomy vs. endoscopic therapy in BE with HGD or IMC outcomes in terms of overall survival were similar.98–100 Each approach has its disadvantages and limitations. It should be underlined that all but EMR ablation techniques have the major disadvantage in that they destroy tissue, and histopathologic evaluation is not possible. Furthermore, the risk of buried BE under the re-epithelized surface remains a concern because of risk of carcinoma development. Bronner et al found that this was not a safety concern for patients who underwent PDT therapy.101 The multimodality endoscopic approach, combining EMR and RFA, CSA, or other ablative interventions for eradicating the remaining high-risk tissue is a promising option to optimize treatment.
FUTURE DIRECTIONS
Questions to Be Addressed
Today none of the biomarkers reported have been introduced into daily clinical practice. According to the Early Detection Research Network, five phases are needed to confirm preliminary results from case studies and develop cancer-screening biomarkers.102 Many questions still need to be addressed; GERD is so prevalent, therefore, we should be able to identify who is susceptible to BE. Furthermore, in patients with BE, we should be able to identify individuals highly susceptible to developing dysplasia and EAC.
Pathways to the Target
Better understanding of tumor biology and the need for a more efficacious therapy has led to the development of specific agents to target carcinogenesis/neoplastic progression. Given the success of monoclonal antibodies and tyrosine kinase inhibitors against solid or hematologic tumors there is optimism that some of these approaches could be applied to premalignant conditions such as BE.
Cyclin D1
Cyclin D1 proto-oncogene is a regulator of the G1-S phase cell cycle transition is found to be expressed in 46% of BE and in 64% of esophageal or gastroesophageal junction adenocarcinomas.103,104 Various studies suggest a role in esophageal tumorigenesis.105,106 Furthermore, in our previous study, Cyclin D1 A870G polymorphism was related with younger age of onset of EAC, greater frequency of distant metastasis, and increased levels of nuclear Cyclin D1 expression.107 Nuclear CD1 alternate form drives cellular transformation and cancer progression. This polymorphism was also related with genomic instability and poor outcome in EAC.34
Nuclear Factor Kappa B (NF-κB)
NF-κB is an important transcription factor that regulates important functions such as proliferation, cell survival, apoptosis, invasion, angiogenesis, and metastasis. Normally it is inactivated and is located in cell cytoplasm in a heterotrimer form consisting of p50–p65 and IκBα subunits. Upon activation, the p50–p65 heterodimer is translocated into the nucleus and binds to the promoters of NF-κB-related genes.108 NF-κB is activated and overexpressed along M-D-A sequence and associated with CD1 nuclear expression.109 NF-κB is an adverse prognostic factor for chemo-radiation efficacy in EC and overall a poor prognosis factor.110,111
Gastrin Signaling
Gastrin is a hormone that mainly regulates gastric acid secretion. Various studies have shown that in patients with BE treated with proton pump inhibitors an elevation of fasting and postprandial serum gastrin levels is assessed.112 Gastrin could have a role in carcinogenesis promotion initially binding to the cholecystokinin receptor (CCK2R).113 Through CCK2R stimulation and subsequent epidermal growth factor (EGF) expression COX-2 expression could be induced.114 COX-2 is expressed in 75% in patients with BE without dysplasia and 100% in HGD and EC patients and is related with neoplastic progression.115 Furthermore, CCK2R activation also participates in proapoptotic factors' inactivation.114 Gastrin-signaling activity in tumorigenesis promotion has been previously described in in vitro experiments; however, clear clinical evidence does not yet exist. Whether PPIs could counterbalance the effect of hypergastrinemia with their ability to suppress acid-reflux signaling is a matter of conjecture. The combined use of gastrin's signaling inhibitors along with PPIs might be an interesting option.
Growth Factors—Receptors
Epidermal growth factor is overexpressed in BE's progression to EAC and is related with matrix metalloproteinase production.116 Its gene amplification is observed as a late event in esophageal carcinogenesis and is related to poor prognosis.117,118 ErbB-2 gene amplification is also a late event in disease progression related to aggressive phenotype.106 Vascular endothelial growth factors (VEGFs) and VEGF receptors are observed in BE and neoplastic lesions and are associated with angiogenesis process.119 Their prognostic role in EC is not yet identified.108 Furthermore, HGF receptor c-Met is overexpressed during this process, in BE with dysplasia and EA, compared to normal esophagus epithelium.120,121 Herrera et al also showed that c-Met dysregulation can occur as an early event in EAC tumorigenesis.122 These findings suggest that it may be an attractive target for chemoprevention or targeted therapy against EAC. In addition, inhibition of COX-2 has been shown to downregulate c-Met expression and decrease the frequency of BE malignant transformation and could be an option for preventive therapy.123
Future efforts should be directed toward defining the high-risk population through biomarkers.
Footnotes
Supported in part by Dallas, Park, Caporella, Sultan, Smith, and Cantu Family funds and by the Rivercreek Foundation. Also supported by the Multidisciplinary Research Program Grant from UT MD Anderson Cancer Center and NHI-NCI grants (CA142072, CA127672, CA129906, CA111922).
Disclosures of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
REFERENCES
- 1. Parkin DM: International variation. Oncogene 23:6329–6340, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Jemal A, Siegel R, Ward E, et al. : Cancer statistics, 2009. CA Cancer J Clin 59:225–249, 2009 [DOI] [PubMed] [Google Scholar]
- 3. Tew WP, Kelsen DP, Ilson DH: Targeted therapies for esophageal cancer. Oncologist 10:590–601, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Enginger PC, Mayer RJ: Esophageal cancer. N Engl J Med 349:2241–2252, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Ekman S, Dreilich M, Lennartsson J, et al. : Esophageal cancer: current and emerging therapy modalities. Expert Rev Anticancer Ther 8:1433–1448, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Devesa SS, Blot WJ, Fraumeni JF, Jr: Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer 83:2049–2053, 1998 [PubMed] [Google Scholar]
- 7. Barrett NR: Chronic peptic ulcer of the oesophagus and “oesophagitis.” Br J Surg 38:175–182, 1950 [DOI] [PubMed] [Google Scholar]
- 8. Mueller J, Werner M, Stolte M: Barrett's esophagus: histopathologic definitions and diagnostic criteria. World J Surg 28:148–154, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Sharma P, McQuaid K, Dent J, et al. : A critical review of the diagnosis and management of Barrett's esophagus: the AGA Chicago Workshop. Gastroenterology 127:310–330, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Playford RJ: New British Society of Gastroenterology (BSG) guidelines for the diagnosis and management of Barrett's oesophagus. Gut 55:310–442, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ronkainen J, Aro P, Storskrubb T, et al. : Prevalence of Barrett's esophagus in the general population: an endoscopic study. Gastroenterology 129:1825–1831, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Rex DK, Cummings OW, Shaw M, et al. : Screening for Barrett's esophagus in colonoscopy patients with and without heartburn. Gastroenterology 125:1670–1677, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Shaheen NJ, Richter JE: Barrett's oesophagus. Lancet 373:850–861, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Shaheen NJ, Crosby MA, Bozymski EM, et al. : Is there publication bias in the reporting of cancer risk in Barrett's esophagus? Gastroenterology 119:333–338, 2000 [DOI] [PubMed] [Google Scholar]
- 15. Solaymani-Dodaran M, Logan RF, West J, et al. : Risk of oesophageal cancer in Barrett's oesophagus and gastro-oesophageal reflux. Gut 53:1070–1074, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reid BJ, Blount PL, Rubin CE, et al. : Flow cytometric and histological progression to malignancy in Barrett's esophagus: prospective endoscopic surveillance of a cohort. Gastroenterology 102:1212–1219, 1992 [PubMed] [Google Scholar]
- 17. Skacel M, Petras RE, Gramlich TL, et al. : The diagnosis of low-grade dysplasia in Barrett's esophagus and its implications for disease progression. Am J Gastroenterol 95:3383–3387, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Lim CH, Treanor D, Dixon MF, et al. : Low-grade dysplasia in Barrett's esophagus has a high risk of progression. Endoscopy 39:581–587, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Rastogi A, Puli S, El-Serag HB, et al. : Incidence of esophageal adenocarcinoma in patients with Barrett's esophagus and high-grade dysplasia: a meta-analysis. Gastrointest Endosc 67:394–398, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Buttar NS, Wang KK, Sebo TJ, et al. : Extent of high-grade dysplasia in Barrett's esophagus correlates with risk of adenocarcinoma. Gastroenterology 120:1630–1639, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Spechler SJ: Clinical practice: Barrett's esophagus. N Engl J Med 346:836–842, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Abrams JA, Fields S, Lightdale CJ, et al. : Racial and ethnic disparities in the prevalence of Barrett's esophagus among patients who undergo upper endoscopy. Clin Gastroenterol Hepatol 6:30–34, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Corley DA, Kubo A, Levin TR, et al. : Abdominal obesity and body mass index as risk factors for Barrett's esophagus. Gastroenterology 133:34–41, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Dvorak K, Payne CM, Chavarria M, et al. : Bile acids in combination with low pH induce oxidative stress and oxidative DNA damage: relevance to the pathogenesis of Barrett's oesophagus. Gut 56:763–771, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wong A, Fitzgerald RC: Epidemiologic risk factors for Barrett's esophagus and associated adenocarcinoma. Clin Gastroenterol Hepatol 3:1–10, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Avidan B, Sonnenberg A, Schnell TG, et al. : Gastric surgery is not a risk for Barrett's esophagus or esophageal adenocarcinoma. Gastroenterology 121:1281–1285, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Sharma P, Sampliner R: GERD, DGER or both in Barrett's esophagus? Am J Gastroenterol 92:903–904, 1997 [PubMed] [Google Scholar]
- 28. Sodhani P, Gupta S, Prakash S, et al. : Columnar and metaplastic cells in vault smears: cytologic and colposcopic study. Cytopathology 10:122–126, 1999 [DOI] [PubMed] [Google Scholar]
- 29. Schmidt PH, Lee JR, Joshi V, et al. : Identification of a metaplastic cell lineage associated with human gastric adenocarcinoma. Lab Invest 79:639–646, 1999 [PMC free article] [PubMed] [Google Scholar]
- 30. Wallner B, Sylvan A, Stenling R, et al. : The esophageal Z-line appearance correlates to the prevalence of intestinal metaplasia. Scand J Gastroenterol 35:17–22, 2000 [DOI] [PubMed] [Google Scholar]
- 31. Körbling M, Katz RL, Khanna A, et al. : Hepatocytes and epithelial cells of donor origin in recipients of peripheral-blood stem cells. N Engl J Med 346:738–746, 2002 [DOI] [PubMed] [Google Scholar]
- 32. Sarosi G, Brown G, Jaiswal K, et al. : Bone marrow progenitor cells contribute to esophageal regeneration and metaplasia in a rat model of Barrett's esophagus. Dis Esoph 21:43–50, 2008 [DOI] [PubMed] [Google Scholar]
- 33. Kala Z, Dolina J, Marek F, et al. : Polymorphisms of glutathione S-transferase M1, T1 and P1 in patients with reflux esophagitis and Barrett's esophagus. J Hum Genet 52:527–534, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Izzo JG, Wu TT, Wu X, et al. : Cyclin D1 guanine/adenine 870 polymorphism with altered protein expression is associated with genomic instability and aggressive clinical biology of esophageal adenocarcinoma. J Clin Oncol 25:698–707, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Pan J, Lin J, Izzo JG, et al. : Genetic susceptibility to esophageal cancer: the role of the nucleotide excision repair pathway. Carcinogenesis 30:785–792, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fahmy N, King JF: Barrett's esophagus: an acquired condition with genetic predisposition. Am J Gastroenterol 88:1262–1265, 1993 [PubMed] [Google Scholar]
- 37. Eng C, Spechler SJ, Ruben R, et al. : Familial Barrett esophagus and adenocarcinoma of the gastroesophageal junction. Cancer Epidemiol Biomarkers Prev 2:397–399, 1993 [PubMed] [Google Scholar]
- 38. Chak A, Lee T, Kinnard MF, et al. : Familial aggregation of Barrett's oesophagus, oesophageal adenocarcinoma, and oesophagogastric junctional adenocarcinoma in Caucasian adults. Gut 51:323–328, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chak A, Ochs-Balcom H, Falk G, et al. : Familiality in Barrett's esophagus, adenocarcinoma of the esophagus, and adenocarcinoma of the gastroesophageal junction. Cancer Epidemiol Biomarkers Prev 15:1668–1673, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Chak A, Faulx A, Kinnard M, et al. : Identification of Barrett's esophagus in relatives by endoscopic screening. Am J Gastroenterol 99:2107–2114, 2004 [DOI] [PubMed] [Google Scholar]
- 41. Poynton AR, Walsh TN, O'Sullivan G, et al. : Carcinoma arising in familial Barrett's esophagus. Am J Gastroenterol 91:1855–1856, 1996 [PubMed] [Google Scholar]
- 42. Jochem VJ, Fuerst PA, Fromkes JJ: Familial Barrett's esophagus associated with adenocarcinoma. Gastroenterology 102:1400–1402, 1992 [PubMed] [Google Scholar]
- 43. Souza RF, Krishnan K, Spechler SJ: Acid, bile, and CDX: the ABCs of making Barrett's metaplasia. Am J Physiol Gastrointest Liver Physiol 295:G211–G218, 2008 [DOI] [PubMed] [Google Scholar]
- 44. Pahl HL: Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene 18:6853–6866, 1999 [DOI] [PubMed] [Google Scholar]
- 45. Keenan ID, Sharrard RM, Isaacs HV: FGF signal transduction and the regulation of Cdx gene expression. Dev Biol 299:478–488, 2006 [DOI] [PubMed] [Google Scholar]
- 46. van Baal JW, Milano F, Rygiel AM, et al. : A comparative analysis by SAGE of gene expression profiles of Barrett esophagus, normal squamous esophagus, and gastric cardia. Gastroenterology 129:1274–1281, 2005 [DOI] [PubMed] [Google Scholar]
- 47. Ioannides AS, Henderson DJ, Spitz L, et al. : Role of Sonic hedgehog in the development of the trachea and oesophagus. J Pediatr Surg 38:29–36, 2003 [DOI] [PubMed] [Google Scholar]
- 48. Shimizu T, Bae YK, Muraoka O, et al. : Interaction of Wnt and caudal-related genes in zebra Wsh posterior body formation. Dev Biol 279:125–141, 2005 [DOI] [PubMed] [Google Scholar]
- 49. Roman S, Pétré A, Thépot A, et al. : Downregulation of p63 upon exposure to bilesalts and acid in normal and cancer esophageal cells in culture. Am J Physiol Gastrointest Liver Physiol 293:G45–G53, 2005 [DOI] [PubMed] [Google Scholar]
- 50. Smith G, Carey FA, Beattie J, et al. : Mutations in APC, Kirsten-ras, and p53–alternative genetic pathways to colorectal cancer. Proc Natl Acad Sci U S A 99:9433–9438, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pardal R, Clarke MF, Morrison SJ: Applying the principles of stem-cell biology to cancer. Nat Rev Cancer 3:895–902, 2003 [DOI] [PubMed] [Google Scholar]
- 52. Wicha MS, Liu S, Dontu G: Cancer stem cells: an old idea—a paradigm shift. Cancer Res 66:1883–1890, 2006 [DOI] [PubMed] [Google Scholar]
- 53. Taipale J, Beachy PA: The hedgehog and Wnt signaling pathways in cancer. Nature 411:349–354, 2001 [DOI] [PubMed] [Google Scholar]
- 54. Reya T, Duncan AW, Ailles L, et al. : A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature 423:409–414, 2003 [DOI] [PubMed] [Google Scholar]
- 55. Lessard J, Sauvageau G: Bmi-1 determines the proliferative capacity of normal and leukaemic stem cells. Nature 423:255–260, 2003 [DOI] [PubMed] [Google Scholar]
- 56. Varnum-Finney B, Xu L, Brashem-Stein C, et al. : Pluripotent, cytokine-dependent, hematopoietic stem cells are immortalized by constitutive Notch1 signaling. Nat Med 6:1278–1281, 2000 [DOI] [PubMed] [Google Scholar]
- 57. Bonnet D, Dick JE: Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med 3:730–737, 1997 [DOI] [PubMed] [Google Scholar]
- 58. Al-Hajj M, Wicha MS, Benito-Hernandez A, et al. : Prospective identification of tumorigenicbreast cancer cells. Proc Natl Acad Sci U S A 100:3983–3988, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Singh SK, Clarke ID, Terasaki M, et al. : Identification of a cancer stem cell in human brain tumors. Cancer Res 63:5821–5828, 2003 [PubMed] [Google Scholar]
- 60. Okumura T, Shimada Y, Imamura M, et al. : Neurotrophin receptor p75(NTR) characterizes humanesophageal keratinocyte stem cells in vitro. Oncogene 22:4017–4026, 2003 [DOI] [PubMed] [Google Scholar]
- 61. Okumura T, Tsunoda S, Mori Y, et al. : The biological role of the low-affinity p75 neurotrophin receptor in esophageal squamous cell carcinoma. Clin Cancer Res 12:5096–5013, 2006 [DOI] [PubMed] [Google Scholar]
- 62. Huang SD, Yuan Y, Liu XH, et al. : Self-renewal and chemotherapy resistance of p75NTR positive cells in esophageal squamous cell carcinomas. BMC Cancer 9:5096–9, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Nowell PC: The clonal evolution of tumor cell populations. Science 194:23–28, 1976 [DOI] [PubMed] [Google Scholar]
- 64. Barrett MT, Sanchez CA, Galipeau PC, et al. : Allelic loss of 9p21 and mutation of the CDKN2/p16 gene develop as early lesions during neoplastic progression in Barrett's esophagus. Oncogene 13:1867–1873, 1996 [PubMed] [Google Scholar]
- 65. Maley CC, Galipeau PC, Li X, et al. : Selectively advantageous mutations and hitchhikers in neoplasms: p16 lesions are selected in Barrett's esophagus. Cancer Res 64:3414–3427, 2004 [DOI] [PubMed] [Google Scholar]
- 66. Galipeau PC, Prevo LJ, Sanchez CA, et al. : Clonal expansion and loss of heterozygosity at chromosomes 9p and 17p in premalignant esophageal (Barrett's) tissue. J Natl Cancer Inst 91:2087–2095, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hittelman WN: Genetic instability in epithelial tissues at risk for cancer. Ann NY Acad Sci 952:1–12, 2001 [DOI] [PubMed] [Google Scholar]
- 68. Wistuba II, Meyerson M: Chromosomal deletions and progression of premalignant lesions: less is more. Cancer Prev Res (Phila Pa) 1:404–408, 2008 [DOI] [PubMed] [Google Scholar]
- 69. Li X, Galipeau PC, Sanchez CA, et al. : Single nucleotide polymorphism-based genome-wide chromosome copy change, loss of heterozygosity, and aneuploidy in Barrett's esophagus neoplastic progression. Cancer Prev Res (Phila Pa) 1:413–423, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lai LA, Paulson TG, Li X, et al. : Increasing genomic instability during premalignant neoplastic progression revealed through high resolution array-CGH. Genes Chromosomes Cancer 46:532–542, 2007 [DOI] [PubMed] [Google Scholar]
- 71. Paulson TG, Maley CC, Li X, et al. : Chromosomal instability and copy number alterations in Barrett's esophagus and esophageal adenocarcinoma. Clin Cancer Res 15:3305–3314, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Maley CC, Galipeau PC, Finley JC, et al. : Genetic clonal diversity predicts progression to esophageal adenocarcinoma. Nat Genet 38:468–473, 2006 [DOI] [PubMed] [Google Scholar]
- 73. Leedham SJ, Preston SL, McDonald SA, et al. : Individual crypt genetic heterogeneity and the origin of metaplastic glandular epithelium in human Barrett's oesophagus. Gut 57:1041–1048, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Galipeau PC, Li X, Blount PL, et al. : NSAIDs modulate CDKN2A, TP53 and DNA content risk for progression to esophageal adenocarcinoma. PLoS Med 4:e67, 342–354, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wang KK, Sampliner RE: Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett's esophagus. Am J Gastroenterol 103:788–797, 2008 [DOI] [PubMed] [Google Scholar]
- 76. Rice TW, Falk Gw, Achkar E, et al. : Surgical management of high-grade dysplasia in Barrett's esophagus. Am J Gastroenterol 88:1811–1812, 1993 [PubMed] [Google Scholar]
- 77. Zaninotto G, Parenti AR, Ruol A, et al. : Oesophageal resection for high-grade dysplasia in Barrett's oesophagus. Br J Surg 87:1102–1105, 2000 [DOI] [PubMed] [Google Scholar]
- 78. Peters JH, Clark GW, Ireland A, et al. : Outcome of adenocarcinoma arising in Barrett's esophagus in endoscopically surveyed and nonsurveyed patients. J Thorac Cardiovasc Surg 108:813–821, 1994 [PubMed] [Google Scholar]
- 79. Birkmeyer JD, Siewers AE, Finlayson EV, et al. : Hospital volume and surgical mortality in the United States. N Engl J Med 346:1128–1137, 2002 [DOI] [PubMed] [Google Scholar]
- 80. Chang AC, Ji H, Birkmeyer NJ, et al. : Outcomes after transhiatal and transthoracic esophagectomy for cancer. Ann Thorac Surg 85:424–429, 2008 [DOI] [PubMed] [Google Scholar]
- 81. Nguyen NT, Follette DM, Wolfe BM, et al. : Comparison of minimally invasive esophagectomy with transthoracic and transhiatal esophagectomy. Arch Surg 135:920–925, 2000 [DOI] [PubMed] [Google Scholar]
- 82. Stein HJ, Feith M, Bruecher BL, et al. : Early esophageal cancer: pattern of lymphatic spread and prognostic factors for long-term survival after surgical resection. Ann Surg 242:566–575, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Gossner L, May A, Stolte M, et al. : KTP laser destruction of dysplasia and early cancer in columnar-lined Barrett's esophagus. Gastrointest Endosc 49:8–12, 1999 [DOI] [PubMed] [Google Scholar]
- 84. Weston AP, Sharma P: Neodymium: yttrium-aluminum garnet contact laser ablation of Barrett's high grade dysplasia and early adenocarcinoma. Am J Gastroenterol 97:2998–3006, 2002 [DOI] [PubMed] [Google Scholar]
- 85. Overholt BF, Panhepour M, Haydek JM: Photodynamic therapy for Barrett's esophagus: follow-up in 100 patients. Gastrointest Endosc 49:1–7, 1999 [DOI] [PubMed] [Google Scholar]
- 86. Overholt BF, Wang KK, Burdick JS, et al. : Five-year efficacy and safety of photodynamic therapy with Photofrin in Barrett's high-grade dysplasia. Gastrointest Endosc 66:460–468, 2007 [DOI] [PubMed] [Google Scholar]
- 87. Sampliner RE, Faigel D, Fennerty MB, et al. : Effective and safe endoscopic reversal of nondysplastic Barrett's esophagus with thermal electrocoagulation combined with high-dose acid inhibition: a multicenter study. Gastrointest Endosc 53:554–558, 2001 [DOI] [PubMed] [Google Scholar]
- 88. Kovacs BJ, Chen YK, Lewis TD, et al. : Successful reversal of Barrett's esophagus with multipolar electrocoagulation despite inadequate acid suppression. Gastrointest Endosc 49:547–553, 1999 [DOI] [PubMed] [Google Scholar]
- 89. Attwood SE, Lewis CJ, Caplin S, et al. : Argon beam plasma coagulation as therapy for high-grade dysplasia in Barrett's esophagus. Clin Gastroenterol Hepatol 1:258–263, 2003 [PubMed] [Google Scholar]
- 90. Bright T, Watson DI, Tam W, et al. : Randomized trial of argon plasma coagulation versus endoscopic surveillance for barrett esophagus after antireflux surgery: late results. Ann Surg 246:1016–1020, 2007 [DOI] [PubMed] [Google Scholar]
- 91. Roorda AK, Marcus SN, Triadafilopoulos G: Early experience with radiofrequency energy ablation therapy for Barrett's esophagus with and without dysplasia. Dis Esophagus 20:516–522, 2007 [DOI] [PubMed] [Google Scholar]
- 92. Fleischer DE, Overholt BF, Sharma VK, et al. : Endoscopic ablation of Barrett's esophagus: a multicenter study with 2.5-year follow-up. Gastrointest Endosc 68:867–876, 2008 [DOI] [PubMed] [Google Scholar]
- 93. Shaheen NJ, Sharma P, Overholt BF, et al. : Radiofrequency ablation in Barrett's esophagus with dysplasia. N Engl J Med 360:2277–2288, 2009 [DOI] [PubMed] [Google Scholar]
- 94. Ell C, May A, Gossner L, et al. : Endoscopic mucosal resection of early cancer and high-grade dysplasia in Barrett's esophagus. Gastroenterology 118:670–677, 2000 [DOI] [PubMed] [Google Scholar]
- 95. Larghi A, Lightdale CJ, Ross AS, et al. : Long-term follow-up of complete Barrett's eradication endoscopic mucosal resection (CBE-EMR) for the treatment of high grade dysplasia and intramucosal carcinoma. Endoscopy 39:1086–1091, 2007 [DOI] [PubMed] [Google Scholar]
- 96. Dumot JA, Vargo JJ, 2nd, Falk GW, et al. : An open-label, prospective trial of cryospray ablation for Barrett's esophagus high-grade dysplasia and early esophageal cancer in high-risk patients. Gastrointest Endosc 70:635–644, 2009 [DOI] [PubMed] [Google Scholar]
- 97. Greenwald BD, Dumot JA, Horwhat JD, et al. : Safety, tolerability, and efficacy of endoscopic low-pressure liquid nitrogen spray cryotherapy in the esophagus. Dis Esophagus 23:13–19, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Prasad GA, Wang KK, Buttar NS, et al. : Long-term survival following endoscopic and surgical treatment of high-grade dysplasia in Barrett's esophagus. Gastroenterology 132:1226–1233, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Schembre DB, Huang JL, Lin OS, et al. : Treatment of Barrett's esophagus with early neoplasia: a comparison of endoscopic therapy and esophagectomy. Gastrointest Endosc 67:595–601, 2008 [DOI] [PubMed] [Google Scholar]
- 100. Prasad GA, Wu TT, Wigle DA, et al. : Endoscopic and surgical treatment of mucosal (T1a) esophageal adenocarcinoma in Barrett's esophagus. Gastroenterology 137:815–823, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Bronner MP, Overholt BF, Taylor SL, et al. : Squamous overgrowth is not a safety concern for photodynamic therapy for Barrett's esophagus with high-grade dysplasia. Gastroenterology 136:56–64, 2009 [DOI] [PubMed] [Google Scholar]
- 102. Pepe MS, Etzioni R, Feng Z, et al. : Phases of biomarker development for early detection of cancer. J Natl Cancer Inst 93:1054–1061, 2001 [DOI] [PubMed] [Google Scholar]
- 103. Sherr CJ: Cancer cell cycles. Science 274:1672–1677, 1996 [DOI] [PubMed] [Google Scholar]
- 104. Arber N, Lightdale C, Rotterdam H, et al. : Increased expression of the cyclin D1 gene in Barrett's esophagus. Cancer Epidemiol Biomarkers Prev 5:457–459, 1999 [PubMed] [Google Scholar]
- 105. Arber N, Gammon MD, Hibshoosh H, et al. : Overexpression of cyclin D1 occurs in both squamous carcinomas and adenocarcinomas of the esophagus and in adenocarcinomas of the stomach. Hum Pathol 30:1087–1092, 1999 [DOI] [PubMed] [Google Scholar]
- 106. Bani-Hani K, Martin IG, Hardie LJ, et al. : Prospective study of cyclin D1 overexpression in Barrett's esophagus: association with increased risk of adenocarcinoma. J Natl Cancer Inst 92:1316–1321, 2000 [DOI] [PubMed] [Google Scholar]
- 107. Izzo JG, Malhotra U, Wu TT, et al. : Impact of cyclin D1 A870G polymorphism in esophageal adenocarcinoma tumorigenesis. Semin Oncol 32(6 Suppl 9):S11–S15, 2005 [DOI] [PubMed] [Google Scholar]
- 108. Aggarwal BB: Nuclear factor-kappaB: the enemy within. Cancer Cell 6:203–208, 2004 [DOI] [PubMed] [Google Scholar]
- 109. Izzo JG, Luthra R, Wu TT, et al. : Molecular mechanisms in Barrett's metaplasia and its progression. Semin Oncol 34(Suppl 1):S2–S6, 2007 [DOI] [PubMed] [Google Scholar]
- 110. Luthra R, Wu TT, Luthra MG, et al. : Gene expression profiling of localized esophageal carcinomas: Association with pathologic response to preoperative chemoradiation. J Clin Oncol 24:259–267, 2006 [DOI] [PubMed] [Google Scholar]
- 111. Izzo JG, Malhotra U, Wu TT, et al. : Association of activated transcription factor nuclear factor kappa b with chemoradiation resistance and poor outcome in esophageal carcinoma. J Clin Oncol 24:748–754, 2006 [DOI] [PubMed] [Google Scholar]
- 112. Iwao T, Toyonaga A, Kuboyama S, et al. : Effects of omeprazole and lansoprazole on fasting and postprandial serum gastrin and serum pepsinogen A and C. Hepatogastroenterology 42:677–682, 1995 [PubMed] [Google Scholar]
- 113. Haigh CR, Attwood SE, Thompson DG, et al. : Gastrin induces proliferation in Barrett's metaplasia through activation of the CCK2 receptor. Gastroenterology 124:615–625, 2003 [DOI] [PubMed] [Google Scholar]
- 114. Abdalla SI, Lao-Sirieix P, Novelli MR, et al. : Gastrin-induced cyclooxygenase-2 expression in Barrett's carcinogenesis. Clin Cancer Res 10:4784–4792, 2004 [DOI] [PubMed] [Google Scholar]
- 115. Shirvani VN, Ouatu-Lascar R, Kaur BS, et al. : Cyclooxygenase 2 expression in Barrett's esophagus and adenocarcinoma: ex vivo induction by salts and acid exposure. Gastroenterology 118:487–496, 2000 [DOI] [PubMed] [Google Scholar]
- 116. Murray GI, Duncan ME, O'Neil P, et al. : Matrix metalloproteinase-1 is associated with poor prognosis in esophageal cancer. J Pathol 185:256–261, 1998 [DOI] [PubMed] [Google Scholar]
- 117. Peters CJ, Fitgerald RC: Systematic review: the application of molecular pathogenesis to prevention and treatment of oesophageal adenocarcinoma. Aliment Pharmacol Ther 25:1253–1269, 2007 [DOI] [PubMed] [Google Scholar]
- 118. AI-Kasspooles M, Moore JH, Orringer MB, et al. : Amplification and overexpression of the EGFR and erbB-2 genes in human esophageal adenicarcinomas. Int J Cancer 54:213–219, 1993 [DOI] [PubMed] [Google Scholar]
- 119. Auvinen MI, Sihvo EI, Ruohtula T, et al. : Incipient angiogenesis in Barrett's epithelium and lymphangiogenesis in Barrett's adenocarcinoma. J Clin Oncol 20:2971–2979, 2002 [DOI] [PubMed] [Google Scholar]
- 120. Anderson MR, Harrison R, Atherfold PA, et al. : Met receptor signaling: a key effector in esophageal adenocarcinoma. Clin Cancer Res 15:5936–5943, 2006 [DOI] [PubMed] [Google Scholar]
- 121. Watson GA, Zhang X, Stang MT, et al. : Inhibition of c-Met as a therapeutic strategy for esophageal adenocarcinoma. Neoplasia 8:949–955, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Herrera LJ, El-Hefnawy, Quiroz de Oliveira PE, et al. : The HGF receptor c-Met is overexpressed in esophageal adenocarcinoma. Neoplasia 7:75–84, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Gupta RA, DuBois RN: Cyclooxygenase-2 inhibitor therapy for the prevention of esophageal adenocarcinoma in Barrett's esophagus. J Natl Cancer Inst 94:406–407, 2002 [DOI] [PubMed] [Google Scholar]