Abstract
Human CD34+ progenitor-derived Langerhans-type dendritic cells (LCs) are more potent stimulators of T-cell immunity against tumor and viral antigens in vitro than are monocyte-derived DCs (moDCs). The exact mechanisms have remained elusive until now, however. LCs synthesize the highest amounts of IL-15R-α mRNA and protein, which binds IL-15 for presentation to responder lymphocytes, thereby signaling the phosphorylation of signal transducer and activator of transcription 5 (pSTAT5). LCs electroporated with Wilms tumor 1 (WT1) mRNA achieve sufficiently sustained presentation of antigenic peptides, which together with IL-15R-α/IL-15, break tolerance against WT1 by stimulating robust autologous, WT1-specific cytolytic T-lymphocytes (CTLs). These CTLs develop from healthy persons after only 7 days' stimulation without exogenous cytokines and lyse MHC-restricted tumor targets, which include primary WT1+ leukemic blasts. In contrast, moDCs require exogenous rhuIL-15 to phosphorylate STAT5 and attain stimulatory capacity comparable to LCs. LCs therefore provide a more potent costimulatory cytokine milieu for T-cell activation than do moDCs, thus accounting for their superior stimulation of MHC-restricted Ag-specific CTLs without need for exogenous cytokines. These data support the use of mRNA-electroporated LCs, or moDCs supplemented with exogenous rhuIL-15, as vaccines for cancer immunotherapy to break tolerance against self-differentiation antigens shared by tumors.
Introduction
The dendritic cell (DC) hematopoietic lineage comprises heterogeneous subsets that share the capacity for potent initiation and control of innate and adaptive immunity,1–5 but with sufficient plasticity to orchestrate the quantity and quality of lymphocyte responses. Indeed, defined subsets of human DCs generated with cytokines in vitro also yield sufficient numbers with counterparts in vivo to discern distinct consequences to T cells interacting with one or another subtype.6–12
Human Langerhans-type DCs derived from CD34+ hematopoietic progenitor cells (HPCs) have consistently proven superior to other conventional DC subsets as stimulators of CD8+ cytolytic T lymphocytes (CTLs) in vitro.9,11 This has led some investigators to advocate inclusion of CD34+ HPC-derived Langerhans cells (LCs) in DC vaccines13,14 rather than relying solely on the more commonly used monocyte-derived DCs (moDCs). The mechanisms underlying LC potency have remained elusive, but IL-15 has emerged as an important cytokine mediator.9,14 First, LCs' potent stimulation of CD8+ CTLs occurs in the complete absence of IL-12p70,9,15 which has proven more critical for the activation of NK cells15 than for CD8+ CTLs.9 Despite several shared functions, IL-15 also has important contrasting roles with IL-2.16–18 IL-2 controls autoreactive T cells through their activation-induced cell death, or apoptosis, and the maintenance and expansion of Tregs. In contrast, IL-15 is critical to the generation of durable, high-avidity, memory CD8+ T cells, which could include autoimmune populations with specificity for self-differentiation tumor antigens.16–18
LCs secrete more IL-15 than any other conventional DC subtype,9,15 but investigators have not previously identified IL-15R-α on the surface of LCs. This α-subunit is required for transport of IL-15 to the cell membrane to bind β (CD122) and γ (CD132) chains on responding lymphocytes. Only then is the biologically active, heterotrimeric receptor-cytokine complex complete, supporting subsequent lymphocyte signaling and activation.16–18
We therefore undertook alternative approaches to define the expression of IL-15R-α by resident populations of primary human LCs emigrating from cultured human epidermal sheets, as well as LCs, dermal-interstitial DCs (DDC-IDCs), and moDCs generated with recombinant human cytokines in vitro.9 Eliminating exposure to IL-15 during DC development allowed comparison of tumor-specific CTLs stimulated by LCs with those elicited by moDCs, as opposed to evaluating IL-15–induced moDCs with Langerhans-like properties.19,20 This also facilitated evaluation of early T-cell signaling events in response to LC or moDC stimulation, under conditions of limited exogenous IL-15 or IL-15R-α blockade. Finally, we compared the capacity of LCs and moDCs to stimulate CTLs from healthy persons in vitro against Wilms tumor protein 1 (WT1). Our findings link the expression of full-length antigen-derived epitopes and the IL-15/IL-15R-α complex by human LCs with their capacity to overcome tolerance by potent stimulation of CTLs against a self-differentiation tumor antigen like WT1. These data provide a sound rationale for the evaluation of LCs, or moDCs obligatorily supplemented with exogenous IL-15, in clinical trials of active immunotherapy against human cancer.
Methods
Media and noncytokine supplements
Complete RPMI 1640 included 10mM HEPES, 1% penicillin/streptomycin (Media Lab, Memorial Sloan-Kettering Cancer Center [MSKCC]), 50μM 2-mercaptoethanol (Invitrogen), 1% l-glutamine (Invitrogen), and 1% or 10% volume/volume heat-inactivated, pooled normal human serum (NHS; Atlanta Biologicals). X-VIVO 15 (BioWhittaker; Lonza Walkersville) was used as manufactured without additives. All media and reagents were endotoxin-free.
Human cells, media, and cytokines
Collection and use of human biospecimens adhered to protocols approved by the Institutional Review and Privacy Board of Memorial Hospital, MSKCC. Resident LCs were collected from excess skin obtained from patients undergoing reconstructive surgery. Initially, sheets of full thickness skin were cut into 4- to 6-mm pieces and partially digested in Dispase (StemCell Technologies) at 4°C for 8 to 10 hours. Epidermal sheets were then carefully peeled from the dermis, washed with cold PBS (MSKCC Media Laboratory), and floated on 250 μL of complete RPMI-10% NHS in a 48-well tissue culture plate (Corning Life Sciences). To improve yield, dermatomal sheets were obtained directly from reconstructive surgery cases, from which multiple 8-mm punch biopsies were taken. These were floated separately in 16-mm wells of a 24-well plate (Corning Life Sciences) in 1 mL of complete RPMI-10% NHS. GM-CSF 1000 IU/mL was added or not to maintain LC viability21 after either method to obtain epidermal sheets, but no other cytokines were added for maturation or activation. Epidermal sheets and cells were never exposed to xenogeneic serum (eg, FCS). Cells that emigrated from the epidermis were collected after 40 to 48 hours.
G-CSF–elicited CD34+ HPCs from healthy donors undergoing leukopheresis for allogeneic hematopoietic stem cell transplantation were used to generate LCs and DDC-IDCs. These donors (before any GCSF exposure) and healthy volunteers also provided PBMCs for the generation of moDCs. Media, media supplements, cytokines, and commercial sources were exactly as published9 (see erratum for correct FLT-3-ligand dose22).
In brief, however, CD34+ HPCs were cultured in serum-free X-VIVO 15, supplemented with GM-CSF and TNF-α, to which c-kit-ligand and FLT-3-ligand were added for only the first 5 to 6 days of a 10- to 12-day culture. In addition, for the respective generation of LCs or DDC-IDCs from CD34+ HPCs, either TGF-β or IL-4 was added throughout the entire culture period. For the generation of moDCs, tissue culture plastic adherent CD14+ monocytes were cultured in complete RPMI-1% NHS with GM-CSF and IL-4 for 5 to 6 days. Each of these DC subtypes underwent terminal maturation for an additional 48 hours using a combination of TNF-α, IL-1β, IL-6, and prostaglandin E2. Each of the resulting DC subtypes was uniformly CD14−, CD11c+, CD80+, CD86bright, and CD83+. The CD34+ HPC-derived LCs were unique among the other DC subtypes for the absence of CD11b and asynchronous expression of Langerin (CD207), which decreased with terminal activation and maturation.9 Although we did not specifically evaluate EpCAM, the phenotype of CD34+ HPC-derived LCs generated under similar culture conditions in vitro has recently been shown to correspond to LCs isolated from human epidermis.23 Both CD34+ HPC-derived DDC-IDCs and moDCs expressed CD11b but lacked Langerin.9 Only moDCs expressed CD91, the α-2-macroglobulin receptor, or significant amounts of CD52.9 We did not study monocyte-derived LCs,24,25 which express little Langerin or Birbeck granules and paradoxically secrete IL-12p70 in contrast to primary or CD34+ HPC-derived LCs.9,15,26
T cells were tissue culture plastic nonadherent PBMCs, further purified by nonadherence and elution from nylon wool columns (Polysciences). Purity was more than 95% based on CD3 expression.
All cells were used either fresh or thawed after cryopreservation.27 Viable recovery was more than 90%, without any compromise of phenotype or activity.
Peptides
HLA-A*0201–restricted, native WT1 peptide (RMFPNAPYL126-134; Sigma-Genosys)28 was used to verify antigen-specific CTL targeting. Synthetic influenza matrix peptide (fluMP58-66 GILGFVFTL; Research Genetics, Invitrogen) served as a positive recall antigen for HLA-A*0201–restricted responses.
Immunofluorescence microscopy and analysis
Resident LCs from primary epidermal cell émigrés, cytokine-generated CD34+ HPC-derived LCs, and cytokine-differentiated moDCs, the latter two subjected to immunomagnetic selection for high HLA-DR expression (MiniMACS; Miltenyi Biotec), were cytocentrifuged (10 000 cells/slide) at 91.45g for 5 minutes (Cytospin 3; Shandon). Excess medium was aspirated, after which the cells underwent fixation and permeabilization in 3% paraformaldehyde for 20 minutes at 4°C, followed by air drying and storage at room temperature or direct staining.
Immunofluorescent staining used polyclonal goat anti–human IL-15R-α (R&D Systems) followed by secondary rhodamine Red-X-conjugated donkey anti–goat IgG (H + L) (Jackson ImmunoResearch Laboratories). Resident epidermal LC émigrés were additionally stained with monoclonal mouse anti–human HLA-DR (clone L243; BioLegend) followed by AlexaFluor-647 rabbit anti–mouse IgG (H + L; Invitrogen).29 LCs and moDCs generated in vitro had already been purified by immunomagnetic selection for high HLA-DR expression and so did not require additional anti–HLA-DR staining for identification. Actin staining of these latter two populations used bodipy-conjugated phallacidin (Invitrogen).
Slides were visualized through an ×100 1.4 NA oil immersion lens with an inverted Olympus IX-70 microscope (Delta Vision Image Restoration Microscope; Applied Precision/Olympus) and Photometrics CoolSnap QE camera. Alternatively, an inverted Leica LX microscope and a Leica DFC 350 FX camera were used. Serial optical sections (0.2 μm; 40-60 sections) were acquired for all labelings. Images were deconvoluted using DeltaVision SoftWoRx Version 3.4.4 software or Huygens 1.1.4 software (Montpellier RIO Imaging). Fluorescent intensity per unit of volume of the image-reconstructed cells yielded 3-dimensional units called voxels, which were analyzed with ImageJ Version 1.41n (NIH). These were averaged from the means of at least 20 cells in randomly selected fields per slide from 3 independent experiments.
Quantitative RT-PCR for measurement of IL-15R-α mRNA transcripts
Immunomagnetic selection for high HLA-DR expression (MiniMACS, Miltenyi Biotec) provided purified populations of cytokine-generated, immature and mature LCs, DDC-IDCs, and moDCs.9 Real-time RT-PCR quantified IL-15R-α mRNA transcripts by normalizing the average copy number of IL-15R-α cDNA to the 18S rRNA housekeeping cDNA within each DC subtype. Assays-on-Demand Gene Expression probes for IL-15R-α (Hs00542604_m1, Applied Biosystems) detected mRNA transcripts based on cDNA transcribed from 0.003 mg of Trizol (Invitrogen)–isolated RNA. An 18S rRNA specific probe (Hs99999901_s1; Applied Biosystems) served as an internal housekeeping control. Thermal cycler parameters were 2 minutes at 50°C, 10 minutes at 95°C, 40 cycles of 95°C for 15 seconds, and 60°C for 1 minute. Relative gene expression data were calculated from quadruplicate samples by normalizing the average copy number of IL-15R-α cDNA to the 18S rRNA housekeeping cDNA within each DC subtype, then comparing that with immature moDCs, which had the lowest transcript levels.
Phosphorylation of STAT5 in responder T cells
A total of 1 × 106 LCs or moDCs from HLA-A*0201–expressing donors were pulsed with fluMP (fluMP58-66 GILGFVFTL, 10μM) in 3 mL complete RPMI in 6-well plates (Corning Life Sciences) for 1 hour at room temperature, then cocultured with autologous T cells for 6 to 7 days9 to generate fluMP-reactive T lymphoblasts. In other cases where HLA typing was not available and Ag-specific responses were not required, concanavalin A 4 μg/mL (conA; Sigma-Aldrich) was added to 106 PBMCs/mL complete RPMI for 72 hours at 37°C to generate T lymphoblasts. T cells were harvested and washed at the end of these culture periods, but a modification for conA T lymphoblasts included washing in 10mM methyl-α-D-mannopyranoside (Calbiochem) to remove residual lectin. T cells were then exposed to a glycine solution (pH 4) at 1 × 106 T cells/mL on ice for 1 minute to strip residual receptor-bound cytokines, after which the reaction was stopped by the addition of 10 mL ice-cold PBS. Thereafter, the T cells were resuspended in complete RPMI and rested for 1 to 2 hours at 37°C.
The absence of phosphorylated STAT5 was confirmed by flow cytometry in these rested T cells before proceeding. For antigen-specific responses, the rested but fluMP-reactive T cells were restimulated with fresh, autologous LCs or moDCs pulsed de novo with fluMP, to which nothing, rhuIL-15 (10 ng/mL; R&D Systems), or anti–IL-15R-α (5 μg/mL; R&D Systems) was added. T/LC or moDC ratios were 10:1 for these restimulations. Cell-free supernatants were also collected after the usual 48-hour maturation of LCs at 1 × 106 cells/3 mL/well of a 6-well tissue culture plate (Corning Life Sciences) in inflammatory cytokines9,30 and compared at 50% and 100% volume/volume with intact autologous LCs (T/LC = 10:1) for their capacity to mediate pSTAT5 in rested conA T-cell blasts.
Cells and media were maintained at 37°C throughout. Cells were gently pelleted at 300g for 20 seconds, incubated for 30 minutes at 37°C, then gently repelleted. Cells were then fixed in 1.6% paraformaldehyde or Cytofix (BD Biosciences), followed by permeabilization with ice-cold 90% methanol, each for 10 minutes. Cells were washed in FACS buffer and then stained with mouse anti–human CD3 FITC (Beckman Coulter) and either rabbit anti–human pSTAT5 (Y694, Clone C11C5, Cell Signaling Technology) and allophycocyanin-conjugated donkey anti–rabbit secondary antibody (Jackson ImmunoResearch Laboratories) or directly conjugated Alexa Fluor 647 anti–human pSTAT5 (pY694, BD Biosciences). T cells were analyzed for STAT5 phosphorylation (pSTAT5) after gating on viable FITC-CD3+ T cells (Cytomics FC500, Beckman Coulter; or LSR II, BD Biosciences).
Electroporation of DCs by mRNA encoding WT1
An EcoRI insert encoding WT1 cDNA, derived from the pUC119 plasmid (Riken Bioresource), was cloned into a pGEM-4Z vector (Promega). The plasmid was transformed in Max Efficiency DH5-α competent cells (Invitrogen) and purified using a Plasmid Maxi Kit (QIAGEN). The pGEM-4Z/WT1 plasmid was linearized with HindIII (New England Biolabs) before mRNA transcription in vitro, which was performed with SP6 RNA polymerase (mMessage mMachine SP6 kit; Ambion). Agarose gel electrophoresis confirmed production of full-length capped mRNA, and spectrophotometry measured mRNA concentration. Immature moDCs9 were electroporated on days 5 to 6 and immature LCs9 on days 10 to 11, after washing and resuspending in OptiMEM (Invitrogen) at 20 × 106 cells/mL. A total of 100 μL of cell suspension was then mixed with 4 μg of WT1 mRNA transcribed in vitro and electroporated in a 2-mm gap cuvette at 300 V for 500 μs, using BTX ECM 830 square-wave electroporator (BTX Harvard).31,32 Electroporated immature moDCs and LCs were recultured in a combination of inflammatory cytokines for terminal maturation and activation.9
CTL assays
Mature WT1 mRNA-electroporated autologous moDCs or LCs were separately added in graded doses to triplicate wells of 1 × 105 T cells in a 96 round-bottomed well plate (Corning Life Sciences). Final volume was 100 μL/well of complete RPMI-10% heat-inactivated, autologous serum. Recombinant IL-15 (10 ng/mL; R&D Systems) was added only to moDC-stimulated cultures where indicated.
Cytolytic activity exerted by responder T lymphocytes was assessed in 51Cr release or colorimetric assays. 51Cr-labeled targets included HLA-A*0201 positive, WT1-expressing cell lines, HLA-A*0201 positive cell lines pulsed or not with the HLA-A*0201 restricted WT1 peptide, or a class I MHC-negative NK cell–sensitive cell line. Peripheral blood or marrow of patients with acute leukemia provided primary blasts as targets for colorimetric cytolytic assays, after semiquantitative PCR (Eppendorf) confirmed WT1 expression using published sequences.28,33
By adding 5 × 103 51Cr-labeled target cells directly to replicate microwells after only 7 days of stimulation by WT1 mRNA-electroporated LCs or moDCs, total cytolytic activity generated per primary culture condition could be compared based on 51Cr release into the supernatants collected after 4 to 6 hours.9 Percent specific lysis was calculated in standard fashion. Lysis of primary leukemic blasts required a colorimetric CTL assay,34 which gave lower background than did spontaneous 51Cr release.
Statistics
Replicate means from more than 3 independent experiments were averaged and SEM calculated as the measure of variability. A stratified t test (stratified by the effector/target or responder/stimulator ratio) was used for the functional assays. The 2-sample t test was used for each comparison within a given time point or condition for all other analyses.
Results
Mature LCs express significantly higher IL-15R-α mRNA transcripts and protein than do mature moDCs
Using flow cytometry, the surface expression of IL-15R-α had been previously documented on nonpermeabilized moDCs but not LCs.15 This was despite LCs' superior stimulation of CTLs compared with moDCs9 and a known requirement for IL-15R-α to transport IL-15 to the cell surface for binding to β (CD122) and γ (CD132) chains on responder lymphocytes.18 This completes the biologically active, heterotrimeric receptor-cytokine complex for lymphocyte activation.16–18
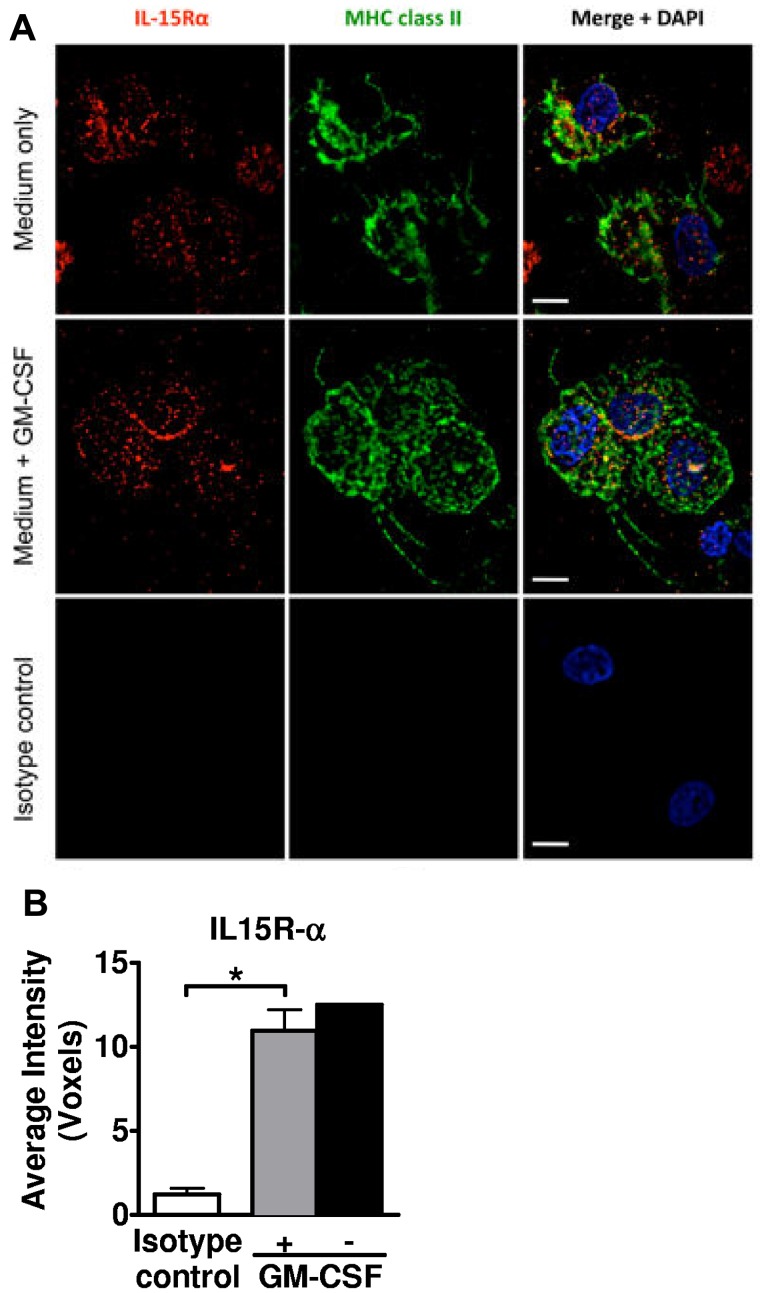
We therefore evaluated primary tissue-resident LCs after emigration from epidermal sheets into suspension culture, which were then cytocentrifuged onto glass slides, permeabilized, and immunostained. Apart from mechanical perturbations, these LCs had no exposure to other maturation stimuli, although we did compare cells cultured with or without GM-CSF for enhanced LC viability.21 Importantly, neither epidermal sheets nor LC émigrés were ever cultured in FCS, which enhanced viability and activation state. Immunofluorescent microscopy identified intracellular and cell membrane expression of IL-15R-α by the HLA-DR+ epidermal LC émigrés (Figure 1). There was no significant difference in intensity of expression between LCs exposed to GM-CSF or not, although the GM-CSF–treated condition appeared to concentrate more of the IL-15R-α at the cell membrane.
Figure 1.
Human primary LCs express IL-15R-α. (A) Epidermal sheets from healthy skin were cultured in complete RPMI-10% NHS. GM-CSF 1000 IU/mL was added or not to maintain LC viability.21 The cells recovered in culture after 40 to 48 hours were cytocentrifuged, fixed and permeabilized, and stained as indicated for IL-15R-α (red), HLA-DR (green), and 4,6-diamidino-2-phenylindole (blue nuclear DNA stain). Scale bars represent 10 μm. (B) At least 20 cells in randomly selected fields from each donor/condition were evaluated to calculate the average voxels, which represent digitalized 3-dimensional image data. Shown in the bar graph are the averaged replicate means from 3 independent experiments ± SEM. *P = .0254. Error bar not shown for the condition without GM-CSF, as lower viability yielded sufficient cells from only 2 independent experiments.
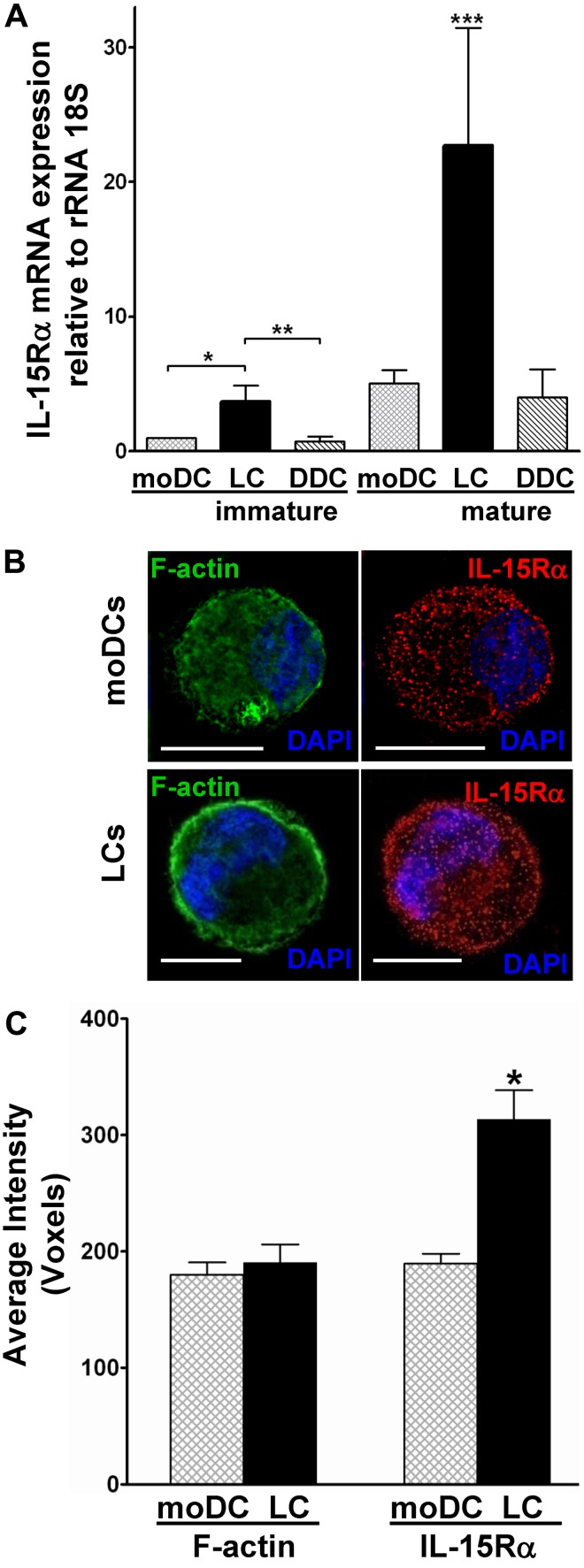
Returning to purified populations of LCs expanded from G-CSF–mobilized, CD34+ HPCs,9 we first compared these with CD34+ HPC-derived DDC-IDCs and moDCs. LC mRNA transcripts for IL-15R-α, quantified by real-time RT-PCR, were significantly higher than transcripts in the other conventional DC subtypes, especially after maturation (Figure 2A).
Figure 2.
Mature CD34+ HPC-derived LCs express the highest levels of IL-15R-α among all conventional DC subtypes. (A) Highly purified populations of cytokine-generated, immature and mature moDCs, CD34+ HPC-derived LCs, and CD34+ HPC-derived DDC-IDCs underwent quantification of IL-15R-α mRNA by real-time RT-PCR. Values were normalized to the housekeeping gene 18S rRNA subunit. Data represent the averages of quadruplicate means ± SEM from 3 independent experiments. *P = .019, immature LCs vs immature moDCs. **P = .0005, immature LCs vs immature DDC-IDCs. ***P = .01, mature LCs vs all other DC subtypes. (B) Cytocentrifuged, fixed, and permeabilized mature moDCs and LCs were stained with polyclonal anti–IL-15R-α followed by rhodamine Red-X–conjugated donkey anti–goat IgG (red). F-actin control was stained by bodipy-conjugated phallacidin (green), and nuclear DNA was stained with 4,6-diamidino-2-phenylindole (DAPI; blue). Cells were examined by immunofluorescent microscopy. Scale bars represent 10 μm. (C) At least 20 cells in randomly selected fields from each experiment were evaluated to calculate the average voxels, which represent digitalized 3-dimensional image data. Shown in the bar graph are the averaged replicate means from 3 independent experiments ± SEM. *P = .0014 for IL-15R-α. P = not significant for F-actin.
We then concentrated solely on CD34+ HPC-derived LCs and the more commonly used moDCs in cancer vaccines. Immunofluorescent microscopy and digital imaging of immunostained, cytocentrifuged cells demonstrated that IL-15R-α protein concentrated along the LC surface membrane (Figure 2B), similar to what we had observed with epidermal LC émigrés cultured in GM-CSF. In contrast, moDCs exhibited a more generalized distribution (Figure 2B). The overall density of IL-15R-α was also significantly higher on mature, activated CD34+ HPC-derived LCs than on mature moDCs (P = .0014; Figure 2C). These mature, activated LCs therefore maintain both the largest pool of mRNA transcripts and the highest protein levels of both intracellular and cell membrane-associated IL-15R-α, compared with other conventional DC subsets.
LCs provide a more potent T-cell costimulatory cytokine environment through endogenous IL-15 than do moDCs
Previous attempts to demonstrate a unique role for LC-derived IL-15 in priming CD8+ T cells documented inhibition after LC blockade by anti–IL-15R-α, but the inhibition was incomplete.14 Plausible explanations included the presence of CD4+ T cells that secreted other cytokines supporting CD8+ CTL expansion, which were not blocked, or inadequate concentrations of the blocking anti–IL-15R-α without a positive control to establish sufficient antibody dose. Other unmeasured factors could also have contributed to CTL stimulation downstream of the initial T-cell activation events, especially over two 7-day rounds of stimulation in vitro that followed priming by peptide-pulsed LC or moDC vaccines in vivo.
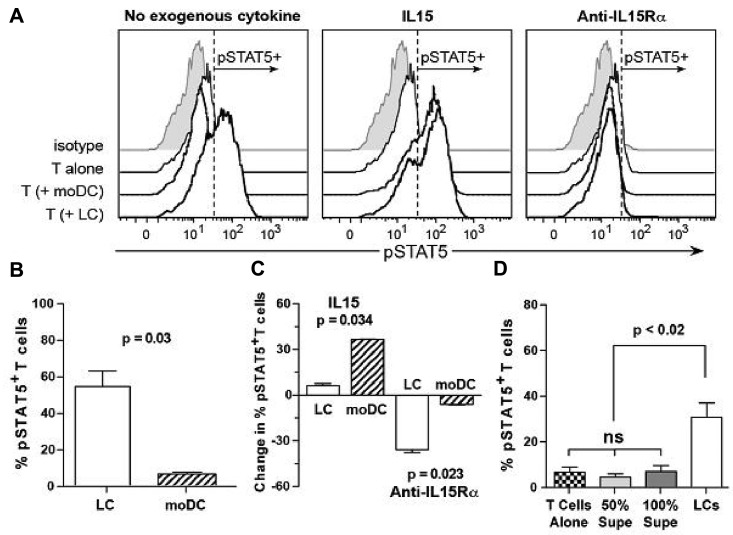
We therefore required a reporter tool for an IL-15–dependent early T-cell activation event. Hence, we assessed phosphorylation of STAT5, which results from signaling by the IL-15–heterotrimeric IL-15R complex via JAK3.35 We also used influenza as a positive recall antigen to avoid any impediments to priming against a self-differentiation tumor antigen. T cells were first stimulated for 6 to 7 days by CD34+ HPC-derived LCs or moDCs presenting fluMP,9 then washed, resuspended, and rested for 1 to 2 hours. The absence of phosphorylated STAT5 was confirmed, after which T cells were restimulated for 30 minutes with fresh, mature LCs or moDCs, each pulsed de novo with fluMP, in the presence or absence of exogenous rhuIL-15 or anti–IL-15R-α. LCs stimulated very strong phosphorylation of STAT5, with no biologic advantage conferred by exogenous rhuIL-15 (Figure 3A-C). This contrasted with moDCs, which had an absolute requirement for exogenous rhuIL-15 to stimulate a comparably robust pSTAT5 response (Figure 3A-C). Anti–IL-15R-α completely inhibited phosphorylation of STAT5, whether IL-15 was provided endogenously by LCs or exogenously to moDCs (Figure 3A). Figure 3C shows the percentage increase or decrease in fluMP-reactive T cells with phosphorylated STAT5 after restimulation by LCs or moDCs in the presence of either exogenous IL-15 or anti–IL-15R-α, relative to restimulation by LCs or moDCs alone. These experiments showed that LCs produced enough IL-15 that the biologic advantage conferred by exogenous IL-15 was limited to and significantly greater for moDCs, which depended on this supplemental IL-15 to stimulate pSTAT5 in fluMP-reactive T cells. Conversely, the effect of anti–IL-15R-α was significantly greater on LCs, because of their endogenous IL-15 production, than it was on moDCs. Finally, to prove the necessity for the complete LC membrane-bound IL-15R-α/IL-15 complex and to exclude the possibility of soluble complexes mediating the observed effects, we found that neither 50% nor 100% volume/volume supernatants from activated and matured LCs reproduced the phosphorylation of STAT5 in T cells stimulated by LCs themselves (Figure 3D).
Figure 3.
CD34+ HPC-derived LCs provide a more potent costimulatory cytokine environment through endogenous IL-15 for CTL activation than do moDCs. (A) Cytokine-generated CD34+ HPC-derived LCs or moDCs were pulsed with fluMP and recultured with purified autologous T cells already primed against fluMP (responder/stimulator ratio = 10:1). Cytokine or receptor blocking conditions are shown above each panel. Stimulation was stopped after 30 minutes at 37°C, and the proportion of T cells that had phosphorylated STAT5 was measured by flow cytometry. One representative experiment of 3 is shown. (B) Using the gating for pSTAT5 in panel A, the means from 3 independent experiments ± SEM are shown after stimulation by LCs or moDCs alone (P = .03; unpaired Student t test). (C) Again, using the pSTAT5 gating in panel A, the change in percentage of pSTAT5+ fluMP-reactive T cells, stimulated by either LCs or moDCs in the presence of IL-15 or anti–IL-15R-α, relative to stimulation by LCs or moDCs alone, is shown (n = 3 independent experiments each, mean ± SEM, P = .034 for the supplemental IL-15 condition, and P = .023 for the anti–IL-15R-α condition, comparing LCs vs moDCs by the unpaired Student t test). (D) The mean percentage ± SEM of antigen-nonspecific, rested conA T lymphoblasts that expressed pSTAT5 after restimulation by autologous LCs (n = 4 independent experiments) or 50% or 100% volume/volume LC-free supernatants (n = 5 independent experiments) was determined by cytofluororaphy using the same gating strategy as in panel A. The paired t test yielded P < .02 for the intact LC stimulation versus either concentration of LC-free supernatants, which were not significantly different from the negative control, T cells alone. ns indicates not significant.
LCs, but not moDCs, stimulate WT1-reactive CTLs in the absence of exogenous rhuIL-15
We further challenged our model by comparing the capacity of CD34+ HPC-derived LCs with moDCs to stimulate CTLs in vitro from healthy volunteers against WT1. This is a strongly conserved self-differentiation antigen shared by many tumors, toward which healthy persons should be tolerant.
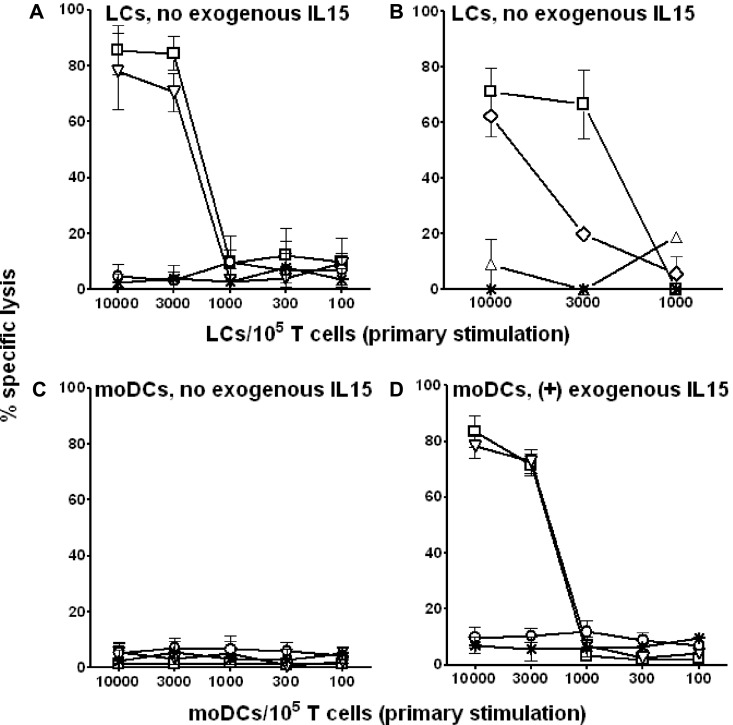
LCs and moDCs underwent electroporation with WT1 mRNA to achieve sustained Ag presentation of epitopes restricted to both class I and II MHC.36 We nevertheless limited these experiments to persons expressing HLA-A*0201 to allow confirmation of class I MHC restriction of the resulting CTL reactivity. After primary stimulation in vitro without exogenous rhuIL-15 for only 7 days, WT1 mRNA-electroporated LCs demonstrated potent stimulation of autologous HLA-A*0201–restricted CTLs from healthy donors against WT1-expressing tumor cell lines or against WT1− tumor cell lines bearing the immunodominant HLA-A*0201–restricted WT1 peptide (Figure 4A). Importantly, these CTLs also lysed primary WT1-positive blasts from HLA-A*0201–positive patients with acute myeloid leukemia (AML; Figure 4B). IL-15/IL-15R-α interaction was critical to LC stimulation of WT1-specific CTL because blocking LCs with anti–IL-15R-α during the 7-day priming of T-cell responders completely abrogated lysis of a WT1+ HLA-A*0201+ control target (P = .001; Figure 4B). In contrast to LCs, WT1 mRNA-electroporated moDCs, derived from the same persons from whom LCs had been generated, did not stimulate any WT1-specific CTLs without exogenous IL-15 (Figure 4C). Supplementary IL-15, however, supported stimulation of cytolysis, which closely approximated that stimulated by LCs providing endogenous IL-15R-α/IL-15 (Figure 4D).
Figure 4.
In the absence of exogenous rhuIL-15, only WT1 mRNA-electroporated CD34+ HPC-derived LCs stimulate primary WT1-specific CTLs in vitro that kill both immortalized and primary tumor cells. Cytokine-generated, CD34+ HPC-derived LCs (A-B) and moDCs (C-D) from the same healthy, HLA-A*0201+ donors were electroporated with WT1 mRNA, matured by a combination of inflammatory cytokines, and then added in serial doses to triplicate microwells containing 1 × 105 autologous T cells. Exogenous rhuIL-15 (10 ng/mL) was added to the primary cultures stimulated by moDCs only in panel D. A total of 5 × 103 51Cr-labeled target cells were added directly to the primary culture microwells after only 7 days stimulation. 51Cr release was measured in the supernatants collected after 6 hours in panels A, C, and D. WT1+ primary AML blasts from patients expressing HLA-A*0201 were labeled with PKH-26 and evaluated as targets of CTLs stimulated by LCs in the absence of exogenous rhuIL-15 in panel B. These response assessments used a flow cytometry-based assay whereby lysed targets took up an otherwise membrane impermeable DNA stain, TO-PRO-3. Specific lysis was based on the frequency of PKH-26+ TO-PRO3+ relative to PKH-26+ TO-PRO3− events. With either 51Cr or colorimetric labeling, specific lysis has been plotted against the y-axes with respect to the conditions of primary stimulation shown along the x-axes. The cytolytic activity generated per primary culture condition could then be compared between LC (A) and moDC stimulators, the latter without (C) or with (D) exogenous rhuIL-15 supplementation. (A,C-D) Data points are the averages ± SEM of triplicate means from each of 3 independent experiments using 3 different healthy donors. (A,C-D) Target cells were 697 cells (□, HLA-A*0201+, WT1+ cell line); SKLY-16 cells pulsed with HLA-A*0201-restricted WT1 peptide (▿; HLA-A*0201+, WT1− cell line); unpulsed SKLY-16 cells (O; HLA-A*0201+, WT1− cell line); and LCL721.221 cells (*; MHC class I-negative, NK cell sensitive cell line). (B) One representative experiment of 3 independent experiments performed where 697 cells were targeted by T cells stimulated by LCs, blocked (▵) or not (□) by anti–IL-15R-α during T-cell priming. Primary AML blasts (♢; HLA-A*0201+, WT1+) proved susceptible, whereas NK cell-sensitive LCL721.221 cells (*) proved resistant to CTLs stimulated by LCs in the absence of blocking anti–IL-15R-α. *P < .01; **P < .001 for pairwise comparisons between either 697 or SKLY-16 WT1-pulsed cells versus LCL721.221 at the top 2 stimulator doses (A,D). **P < .001 for pairwise comparisons between 697 cells targeted by T cells primed by LCs blocked or not with anti–IL-15R-α, and for AML blasts versus LCL721.221, also at the top 2 stimulator doses (B).
Discussion
These results demonstrate that CD34+ HPC-derived LCs use an endogenous IL-15R-α/IL-15–mediated mechanism, together with effective presentation of a diverse array of antigenic peptides from electroporated WT1 mRNA, to prime potent CTLs and break tolerance against this self-differentiation tumor antigen. Importantly, these CTLs lyse not only tumor cell lines bearing WT1, but also WT1-positive leukemic blasts from patients. LC activation of T cells leads to IL-15R-α/IL-15–mediated intracellular signaling involving phosphorylation of STAT5 in the responder T cells. This is in complete contrast with moDCs, which synthesize significantly lower levels of IL-15R-α and have an absolute requirement for exogenous IL-15 to phosphorylate STAT5 or attain CTL stimulatory capacity approximating that of LCs.
CD34+ HPC-derived LCs express the highest levels of mRNA and protein for the IL-15R-α-receptor, compared with all other conventional DC subtypes generated with cytokines in vitro. Although our methods could not determine the amount of receptor protein on the cytoplasmic versus external side of the cell membrane, previous evaluations by flow cytometry of nonpermeabilized cells using the same reagents had detected surface IL-15R-α on moDCs but not LCs,15 thus indicating that LCs must reserve most or all of their IL-15R-α intracellularly. This, together with their large pool of mRNA for IL-15R-α, should afford LCs great flexibility in response to inflammation for rapid translation of the functional receptor protein and optimal transport of bound IL-15 to the cell surface and immune synapses with responder lymphocytes. Digital imaging by fluorescent microscopy supports this conclusion, based on the much higher expression of IL-15R-α by CD34+ HPC-derived LCs exposed to inflammatory cytokines, compared with resident epidermal LC émigrés from healthy skin. It is nevertheless important to note that, apart from inflammatory maturation in vitro, the phenotype of CD34+ HPC-derived LCs, generated under culture conditions similar to our own, corresponds closely to LCs isolated from human epidermis,23 but not to so-called monocyte-derived LCs.24,25 Until investigators develop the means to target LCs in situ and selectively activate their migration to and stimulatory capacity in draining lymph nodes, however, well-defined LCs generated in vitro remain suitable candidates for cell-based vaccines. moDCs express significantly less IL-15R-α, even after inflammatory maturation, but seem capable of stimulating nearly comparable cytolysis, at least in vitro, as long as exogenous IL-15 is provided.
The critical role of IL-15R-α/IL-15 for effective stimulation of potent antitumor CTLs in vitro finally helps explain the superiority of human LCs over moDCs. IL-15 promotes high-avidity CTLs,37 mitigates CD8+ CTL apoptosis,14,38 and counters IL-2–induced Treg development.14 These have useful applications to the induction of cellular immunity against self-differentiation tumor antigens, toward which tolerance otherwise exists. Other common γ-chain cytokines, such as IL-2 and IL-7, can also signal through JAK3 and lead to pSTAT5. Reports in the literature support the production of these and other cytokines by DCs and their subsets, but there is great variation depending on the cytokine conditions and type of serum exposure in culture.
Invoking a significant role for IL-2 in stimulating CTLs and breaking tolerance against a self-differentiation Ag, such as WT1, however, would contradict the well-established and contrasting functions of IL-2 with IL-15, in terms of IL-2's promoting tolerance and countering autoimmunity by expansion of Tregs and induction of apoptosis in activated T cells.17 IL-7 is also unlikely to play a major role here given the cytokine-mediated down-regulation of IL-7R-α on antigen-reactive T cells.39,40 The more compelling unknowns are the transcription factors activated and genes expressed, which link enhanced cytotoxicity with antigen presentation by LCs in the context of IL-15 signaling through pSTAT5. Indeed, such molecular mechanisms are keys to understanding the relative affinities of different cytokines for different JAK-STAT pathways and how IL-15 and other cytokines that signal through a common, proximal JAK3-STAT5 pathway mediate such distinct outcomes in responder lymphocytes.
mRNA electroporation, which facilitates processing and presentation of multiple class I and II MHC-restricted epitopes from the translated protein, also provides a significant advance over loading single peptides on DCs.14 Furthermore, although our studies used HLA-A*0201–expressing individuals to prove MHC restriction of the stimulated T-cell responses, mRNA electroporation allows individuals of any HLA type to process and present peptides tailored to their own MHC molecules.
Our data do not exclude a possible role for moDC-based vaccines supplemented with exogenous rhuIL-15. This obligatory requirement for IL-15, however, may in part account for the suboptimal results in many vaccine trials using moDCs in the absence of adequate IL-15. On the other hand, there is a report of successful remission induction of AML after vaccination with moDCs electroporated with WT1 mRNA.41 LCs were not evaluated or compared with moDCs in this trial. Most intriguing, however, is that clinical responses correlated with elevated levels of activated NK cells, given that myeloid leukemias are especially sensitive to NK cell-mediated lysis.42,43 Only moDCs, by provision of IL-12p70, can stimulate NK cell reactivity.15 LCs secrete no IL-12p70 but support NK cell viability via IL-15.9,15
The logistics are simpler to generate moDCs instead of LCs from their respective precursors using recombinant cytokines in vitro. Human clinical trials of rhuIL-15 are also beginning to evaluate the effect of this cytokine administered directly in vivo (#NCT01021059) or used in the production of peptide-loaded DC vaccines (#NCT01189383). Before investigators embrace either LCs, which provide endogenous IL-15, or moDCs supplemented by or generated in the presence of rhuIL-15,19 an improved understanding of the molecular mechanisms linking IL-15 signaling via pSTAT5 to the development of cytolysis by CD34+ HPC-derived LCs is warranted.
At this juncture, however, our data conclusively establish that CD34+ HPC-derived LCs synthesize significantly greater amounts of IL-15R-α mRNA and protein, as well as IL-15 itself,9,15 compared with other conventional DCs. LCs also provide endogenous IL-15R-α/IL-15, which induces transcriptional events mediated through the JAK3/STAT5 pathway in responding effector lymphocytes. moDCs achieve this only after provision of exogenous rhuIL-15. The impressive CTL stimulatory activity of human LCs, electroporated with mRNA for sustained presentation of a self-differentiation tumor antigen like WT1, should therefore undergo further investigation in early phase clinical trials. These could include either active immunization in vivo or adoptive transfer of tumor antigen-specific CTLs expanded in vitro. Investigators could also compare LC-based vaccines with rhuIL-15–supplemented moDC vaccines or use the two in combination to elicit both adaptive and innate cellular immunity, especially against hematologic malignancies expressing WT1.
Acknowledgments
The authors thank the MSKCC physicians and nurses for support and assistance, the staff of the Blood Bank Donor Room and Cytotherapy Laboratory in obtaining blood and stem cell samples, and the other members of the J.W.Y. laboratory for intellectual input.
This work was supported by the National Cancer Institute, National Institutes of Health (R01-CA083070, R01-CA118974, and R21-CA119528, J.W.Y.; P01-CA23766, J.W.Y., G.H.; U54-CA143798 and R01-AI083408, G.A.-B.; and R01-CA108609, C.M.), the Portuguese GABBA Graduate Student Program of the University of Porto (R.B.d.S.), Swim Across America (F.A. and J.W.Y.), and Mr William H. Goodwin and Mrs Alice Goodwin of the Commonwealth Cancer Foundation for Research and the Experimental Therapeutics Center of MSKCC (J.W.Y.).
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: E.R. and J.W.Y. designed, planned, and performed the experiments and data analyses and wrote the manuscript; E.R. and J.W.C. designed and performed the pSTAT5 experiments; E.T.S.A. and M.J.F. assisted in designing and performing the pSTAT5 experiments; G.A.-B. provided oversight and analysis in the design and performance of pSTAT5 experiments; F.A. assisted with the RT-PCR studies; E.R. and R.B.d.S. performed the immunofluorescence microscopy studies; C.M. provided oversight with the immunofluorescence microscopy studies; B.M. provided human epidermis samples and assisted with their analysis; B.C.B., D.J.C., E.T.S.A., and M.J.F. isolated and evaluated LCs from normal human epidermis; G.H. provided statistical design and analysis; B.C.B. and D.J.C. provided editorial assistance; and J.W.Y. rewrote and revised the final versions of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for E.R. is Multidisciplinary Oncology Center, University Hospital of Lausanne, Lausanne, Switzerland. The current affiliation for R.B.d.S. and C.M. is Viral Immunobiology, Institute of Experimental Immunology, University of Zürich, Zürich, Switzerland. The current affiliation for B.C.B. is Department of Blood & Marrow Transplantation, H. Lee Moffitt Cancer Center, University of South Florida, Tampa, FL.
Correspondence: James W. Young, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, New York, NY 10065; e-mail: youngjw@mskcc.org.
References
- 1.Palucka K, Banchereau J, Mellman I. Designing vaccines based on biology of human dendritic cell subsets. Immunity. 2010;33(4):464–478. doi: 10.1016/j.immuni.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Merad M, Ginhoux F, Collin M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat Rev Immunol. 2008;8(12):935–947. doi: 10.1038/nri2455. [DOI] [PubMed] [Google Scholar]
- 3.Merad M, Manz MG. Dendritic cell homeostasis. Blood. 2009;113(15):3418–3427. doi: 10.1182/blood-2008-12-180646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steinman RM. Lasker Basic Medical Research Award. Dendritic cells: versatile controllers of the immune system. Nat Med. 2007;13(10):1155–1159. doi: 10.1038/nm1643. [DOI] [PubMed] [Google Scholar]
- 5.Rossi M, Young JW. Human dendritic cells: potent antigen-presenting cells at the crossroads of innate and adaptive immunity. J Immunol. 2005;175(3):1373–1381. doi: 10.4049/jimmunol.175.3.1373. [DOI] [PubMed] [Google Scholar]
- 6.Szabolcs P, Avigan D, Gezelter S, et al. Dendritic cells and macrophages can mature independently from a human bone marrow-derived, post-colony-forming unit intermediate. Blood. 1996;87(11):4520–4530. [PubMed] [Google Scholar]
- 7.Caux C, Vanbervliet B, Massacrier C, et al. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to GM-CSF+TNF alpha. J Exp Med. 1996;184(2):695–706. doi: 10.1084/jem.184.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caux C, Massacrier C, Vanbervliet B, et al. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to granulocyte-macrophage colony-stimulating factor plus tumor necrosis factor alpha: II. Functional analysis. Blood. 1997;90:1458–1470. [PubMed] [Google Scholar]
- 9.Ratzinger G, Baggers J, de Cos MA, et al. Mature human Langerhans cells derived from CD34+ hematopoietic progenitors stimulate greater cytolytic T lymphocyte activity in the absence of bioactive IL-12p70, by either single peptide presentation or cross-priming, than do dermal-interstitial or monocyte-derived dendritic cells. J Immunol. 2004;173:2780–2791. doi: 10.4049/jimmunol.173.4.2780. (Erratum in J Immunol. 2005;2174:3818.) [DOI] [PubMed] [Google Scholar]
- 10.Bogunovic M, Ginhoux F, Wagers A, et al. Identification of a radio-resistant and cycling dermal dendritic cell population in mice and men. J Exp Med. 2006;203(12):2627–2638. doi: 10.1084/jem.20060667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klechevsky E, Morita R, Liu M, et al. Functional specializations of human epidermal Langerhans cells and CD14+ dermal dendritic cells. Immunity. 2008;29(3):497–510. doi: 10.1016/j.immuni.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haniffa M, Ginhoux F, Wang XN, et al. Differential rates of replacement of human dermal dendritic cells and macrophages during hematopoietic stem cell transplantation. J Exp Med. 2009;206(2):371–385. doi: 10.1084/jem.20081633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banchereau J, Palucka AK, Dhodapkar M, et al. Immune and clinical responses in patients with metastatic melanoma to CD34(+) progenitor-derived dendritic cell vaccine. Cancer Res. 2001;61(17):6451–6458. [PubMed] [Google Scholar]
- 14.Romano E, Rossi M, Ratzinger G, et al. Peptide-loaded Langerhans cells, despite increased IL-15 secretion and T-cell activation in vitro, elicit anti-tumor T-cell responses comparable to peptide-loaded monocyted-derived dendritic cells in vivo. Clin Cancer Res. 2011;17(7):1984–1997. doi: 10.1158/1078-0432.CCR-10-3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Munz C, Dao T, Ferlazzo G, de Cos MA, Goodman K, Young JW. Mature myeloid dendritic cell subsets have distinct roles for activation and viability of circulating human natural killer cells. Blood. 2005;105(1):266–273. doi: 10.1182/blood-2004-06-2492. [DOI] [PubMed] [Google Scholar]
- 16.Lodolce JP, Burkett PR, Boone DL, Chien M, Ma A. T cell-independent interleukin 15R-alpha signals are required for bystander proliferation. J Exp Med. 2001;194(8):1187–1194. doi: 10.1084/jem.194.8.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6(8):595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- 18.Mortier E, Woo T, Advincula R, Gozalo S, Ma A. IL-15R-alpha chaperones IL-15 to stable dendritic cell membrane complexes that activate NK cells via trans presentation. J Exp Med. 2008;205(5):1213–1225. doi: 10.1084/jem.20071913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohamadzadeh M, Berard F, Essert G, et al. Interleukin 15 skews monocyte differentiation into dendritic cells with features of Langerhans cells. J Exp Med. 2001;194(7):1013–1020. doi: 10.1084/jem.194.7.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dubsky P, Saito H, Leogier M, et al. IL-15-induced human DC efficiently prime melanoma-specific naive CD8+ T cells to differentiate into CTL. Eur J Immunol. 2007;37(6):1678–1690. doi: 10.1002/eji.200636329. [DOI] [PubMed] [Google Scholar]
- 21.Witmer-Pack MD, Olivier W, Valinsky J, Schuler G, Steinman RM. Granulocyte/macrophage colony-stimulating factor is essential for the viability and function of cultured murine epidermal Langerhans cells. J Exp Med. 1987;166:1484–1498. doi: 10.1084/jem.166.5.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ratzinger G, Baggers J, de Cos MA, et al. Mature human Langerhans cells derived from CD34+ hematopoietic progenitors stimulate greater cytolytic T lymphocyte activity in the absence of bioactive IL-12p70, by either single peptide presentation or cross-priming, than do dermal-interstitial or monocyte-derived dendritic cells [erratum]. J Immunol. 2005;174(6):3818. doi: 10.4049/jimmunol.173.4.2780. [DOI] [PubMed] [Google Scholar]
- 23.Eisenwort G, Jurkin J, Yasmin N, Bauer T, Gesslbauer B, Strobl H. Identification of TROP2 (TACSTD2), an EpCAM-like molecule, as a specific marker for TGF-[beta]1-dependent human epidermal Langerhans cells. J Invest Dermatol. 2011;131(10):2049–2057. doi: 10.1038/jid.2011.164. [DOI] [PubMed] [Google Scholar]
- 24.Geissmann F, Prost C, Monnet J-P, Dy M, Brousse N, Hermine O. Transforming growth factor beta 1, in the presence of granulocyte/macrophage colony-stimulating factor and interleukin 4, induces differentiation of human peripheral blood monocytes into dendritic Langerhans cells. J Exp Med. 1998;187:961–966. doi: 10.1084/jem.187.6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Aar AMG, Sylva-Steenland RMR, Bos JD, Kapsenberg ML, de Jong EC, Teunissen MBM. Cutting edge: loss of TLR2, TLR4, and TLR5 on Langerhans cells abolishes bacterial recognition. J Immunol. 2007;178(4):1986–1990. doi: 10.4049/jimmunol.178.4.1986. [DOI] [PubMed] [Google Scholar]
- 26.de Saint-Vis B, Fugier-Vivier I, Massacrier C, et al. The cytokine profile expressed by human dendritic cells is dependent on cell subtype and mode of activation. J Immunol. 1998;160:1666–1676. [PubMed] [Google Scholar]
- 27.Maecker HT, Moon J, Bhatia S, et al. Impact of cryopreservation on tetramer, cytokine flow cytometry, and ELISPOT. BMC Immunol. 2005;6:17. doi: 10.1186/1471-2172-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pinilla-Ibarz J, May RJ, Korontsvit T, et al. Improved human T-cell responses against synthetic HLA-0201 analog peptides derived from the WT1 oncoprotein. Leukemia. 2006;20(11):2025–2033. doi: 10.1038/sj.leu.2404380. [DOI] [PubMed] [Google Scholar]
- 29.Brilot F, Strowig T, Roberts SM, Arrey F, Munz C. NK cell survival mediated through the regulatory synapse with human DCs requires IL-15R-alpha. J Clin Invest. 2007;117(11):3316–3329. doi: 10.1172/JCI31751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jonuleit H, Kuhn U, Muller G, et al. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur J Immunol. 1997;27:3135–3142. doi: 10.1002/eji.1830271209. [DOI] [PubMed] [Google Scholar]
- 31.Van Tendeloo VF, Ponsaerts P, Lardon F, et al. Highly efficient gene delivery by mRNA electroporation in human hematopoietic cells: superiority to lipofection and passive pulsing of mRNA and to electroporation of plasmid cDNA for tumor antigen loading of dendritic cells. Blood. 2001;98(1):49–56. doi: 10.1182/blood.v98.1.49. [DOI] [PubMed] [Google Scholar]
- 32.Su Z, Dannull J, Yang BK, et al. Telomerase mRNA-transfected dendritic cells stimulate antigen-specific CD8+ and CD4+ T cell responses in patients with metastatic prostate cancer. J Immunol. 2005;174(6):3798–3807. doi: 10.4049/jimmunol.174.6.3798. [DOI] [PubMed] [Google Scholar]
- 33.Boublikova L, Kalinova M, Ryan J, et al. Wilms' tumor gene 1 (WT1) expression in childhood acute lymphoblastic leukemia: a wide range of WT1 expression levels, its impact on prognosis and minimal residual disease monitoring. Leukemia. 2006;20(2):254–263. doi: 10.1038/sj.leu.2404047. [DOI] [PubMed] [Google Scholar]
- 34.Ferlazzo G, Pack M, Thomas D, et al. Distinct roles of IL-12 and IL-15 in human natural killer cell activation by dendritic cells from secondary lymphoid organs. Proc Natl Acad Sci U S A. 2004;101(47):16606–16611. doi: 10.1073/pnas.0407522101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghoreschi K, Laurence A, O'Shea JJ. Janus kinases in immune cell signaling. Immunol Rev. 2009;228(1):273–287. doi: 10.1111/j.1600-065X.2008.00754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Su Z, Dannull J, Heiser A, et al. Immunological and clinical responses in metastatic renal cancer patients vaccinated with tumor RNA-transfected dendritic cells. Cancer Res. 2003;63(9):2127–2133. [PubMed] [Google Scholar]
- 37.Oh S, Perera LP, Burke DS, Waldmann TA, Berzofsky JA. IL-15/IL-15R-alpha-mediated avidity maturation of memory CD8+ T cells. Proc Natl Acad Sci U S A. 2004;101(42):15154–15159. doi: 10.1073/pnas.0406649101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oh S, Perera LP, Terabe M, Ni L, Waldmann TA, Berzofsky JA. IL-15 as a mediator of CD4+ help for CD8+ T cell longevity and avoidance of TRAIL-mediated apoptosis. Proc Natl Acad Sci U S A. 2008;105(13):5201–5206. doi: 10.1073/pnas.0801003105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vera JF, Hoyos V, Savoldo B, et al. Genetic manipulation of tumor-specific cytotoxic T lymphocytes to restore responsiveness to IL-7. Mol Ther. 2009;17(5):880–888. doi: 10.1038/mt.2009.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park J-H, Yu Q, Erman B, et al. Suppression of IL-7Ralpha transcription by IL-7 and other prosurvival cytokines: a novel mechanism for maximizing IL-7-dependent T cell survival. Immunity. 2004;21(2):289–302. doi: 10.1016/j.immuni.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 41.Van Tendeloo VF, Van de Velde A, Van Driessche A, et al. Induction of complete and molecular remissions in acute myeloid leukemia by Wilms' tumor 1 antigen-targeted dendritic cell vaccination. Proc Natl Acad Sci U S A. 2010;107(31):13824–13829. doi: 10.1073/pnas.1008051107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller JS, Soignier Y, Panoskaltsis-Mortari A, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105(8):3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 43.Hsu KC, Keever-Taylor CA, Wilton A, et al. Improved outcome in HLA-identical sibling hematopoietic stem-cell transplantation for acute myelogenous leukemia predicted by KIR and HLA genotypes. Blood. 2005;105(12):4878–4884. doi: 10.1182/blood-2004-12-4825. [DOI] [PMC free article] [PubMed] [Google Scholar]