Abstract
Disparities in cancer burden by race/ethnicity have been reported, primarily in adults with cancer. However, there appear to be gaps in the pediatric oncology literature with regards to a comprehensive overview on this topic. Extant literature is used to highlight the results of studies focusing on racial and ethnic disparities in outcome observed in selected childhood cancers. A comprehensive approach is utilized to understand possible underlying causes of disparities in cancer outcomes, and to highlight the gaps that currently exist. This review helps define areas of future research that could help develop targeted, disease-specific approaches to eliminate the disparities.
Keywords: Childhood Cancer, Health Disparities, Survival, Survivorship
INTRODUCTION
Disparities in cancer burden by race/ethnicity and socioeconomic status exist in adults with cancer.1,2 Factors such as poverty, inadequate education, and lack of health insurance appear to be as important as biological differences. Practically speaking, elimination of disparities is defined as reduction in cancer mortality and improvement in survival among the disadvantaged. In 2003, the Institute of Medicine published a comprehensive review of racial and ethnic disparities in healthcare.3 Their summary indicates that income, education, and health insurance coverage influence access to appropriate care, impacting early detection, treatment and palliative care. Furthermore, social inequities, such as the legacy of racial discrimination in the United States, can still influence the interactions between patients and physicians.3 Finally, cultural factors may play a role in health behaviors, attitudes toward illness, and belief in modern medicine versus alternative forms of healing.1,2,4 The US Department of Health and Human Services Healthy People 2010 Initiative has committed the nation to the goal of eliminating health disparities,5 and elimination of disparities in cancer burden is one of the overarching themes of the American Cancer Society 2015 goals.6 However, while disparities in chronic diseases burden (including cancer) have been extensively studied in the adult population, there appear to be gaps in the pediatric oncology literature with regards to a comprehensive overview on this topic.
For the pediatric population, survival outcomes by race and ethnicity have been described more extensively for hematological malignancies, than for non-hematologic solid tumors. The published studies have utilized population-based public datasets, such as Surveillance Epidemiology and End Results (SEER), data from cooperative group clinical trials as well as trials conducted at single institutions; each of these sources carries its own strengths and limitations. Thus, while the SEER Program allows access to population-based data, it lacks clinical and therapeutic details. Single institution studies prevent the ability to generalize the findings. Cooperative group trials overcome the limitations of the population-based and single-institution studies, because of the availability of details regarding therapeutic exposures and disease characteristics, as well as the ability to generalize the findings from cooperative group trials.
Survival trends for primary cancers in children and adolescents, birth to 19 years, evaluated using SEER Program data demonstrate that while the 5-year survival rates have increased significantly overall from 63% (1975-1979) to 79% (1995-1999) (p<0.0001), Hispanic and black children and adolescents have poorer 5-year survival rates than their non-Hispanic white counterparts (74% and 73% vs. 81%, respectively, p<0.0001).7
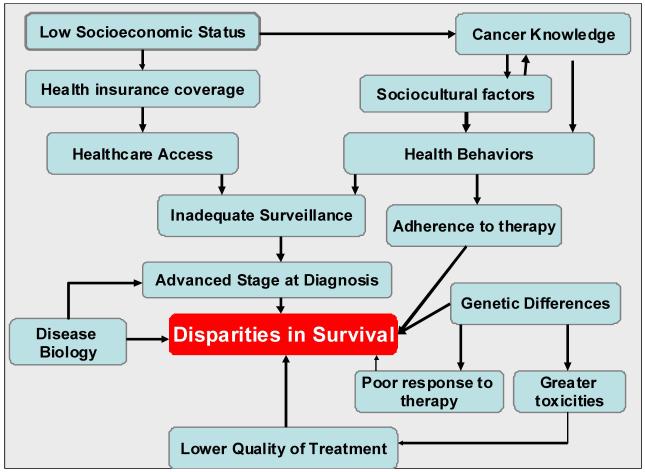
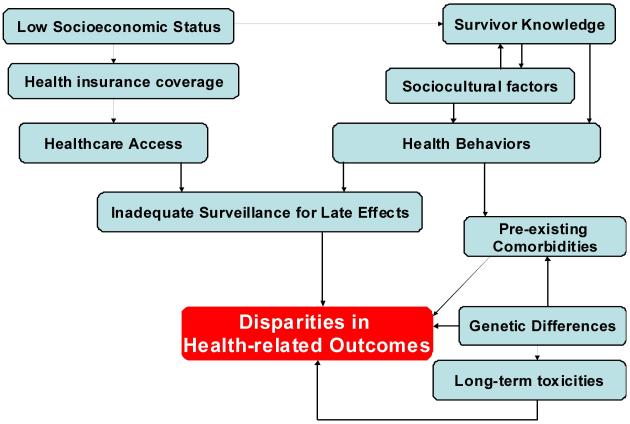
This review focuses on the racial and ethnic disparities in outcome observed in selected childhood cancers (acute lymphoblastic leukemia [ALL], acute myeloid leukemia [AML], Hodgkin lymphoma [HL], neuroblastoma [NBL], central nervous system [CNS] tumors, and rhabdomyosarcoma [RMS]). The author presents models to describe possible causes of disparities in disease-free survival (Figure 1) as well as long-term outcomes (Figure 2) in children with cancer, using extant data to support the causes postulated in the models.
Figure 1.
Potential Causes of Disparities in Disease-Free Survival in Patients with Newly Diagnosed Cancer.
Figure 2.
Potential Causes of Disparities in Health-related Outcome in Long-term Survivors of Childhood Cancer.
Acute Lymphoblastic Leukemia
Several groups have published reports on ethnic and racial differences in survival after childhood ALL (Table I). Reports include population-based cohorts from the SEER Program, patients treated on therapeutic protocols run by cooperative groups, and single-institution studies. In general, these studies report poorer outcomes experienced by black children compared with whites,8-10 with the exception of one single-center study demonstrating no racial difference in survival in children treated with contemporary multimodality therapy.11
Table 1.
Survival in Children and Adolescents with Cancer by Race and Ethnicity.
| Study | Cohort size | Whites | Blacks | Hispanics | Asians |
|---|---|---|---|---|---|
| Acute lymphoblastic leukemia | |||||
| SEER database 5-year overall survival8 |
4,952 | 84% | 75% | 72% | 81% |
| Cooperative group 5-year EFS9 |
8,447 | 72.8%±0.6% | 61.5%±2.2% | 65.9%±1.5% | 75.1%±3.5% |
| Cooperative group 5-year overall survival10 |
5,086 | 81.9% 0.6% | 68.6% 2.1% | 74.9% 2% | – |
| Single institution 5-year EFS11 |
412 | 79.4% (74.7-84.1) | 80.7% (70.3-91.1) | — | — |
| Acute myeloid leukemia | |||||
| Cooperative group 5-year overall survival15 |
791 | 48%±4% | 34%±10% | 37%±9% | – |
| Cooperative group 5-year overall survival15 |
850 | 60%±4% | 45%±12% | 51%±8% | — |
| Single institution 5-year EFS16 |
287 | 31.2%± 3.5% | 29.3%±6.4% | — | — |
| Hodgkin lymphoma | |||||
| Single institution 5-year EFS18 |
327 | 84%±2.4% | 71%±6.1% | — | — |
| Rhabdomyosarcoma | |||||
| Cooperative group 5-year EFS19 |
2057 | 66% | 61% | ||
| Neuroblastoma | |||||
| Cooperative group 5-year EFS26 |
3,539 | 67% (65%-69%) | 56% (50%-625) | 69% (63%-74%) | 62% (51%-71%) |
Population-based
Cases were identified from the SEER Program, and included 4,952 children diagnosed with ALL between 1973 and 1999 at age 19 years or younger.8 For children diagnosed with ALL between 1990 and 1999, the 5-year survival was 84% for whites, 81% for Asians/Pacific Islanders, 75% for blacks, and 72% for American Indian/Alaskans and Hispanics. After adjusting for era, age at diagnosis, and gender, blacks, Hispanics, and American Indians/Alaskans had hazard ratios of 1.5, 1.8, and 1.9 respectively, compared with whites.
Cooperative group trials
A retrospective study of children placed on Children’s Cancer Group (CCG) therapeutic protocols was undertaken to determine outcomes by racial and ethnic backgrounds of patients treated with contemporary risk-based therapy.9 In total, 8,447 children (white, [n=6703]; Hispanic [n=1,071]; black [n=506], Asian [n=167]) with newly diagnosed ALL between 1983 and 1995 were observed for a median of 6.5 years. 5-year disease-free survival (DFS) differed by race/ethnicity (Asians: 75.1%±3.5%; whites: 72.8%±0.6%; Hispanic: 65.9%±1.5%; and blacks: 61.5%±2.2%, p<0.0001). Remission rates were comparable among the groups (97%-99%), but relapse rates were significantly different, resulting in the observed difference in DFS. After adjusting for age at diagnosis, white count at presentation, and chromosomal abnormalities, blacks and Hispanics had a worse outcome and Asians had a superior outcome, compared to whites. There was an overrepresentation of black and Hispanic patients among the low-parental education and low-income categories. Inclusion of these variables in the multivariable model did not alter the worse outcome for black children when compared with whites, but mitigated the difference between Hispanics and whites. To take into consideration improved survival rates over time, the investigators stratified the cohort into an “early treatment era” (1983 to 1989) and a “recent treatment era” (1990 to 1995). In contrast to a single institution study,12 there continued to be significant survival differences by race/ ethnicity for patients treated in recent treatment era.
A retrospective analysis of 5,086 children (4061 white, 518 black, and 507 Hispanic) placed on Pediatric Oncology Group (POG) therapeutic trials between 1981 and 1994 revealed the following 5-year overall survival rates: whites 81.9%±0.8%; Hispanics: 74.9±2.0%; blacks: 68.6±2.1%.10 After adjusting for age at diagnosis, white cell count at presentation, gender, era of treatment, and blast ploidy, blacks had 42% excess mortality, and Hispanics 33% excess mortality compared with whites.
Single institution studies
Pui et al. conducted a retrospective analysis in patients treated on therapeutic protocols at a single institution.12 Analysis was stratified by treatment era, defined by time points at which significantly improved outcome was demonstrated for specific tumor types. During the early treatment era, a significant difference was seen for ALL, with 10-year event-free survival (EFS) of 34% for blacks and 57% for whites. However, during the recent treatment era, there were no significant differences in treatment outcomes by race (5-year EFS: whites: 80%; blacks: 77%). The authors concluded that with equal access to effective contemporary treatment, black children fare as well as white children with protocol-based therapy at a pediatric oncology research center. The investigators then extended the analysis to 412 children (68 blacks, and 338 whites) with newly diagnosed ALL.11 These children were diagnosed between 1991 and 1998 and placed on therapeutic trials, regardless of race/ethnicity, or ability to pay for medical care. There was no difference in the 5-year EFS (whites: 80.6% vs. blacks: 79.4%). Adjustment for high-risk features confirmed the absence of racial effects on DFS.
Thus, reports based on population-based data and cooperative group trials demonstrate a significant difference in outcome by race/ ethnicity. However, single-institution studies fail to do so, a phenomenon that could be explained by the unique characteristics of the single institution in its ability to provide close individual care to patients irrespective of the patients’ ability to pay, consistent with the belief that sociodemographic factors play a role in the observed differences in survival.
Acute myeloid leukemia
Pediatric AML therapy includes intensive chemotherapy and hematopoietic cell transplantation (HCT) for patients in first remission with matched related donors.13 Clinical characteristics such as white cell count at diagnosis and blast cytogenetics only partially explain the heterogeneity in AML outcome.14 Since, pediatric AML therapy occurs primarily in the inpatient setting, the role of adherence in explaining variability is minimized. Thus, pediatric AML therapy is a good clinical model to evaluate the role of disease biology in the ethnic differences in disease outcome. Two studies have examined ethnic differences in survival, a cooperative group study and a single institution study. The details are outlined below.
Cooperative group trials
Aplenc et al. evaluated differences in outcome by race/ ethnicity among 791 children placed on CCG 2891 trial; they subsequently confirmed the findings in 850 children treated on CCG 2961 trial (Table I).15 Hispanics and blacks treated with chemotherapy on CCG 2891 had significantly inferior overall survival (OS) compared with whites (37%±9% vs. 48%±4%, p=0.016; and 34%±10% vs. 48%±4%, p=0.007, respectively). Analyses of CCG 2961 confirmed that blacks had significantly decreased OS rates compared with whites (45%±12% vs. 60%±4%, p=0.007); the difference in OS between Hispanics and whites approached statistical significance (51%±8% vs. 60%±4%, p=0.065). Significantly fewer black children had related donors for both CCG 2891 and 2961 trials. For CCG 2891, patients undergoing allogeneic or autologous HCT had comparable outcomes to those of white children. For CCG 2961, survival was superior in children with available allogeneic donors than in those without such donors for all ethnic groups. In contrast, OS and DFS were inferior in black children without donors than in white children without donors. Multivariable analysis for either CCG 2891 or 2961 did not take into account availability of allogeneic donors.
Single-institution reports
Rubnitz et al. compared clinical characteristics, biological features, and outcomes between 229 whites and 58 blacks with AML treated on consecutive clinical protocols between 1980 and 2002 at a single institution (Table I),16 and observed no statistically significant differences in clinical characteristics, FAB subtypes, cytogenetic features or outcomes. The 5-year survival estimate was 39.2%±3.6% for whites and 33.8%±6.5% for blacks. However, on the most recent trial (AML-97), there was a trend towards inferior outcome among blacks: the 5-year survival estimates were 55.6%±12.3% and 27.3%±13.5% for whites and blacks respectively.
Hodgkin lymphoma
Prognostic factors significantly associated with risk of relapse in children treated for HL include presence of B symptoms, advanced stage of disease, bulky mediastinal mass, low hemoglobin concentration, and high erythrocyte sedimentation rate at diagnosis. Limited information exists regarding the existence of differential survival rates by race and ethnicity. Two studies outline the current reports in literature – a population-based study and a single institution study.
Population-based
Data from SEER between 1975 and 1995 indicate that white children with HL have a higher 5-year survival than do blacks.17 However, this study is limited by the lack of details regarding therapeutic exposures.
Single-institution
Metzger et al. report the results on a retrospective analysis of 327 children and adolescents diagnosed with HL between 1990 and 2005 (Table I).18 Patients were treated with risk-directed multimodal therapy regardless of race, ethnicity, or ability to pay. The 262 whites and 65 blacks did not differ significantly in presenting features, or clinical characteristics. More blacks (71% vs. 45%) resided in poor counties (p<0.001). While blacks and whites were equally likely to have progressive disease or early relapse, blacks were 3.7 times (95% CI, 1.7-8.0) more likely to relapse 12 or more months after diagnosis. 5-year DFS was 71%±6.1% for blacks and 84%±2.4% for whites (p=0.01).
Rhabdomyosarcoma
Survival for children with RMS has improved significantly during the past 30 years, primarily attributable to advances in therapy and supportive care. The Intergroup Rhabdomyosarcoma Study Group (IRSG) designed and conducted four consecutive clinical trials that demonstrated a tripling of survival rates from 25% in 1970 to 75% now. Disease stage, group, age, and histology served as good prognostic factors. Race/ethnicity is another potentially important patient characteristic that may carry prognostic significance, and is detailed below.
Cooperative group trials
Baker et al. conducted a retrospective cohort analysis of patients treated on IRSG protocols between 1984 and 1997 (Table I).19 Clinical features and outcomes of 336 blacks and 286 children from other ethnic minorities were compared with those of 1,721 whites. All racial/ethnic groups enjoyed similar 5-year DFS (blacks: 61%, other ethnic groups: 61% and whites: 66%, p=0.15). Compared with whites, non-white patients more often had invasive T2 tumors (p=0.03), advanced stage tumors (stage 2 or 3, p=0.003), large tumors (>5 cm, p<0.006), and tumors with positive regional nodes (N1, p=0.002). After adjustment for T stage, risk category, and age, the investigators found that compared with whites, the relative risk of failure was 1.14 for blacks, and 1.2 for other ethnic minority patients, values that were not significantly different. Thus, patients from ethnic minority groups more often had larger, invasive tumors with positive lymph nodes. Nevertheless, they have benefited equally as whites from the progress in therapy of RMS.
Neuroblastoma
NBL has remarkable clinical heterogeneity and widely varying survival rates, depending on clinical features and biological characteristics of the tumor. Risk groups have been defined based on these prognostic and clinical biological markers,20,21 and modern treatment strategies are tailored accordingly. This approach has led to substantial improvement in outcome of children with low- and intermediate-risk disease,22-24 as well as for children with high-risk disease; however, long-term survival remains poor for the latter group, at less than 40%.20,25 Differences in survival by race and ethnicity had not been described until recently, and are detailed below.
Cooperative group
Henderson et al. investigated the racial/ethnic differences in clinical and biological risk factors, and outcome of NBL patients enrolled on COG ANBL00B1 between 2001 and 2009 (Table I).26 A total of 3,539 patients (white: 72%; black, 12%; Hispanic 12%; Asian 4%; and Native American <1%) were included. The 3-year OS was 82% (95% CI, 80%-83%) for whites, 82% (77%-86%) for Hispanics, 76% (70%-80%) for blacks, 70% (58%-79%) for Asians and 51% (28%-71%) for Native Americans. Compared with white children, blacks (p<0.001) and Native Americans (p=0.04) had a higher prevalence of high-risk disease, and significantly worse DFS (p=0.01, and 0.002, respectively). Adjustment for risk group abrogated these differences. However, examination of patients with high-risk disease revealed a higher prevalence of late-occurring events (>2 years from diagnosis) among blacks compared with whites (HR=1.5, 95% CI, 1.0-2.3, p=0.04). The authors concluded that the higher prevalence of late-occurring events among blacks with high-risk disease suggests that this population may be more resistant to chemotherapy.
Central Nervous System tumors
Mortality rates among children with CNS tumors exceed those among children with ALL by more than 3-fold. Survival of children after diagnosis with a CNS tumor is highly dependent on age at diagnosis, histological subtype, location of the tumor in the brain, and treatment. Limited data exist regarding the role of race and ethnicity in determining disease outcome.
Population-based studies
Barnholtz-Sloan et al. assessed racial/ethnic differences in survival of children with CNS tumors, focusing on Hispanics, Asians, blacks and whites. Subjects were identified through the SEER Program and included 2,799 children, 19 years of age or younger at diagnosis of primary malignant CNS tumors diagnosed between 1973 and 1996. Racial and ethnic differences in distribution of histological subtypes were observed. OS was similar for Hispanics, blacks, and Asians, when compared with whites.27 Furthermore, the adjusted hazard ratio did not differ by race and ethnicity (Hispanics: HR=0.95, 95% CI, 0.7-1.2; African-Americans: HR=1.02, 95% CI, 0.8-1.2; Asians: HR=1.12, 95% CI, 0.7-1.8; referent group: non-Hispanic whites).
As detailed in the sections above, and summarized in Table I, disparities in outcome exist in the pediatric oncologic population in the United States. There is therefore a critical need to understand the underlying causes of these differences in outcome by race and ethnicity. The detail with which this topic has been explored for a particular disease type varies from fairly extensive for ALL, to minimal for CNS tumors, to non-existent for non-Hodgkin lymphoma and Wilms tumor, bone tumors, and other rare childhood tumors. As discussed above, understanding ethnic/ racial differences in survival in patients placed on cooperative group trials afford an advantage over single institution studies or population-based studies, because of the availability of therapeutic exposures and clinical details for large, geographically and socioeconomically diverse populations treated uniformly. Nonetheless, data presented above allow one to make the following observations: the underlying causes of observed ethnic/racial differences are as diverse as the diagnoses, and, therefore, an attempt at understanding the underlying causes would require a tailored, disease-specific approach to incorporating the factors outlined in this review.
Considerable debate exists on whether race and ethnicity are primarily social or biologic constructs.28 Unlike a biologic category such as sex, racial and ethnic categories are determined through geographic, social, and cultural factors, and, as such are potentially dynamic. Even though these factors are not biologically determined, racial or ethnic groups do differ from each other genetically, with attendant biologic implications. Race, therefore, is a composite term and represents an interaction between genetic or biologic factors, and socioeconomic, sociocultural, and environmental factors that inform, and are in turn informed by, cancer knowledge, health behaviors, and health care access across the entire trajectory of disease. These issues are discussed as they apply to newly diagnosed patients, as well as to long-term cancer survivors.
POSSIBLE REASONS FOR ETHNIC/ RACIAL DIFFERENCES IN DISEASE-FREE SURVIVAL
A clear understanding of the underlying causes of racial/ethnic differences in disease-free survival in children with cancer, could inform a focused and targeted approach to developing interventions to reduce these disparities. Figure 1 proposes a model where disparities in survival arise from a complex interplay of economic, social, cultural, disease biology, and pharmacogenetic factors. The present state of knowledge in the pediatric oncology literature addressing each of these factors is discussed below.
Socioeconomic status (SES) and health insurance coverage
The influence of race and ethnicity on survival appears to be closely linked with SES. Early diagnosis, ready access to quality health care, and sufficient time and energy to maintain adherence with treatment are all closely linked with SES, ethnicity, and survival, as has been demonstrated in studies of mortality in adults with chronic health conditions.29 In the United States, SES continues to be closely linked with race and ethnicity in children with cancer. Thus, black children with ALL were less likely to have private insurance (34% vs. 57%) and more likely to have public insurance (54% vs. 23%),11 and parents of Hispanic and black children were more likely to have received less than high school education (p<0.001), and were less likely to have annual household income exceeding $30,000 (p<0.001).9 While inclusion of SES in the multivariate model did not alter the worse outcome for blacks with ALL, when compared with whites, the difference between Hispanics and whites was mitigated. These examples notwithstanding, studies focusing on the impact of SES on childhood cancer outcomes are few and far between. There is therefore, a critical need to include these variables in reports on outcomes of therapeutic trials.
Access to care
Diagnosis delays
Timely access to quality healthcare is critical in optimizing survival, since it allows an opportunity for appropriate intervention while the disease burden is in its early stages.30 Diagnosis delay can be grouped into three categories, depending on the underlying cause: patient- and/or parent-related, disease-related, and healthcare-related. Factors associated with diagnosis delay include older age at diagnosis, lower parental education, type of cancer (HL, renal tumors, CNS tumors), presentation of symptoms, and the first medical specialty consulted (longer delays for those who visited a family physician rather than a pediatrician). Race/ ethnicity were not associated with diagnosis delays and access to healthcare services.
Enrollment on cooperative group trials
COG, an NCI-funded cooperative group conducts clinical trials for the treatment of childhood cancer. Contemporary, risk-based therapy, offered in the setting of clinical trials developed by COG has played a large role in the success in survival in children with cancer. For the vast majority of cancer diagnoses, children treated at pediatric oncology centers are reported to have a significant survival advantage compared with those treated elsewhere, and children treated on standardized protocols appear to fare better than those who are not.31,32 A valid concern relates to existence of ethnic or racial differences in enrollment on cooperative group trials that could potentially result in the observed disparities in cancer outcome. An earlier study had shown that age-adjusted registration rates were comparable across all racial/ ethnic groups: non-Hispanic whites (59.2%), followed by Asians (58.6%), blacks (57.7%) and Hispanics (54.2%).33 However, a recent study compared the observed proportions of US children enrolled on COG clinical trials from 2000 to 2003 with expected proportions based on SEER data,34 and showed that blacksand Hispanics were under-represented in cooperative group trials. The reason(s) for the observed differences in enrollment are not clear, but need to be explored.
Knowledge about cancer diagnosis, treatment and toxicities
A lack of knowledge regarding cancer diagnosis, treatment, and potential acute toxicities could prevent active participation by the patient/parents in the care of the child with cancer, including delay in diagnosis. While, information is emerging regarding the lack of knowledge among cancer survivors (see sections below), there is limited information in the published literature on this topic in children with newly diagnosed cancer.
Cancer surveillance
Cancer surveillance typically encompasses surveillance for primary cancer recurrence as well as screening for therapy-related second cancers. There are no reports in the literature describing the surveillance patterns for primary cancer recurrence by race/ethnicity.
Risky health behaviors
Similarly, risky health behaviors, such as smoking, binge or heavy drinking, and physical inactivity, can adversely affect survival. Again, there is a paucity of information regarding this topic in the current literature, although the health behaviors of childhood cancer survivors have been examined and are detailed in the sections below.
Disease Biology
Underlying differences in disease biology could play an equally important role in the observed difference in survival by race and ethnicity. This section describes ethnic/ racial differences in disease characteristics at presentation and in cytogenetic characteristics of the malignancy among children with ALL, AML, HL, RMS, and NBL.
Acute lymphoblastic leukemia
Several studies have demonstrated that high white cell count and older age at presentation contribute to an overrepresentation of black children in the high-risk group.8-10 Aldrich et al. compared the cytogenetic profile between Hispanics and whites with ALL,35 and demonstrated no differences in the frequency of 11q23/MLL gene rearrangements. However, among B-lineage ALL patients, the percentage of TEL-AML1 translocations was significantly lower in Hispanics (13%) than in whites (24%, p=0.01). However, these findings need to be confirmed in larger studies.
Acute myeloid leukemia
Rubnitz et al. did not identify any difference in distribution of clinical characteristics, FAB subtype or cytogenetic features between white and black patients with AML.16 Hispanic patients were significantly less likely than others to have CNS involvement (p=0.03), and to have M1 or M2 morphology (p=0.01). Aplenc et al. found that white children were more likely to have chromosome 11 abnormalities in leukemic blasts.15 The proportion of patients with cytogenetic abnormalities t(8;21), inv(16), and del(7) did not differ among the ethnic groups, and no differences were observed in age or white cell counts at presentation.
Hodgkin lymphoma
Metzger et al. demonstrated an overrepresentation of patients with certain high-risk features among black children with HL (advanced stage disease: 46% vs. 35%; low hemoglobin levels: 31% vs. 24%; high ESR: 38% vs. 29%). However, these differences did not reach statistical significance because of the small number of patients (blacks: n=65).18
Rhabdomyosarcoma
Baker et al. reported that non-white patients with RMS were more likely to present with invasive T2 tumors (p=0.03), tumors with positive regional lymph nodes (N1, p=0.002), large tumors (>5cm, p=0.006) and tumors which were stage 2 or 3 (p=0.03) compared with whites.19
Neuroblastoma
Henderson et al. showed that black children with NBL were diagnosed at an older age (p<0.001), had a higher prevalence of stage 4 disease (p=0.001) and unfavorable histology tumors (p<0.001), when compared with whites.26 Thus blacks were more likely to present with high-risk disease than whites (57% vs. 44%, p<0.001). However, frequency of MYCN amplification or ploidy did not differ between blacks and whites. The prevalence of high-risk disease among Hispanics did not differ from whites.
These studies demonstrate that there may be evidence for ethnic/ racial differences in disease biology that could account for the observed disparities in survival. However, these studies have relied on retrospective cohorts and in large part, on clinical characteristics to describe the differences in disease biology. There is a need for a more sophisticated molecular understanding for differences in disease biology that could provide leads to developing more targeted therapies in order to reduce the disparities in survival.
Pharmacogenetics and acute toxicity
Patients with ALL are treated with 6-mercaptopurine (6MP) during the maintenance or continuation phase of therapy to ensure durable remissions; those with homozygous deficiency in thiopurine S-methyltransferase (TPMT) enzyme activity have an extreme sensitivity to 6MP as a result of the accumulation of higher intracellular concentrations of thioguanine nucleotides (TGNs). Red cell concentrations of TGNs are inversely related to TPMT enzyme activity (p<0.01).36 The cumulative incidence of 6MP dose reductions due to toxicity is highest among patients homozygous for mutant TPMT (100%), intermediate among homozygous patients (35%) and lowest among wild-type patients (7%, p<0.001). Thus genetic polymorphisms in TPMT are an important determinant of 6MP toxicity, even among patients who are heterozygous for this trait. Furthermore, lower activity of TPMT is associated with better outcome.37,38 The frequency and distribution of mutant alleles in different ethnic groups has been described, with differences reported between white, black, and Asian populations.39-43 These differences may be important in elucidating the observed racial/ethnic differences in outcomes.
Glutathione S-transferase (GST) genes are involved in the metabolism of some classes of chemotherapy drugs. GSTT1, GSTP1, and GSTM1 genotypes are polymorphic in humans, and the phenotypic absence of the enzyme activity is caused by a homozygous inherited deletion of the gene. In a case-control study, Stanulla et al. investigated the association between polymorphisms within GSTT1, GSTM1, and GSTP1 genes and risk of relapse in childhood ALL. The null genotype for GSTM1 and GSTT1 conferred a 2-fold and 2.8-fold reduction in risk of relapse, respectively, relative to the presence of the GSTM1 and GSTT1 gene. These findings suggest that polymorphisms within genes of the GST superfamily may be associated with risk of relapse in childhood ALL.44
Adherence to therapy
Poor adherence to medication accounts for substantial worsening of disease, death, and increased healthcare costs.45 Of all medication-related hospital admissions in the US, 33% to 69% are due to poor medication adherence, with a resultant cost of approximately $100 billion a year.45 Treatment of childhood ALL includes a maintenance phase composed of oral administration of antimetabolites (6MP and methotrexate) for prolonged periods. Previous studies have shown that low systemic exposure to oral 6MP during maintenance adversely affects prognosis.46 It is speculated that genetic differences in metabolism of these drugs might explain the ethnic influence on treatment outcome (described under section on pharmacogenetics). An alternative mechanism for the observed differences in survival by ethnicity could be related to variable exposure to 6MP during maintenance resulting from differences in adherence to prescribed therapy. Adherence to therapeutic protocol is a complex health behavior determined by a variety of socioeconomic, individual, familial, and cultural factors. In multicultural and minority populations, the issue of communication may play an even larger role because of linguistic and contextual barriers that preclude effective provider-patient communications. This has been demonstrated in cardiovascular disease, which disproportionately affects minority populations47, and in HIV-infected patients, where nonwhite patients were more likely to be non-adherent than white patients48. Therefore, when treating patients from multiple cultural and ethnic backgrounds, it is important to explore carefully the beliefs held by the patients regarding illness causality and treatment expectations.
There are data in the literature confirming that lack of adherence in pediatric ALL patients is a clinically important problem in 10% to 33% of the patients studied.49-51 The reason(s) for non-adherence are not clear. There are reports suggesting that adolescents are more likely to be non-adherent.52 Other risk factors include family size (lower adherence in larger families) and time on treatment (adherence drifts over time).52 Frequency of clinic visits also seems to play a role; more frequent visits increase adherence.53 The influence of treatment side-effects remains uncertain; only 5 to 10% of the patients cite side-effects as the reason for non-adherence.54
Evidence that poor adherence to protocol is related to disease outcomes is persuasive, albeit indirect. There is a substantial shortfall in the proportion of children achieving long-term DFS where there is poverty, malnutrition, poor communication between parents and health care professionals, or low levels of parental education.50,52 Remission rates are broadly comparable, but relapse rates are higher. Many patients default on outpatient care. Persuading families that maintenance treatment is important when the child appears “cured” is difficult, and in some countries 25% to 40% of families fail to attend clinics at all during the maintenance phase.50 Even when children are adherent with appointments and collect their drugs, unexpected relapses arise more often in children who tolerate full doses of oral antimetabolites than in those who develop cytopenias.52 Receiving a higher proportion of maintenance therapy has been associated with improved outcomes.55,56 Also, children on maintenance treatment who have lower than average concentrations of intracellular 6MP metabolites are at a greater risk of relapse, independent of other prognostic factors.57 However, ethnic and racial differences in adherence have not been explored systematically to draw meaningful conclusions regarding impact of non-adherence on ethnic and racial differences in outcome of children with ALL, or in other disease groups that rely on oral therapy for disease outcomes, and are the subject of a current COG-wide investigation.
POSSIBLE REASONS FOR ETHNIC/ RACIAL DIFFERENCES IN HEALTH-RELATED OUTCOMES IN LONG-TERM SURVIVORS
The cumulative incidence of severe or life-threatening chronic health conditions exceeds 40% for children with cancer surviving 30 years from primary diagnosis. These chronic health conditions place the survivors at an increased risk of premature death. In fact, increases in cause-specific mortality are described for patients with second malignancies (standardized mortality ratio [SMR]: 15.2), cardiac (SMR: 7.0) and pulmonary causes (SMR: 8.8). Thus, it is imperative that cancer survivors be followed life-long, with institution of standardized screening for early identification of long-term complications. However, long-term standardized care of these survivors depends could vary by race/ ethnicity, contributing to the observed disparity in long-term survival.
Knowledge about cancer diagnosis, treatment and late toxicities
A lack of knowledge regarding treatment and potential late toxicities could prevent active participation by the patient/parents in the long-term care of the survivor. Kadan-Lottick et al. conducted a cross-sectional survey of 635 consecutive adult survivors of childhood cancer, assessing knowledge of their cancer diagnosis, and associated therapies.58 Overall 72% accurately reported their diagnosis with precision. Participants’ accuracy for reporting their treatment history was 94% for chemotherapy, 89% for radiation, and 93% for splenectomy. Among those who received radiation, 70% recalled site of radiation. Participants who had received less than high school education were 6.7-fold more likely to inaccurately report diagnosis, or treatment. The report however, did not examine race/ ethnicity as a variable in knowledge about cancer diagnosis or treatment received.
Surveillance for long-term toxicities
Castellino et al. reported racial/ ethnic differences in adherence to cancer screening guidelines in adult survivors of childhood cancer including 7,892 whites, 503 Hispanics, and 443 blacks,59 and showed that black females were more likely (OR=1.6, 95% CI, 1.1-2.4) and Hispanic females less likely (OR=0.7, 95% CI, 0.5-1.0) to have had a recent Pap smear compared with whites. Black females were more likely to report breast self-exams compared with their white counterparts. These comparisons were adjusted for income, education, and health insurance.
Risky health behaviors
An evaluation of risky health behaviors such as smoking, binge or heavy drinking, and physical inactivity, by race/ethnicity revealed that Hispanic and black adult survivors of childhood cancer were less likely to be current smokers.59 Furthermore, blacks were less likely to indulge in binge or heavy drinking. Finally, Hispanic males were more likely to report physical inactivity as compared with their white counterparts.
Pharmacogenetics and late treatment-related outcomes
Research on survivorship issues has clearly demonstrated well-established associations between specific therapeutic exposures and adverse outcomes such as subsequent malignant neoplasms, cardiopulmonary dysfunction, avascular necrosis, endocrinopathies, and neurocognitive disorders. It is increasingly recognized that for each given therapeutic exposure, marked heterogeneity exists in the prevalence and severity of many of the long-term adverse outcomes experienced by the survivors. There is emerging data to suggest that genetic susceptibility could play a role in modifying individual response to therapeutic exposures. Using a biologically plausible candidate gene approach, investigators have begun to identify polymorphisms that could alter metabolic pathways of therapeutic agents associated with specific adverse events. Many of these genetic variants when fully established, could potentially play an important role in understanding the pathogenesis of the subsequent therapy-related adverse events, and facilitate implementation of targeted prevention strategies. The current knowledge regarding established associations between therapeutic exposures and these genomic variables has been reviewed recently.60 While understanding the molecular underpinning of treatment-related adverse events will allow a better understanding of the pathogenesis of these life-threatening complications, it will be equally important to explore whether any racial/ethnic differences exist in the frequencies of the at risk variants that would place a sub-population at a particularly high risk of complications, impacting long-term survival.
CONCLUSION
While survival rates have improved across all groups with recent therapy, there continue to racial and ethnic differences in overall and event-free survival among children with cancer receiving contemporary, risk-based therapy. These racial and ethnic differences in outcome are likely not only due to genetic differences in disease biology; socioeconomic status is strongly correlated with race/ethnicity, and is a robust predictor of access to and quality of healthcare, which in turn, may be associated with outcome. Thus evaluation of whether genetic differences underlie racial disparities in outcome should take into account socioeconomic status and access to care, and vice versa. The poorer outcome in blacks and Hispanics is likely due to a combination of differences in disease biology, pharmacogenetic differences, socioeconomic and sociocultural factors that influence access to care and adherence to therapy, as well as self-advocacy in preventing toxicities that would influence outcomes. However, the role played by any of these factors likely differs by primary diagnosis, and hence future studies need to identify the specific cause of disparities by cancer diagnoses, such that informed and tailored interventions can be strategized.
Acknowledgments
Supported in part by the National Cancer Institute of the National Institutes of Health (R01 CA 096670 [S Bhatia] and U10 CA098543-01 [Reaman])
REFERENCES
- 1.Freeman HP. Cancer in the socioeconomically disadvantaged. CA Cancer J Clin. 1989;39:266–88. doi: 10.3322/canjclin.39.5.266. [DOI] [PubMed] [Google Scholar]
- 2.Freeman HP. Cancer in the economically disadvantaged. Cancer. 1989;64(Suppl 1):324–34. doi: 10.1002/1097-0142(19890701)64:1+<324::aid-cncr2820641334>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 3.Unequal treatment: Confronting Racial and Ethnic Disparities in Healthcare. The National Academies Press; Washington DC: 2003. [PubMed] [Google Scholar]
- 4.Freeman HP. Commentary on the meaning of race in science and society. Cancer Epidemiol Biomarkers Prev. 2003;12:232S–236S. [PubMed] [Google Scholar]
- 5.UDoHaH, editor. Tracking Healthy People 2010, in Service. US Government Printing Office; Washington DC: 2000. [Google Scholar]
- 6.Byes T, Mouchawar J, Marks J, et al. The American Cancer Society challenge goals. How far can cancer rates decline in the US by the year 2015? Cancer. 1999;86:715–27. [PubMed] [Google Scholar]
- 7.Linabery AM, Ross JA. Childhood and adolescent cancer survival in the US by race and ethnicity for the diagnostic period 1975-1999. Cancer. 2008;113:2575–96. doi: 10.1002/cncr.23866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kadan-Lottick N, Ness KK, Bhatia S, et al. Survival variability by race and ethnicity in childhood acute lymphoblastic leukemia. JAMA. 2003;290:2008–14. doi: 10.1001/jama.290.15.2008. [DOI] [PubMed] [Google Scholar]
- 9.Bhatia S, Sather HN, Heerema NA, et al. Racial and ethnic differences in survival of children with acute lymphoblastic leukemia. Blood. 2002;100:1957–64. doi: 10.1182/blood-2002-02-0395. [DOI] [PubMed] [Google Scholar]
- 10.Pollock BH, DeBaun MR, Camitta BM, et al. Racial differences in the survival of childhood P-precursor acute lymphoblastic leukemia: A Pediatric Oncology Group Study. J Clin Oncol. 2000;18:813–23. doi: 10.1200/JCO.2000.18.4.813. [DOI] [PubMed] [Google Scholar]
- 11.Pui C-H, Sandlund JT, Pei D, et al. Results of therapy for acute lymphoblastic leukemia in black and white children. JAMA. 2003;290:2001–7. doi: 10.1001/jama.290.15.2001. [DOI] [PubMed] [Google Scholar]
- 12.Pui C-H, Boyett JM, Hancock ML, et al. Outcome of treatment for childhood cancer in black compared with white children: The St. Jude Children’s Research Hospital experience, 1962-1992. JAMA. 1995;273:633–7. [PubMed] [Google Scholar]
- 13.Woods WG, Neudorf S, Gold S, et al. A comparison of allogeneic bone marrow transplantation, autologous bone marrow transplantation, and aggressive chemotherapy in children with acute myeloid leukemia in remission. Blood. 2001;97:56–62. doi: 10.1182/blood.v97.1.56. [DOI] [PubMed] [Google Scholar]
- 14.Meshinchi S, Smith FO, Arceci RJ. Prognostic factors and risk-based therapy in pediatric acute myeloid leukemia. Curr Oncol Rep. 2003;5:489–97. doi: 10.1007/s11912-003-0010-1. [DOI] [PubMed] [Google Scholar]
- 15.Aplenc R, Alonzo TA, Gerbing RB, et al. Ethnicity and survival in childhood acute myeloid leukemia: a report from the Children’s Oncology Group. Blood. 2006;108:74–80. doi: 10.1182/blood-2005-10-4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rubnitz JE, Lensing S, Razzouk BI, et al. Effect of race on outcome of white and black children with acute myeloid leukemia: The St. Jude Experience. Pediatr Blood Cancer. 2007;48:10–15. doi: 10.1002/pbc.20878. [DOI] [PubMed] [Google Scholar]
- 17.Percy CL, Smith MA, Linet M, et al. Lymphomas and reticuloendothelial neoplasms. In: Reis LA, Smith MA, Gurney JG, et al., editors. Cancer incidence and survival among children and adolescents: United States SEER Program 1975-1995. National Cancer Institute; Bethesda MD: 1999. NIH publication 99-4649. [Google Scholar]
- 18.Metzger ML, Castellino SM, Hudson MM, et al. Effect of race on outcome of pediatric patients with Hodgkin’s lymphoma. J Clin Oncol. 2008;26:1282–88. doi: 10.1200/JCO.2007.14.0699. [DOI] [PubMed] [Google Scholar]
- 19.Baker KS, Anderson JR, Lobe TE, et al. Children from ethnic minorities have benefited equally as other children from contemporary therapy for rhabdomyosarcoma: A report from the Intergroup Rhabdomyosarcoma Study Group. J Clin Oncol. 2002;20:4428–33. doi: 10.1200/JCO.2002.11.131. [DOI] [PubMed] [Google Scholar]
- 20.Maris JM, Hogarty MD, Bagatell R, et al. Neuroblastoma. Lancet. 2007;369:2106–20. doi: 10.1016/S0140-6736(07)60983-0. [DOI] [PubMed] [Google Scholar]
- 21.Cohn SL, Pearson AD, London WB, et al. The International Neuroblastoma Risk Group (INRG) classification system: an INRG Task Force report. J Clin Oncol. 2009;27:289–97. doi: 10.1200/JCO.2008.16.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmidt ML, Lukens JN, Seeger RC, et al. Biologic factors determine prognosis in infants with stage IV neuroblastoma: a prospective Children’s Cancer Group Study. J Clin Oncol. 2000;18:1260–1268. doi: 10.1200/JCO.2000.18.6.1260. [DOI] [PubMed] [Google Scholar]
- 23.Bagatell R, Rumcheva P, London WB, et al. Outcomes of children with intermediate-risk neuroblastoma after treatment stratified by MYCN status and tumor cell ploidy. J Clin Oncol. 2005;23:8819–27. doi: 10.1200/JCO.2004.00.2931. [DOI] [PubMed] [Google Scholar]
- 24.De Bernardi B, Gerrard M, Boni L, et al. Excellent outcome with reduced treatment for infants with disseminated neuroblastoma without MYCN gene amplification. J Clin Oncol. 2009;27:1034–40. doi: 10.1200/JCO.2008.17.5877. [DOI] [PubMed] [Google Scholar]
- 25.Matthay KK, Reynolds CP, Seeger RC, et al. Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: a Children’s Oncology Group Study. J Clin Oncol. 2009;27:1007–13. doi: 10.1200/JCO.2007.13.8925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henderson TO, Bhatia S, Pinto N, et al. Racial and ethnic disparities in risk and survival in children with neuroblastoma: A Children’s Oncology Group (COG) Study. J Clin Oncol. 2010 doi: 10.1200/JCO.2010.29.6103. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barnholtz-Sloan JS, Severson RK, Stanton B, et al. Pediatric brain tumors in non-Hispanics, Hispanics, African-Americans and Asians: Differences in survival after diagnosis. Cancer Causes and Control. 2005;16:587–92. doi: 10.1007/s10552-004-7843-2. [DOI] [PubMed] [Google Scholar]
- 28.Kaufman JS, Cooper RS. Considerations for use of racial/ethnic classification in etiologic research. Am J Epidemiol. 2001;154:291–8. doi: 10.1093/aje/154.4.291. [DOI] [PubMed] [Google Scholar]
- 29.Pappas G, Queen S, Hadden W, et al. The increasing disparity in mortality between socioeconomic groups in the United States, 1960 to 1986. N Engl J Med. 1993;324:103–9. doi: 10.1056/NEJM199307083290207. [DOI] [PubMed] [Google Scholar]
- 30.Dang-Tan T, Franco EL. Diagnosis delays in childhood cancer. A review. Cancer. 2007;110:703–13. doi: 10.1002/cncr.22849. [DOI] [PubMed] [Google Scholar]
- 31.Stiller CA. Centralization of treatment and survival rates for cancer. Arch Dis Child. 1988;63:23–30. doi: 10.1136/adc.63.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stiller CA, Draper GJ. Treatment center size, entry to trials and survival in acute lymphoblastic leukemia. Arch Dis Child. 1989;64:657–61. doi: 10.1136/adc.64.5.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu L, Krailo M, Reaman GH, et al. Childhood cancer patients’ access to cooperative group cancer programs: a population-based study. Cancer. 2003;97:1339–45. doi: 10.1002/cncr.11192. [DOI] [PubMed] [Google Scholar]
- 34.Lund MJ, Eliason MT, Haight AE, et al. Racial/ ethnic diversity in Children’s Oncology Group trials. Cancer. 2009;115:3808–16. doi: 10.1002/cncr.24437. [DOI] [PubMed] [Google Scholar]
- 35.Aldrich MC, Zhang LP, Weimels JL, et al. Cytogenetics of Hispanic and white children with acute lymphoblastic leukemia in California. Cancer Epidemiol Biomarkers Prev. 2006;15:578–81. doi: 10.1158/1055-9965.EPI-05-0833. [DOI] [PubMed] [Google Scholar]
- 36.Relling MV, Hancock ML, Rivera GK, et al. Mercaptopurine therapy intolerance and heterozygosity at the thiopurine S-methyltransferase gene locus. J Natl Cancer Inst. 1999;91:2001–8. doi: 10.1093/jnci/91.23.2001. [DOI] [PubMed] [Google Scholar]
- 37.Lennard L, Lilleyman J, Loon JV, et al. Genetic variation in response to 6-mercaptopurine for childhood lymphoblastic leukemia. Lancet. 1990;336:225–9. doi: 10.1016/0140-6736(90)91745-v. [DOI] [PubMed] [Google Scholar]
- 38.Relling MV, Hancock ML, Boyett JM, et al. Prognostic importance of 6-mercaptopurine dose intensity in acute lymphoblastic leukemia. Blood. 1999;93:2817–23. [PubMed] [Google Scholar]
- 39.Collie-Duguid ES, Pritchard SC, Powrie RH, et al. The frequency and distribution of thiopurine methyltransferase alleles in Caucasian and Asian populations. Pharmacogenetics. 1999;9:37–42. doi: 10.1097/00008571-199902000-00006. [DOI] [PubMed] [Google Scholar]
- 40.Hon YY. Polymorphism of the thiopurine S-methyltransferase gene in African-Americans. Hum Mol Genet. 1999;8 doi: 10.1093/hmg/8.2.371. [DOI] [PubMed] [Google Scholar]
- 41.Mcleod H, Lin J, Scott E, et al. Thiopurine methyltransferase activity in American white and American black subjects. Clin Pharmacol Ther. 1994;55:15–20. doi: 10.1038/clpt.1994.4. [DOI] [PubMed] [Google Scholar]
- 42.Mcleod H, Pritchard S, Githanga J, et al. Ethnic differences in thiopurine methyltransferase pharmacogenetics: evidence for allele-specificity in Caucasian and Kenyan subjects. Pharmacogenetics. 1999;9:773–6. doi: 10.1097/00008571-199912000-00012. [DOI] [PubMed] [Google Scholar]
- 43.Cooper SC, Ford LT, Berg JD, et al. Ethnic variation of thiopurine S-methyltransferase activity: a large, prospective population study. Pharmacogenomics. 2008;9:303–9. doi: 10.2217/14622416.9.3.303. [DOI] [PubMed] [Google Scholar]
- 44.Stanulla M, Schrappe M, Brechlin AM, et al. Polymorphisms within glutathione S-transferase genes (GSTM1, GSTT1, GSTP1) and risk of relapse in childhood B-cell precursor acute lymphoblastic leukemia: a case-control study. Blood. 2000;95:1222–8. [PubMed] [Google Scholar]
- 45.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–97. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 46.Koren G, Ferrazini G, Sulh H, et al. Systemic exposure to mercaptopurine as a prognostic factor in acute lymphocytic leukemia in children. N Engl J Med. 1990;323:17–21. doi: 10.1056/NEJM199007053230104. [DOI] [PubMed] [Google Scholar]
- 47.Betancourt JR, Carrillo JE, Green AR. Hypertension in multicultural and minority populations: linking communication to compliance. Curr Hypertens Res. 1999;1:482–8. doi: 10.1007/BF03215777. [DOI] [PubMed] [Google Scholar]
- 48.Singh N, Berman SM, Swindell S, et al. Adherence to human immunodeficiency virus-infected patients to anti-retroviral therapy. Clin Infect Dis. 1999;29:824–30. doi: 10.1086/520443. [DOI] [PubMed] [Google Scholar]
- 49.Lennard L, Welch J, Lilleyman JS. Intracellular metabolites of mercaptopurine in children with lymphoblastic leukaemia: a possible indicator of non-compliance? Br J Cancer. 1995;72:1004–6. doi: 10.1038/bjc.1995.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Davies HA, Lennard L, Lilleyman JS. Variable mercaptopurine metabolism in children with leukaemia: a problem of non-compliance? BMJ. 1993;306:1239–40. doi: 10.1136/bmj.306.6887.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lau RC, Matsui D, Greenberg M, et al. Electronic measurement of compliance with mercaptopurine in pediatric patients with acute lymphoblastic leukemia. Med Pediatr Oncol. 1998;30:85–90. doi: 10.1002/(sici)1096-911x(199802)30:2<85::aid-mpo3>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 52.Tebbi CK, Cummings KM, Zevon MA, et al. Compliance of pediatric and adolescent cancer patients. Cancer. 1986;58:1179–84. doi: 10.1002/1097-0142(19860901)58:5<1179::aid-cncr2820580534>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 53.Smith SD, Rosen D, Trueworthy RC, et al. A reliable method of evaluating drug compliance in children with cancer. Cancer. 1979;43:169–73. doi: 10.1002/1097-0142(197901)43:1<169::aid-cncr2820430125>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 54.MacDougall LG, McElligot SE, Rose E, et al. Pattern of 6-mercaptopurine urinary excretion in children with acute lymphoblastic leukemia: urinary assays as a measure of compliance. Ther Drug Monit. 1992;14:371–5. doi: 10.1097/00007691-199210000-00005. [DOI] [PubMed] [Google Scholar]
- 55.Welch JC, Lilleyman JS. Mercaptopurine dose escalation and its effect on drug tolerance in childhood lymphoblastic leukemia. Cancer Chemother Pharmacol. 1996;38:113–6. doi: 10.1007/s002800050457. [DOI] [PubMed] [Google Scholar]
- 56.Dibenedetto SP, Guardabasso V, Ragusa R, et al. 6-Mercaptopurine cumulative dose: a critical factor of maintenance therapy in average risk childhood acute lymphoblastic leukemia. Pediatr Hematol Oncol. 1994;11:251–8. doi: 10.3109/08880019409141668. [DOI] [PubMed] [Google Scholar]
- 57.Lennard L, Lilleyman JS. Mercaptopurine metabolism and risk of relapse in childhood lymphoblastic leukemia. Lancet. 1994;343:1188–90. doi: 10.1016/s0140-6736(94)92400-7. [DOI] [PubMed] [Google Scholar]
- 58.Kadan-Lottick N, Robison LL, Gurney JG, et al. Childhood cancer survivors’ knowledge about their past diagnosis and treatment Childhood Cancer Survivor Study. JAMA. 2002;287:1832–9. doi: 10.1001/jama.287.14.1832. [DOI] [PubMed] [Google Scholar]
- 59.Castellino SM, Casillas J, Hudson MM, et al. Minority adult survivors if childhood cancer: A comparison of long-term outcomes, health care utilization, and health-related behaviors from the Childhood Cancer Survivor Study. J Clin Oncol. 2005;23:6499–507. doi: 10.1200/JCO.2005.11.098. [DOI] [PubMed] [Google Scholar]
- 60.Armenian SH, Bhatia S. Chronic health conditions in childhood cancer survivors: Is it all treatment-related - or do genetics play a role? J Gen Intern Med. 2009;24:395–400. doi: 10.1007/s11606-009-0995-8. [DOI] [PMC free article] [PubMed] [Google Scholar]