Abstract
Autoimmune hepatitis is a rare but life threatening autoimmune disease of the liver of unknown etiology1,2. In the past many attempts have been made to generate an animal model that reflects the characteristics of the human disease 3-5. However, in various models the induction of disease was rather complex and often hepatitis was only transient3-5. Therefore, we have developed a straightforward mouse model that uses the major human autoantigen in type 2 autoimmune hepatitis (AIH-2), namely hCYP2D6, as a trigger6. Type 1 liver-kidney microsomal antibodies (LKM-1) antibodies recognizing hCYP2D6 are the hallmark of AIH-27,8. Delivery of hCYP2D6 into wildtype FVB or C57BL/6 mice was by an Adenovirus construct (Ad-2D6) that ensures a direct delivery of the triggering antigen to the liver. Thus, the ensuing local inflammation generates a fertile field9 for the subsequent development of autoimmunity. A combination of intravenous and intraperitoneal injection of Ad-2D6 is the most effective route to induce a long-lasting autoimmune damage to the liver (section 1). Here we provide a detailed protocol on how autoimmune liver disease is induced in the CYP2D6 model and how the different aspects of liver damage can be assessed. First, the serum levels of markers indicating hepatocyte destruction, such as aminotransferases, as well as the titers of hCYP2D6 antibodies are determined by sampling blood retroorbitaly (section 2). Second, the hCYP2D6-specific T cell response is characterized by collecting lymphocytes from the spleen and the liver. In order to obtain pure liver lymphocytes, the livers are perfused by PBS via the portal vein (section 3), digested in collagen and purified over a Percoll gradient (section 4). The frequency of hCYP2D6-specific T cells is analyzed by stimulation with hCYP2D6 peptides and identification of IFNγ-producing cells by flow cytometry (section 5). Third, cellular infiltration and fibrosis is determined by immunohistochemistry of liver sections (section 6). Such analysis regimen has to be conducted at several times after initiation of the disease in order to prove the chronic nature of the model. The magnitude of the immune response characterized by the frequency and activity of hCYP2D6-specific T and/or B cells and the degree of the liver damage and fibrosis have to be assessed for a subsequent evaluation of possible treatments to prevent, delay or abrogate the autodestructive process of the liver.
Keywords: Medicine, Issue 60, autoimmunity, liver, autoantigen, fibrosis, perfusion
Protocol
1. Intravenous injection of Ad-2D6 into ophthalmic sinus
Under sterile conditions, dilute the Ad-2D6 virus stock solution to a concentration of 5 x 109 pfu/ml in RPMI and keep the Ad-2D6 virus solution on ice. Injection of two doses of 5 x 108 pfu (intravenous and intraperitoneal) has proven to result in the most reliable outcome of autoimmune hepatitis in mice of the FVB strain.
Anesthetize mouse with 4% isoflurane in an anesthesia chamber. Pinch the hind leg to make sure the animal is anesthetized.
Hold the mouse by the back of the neck and tighten the loose skin of the head with thumb and middle finger. Like this, the eye protrudes slightly by the traction to the skin adjacent to the eye.
Place the needle vertically into the medial canthus (junction of eyelids closest to the animal's nose) and slowly inject 100 μl Ad-2D6 virus solution. It is important not to apply too much pressure, to avoid damage of the vessels and trachea.
Slowly withdraw the needle to avoid spillage and close the eyelids.
Injection worked well if the virus solution does not leak out trough the nose and the eye slides back to its initial position.
Immediately following the intravenous injection, perform a standard intraperitoneal injection of the second 100 μl of the Ad-2D6 virus solution.
Alternatively, the intravenous injection can be executed via the tail vein. However, injection via the ophthalmic sinus is much easier to perform and minimizes the loss of virus solution. It has been demonstrated that these two techniques can be used interchangeably and that both routes are equally effective 10.
Infected mice are monitored for adverse effects and obvious signs of suffering and pain, such as loss of appetite, loss of weight, loss of mobility, failure to groom, rough hair coat, hunched appearance, as well as licking, biting, scratching, or shaking a particular area (i.e. site of injection). Although the mice display a massive damage to the liver in the CYP2D6 model, the mice seem healthy and show no signs of suffering, pain or stress. Nevertheless, indicators of severe liver failure, such as serum aminotransferases are determined on a regular basis or are part of the performed experiments. Serum aminotransferase levels of >1000 U/l should only appear as a direct result of the virus infection, but not chronically. If the serum aminotransferase levels are chronically above 1000 U/l (severity limit) the mice are sacrificed.
2. Mouse eye bleeding
Anesthetize mouse with 4% isoflurane in an anesthesia chamber. Pinch the hind leg to make sure the animal is anesthetized.
Hold the mouse by the back of the neck and tighten the loose skin of the head with thumb and middle finger. Like this, the eye protrudes slightly by the traction to the skin adjacent to the eye.
Place the tip of the capillary tube at the lower or inner corner of the eye and gently but firmly slid alongside the eyeball to the ophthalmic venous sinus. The venous capillaries rupture and the resulting hemorrhage fills the orbital cavity.
Slightly withdraw the capillary tube to free the tip so that the accumulated blood is drawn into the tube.
When enough blood is collected, the tube is withdrawn and the eyelids closed. Bleeding stops upon withdrawal of the tube and reestablishment of normal ocular pressure upon the venous complex.
Blood in the capillary tube is transferred to a Microtainer tube and incubated for 30 min on ice.
The Microtainer tube is centrifuged at 7500 x g for 2 min at 4 °C.
The supernatant corresponds to the serum which is transferred to a fresh tube and stored at -80 °C.
Serum can be used to determine antibody titer by ELISA. Indicators of liver damage such as the serum levels of the hepatic enzymes alanine aminotransferase (ALT/GPT) and aspartate aminotransferase (AST/GOT) can be assessed using the according test strips from Roche Diagnostics (Mannheim, Germany) and a Reflotron Plus analyzer (Roche Diagnostics, Mannheim, Germany).
Alternatively, blood sampling can be done via the tail vein or via the superficial temporal vein11.
3. Mouse liver perfusion
Remark: This step is important to avoid a contamination of isolated liver lymphocytes by blood lymphocytes
Euthanize the mouse by lethal doses of CO2 followed by cervical dislocation.
Mount the animal laying on its back on the styrofoam board. Use 23 G needles to pin extremities.
Moisturize and disinfect the mouse with 70 % EtOH
About 1 cm away from hind legs, make an incision through the skin and the muscle layer. Cut on both sides of the animal working up to the diaphragm.
When the diaphragm is reached the skin can be flipped backwards.
Cut the diaphragm away from the rips then cut the vena cava.
Move the stomach and the intestines off to the side, exposing the portal vein.
In the portal vein insert a 27 G needle connected to a 5 ml syringe filled with cold PBS. Let the PBS slowly wash the blood out of the liver.
Dissect the liver by grabbing onto the diaphragm and cutting the diaphragm away from the body.
Transfer the liver to a petri dish and remove the diaphragm, the gallbladder and any other tissue still connected to the liver
Transfer the liver to 10 ml PBS in a 50 ml tube on ice or embed in OCT compound.
4. Isolation of liver lymphocytes
- Prepare the following stock solutions:
- Collagenase IV stock (100 mg/ml) in PBS. (Make small aliquots and store at -20 °C).
- DNase stock (25 mg/ml) in PBS. (Make small aliquots and store at -20 °C).
- Collagenase buffer: PBS containing 0.2 mg/ml collagenase IV, 0.02 mg/ml DNase and 5% FCS; for 1 liver, mix 9.5 ml ice-cold PBS, 20 μl collagenase IV (100 mg/ml stock), 8 μl DNase (25 mg/ml stock), and 500 μl heat-inactivated FCS.
- 35 % Percoll; mix 175 ml Percoll, 20 ml 10 x PBS stock; 305 ml PBS.
- RPMIcomplete = RPMI containing 10% heat-inactivated FCS, 100 μ/ml penicillin, 100 μg/ml streptomycin, 2 mM L-glutamine.
Transfer the perfused liver (see section 3) to 10 ml of fresh PBS in a petri dish on ice and cut into small pieces using scissors.
Transfer into a 70 μm cell strainer and squeeze liver junks through with a glass pestle or a pestle from a 2 ml syringe. Carefully add 10 ml cold collagenase buffer and press through strainer. Collect suspension and filter through strainer two times more.
Transfer suspension to fresh 50 ml tube on ice. Process next liver.
Incubate liver cell suspension at 37 °C for 60 min, mix gently every 15 min.
Centrifuge at 30 x g for 3 min at 4 °C.
Transfer supernatant to a fresh tube, leaving 5 mm fluid above pellet
Centrifuge at 650 x g for 10 min at 4 °C.
Discard supernatant, leaving 3 mm above pellet
Resuspend pellet in 20 ml Percoll buffer
Centrifuge at 600 x g for 20 min at 4 °C.
Discard supernatant and resuspend pellet by flicking the tube.
Wash pellet 1 x with PBS.
Wash pellet 1 x with RPMIcomplete
Resuspend pellet in 3 ml RPMIcomplete and count cells in a 1:10 dilution.
Resuspend cells at ~107 cells/ml in RPMIcomplete and transfer tube to ice.
5. Intracellular cytokine staining (ICCS)
5.1 Stimulation:
Prepare liver lymphocytes at ~107 cells/ml in RPMIcomplete as described. Plate 100 μl (106 cells)/well into a flat 96-well plate which is not tissue-culture treated.
Add 50μl RPMIcomplete containing 2μg/ml Brefeldin A and then add 50μl RPMIcomplete containing 2 μg/ml stimulating CYP2D6 peptide (i.e. the immunodominant CD4 epitope CYP2D641-60 PGLGNLLHVDFQNTPYCFDQ12 or the immunodominant CD8 epitope CYP2D6193-212 RRFEYDDPRFLRLLDLAQEG12). Mix by pipetting.
Incubate for 5 hrs at 37°C (optimal stimulation time but overnight incubation works as well).
5.2. Staining:
- Prepare the following stock solutions:
- FACS buffer: PBS containing 1% fetal calf serum (FCS).
- Fixation/Permeabilization buffer: FACS buffer containing 0.1% saponin and 4% paraformaldehyde
- FACS/saponin wash buffer: FACS buffer containing 0.1% saponin
- FACS/PFA buffer: FACS buffer containing 1% paraformaldehyde (PFA)
Transfer cells into V-bottom microtiter plate (96-well) and spin at 460 x g for 3 min at 4 °C. Discard medium and vortex plate
Add 150 μl FACS buffer and centrifuge at 460 x g for 3 min at 4 °C. Discard medium and vortex plate. Repeat wash step.
Block surface FcR if necessary (when using secondary antibodies) with 1 μg/ml αCD16/32 cocktail (FcR block) in FACS buffer for 15 min at 4 °C and wash 2 x with 150 μl staining buffer (460 x g for 2 min at 4 °C).
Stain for surface molecules: i.e. Anti-CD8a-FITC mAb 10 μg/ml in 50 μl FACS buffer for 30 min at 4 °C in the dark.
Add 100 μl FACS buffer and spin at 460 x g for 3 min at 4 °C.
Wash cells 2 x with 150 μl FACS buffer (460 x g; 3 min; 4 °C). Vortex plate.
Fix/permeabilize cells with 100 μl fixation/permeabilization buffer for 10 min at RT.
Spin at 460 x g for 7 min at 4 °C. Note that it is important to extend the centrifugation time to 7 min, since the fixation/permeabilzation steps changes the overall density of the cells. Vortex plate.
Wash 2 x with 150 μl FACS/saponin wash buffer (460 x g; 7 min; 4 °C). Vortex plate.
Stain for intracellular molecules: i.e. Anti-IFNγ-PE mAb 10 μg/ml in 50 μl FACS/saponin wash buffer for 30 min at 4 °C.
Add 100 μl FACS/saponin wash buffer and spin at 460 x g for 7 min at 4 °C.
Wash cells 2 x with 150 μl FACS/saponin wash buffer (460 x g; 7 min; 4 °C). Vortex plate.
Wash cells 1 x with FACS buffer (460 x g; 7 min; 4 °C). Vortex plate.
Resuspend cells in 200 μl FACS/PFA buffer, transfer into tubes, and store at 4 °C in the dark until acquisition of data by flow cytometry. We normally use a FACSCanto II or a FACSCalibur (BD Biosciences, Heidelberg, Germany).
6. Immunohistochemistry
6.1. H&E staining for the assessment of general liver pathology:
Harvest liver, immerse in Tissue-Tek O.C.T. in a disposable base mold and quick-freeze on dry ice.
Cut 7 μm liver tissue sections using a Leica cryostat set at -17 °C and mount the tissue sections on Superfrost Plus microscope slides. Store slides at -20 °C until further use.
Fix cryosections in pre-cooled EtOH for 15 min at -20 °C. Let dry for 10 min.
Wash 2 x in distilled water for 2 min at room temperature (the following steps, 6.1.5 - 6.1.13, are performed at room temperature).
Stain in Meyer's Hematoxylin solution for 8 min.
Wash in warm (30 °C) tap water for 10 min. Change water several times.
Rinse in distilled water.
Rinse in 95% EtOH for 20 sec.
Counterstain in Eosin G/Y solution for 40 sec.
Dehydrate 2 x in 95% EtOH for 5 min per incubation.
Dehydrate 2 x in 100% EtOH for 5 min per incubation.
Clear in xylene 2 x for 5 min per clearing.
Mount in Roti-Histokitt.
6.2. Immunohistochemistry for collagen I deposition:
Harvest liver, immerse in Tissue-Tek OCT. in a disposable base mold and quick-freeze on dry ice.
Cut 7 μm liver tissue sections using a Leica cryostat set at -17 °C and mount the tissue sections on Superfrost Plus microscope slides. Store slides at -20 °C until further use.
Fix cryosections in cold EtOH for 15 min at -20 °C. Let dry for 10 min.
Wash 2 x in PBS for 2 min at room temperature.
Incubate in PBS containing 0.3% H2O2 and 0.1% Na-azide for 10 min at room temperature.
Wash 2 x in PBS for 2 min at room temperature.
Block with an avidin-biotin blocking kit according the manufacturer's guidelines.
Block with 10 % FCS in PBS (FCS/PBS) for 30 min at room temperature.
The primary antibody is a rabbit anti-mouse collagen I antibody which is diluted 1:200 in 10 % FCS/PBS. Incubate sections with primary antibody for 2 hrs at room temperature.
Wash sections 3 x in PBS for 4 min at room temperature.
Dilute biotinylated anti-rabbit antibody to 1:500 and incubate with the sections for 1.5 hr at room temperature.
Wash sections 3 x in PBS for 4 min at room temperature.
Color reaction is obtained by sequential incubation with avidin-peroxidase conjugate and diaminobenzidine-hydrogen peroxide according the manufacturer's guidelines.
Counterstain in Meyer's Hematoxylin solution for 5 min, wash 2 x in PBS for 2 min at room temperature and mount in Aquatex.
7. Representative Results
Infection of mice with Ad-2D6 induced two distinct stages of liver damage. In the first days after infection, virus infection of the liver causes an acute increase of serum aminotransferase levels, an indicator of hepatocyte death6. This first, acute stage occurs independent of the expression of hCYP2D6 and can also be observed after infection with an empty control virus (Ad-Control) or a virus expressing green fluorescence protein (Ad-GFP). In contrast, the second stage is autoimmune-mediated and dependent on the expression of the triggering molecule hCYP2D6. This autoimmune stage occurs within 2-4 weeks post-infection and persists for several months6.
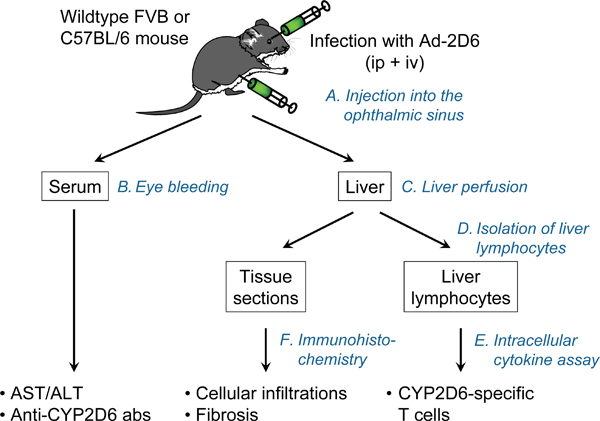
Figure 1. The CYP2D6 model. Wildtype FVB or C57BL/6 mice are injected with two doses of Ad-2D6 (intravenous and intraperitoneal). At a time of interest the liver and the serum are collected and assessed for liver damage and formation of hCYP2D6-specific antibodies and T cells.
The following features are characteristic for the persistent autoimmune hepatitis developing after Ad-2D6-infection of wildtype FVB or C57BL/6 mice in the CYP2D6 model: First, the liver shows an aberrantly changed morphology. In particular, the liver lobes are fused, hyperplasic nodules appear scattered over the entire liver and a massive capsular fibrosis is visible (figure 2).
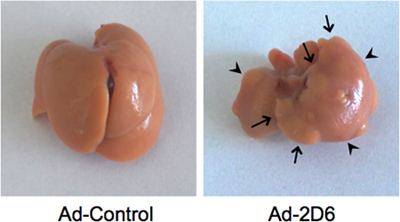
Figure 2. Liver morphology. Typical Ad-2D6-induced liver damage as detected at week 4 post-infection. Note that the individual liver lobes are fused (arrowheads). Hyperplasic nodules appear scattered over the entire liver (arrows). Infection with a control adenovirus not expressing hCYP2D6 has no effect on the liver morphology.
Second, extensive and persistent cellular infiltrations appear in the peri-portal and parenchymal regions of the livers of Ad-2D6-infected but not Ad-Control-infected mice (figure 3A). Fibrosis predominantly develops in the subcapsular region with some collagen bundles protruding into the parenchyma (figure 3B).
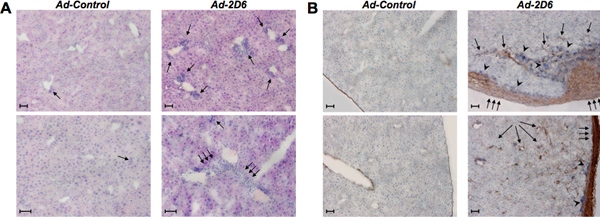
Figure 3. Liver histology. A: H&E staining of liver tissue section of FVB mice infected with either Ad-Control or Ad-2D6 at week 4 post-infection. Note that significant infiltrations of mononuclear cells are only present in Ad-2D6 infected mice (single arrows). Triple arrows indicate larger cellular infiltrations in the liver parenchyma bridging between neighboring portal tracts. B: Immunohistochemical representation of liver fibrosis at week 4 after infection with Ad-2D6 or Ad-control. The liver sections have been stained for collagen using an anti-collagen I antibody. Note multiple layer of collagen disposition under the liver capsule (triple arrows) with some bundles protruding into the parenchyma (single arrows) and the presence of large clusters of infiltrating cells (arrowheads). Two representative liver sections are displayed for each condition. Size bars: 100μm.
A third feature is the generation of high titers of anti-CYP2D6 antibodies, which have a similar epitope specificity to human LKM-1 antibodies 6,13. Last, hCYP2D6-specific CD4 and CD8 T cells are generated that predominantly home to the liver. Such hCYP2D6-specific T cells can be detected by stimulation with immunodominant hCYP2D6 peptides and subsequent measurement of IFNγ expression by an intracellular cytokine staining (ICCS) (figure 4). hCYP2D6-specific CD8 T cells kill Ad-2D6-infected target cells in vivo 12.
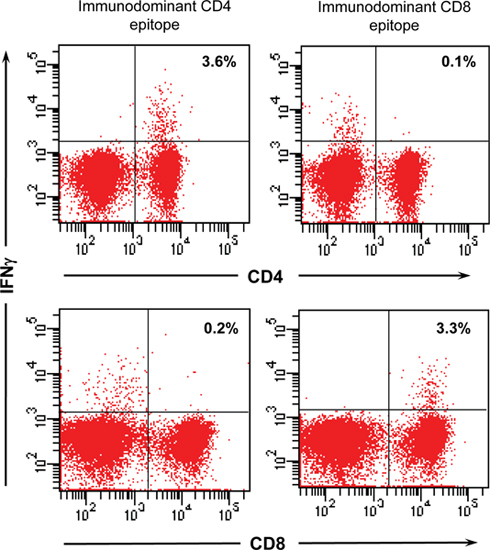
Figure 4. hCYP2D6-specific T cells. Flow cytometric analysis of hCYP2D6-specific T cells. Liver lymphocytes have been isolated at week 4 after Ad-2D6-infection and were stimulated with either the immunodominant CD4 or CD8 hCYP2D6 epitope overnight. Generation of IFNγ was detected by intracellular cytokine staining and analyzed by flow cytometry using a FACS Canto II (BD Biosciences, Heidelberg, Germany). The percentage of hCYP2D6-specific CD4 and CD8 cells is indicated.
Discussion
In previous studies, experimental hepatitis was often reported to be only transient and many current models for autoimmune liver disease depend on a rather complex disease induction protocols (for reviews see 3,5). For example, several models use transgenic mice expressing specific target antigens and adoptively transferred target antigen-specific, mostly TcR-transgenic, T cells14,15. Often an additional infection with livertropic viruses, bacteria or parasites is necessary to induce disease16-18. Alternatively, DNA-vaccination with plasmids encoding for target antigens, including CYP2D619, is used to induce hepatitis. However, an additional vaccination with plasmids encoding for pro-inflammatory cytokines, such as IL-12, is required20. Unfortunately, with a few exceptions15,20 hepatitis is only transient.
The CYP2D6 model6,21 uses a straightforward method to induce autoimmune hepatitis by simply infecting mice with an Adenovirus expressing hCYP2D6, the major autoantigen in AIH-27,8. Delivery of CYP2D6 by an adenovirus construct guarantees both a direct targeting of the liver as well as a local inflammation that promotes the breakdown of tolerance. It is important to note however that both the virus titer as well as the route of administration is crucial to this model. On the one hand, Ad-2D6 is a replication deficient virus, thus, a sufficient high titer of virus is required to generate a critical amount of antigen within the liver. On the other hand, too high a titer might result in a fatal acute liver failure. In addition, it has been demonstrated previously that infection of mice by high titers of adenovirus leads to a functional exhaustion of the immune response22. In our model, we used a combination of intravenous and intraperitoneal infection in order to obtain both chronic cellular infiltration as well as extensive fibrosis. We have observed that intravenous infection alone still causes massive cellular infiltration, but no fibrosis of the subcapsular region, indicating that peritoneal inflammation due to the intraperitoneal injection might be involved in the continuous subcapsular accumulation of collagen (Hintermann & Christen, manuscript in preparation). However, it is important to note that the observed hepatic fibrosis is antigen-specific, since we did not detect activation of hepatic stellate cells and collagen deposition after infection with equal virus-titers of Ad-GFP or Ad-Control23.
The CYP2D6 model reflects many aspects of human AIH1,2, such as chronic hepatitis characterized by persistent cellular infiltration and liver fibrosis6. In addition, the presence of high-titer anti-CYP2D6 antibodies with similar epitope specificity and spreading13 to LKM-1 antibodies found in AIH-2 patients indicates the presence of a common chronic autoimmune reactivity. CYP2D6 is the major antigen in AIH-2, but it is not recognized by patients diagnosed with the more frequent type 1 AIH. In addition, patients suffering from other liver diseases with autoimmune etiology, such as primary biliary cirrhosis (PBC) or primary sclerosing cholangitis (PSC), generate hallmark autoantibodies with specificity to distinct liver autoantigens and their livers show different pathological features centering on small (PBC) or large (PSC) bile ducts. Nevertheless, the CYP2D6 models offers the opportunity to analyze immunopathogenic mechanisms involved in chronic hepatic inflammatory processes as occurring during autoimmune liver diseases, to identify key players that drive the autoimmune destruction of hepatocytes and to evaluate possible therapeutic interventions to cure the disease.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This work is supported by the Goethe University Hospital Frankfurt and a grant of the German Research Foundation to U.C.
References
- Manns MP. Diagnosis and management of autoimmune hepatitis. Hepatology. 2010;51:2193–2213. doi: 10.1002/hep.23584. [DOI] [PubMed] [Google Scholar]
- Czaja AJ, Manns MP. Advances in the Diagnosis, Pathogenesis and Management of Autoimmune Hepatitis. Gastroenterology. 2010;4:429–443. doi: 10.1053/j.gastro.2010.04.053. [DOI] [PubMed] [Google Scholar]
- Christen U, Hintermann E, Jaeckel E. New animal models for autoimmune hepatitis. Semin. Liver Dis. 2009;29:262–272. doi: 10.1055/s-0029-1233536. [DOI] [PubMed] [Google Scholar]
- Christen U, Holdener M, Hintermann E. Animal models for autoimmune hepatitis. Autoimmun. Rev. 2007;6:306–311. doi: 10.1016/j.autrev.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Czaja AJ. Animal models of autoimmune hepatitis. Expert Rev. Gastroenterol. Hepatol. 2010;4:429–443. doi: 10.1586/egh.10.42. [DOI] [PubMed] [Google Scholar]
- Holdener M. Breaking tolerance to the natural human liver autoantigen cytochrome P450 2D6 by virus infection. J. Exp. Med. 2008;205:1409–1422. doi: 10.1084/jem.20071859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns MP, Griffin KJ, Sullivan KF, Johnson EF. LKM-1 autoantibodies recognize a short linear sequence in P450IID6, a cytochrome P-450 monooxygenase. J. Clin. Invest. 1991;88:1370–1378. doi: 10.1172/JCI115443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanger UM, Hauri HP, Loeper J, Homberg JC, Meyer UA. Antibodies against human cytochrome P-450db1 in autoimmune hepatitis type II. Proc. Natl. Acad. Sci. U. S. A. 1988;85:8256–8260. doi: 10.1073/pnas.85.21.8256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Herrath MG, Fujinami RS, Whitton JL. Microorganisms and autoimmunity: making the barren field fertile. Nat. Rev. Microbiol. 2003;1:151–157. doi: 10.1038/nrmicro754. [DOI] [PubMed] [Google Scholar]
- Yardeni T, Eckhaus M, Morris HD, Huizing M, Hoogstraten-Miller S. Retro-orbital injections in mice. Lab. Anim. (NY) 2011;40:155–160. doi: 10.1038/laban0511-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes N. Morbidity and mortality rates associated with serial bleeding from the superficial temporal vein in mice. Lab. Anim. (NY) 2010;39:236–240. doi: 10.1038/laban0810-236. [DOI] [PubMed] [Google Scholar]
- Ehser J. Molecular mimicry rather than identity beaks T cell tolerance in the CYP2D6 mouse model for human autoimmune hepatitis. 2011. Forthcoming. [DOI] [PubMed]
- Hintermann E. Epitope spreading of the anti-CYP2D6 antibody response in patients with autoimmune hepatitis and in the CYP2D6 mouse model. J. Autoimmun. 2011. Forthcoming. [DOI] [PubMed]
- Ando K. Class I-restricted cytotoxic T lymphocytes are directly cytopathic for their target cells in vivo. J. Immunol. 1994;152:3245–3253. [PubMed] [Google Scholar]
- Zierden M, Kuhnen E, Odenthal M, Dienes HP. Effects and regulation of autoreactive CD8+ T cells in a transgenic mouse model of autoimmune hepatitis. Gastroenterology. 2010;139:975–986. doi: 10.1053/j.gastro.2010.05.075. [DOI] [PubMed] [Google Scholar]
- Limmer A. Failure to induce organ-specific autoimmunity by breaking of tolerance: importance of the microenvironment. Eur. J. Immunol. 1998;28:2395–2406. doi: 10.1002/(SICI)1521-4141(199808)28:08<2395::AID-IMMU2395>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Voehringer D. Break of T cell ignorance to a viral antigen in the liver induces hepatitis. J. Immunol. 2000;165:2415–2422. doi: 10.4049/jimmunol.165.5.2415. [DOI] [PubMed] [Google Scholar]
- Derkow K. Differential priming of CD8 and CD4 T-cells in animal models of autoimmune hepatitis and cholangitis. Hepatology. 2007;46:1155–1165. doi: 10.1002/hep.21796. [DOI] [PubMed] [Google Scholar]
- Lapierre P, Djilali-Saiah I, Vitozzi S, Alvarez F. A murine model of type 2 autoimmune hepatitis: Xenoimmunization with human antigens. Hepatology. 2004;39:1066–1074. doi: 10.1002/hep.20109. [DOI] [PubMed] [Google Scholar]
- Djilali-Saiah I, Lapierre P, Vittozi S, Alvarez F. DNA vaccination breaks tolerance for a neo-self antigen in liver: a transgenic murine model of autoimmune hepatitis. J. Immunol. 2002;169:4889–4896. doi: 10.4049/jimmunol.169.9.4889. [DOI] [PubMed] [Google Scholar]
- Christen U, Hintermann E, Holdener M, von Herrath MG. Viral triggers for autoimmunity: is the 'glass of molecular mimicry' half full or half empty. J. Autoimmun. 2010;34:38–44. doi: 10.1016/j.jaut.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs P, Scandella E, Odermatt B, Ludewig B. Rapid functional exhaustion and deletion of CTL following immunization with recombinant adenovirus. J. Immunol. 2005;174:4559–4566. doi: 10.4049/jimmunol.174.8.4559. [DOI] [PubMed] [Google Scholar]
- Hintermann E, Bayer M, Pfeilschifter J, Christen U. Adenoviral delivery of the liver autoantigen cytochrome P450 2D6 chronically activates hepatic stellate cells and induces fibrosis. Manuscript Submitted. 2011.


