Abstract
Acetate kinase, a member of the acetate and sugar kinase-Hsp70-actin (ASKHA) enzyme superfamily1-5, is responsible for the reversible phosphorylation of acetate to acetyl phosphate utilizing ATP as a substrate. Acetate kinases are ubiquitous in the Bacteria, found in one genus of Archaea, and are also present in microbes of the Eukarya6. The most well characterized acetate kinase is that from the methane-producing archaeon Methanosarcina thermophila7-14. An acetate kinase which can only utilize PPi but not ATP in the acetyl phosphate-forming direction has been isolated from Entamoeba histolytica, the causative agent of amoebic dysentery, and has thus far only been found in this genus15,16.
In the direction of acetyl phosphate formation, acetate kinase activity is typically measured using the hydroxamate assay, first described by Lipmann17-20, a coupled assay in which conversion of ATP to ADP is coupled to oxidation of NADH to NAD+ by the enzymes pyruvate kinase and lactate dehydrogenase21,22, or an assay measuring release of inorganic phosphate after reaction of the acetyl phosphate product with hydroxylamine23. Activity in the opposite, acetate-forming direction is measured by coupling ATP formation from ADP to the reduction of NADP+ to NADPH by the enzymes hexokinase and glucose 6-phosphate dehydrogenase24.
Here we describe a method for the detection of acetate kinase activity in the direction of acetate formation that does not require coupling enzymes, but is instead based on direct determination of acetyl phosphate consumption. After the enzymatic reaction, remaining acetyl phosphate is converted to a ferric hydroxamate complex that can be measured spectrophotometrically, as for the hydroxamate assay. Thus, unlike the standard coupled assay for this direction that is dependent on the production of ATP from ADP, this direct assay can be used for acetate kinases that produce ATP or PPi.
Keywords: Molecular Biology, Issue 58, Acetate kinase, acetate, acetyl phosphate, pyrophosphate, PPi, ATP
Protocol
The overall scheme of this protocol is outlined in Figure 1.
1. Solution Preparation for Standard Curves and Assays
Prepare 100 mL of a 2 mol/L solution of hydroxylamine-HCl. Weigh out 13.9 g of hydroxylamine hydrochloride (MW 69.49 g/mol) and dissolve in approximately 50 mL distilled-deionized water (ddH2O). Adjust the pH to 7.0 using potassium hydroxide pellets or a concentrated solution. Bring the final volume to 100 mL. The solution can be stored at room temperature for up to 30 days or at 4 °C for up to 90 days.
Prepare 100 mL of a ferric chloride/ hydrochloric acid solution. Weigh out 13.5 g ferric chloride (MW 270.32 g/mol) and dissolve in approximately 50 mL ddH2O. Add 41.3 mL concentrated hydrochloric acid (12.1 mol/L) and bring to a final volume of 100 mL. The final concentration of ferric chloride will be 0.5 mol/L and the final concentration of hydrochloric acid will be 5 mol/L. The solution is stored at room temperature.
Prepare 100 mL of a 1.84 mol/L solution of trichoroacetic acid. Weigh out 30.0 g of trichloroacetic acid (MW 163.39 g/mol), dissolve in ddH2O and bring the final volume to 100 mL. The solution is stored at room temperature.
Prepare 5 mL of a 0.1 mol/L acetyl phosphate solution. Weigh out 0.092 g acetyl phosphate (MW 184.06 g/mol), dissolve in ddH2O and bring the final volume to 5 mL. Aliquot the solution to microcentrifuge tubes (0.25 mL per tube) and freeze at -20 °C until use. Thawed solution should be placed on ice and can be used for approximately 1 hour. Acetyl phosphate solution that has been thawed should not be refrozen and re-used.
Prepare 5 mL of a 0.1 mol/L solution of ADP. Weigh out 0.24 g of ADP (MW 472.5 g/mol), dissolve in ddH2O, and bring the final volume to 5 mL. Aliquot the solution in microcentrifuge tubes (0.5 mL per tube) and freeze at -20° C until use. Keep thawed solution on ice until use.
Prepare 50 mL of a 1 mol/L solution of Tris. Weigh out 6.06 g Tris (MW 121.14 g/mol), and dissolve in 40 mL ddH2O. Adjust the pH of the solution to 7.0 using hydrochloric acid and bring final volume to 50 mL. Store solution at room temperature.
Prepare 10 mL of a 1 mol/L magnesium chloride solution. Weigh out 2.03 g magnesium chloride (MW 203.31 g/mol), dissolve in ddH2O, and bring final volume to 10 mL. Store the solution at room temperature.
Prepare 25 mL development solution by mixing equal volumes of the ferric chloride/hydrochloric acid solution and the trichloroacetic acid solution. Development solution should be prepared fresh each day and stored at room temperature.
2. Preparation of an Acetyl Phosphate Standard Curve
An acetyl phosphate standard curve is generated using known amounts of acetyl phosphate. A standard curve of six to eight points is suitable. The standards should range from 0.03 μmoles to 0.9 μmoles per 300 μL, which correlates to final concentrations of 0.1 to 3 mmol/L. Dilute the acetyl phosphate stock solution to the appropriate concentrations in 100 mM Tris solution in a final volume of 300 μL. A control sample without acetyl phosphate should also be prepared.
Incubate each standard and the control at 37 °C in a heat block for 1 minute.
Add 50 μL of the hydroxylamine hydrochloride solution to each standard and the control and mix by shaking three times. Place the standard into a 60 °C heat block and incubate for five minutes.
Add 100 μL of development solution to the standards and the control, mix by shaking three times, and allow color to develop for at least one minute at room temperature.
Centrifuge all standards and the control in a microcentrifuge at 18,000 x g for 1 minute.
Measure the samples spectrophotometrically at 540 nm using 1mL plastic cuvettes, using the control as the blank for spectrophotometry.
Generate a standard curve using appropriate data analysis and graphing software (e.g. Kaleidagraph, Synergy Software). R2 values of 0.99 or greater are desirable. The data is plotted as absorbance at 540 nm versus μmoles of acetyl phosphate present.
3. Assay for Acetate Kinase Activity
Using the solutions described, prepare a reaction mix containing a final concentration of 0.1 mol/L Tris buffer, 10 mmol/L magnesium chloride, 5 mmol/L ADP, and 2 mmol/L acetyl phosphate. A 10 mL reaction mixture will require 8.2 mL ddH2O, 1.0 mL Tris solution, 0.1 mL magnesium chloride solution, 0.5 mL ADP solution, and 0.2 mL acetyl phosphate solution. Distribute the reaction mix in 300 μl aliquots to microcentrifuge tubes. For determination of kinetic parameters and optimization of substrate concentrations, one substrate can be varied and the other held at a constant concentration in the reactions.
Pre-incubate the reactions for 1 minute at 37 °C in a heat block.
Add a desired amount of enzyme to the reaction and immediately return the tube to the 37 °C heat block. Also include a control reaction to which no enzyme is added. If several reactions are performed, add enzyme to the tubes at specific time intervals. Be sure to keep track of time. (NOTE: The amount of enzyme to be used will need to be optimized to ensure that all of the acetyl phosphate or ADP substrate will not be depleted - see Discussion section)
After 5 minutes, add 50μl of the hydroxylamine hydrochloride solution to each reaction and the control, mix, and place the tubes in a 60 °C heat block. If several reactions are being performed, this should be done at the same time interval as the enzyme was added.
Incubate the reactions and the control for 5 minutes at 60 °C.
Add 100 μL of the development solution to each reaction and the control, mix, and place in a tube rack on the lab bench. Allow color to develop for at least 1 minute.
Centrifuge all tubes in a microcentrifuge at 18,000 x g for 1 minute.
Measure samples spectrophotometrically at 540 nm using 1mL plastic cuvettes and determine the amount of acetyl phosphate present by comparison to the acetyl phosphate standard curve.
The amount of acetyl phosphate substrate depleted can be determined by subtracting the amount of acetyl phosphate remaining after the enzymatic reaction from the amount of acetyl phosphate present in the control lacking enzyme. Perform data analysis using appropriate data and graphing software (e.g. Kaleidagraph, Synergy Software). R2 values of greater than 0.97 are desirable.
4. Representative Results:
The purpose of this assay is to measure acetate kinase activity in the direction of acetate formation. This is done by measuring consumption of the acetyl phosphate substrate, as there is no easy, direct measurement for acetate production. Acetyl phosphate remaining after a given time during the enzymatic reaction is converted to acetyl hydroxamate and subsequently to ferric hydroxamate complex, which has a reddish color (Figure 2) that can be measured at 540 nm. The standard curve plotting absorbance versus μmoles acetyl phosphate present in the standards is used to determine the amount of acetyl phosphate remaining in each reaction. The standard curve should be linear through the zero point. A representative standard curve shown in Figure 3 has a slope of 1.2332 absorbance units per μmole acetyl phosphate present and an R2 value of 0.99, indicating the data fits the linear equation well. The equation for the linear fit to the standard curve data is used for calculating the quantity of acetyl phosphate remaining in the reaction. The μmoles of acetyl phosphate consumed is determined as the difference between the μmoles of acetyl phosphate present in the control reaction lacking enzyme and the μmoles of acetyl phosphate remaining after the enzymatic reaction.
Purified, recombinant M. thermophila acetate kinase was assayed using the described protocol. For the experiment shown in Figure 4, the concentration of ADP was held constant at 5 mmol/L and the concentration of acetyl phosphate in the reaction was varied to determine the Km for acetyl phosphate. As expected, this experiment demonstrates that the enzyme follows standard Michaelis-Menten-like kinetics for each substrate and produced a hyperbolic saturation curve when plotting activity versus acetyl phosphate concentration. The apparent Km value for acetyl phosphate was 0.27 ± 0.01 mmol/L, which compares favorably to the published Km value of 0.47 ± 0.01 mmol/L using the coupled assay13.
The direct assay can also be used to measure activity of acetate kinases that utilize substrates other than ATP, which is not possible using the standard coupled assay. Purified recombinant E. histolytica acetate kinase16, which produces PPi rather than ATP in the acetate-forming direction, was subjected to this assay using sodium phosphate in place of ADP. The concentration of sodium phosphate in the reaction was held constant at 0.2 mol/L and the acetyl phosphate concentration was varied. As for the M. thermophila acetate kinase, the E. histolytica acetate kinase followed Michaelis-Menten-like kinetics for acetyl phosphate and a hyperbolic saturation curve was observed (Figure 5). The apparent Km value for acetyl phosphate was 0.50 ± 0.006 mmol/L. Reeves and Guthrie15 determined a Km value of 0.06 mmol/L for acetyl phosphate for the native enzyme; however, their enzyme was only partially purified and their assay involved four coupling enzymes which were also only partially purified. Thus, it is difficult to directly compare these values.
Protocol Scheme
Generate an acetyl phosphate standard curve
Dilute enzyme to desired concentration
Prepare the reaction mixture and aliquot 300 μl to microcentrifuge tubes
Preincubate reactions at 37 °C for 1 minute
Start reactions by adding enzyme at specific time intervals
Add hydroxylamine at the same intervals to stop the reactions and incubate at 60 °C for 5 minutes
Add ferric chloride/trichloroacetic acid solution for color development
Centrifuge tubes at 18,000 x g for 1 minute
Measure absorbance at 540 nm
Determine amount of acetyl phosphate consumed by comparison to the standard curve
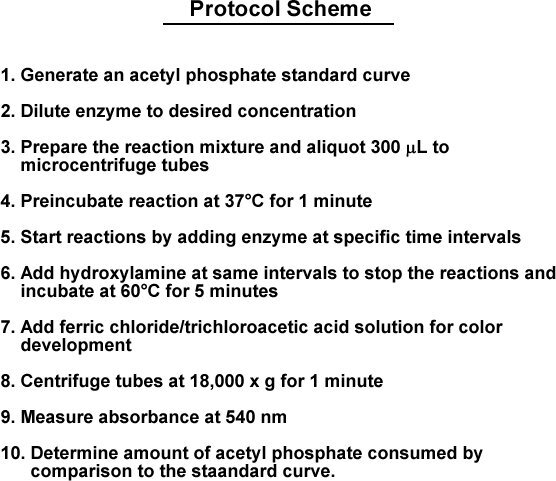 Figure 1.
Protocol Scheme
Figure 1.
Protocol Scheme
 Figure 2.
Acetyl phosphate standards. Increasing concentrations of acetyl phosphate were reacted with hydroxylamine and color was developed as described. The control lacking acetyl phosphate is bright yellow, and reactions containing increasing amounts of acetyl phosphate are successively darker in color. This color change is measured at 540 nm.
Figure 2.
Acetyl phosphate standards. Increasing concentrations of acetyl phosphate were reacted with hydroxylamine and color was developed as described. The control lacking acetyl phosphate is bright yellow, and reactions containing increasing amounts of acetyl phosphate are successively darker in color. This color change is measured at 540 nm.
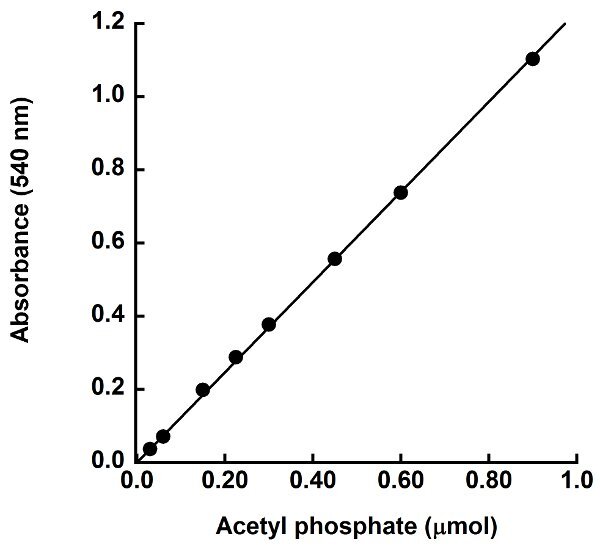 Figure 3. Sample acetyl phosphate standard curve. Varying amounts of acetyl phosphate were reacted with hydroxylamine and the color development solution and absorbance was measured at 540 nm. The absorbance at 540 nm versus μmoles acetyl phosphate was plotted and a linear fit to the data was applied.
Figure 3. Sample acetyl phosphate standard curve. Varying amounts of acetyl phosphate were reacted with hydroxylamine and the color development solution and absorbance was measured at 540 nm. The absorbance at 540 nm versus μmoles acetyl phosphate was plotted and a linear fit to the data was applied.
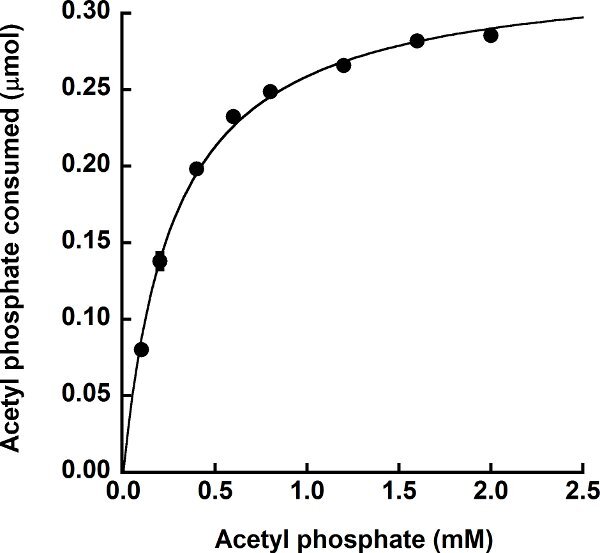 Figure 4.
Utilization of acetyl phosphate by the ATP-producing M. thermophila acetate kinase. Enzymatic activity was determined in the direction of acetate formation in the presence of 5 mmol/L ADP with varying concentrations of acetyl phosphate. The amount of acetyl phosphate consumed was determined as the difference between the starting amount of acetyl phosphate in the reaction and the amount of acetyl phosphate remaining after the enzymatic reaction. Assays were performed in triplicate with error bars shown.
Figure 4.
Utilization of acetyl phosphate by the ATP-producing M. thermophila acetate kinase. Enzymatic activity was determined in the direction of acetate formation in the presence of 5 mmol/L ADP with varying concentrations of acetyl phosphate. The amount of acetyl phosphate consumed was determined as the difference between the starting amount of acetyl phosphate in the reaction and the amount of acetyl phosphate remaining after the enzymatic reaction. Assays were performed in triplicate with error bars shown.
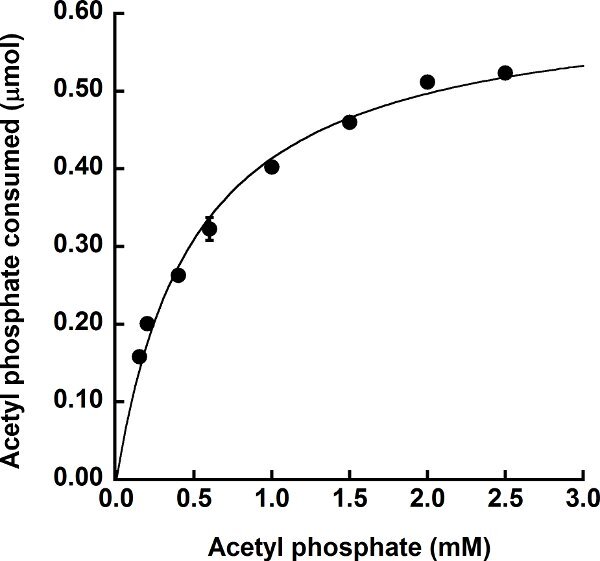 Figure 5.
Utilization of acetyl phosphate by the PPi-producing E. histolytica acetate kinase. Enzymatic activity in the direction of acetate formation was determined in the presence of 0.2 mol/L sodium phosphate with varying concentrations of acetyl phosphate. The amount of acetyl phosphate consumed was determined as the difference between the starting amount of acetyl phosphate in the reaction and the amount of acetyl phosphate remaining after the enzymatic reaction. Assays were performed in triplicate with error bars shown.
Figure 5.
Utilization of acetyl phosphate by the PPi-producing E. histolytica acetate kinase. Enzymatic activity in the direction of acetate formation was determined in the presence of 0.2 mol/L sodium phosphate with varying concentrations of acetyl phosphate. The amount of acetyl phosphate consumed was determined as the difference between the starting amount of acetyl phosphate in the reaction and the amount of acetyl phosphate remaining after the enzymatic reaction. Assays were performed in triplicate with error bars shown.
Discussion
The detection of acetyl phosphate in this assay is dependent upon a sufficient concentration of hydroxylamine hydrochloride and the concentration and acidity of the ferric chloride solution. Alteration of the assay volume will require reconsideration of both of these components. The enzymatic reactions described here were performed at 37°C with the hydroxylamine termination performed at 60 °C for 5 minutes. This higher temperature is critical to allow for rapid conversion of the remaining acetyl phosphate to acetyl hydroxamate. The timing of the color development step and measurement of absorbance is less critical, and can be performed in as little as 5 minutes and up to 15 minutes following the addition of the hydroxylamine solution. The samples should be centrifuged before reading the absorbance, as the acidity of the sample may lead to precipitation of the enzyme and can produce a falsely high reading due to turbidity rather than actual color change. Breakdown of the developed reactions will begin to occur after 10-15 minutes.
The primary complications of this assay for examining enzyme kinetics are determination of the amount of enzyme to use and the length of time to run the first step of the reaction. These must be determined empirically for each enzyme such that the level of activity is not so high that all of the acetyl phosphate substrate is consumed. Careful attention should be given to the percent acetyl phosphate consumption for each reaction point in a kinetic curve. Generally, lower concentrations of the varied component on a kinetic curve should result in acetyl phosphate consumption up to 90 percent or slightly above, while consumption of acetyl phosphate at saturating substrate concentrations should approach 10 percent or slightly less. Once the enzyme concentration is optimized, the substrate range for determination of kinetic constants can then be determined.
Previous methods for measuring acetate kinase activity in the acetate-forming direction involved the use of coupled assays. These methods are problematic with respect to kinetic calculations due to dependence on the activity of the coupling enzyme(s), whose assay conditions (pH, ionic strength, temperature, etc.) may be incompatible with the optimal assay conditions for the enzyme of interest. In addition, use of a direct assay for studies with inhibitors may avoid issues that could arise from inhibition of the coupling enzymes. Overall, this assay should be useful not only for acetate kinase, but for any enzyme that utilizes acetyl phosphate or other activated short chain acyl substrates such as acetyl- or propionyl-CoA, propionyl phosphate, and acetyl-AMP, all of which are reactive with hydroxylamine. In these cases, the standard curve would be generated with the appropriate acyl substrate instead of with acetyl phosphate.
Disclosures
No conflicts of interest declared.
Acknowledgments
This work was supported by NSF award # 0920274 and South Carolina Experiment Station Project (SC-1700340) to KSS. This paper is Technical Contribution No. 5929 of the Clemson University Experiment Station.
References
- Bork P, Sander C, Valencia A. An ATPase domain common to prokaryotic cell cycle proteins, sugar kinases, actin, and hsp70 heat shock proteins. Proc. Natl. Acad. Sci. U. S. A. 1992;89:7290–7294. doi: 10.1073/pnas.89.16.7290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bork P, Sander C, Valencia A. Convergent evolution of similar enzymatic function on different protein folds: the hexokinase, ribokinase, and galactokinase families of sugar kinases. Protein Sci. 1993;2:31–40. doi: 10.1002/pro.5560020104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss KA. Urkinase: structure of acetate kinase, a member of the ASKHA superfamily of phosphotransferases. J. Bacteriol. 2001;183:680–686. doi: 10.1128/JB.183.2.680-686.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley JH. The sugar kinase/heat shock protein 70/actin superfamily: implications of conserved structure for mechanism. Annu. Rev. Biophys. Biomol. Struct. 1996;25:137–162. doi: 10.1146/annurev.bb.25.060196.001033. [DOI] [PubMed] [Google Scholar]
- Holmes KC, Sander C, Valencia A. A new ATP-binding fold in actin, hexokinase and Hsc70. Trends. Cell. Biol. 1993;3:53–59. doi: 10.1016/0962-8924(93)90161-s. [DOI] [PubMed] [Google Scholar]
- Ingram-Smith C, Martin SR, Smith KS. Acetate kinase: not just a bacterial enzyme. Trends. Microbiol. 2006;14:249–253. doi: 10.1016/j.tim.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Aceti DJ, Ferry JG. Purification and characterization of acetate kinase from acetate-grown Methanosarcina thermophila. Evidence for regulation of synthesis. J. Biol. Chem. 1988;263:15444–15448. [PubMed] [Google Scholar]
- Latimer MT, Ferry JG. Cloning, sequence analysis, and hyperexpression of the genes encoding phosphotransacetylase and acetate kinase from Methanosarcina thermophila. J. Bacteriol. 1993;175:6822–6829. doi: 10.1128/jb.175.21.6822-6829.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram-Smith C, Barber RD, Ferry JG. The role of histidines in the acetate kinase from Methanosarcina thermophila. J. Biol. Chem. 2000;275:33765–33770. doi: 10.1074/jbc.M005303200. [DOI] [PubMed] [Google Scholar]
- Miles RD, Iyer PP, Ferry JG. Site-directed mutational analysis of active site residues in the acetate kinase from Methanosarcina thermophila. J. Biol. Chem. 2001;276:45059–45064. doi: 10.1074/jbc.M108355200. [DOI] [PubMed] [Google Scholar]
- Miles RD, Gorrell A, Ferry JG. Evidence for a transition state analog, MgADP-aluminum fluoride-acetate, in acetate kinase from Methanosarcina thermophila. J. Biol. Chem. 2002;277:22547–22552. doi: 10.1074/jbc.M105921200. [DOI] [PubMed] [Google Scholar]
- Ingram-Smith C. Characterization of the acetate binding pocket in the Methanosarcina thermophila acetate kinase. J. Bacteriol. 2005;187:2386–2394. doi: 10.1128/JB.187.7.2386-2394.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorrell A, Lawrence SH, Ferry JG. Structural and kinetic analyses of arginine residues in the active site of the acetate kinase from Methanosarcina thermophila. J. Biol. Chem. 2005;280:10731–10742. doi: 10.1074/jbc.M412118200. [DOI] [PubMed] [Google Scholar]
- Gorrell A, Ferry JG. Investigation of the Methanosarcina thermophila acetate kinase mechanism by fluorescence quenching. Biochemistry. 2007;46:14170–14176. doi: 10.1021/bi701292a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves RE, Guthrie JD. Acetate kinase (pyrophosphate). A fourth pyrophosphate-dependent kinase from Entamoeba histolytica. Biochem. Biophys. Res. Commun. 1975;66:1389–1395. doi: 10.1016/0006-291x(75)90513-6. [DOI] [PubMed] [Google Scholar]
- Fowler ML. Kinetic and structural characterization of the novel PPi-dependent acetate kinase from the parasite Entamoeba histolytica. 2011. Forthcoming.
- Lipmann F. Enzymatic synthesis of acetyl phosphate. J. Biol. Chem. 1944;155:55–70. [Google Scholar]
- Lipmann F, Tuttle LC. A specific micromethod for determination of acyl phosphates. J. Biol. Chem. 1945;159:21–28. [Google Scholar]
- Lipmann F, Jones ME, Black S, Flynn RM. The mechanism of the ATP-CoA-acetate reaction. J. Cell. Physiol. Suppl. 1953;41:109–112. doi: 10.1002/jcp.1030410408. [DOI] [PubMed] [Google Scholar]
- Rose IA, Grunberg-Manago M, Korey SF, Ochoa S. Enzymatic phosphorylation of acetate. J. Biol. Chem. 1954;211:737–756. [PubMed] [Google Scholar]
- Allen SH, Kellermeyer RW, Stjernholm RL, Wood HG. Purification and properties of enzymes involved in the proponic acid fermentation. Journal of Bacteriology. 1964;87:171–187. doi: 10.1128/jb.87.1.171-187.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke PM, Payton MA. An enzymatic assay for acetate in spent bacterial culture supernatants. Anal. Biochem. 1983;130:402–405. doi: 10.1016/0003-2697(83)90607-3. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay S, Hasson MS, Sanders DA. A continuous assay of acetate kinase activity: measurement of inorganic phosphate release generated by hydroxylaminolysis of acetyl phosphate. Bioorg. Chem. 2008;36:65–69. doi: 10.1016/j.bioorg.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima H, Suzuki K, Imahori K. Purification and properties of acetate kinase from Bacillus stearothermophilus. J. Biochem. 1978;84:193–203. doi: 10.1093/oxfordjournals.jbchem.a132108. [DOI] [PubMed] [Google Scholar]


