Abstract
Many devastating inherited eye diseases result in progressive and irreversible blindness because humans cannot regenerate dying or diseased retinal neurons. In contrast, the adult zebrafish retina possesses the robust ability to spontaneously regenerate any neuronal class that is lost in a variety of different retinal damage models, including retinal puncture, chemical ablation, concentrated high temperature, and intense light treatment 1-8. Our lab extensively characterized regeneration of photoreceptors following constant intense light treatment and inner retinal neurons after intravitreal ouabain injection 2, 5, 9. In all cases, resident Müller glia re-enter the cell cycle to produce neuronal progenitors, which continue to proliferate and migrate to the proper retinal layer, where they differentiate into the deficient neurons.
We characterized five different stages during regeneration of the light-damaged retina that were highlighted by specific cellular responses. We identified several differentially expressed genes at each stage of retinal regeneration by mRNA microarray analysis 10. Many of these genes are also critical for ocular development. To test the role of each candidate gene/protein during retinal regeneration, we needed to develop a method to conditionally limit the expression of a candidate protein only at times during regeneration of the adult retina.
Morpholino oligos are widely used to study loss of function of specific proteins during the development of zebrafish, Xenopus, chick, mouse, and tumors in human xenografts 11-14. These modified oligos basepair with complementary RNA sequence to either block the splicing or translation of the target RNA. Morpholinos are stable in the cell and can eliminate or "knockdown" protein expression for three to five days 12.
Here, we describe a method to efficiently knockdown target protein expression in the adult zebrafish retina. This method employs lissamine-tagged antisense morpholinos that are injected into the vitreous of the adult zebrafish eye. Using electrode forceps, the morpholino is then electroporated into all the cell types of the dorsal and central retina. Lissamine provides the charge on the morpholino for electroporation and can be visualized to assess the presence of the morpholino in the retinal cells.
Conditional knockdown in the retina can be used to examine the role of specific proteins at different times during regeneration. Additionally, this approach can be used to study the role of specific proteins in the undamaged retina, in such processes as visual transduction and visual processing in second order neurons.
Keywords: Developmental Biology, Issue 58, Electroporation, morpholino, zebrafish, retina, regeneration
Protocol
1. Morpholino preparation:
All morpholino oligos should be custom-designed and ordered through GeneTools (Philomath, OR). We use the positively-charged lissamine-tagged morpholinos to drive the electroporated morpholinos towards the negative electrode. We tried, unsuccessfully, to target negatively-charged fluorescein-tagged morpholinos to the positive electrode using this technique. Dilute 300 nmol of morpholino into 100 μl of nuclease-free water to yield a 3 mM solution. Aliquot the morpholino into multiple paraffin-sealed microcentrifuge tubes. Store away from light at room temperature.
Determine the morpholino concentration by diluting 5 μl of morpholino (or water used to dilute the morpholino as a blank) in 95 μl of 0.1 N HCl. Set the spectrophotometer wavelength (λ) to 265 nm. Calculate the constant for your morpholino using the following equation: Constant = molecular weight particular to your morpholino X 1000/molar absorbance.
You will find the molecular weight and molar absorbance data on the "Oligo Properties" sheet provided with each morpholino. Analyze the blank to verify baseline readings and take readings of each diluted morpholino. Multiply the absorbance at 265 nm of each morpholino by its determined constant and by the dilution factor to get the concentration in ng/μl. Divide this number by the molecular weight to determine the concentration in mM. Dilute the morpholino, if necessary, to a working concentration.
2. Remove outer corneal layer:
Anesthetize 6-12 month old adult zebrafish in Tricaine or 2-phenoxyethanol at 1.0 mg/ml in zebrafish tank water.
Wrap zebrafish in a moistened piece of paper towel, covering the gills, but leaving the eye exposed. The wrapped fish is placed under a stereoscope and the magnification is increased until the diameter of the eye fills approximately one-third of the visual field (Figure 1).
Using Dumont #5 tweezers, grab the outer cornea near the optic fissure. This tissue is colored in red and is labeled "OC" in Figure 1. There is a slight protrusion or lip indicated by the arrow labeled "CL". Pull at a low angle (i.e. 10 degrees) across the eye to remove the outer cornea. With practice, this can be completed in one motion. If the tweezers slip, re-grab the tissue and again pull at a low angle until removed. A well-attached cornea can cause the eye to be dislodged from the socket when pulled. You can keep the eye from becoming dislodged by steadying with another set of tweezers.
3. Corneal incision and morpholino injection:
Immediately following the removal of the outer cornea, make a small incision in the cornea where the pupil meets the iris (Figure 2). This is done using a sapphire blade scalpel (See Materials).
Load 0.5 μl of the 3.0 mM morpholino solution into a Hamilton syringe equipped with a 33 gauge removable, blunt-end needle (See Materials). Pipette up and down to remove air bubbles in the line. Carefully insert the needle into the vitreous through the incision (Figure 2). Do not insert the needle too far or you will puncture the retina or displace the lens.
Slowly inject the morpholino solution into the vitreal space. Depending on the age of the fish, the vitreal space will hold ~ 0.5 μl. You should visualize uncolored vitreous leaking out of the incision as it is displaced by the morpholino solution. Inject until a small amount of morpholino solution leaks out of the incision. You should clearly visualize the lissamine-tagged morpholino inside the eye.
Following the injection, return the fish to the tank to recover. We usually only inject the left eye, using the right eye as an uninjected control, but electroporation of both eyes is possible.
4. Electroporation:
Following the injection of approximately 10 fish, re-anesthetize an injected fish and wrap it in a moistened piece of paper towel, just covering the gills, but leaving the eye exposed. Transfer the fish to a Petri dish. Put on latex or similar gloves to avoid exposure to the anesthesia. While gently holding the fish to the bottom of the dish, fill the dish with anesthesia (Figure 3, left panel).
A 3-mm-diameter platinum plate electrode (See Materials) localizes the electroporation pulses to approximately one-half of the retina. We typically target the dorsal retina. Gently press the positive electrode downward on the ventral half of the eye. With the outer cornea removed, the eye is easily rotated, exposing the dorsal portion of the eyeball.
While still pressing down on the ventral side of the eye, place the negative electrode near (~1 - 2 mm) the exposed dorsal half of the eye. Be careful not to touch the negative electrode directly to the eye, which will result in damaging the dorsal retina. Once you determine the appropriate distance between the electrodes, use the adjustment screw on the handle of the electrodes to maintain that spread when electroporating. This keeps the negative electrode an optimal distance from the eyeball when the positive electrode is pressed down in position.
Electroporate the eye using a CUY21 Square Wave Electroporator (See Materials). The electroporation parameters should be set to two consecutive 50-msec pulses, at 75 V with a 1-sec pause between pulses.
Return fish to the tank. We typically start our protocol for constant light-induced retinal degeneration immediately following electroporation.
5. Representative results:
For 3-5 days following the electroporation (depending on the stability of the morpholino), the lissamine label can be visualized in all of the retinal layers in a sectioned retina (Figure 4). This serves as a confirmation for the presence of the labeled morpholino.
We can achieve full protein knockdown up to 3-5 days following electroporation, depending of the efficacy of the morpholino (Figure 5). Typically, during light treatment studies, we harvest eyes at 3 or 4 days after morpholino electroporation. This is done by removing the entire eye with Dumont #5B forceps with curved ends (Figure 6; Materials) and processing the tissue for immunohistochemistry 15. As with any knockdown technique, a demonstration of efficient knockdown must be shown in order to validate the phenotype. Therefore, we suggest obtaining antisera for your protein of interest. Alternatively, if and antibody is not available and the morpholino is directed to either the splice acceptor or donor site in the pre-mRNA, it will block efficient splicing of the mRNA. In this case, it is possible to use reverse transcriptase-PCR to amplify the flanking sequences in the morphant RNA and determine the efficiency of blocking the normal splicing pattern.
When the retinas are sectioned from nasal to temporal on the dorsal ventral axis, the shaded blue boxes (Figure 6) represent the areas of the retina that are most often damaged from the electroporation event itself. The shaded pink box represents the area that is targeted for morpholino introduction into retinal cells by the electroporation.
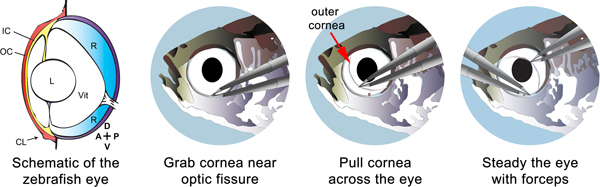 Figure 1. Schematic depicting the removal of the epithelial layers of the cornea. The left panel shows the major structural features of the eye, including the lens (L), the vitreous (Vit), the retina (R), the outer cornea (OC), the inner cornea (IC), and the corneal protrusion (CL). The orientation of the eye is shown in the lower right of the first panel and is labeled anterior (A), posterior (P), dorsal (D), and ventral (V). The red coloration indicates the outer cornea that is removed. The second panel shows how the forceps are used to grasp the corneal protrusion and to pull the outer cornea across the eye at a low angle (third panel). The right panel shows how a second pair of forceps can be used to steady the eye while pulling the cornea.
Figure 1. Schematic depicting the removal of the epithelial layers of the cornea. The left panel shows the major structural features of the eye, including the lens (L), the vitreous (Vit), the retina (R), the outer cornea (OC), the inner cornea (IC), and the corneal protrusion (CL). The orientation of the eye is shown in the lower right of the first panel and is labeled anterior (A), posterior (P), dorsal (D), and ventral (V). The red coloration indicates the outer cornea that is removed. The second panel shows how the forceps are used to grasp the corneal protrusion and to pull the outer cornea across the eye at a low angle (third panel). The right panel shows how a second pair of forceps can be used to steady the eye while pulling the cornea.
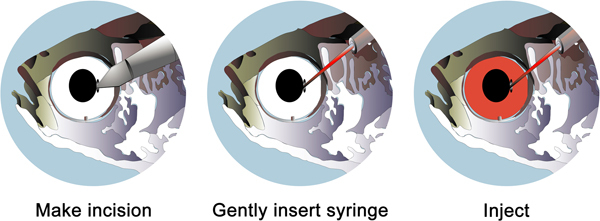 Figure 2. Schematic of the steps involved in the intravitreal injection of morpholino. A small incision is made in the eye where the pupil meets the iris (left panel). The resulting gap should be just large enough for a 33 gauge needle, which is filled with the morpholino solution, to be inserted (middle panel). Slowly inject 0.5 μl of solution into the vitreous (right panel). You should visualize a small amount of vitreal solution come out of the incision as the vitreous is partially replaced by with lissamine-tagged morpholino (right panel).
Figure 2. Schematic of the steps involved in the intravitreal injection of morpholino. A small incision is made in the eye where the pupil meets the iris (left panel). The resulting gap should be just large enough for a 33 gauge needle, which is filled with the morpholino solution, to be inserted (middle panel). Slowly inject 0.5 μl of solution into the vitreous (right panel). You should visualize a small amount of vitreal solution come out of the incision as the vitreous is partially replaced by with lissamine-tagged morpholino (right panel).
 Figure 3. Schematic of the general electroporation procedure. The anesthetized fish, which was intravitreally injected with the morpholino, is gently wrapped in damp paper towel (left panel). The paper towel should cover the gills, but not obstruct the eye (middle panel). Gently press the positive electrode down on the ventral part of the eye, allowing the dorsal half of the eye to rotate out of the socket. Place the negative electrode approximately 1 mm from the dorsal half of the eye and start the electroporation (right panel).
Figure 3. Schematic of the general electroporation procedure. The anesthetized fish, which was intravitreally injected with the morpholino, is gently wrapped in damp paper towel (left panel). The paper towel should cover the gills, but not obstruct the eye (middle panel). Gently press the positive electrode down on the ventral part of the eye, allowing the dorsal half of the eye to rotate out of the socket. Place the negative electrode approximately 1 mm from the dorsal half of the eye and start the electroporation (right panel).
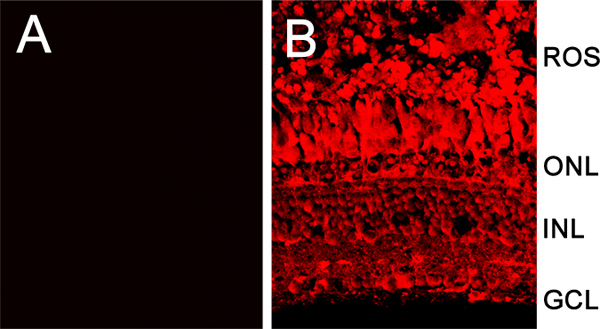 Figure 4. Confocal image comparing a retina electroporated without intravitreally injecting a lissamine-tagged morpholino (A), and a retina intravitreally injected and electroporated with the lissamine-tagged morpholino (B). The lissamine label is found in all the retinal layers and cell types. ROS, rod outer segments; ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer.
Figure 4. Confocal image comparing a retina electroporated without intravitreally injecting a lissamine-tagged morpholino (A), and a retina intravitreally injected and electroporated with the lissamine-tagged morpholino (B). The lissamine label is found in all the retinal layers and cell types. ROS, rod outer segments; ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer.
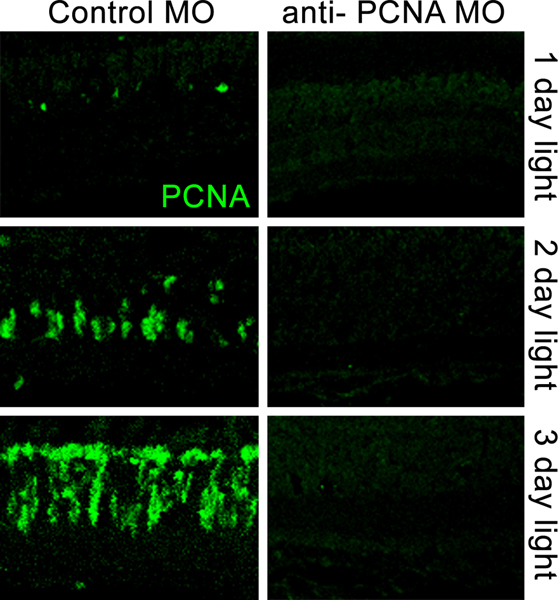 Figure 5. Confocal images comparing PCNA immunolocalization in retinas electroporated with a control morpholino (left panels) and retinas electroporated with an anti-PCNA morpholino (right panels) prior to light-induced photoreceptor cell death. Dark-adapted adult albino zebrafish were placed in constant intense light for 1, 2, or 3 days after electroporation of the morpholino. The control morpholino failed to repress increasing PCNA expression in the light-damaged retinas. The anti-PCNA morpholino efficiently knocked down PCNA protein expression through three days of constant intense light damage.
Figure 5. Confocal images comparing PCNA immunolocalization in retinas electroporated with a control morpholino (left panels) and retinas electroporated with an anti-PCNA morpholino (right panels) prior to light-induced photoreceptor cell death. Dark-adapted adult albino zebrafish were placed in constant intense light for 1, 2, or 3 days after electroporation of the morpholino. The control morpholino failed to repress increasing PCNA expression in the light-damaged retinas. The anti-PCNA morpholino efficiently knocked down PCNA protein expression through three days of constant intense light damage.
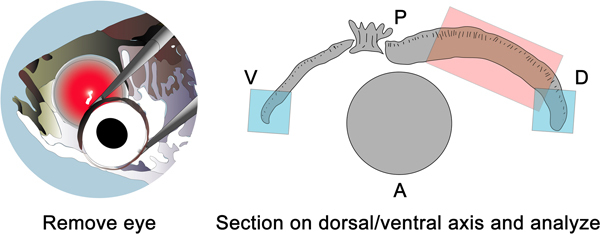 Figure 6. Schematic (left panel) showing enucleation of the eye for processing and cryosectioning, which is done along the dorsal ventral axis. The pink shaded box (right panel) shows the retinal region with the greatest amount of protein knockdown using this technique. The blue shaded boxes represent areas that are most often damaged by the electroporation event. The orientation of the eye is labeled anterior (A), posterior (P), dorsal (D), and ventral (V).
Figure 6. Schematic (left panel) showing enucleation of the eye for processing and cryosectioning, which is done along the dorsal ventral axis. The pink shaded box (right panel) shows the retinal region with the greatest amount of protein knockdown using this technique. The blue shaded boxes represent areas that are most often damaged by the electroporation event. The orientation of the eye is labeled anterior (A), posterior (P), dorsal (D), and ventral (V).
Discussion
Recently, two microarray analyses examined the gene expression changes that occurred during the regeneration of either the light-damaged retina or a surgically-excised retinal patch10, 16. Both studies revealed numerous genes that exhibited significant changes in expression, either increasing or decreasing, that likely are required for different events that occur during retinal regeneration, such as re-entry of the Müller glia into the cell cycle, continued proliferation and migration of the neuronal progenitors to the damaged retinal layer, and the differentiation of the neuronal progenitors into the correct neuronal cell type. While a direct comparison of these two microarray data sets reveal candidate genes that may be involved in these cellular events, ultimately these genes and their encoded proteins must be tested to determine if they are necessary for neuronal regeneration. Here, we describe a powerful loss-of-function approach to conditionally knockdown proteins of interest in the adult zebrafish retina. This technique has been used to study both extracellular and intracellular proteins, as well as signaling molecules, transcription factors and proteins required for DNA replication 17-19. Thus, this method has greatly aided our understanding of the molecular mechanism underlying adult retinal regeneration in zebrafish.
We tested multiple electroporation parameters (voltage, number of pulses, time between pulses) before a successful protocol was achieved. A previous report of electroporating morpholinos into the regenerating zebrafish caudal fin used 10 pulses at a low voltage (15 V) 20. Because the adult zebrafish retina is surrounded by multiple cell layers and is not readily accessible to tweezer electrodes, the parameters used in the fin were unsuccessful at efficiently electroporating morpholinos into the adult retina. Conversely, a high voltage (100 V) often resulted in death to the fish. Recently, 5 pulses of 80 V was described to electroporate plasmid DNA into the newborn mouse pup retina 21. We found that a slightly less intense 2 pulses of 75 V resulted in successful electroporation of the morpholinos into the adult zebrafish retina with survival of 100% of the treated fish. Additionally, we discovered that the removal of the outer most (or external-most) component of the cornea greatly increased the electroporation efficiency. Increasing the number of pulses may eliminate the need for removal of this tissue but may also cause greater tissue damage. Extensive control studies demonstrated that these parameters did not cause any significant retinal cell death or alter the specific cellular responses observed during photoreceptor regeneration 19. Gain-of-function studies are not currently possible using this technique in vivo, due to our inability to consistently electroporate large ribonucleic acids into all retinal cells. However, the success of electroporating plasmids into the mouse retina in vivo and the zebrafish retina in explants suggests it is still a possibility in zebrafish 21, 22.
One of the strengths of this technique is the electroporation appears to efficiently introduce the morpholino into all of the different retinal cell types. However, one potential weakness was that the electroporation efficiently delivered the morpholino into only the dorsal and central retinal regions. A second electroporation event toward the ventral half of the retina results in good targeting to all layers, but often results in damage. This spatial restriction likely was due to the shape and placement of the electrodes. The use of custom-designed cup shaped electrodes that could be placed around the eye may improve this weakness. This spatial restriction of the morpholino delivery limits the use of this technique and precludes the use of an assay such as ERG analysis that would require a global assessment of the retina. It should be noted that as with any morpholino experiment, it is advisable to confirm your results using a second, non-overlapping morpholino to the target mRNA. In some cases, it is possible to also electroporate two different morpholinos simultaneously to knockdown the expression of two different proteins. We demonstrated this by knocking down the expression of both the Pax6a and Pax6b proteins, individually and in combination 18.
Although we demonstrated the use of this technique with our light damage model, it could likely be used to study regeneration in other damage models 2-8 or used to examine the function of proteins in the undamaged retina. For example, electroporation of morpholinos could be used to knockdown the expression of specific channels or signal transduction molecules in ganglion or amacrine cells and then study the function of these signaling pathways in visual processing. However, as morpholinos only affect translation of new protein, one would have to wait until endogenous protein turnover occurs before an assay could be performed. Depending on the stability of the protein, that could range from hours to days 18.
Disclosures
We have nothing to disclose
Acknowledgments
The authors would like to thank the Freimann Life Science Center and Center for Zebrafish Research staff for their care and maintenance of the zebrafish.
References
- Fausett BV, Gumerson JD, Goldman D. The proneural basic helix-loop-helix gene ascl1a is required for retina regeneration. J. Neurosci. 2008;28:1109–1117. doi: 10.1523/JNEUROSCI.4853-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fimbel SM, Montgomery JE, Burket CT, Hyde DR. Regeneration of inner retinal neurons after intravitreal injection of ouabain in zebrafish. J. Neurosci. 2007;27:1712–1724. doi: 10.1523/JNEUROSCI.5317-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P, Ochocinska M, Sieh A, Otteson D. Persistent and injury-induced neurogenesis in the vertebrate retina. Prog. Retin. Eye Res. 2004;23:183–194. doi: 10.1016/j.preteyeres.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Raymond PA, Barthel LK, Bernardos RL, Perkowski JJ. Molecular characterization of retinal stem cells and their niches in adult zebrafish. B.M.C Dev. Biol. 2006;6:36–36. doi: 10.1186/1471-213X-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vihtelic TS, Hyde DR. Light-induced rod and cone cell death and regeneration in the adult albino zebrafish (Danio rerio) retina. J. Neurobiol. 2000;44:289–307. doi: 10.1002/1097-4695(20000905)44:3<289::aid-neu1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Wolburg H. Time- and dose-dependent influence of ouabain on the ultrastructure of optic neurones. Cell Tissue Res. 1975;164:503–517. doi: 10.1007/BF00219941. [DOI] [PubMed] [Google Scholar]
- Wu DM, Schneiderman T, Burgett J, Gokhale P, Barthel L, Raymond PA. Cones regenerate from retinal stem cells sequestered in the inner nuclear layer of adult goldfish retina. Invest. Ophthalmol. Vis. Sci. 2001;42:2115–2124. [PubMed] [Google Scholar]
- Yurco P, Cameron DA. Responses of Muller glia to retinal injury in adult zebrafish. Vision Res. 2005;45:991–1002. doi: 10.1016/j.visres.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Vihtelic TS, Soverly JE, Kassen SC, Hyde DR. Retinal regional differences in photoreceptor cell death and regeneration in light-lesioned albino zebrafish. Exp. Eye Res. 2006;82:558–575. doi: 10.1016/j.exer.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Kassen SC, Ramanan V, Montgomery JE, Burket CT, Liu CG, Vihtelic TS, Hyde DR. Time course analysis of gene expression during light-induced photoreceptor cell death and regeneration in albino zebrafish. Dev. Neurobiol. 2007;67:1009–1031. doi: 10.1002/dneu.20362. [DOI] [PubMed] [Google Scholar]
- Heasman J, Kofron M, Wylie C. Beta-catenin signaling activity dissected in the early Xenopus embryo: a novel antisense approach. Dev. Biol. 2000;222:124–134. doi: 10.1006/dbio.2000.9720. [DOI] [PubMed] [Google Scholar]
- Nasevicius A, Ekker SC. Effective targeted gene 'knockdown' in zebrafish. Nat. Genet. 2000;26:216–220. doi: 10.1038/79951. [DOI] [PubMed] [Google Scholar]
- Coonrod SA, Bolling LC, Wright PW, Visconti PE, Herr JC. A morpholino phenocopy of the mouse mos mutation. Genesis. 2001;30:198–200. doi: 10.1002/gene.1065. [DOI] [PubMed] [Google Scholar]
- London CA, Sekhon HS, Arora V, Stein DA, Iversen PL, Devi GR. A novel antisense inhibitor of MMP-9 attenuates angiogenesis, human prostate cancer cell invasion and tumorigenicity. Cancer Gene Ther. 2003;10:823–832. doi: 10.1038/sj.cgt.7700642. [DOI] [PubMed] [Google Scholar]
- Thummel R, Kassen SC, Enright JM, Nelson CM, Montgomery JE, Hyde DR. Characterization of Muller glia and neuronal progenitors during adult zebrafish retinal regeneration. Exp. Eye Res. 2008;87:433–444. doi: 10.1016/j.exer.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron DA, Gentile KL, Middleton FA, Yurco P. Gene expression profiles of intact and regenerating zebrafish retina. Mol. Vis. 2005;11:775–791. [PubMed] [Google Scholar]
- Craig SE, Thummel R, Ahmed H, Vasta GR, Hyde DR, Hitchcock PF. The zebrafish galectin Drgal1-l2 is expressed by proliferating Muller glia and photoreceptor progenitors and regulates the regeneration of rod photoreceptors. Invest. Ophthalmol. Vis. Sci. 2010;51:3244–3252. doi: 10.1167/iovs.09-4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thummel R, Enright JM, Kassen SC, Montgomery JE, Bailey TJ, Hyde DR. Pax6a and Pax6b are required at different points in neuronal progenitor cell proliferation during zebrafish photoreceptor regeneration. Exp. Eye Res. 2010;90:572–582. doi: 10.1016/j.exer.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thummel R, Kassen SC, Montgomery JE, Enright JM, Hyde DR. Inhibition of Muller glial cell division blocks regeneration of the light-damaged zebrafish retina. Dev. Neurobiol. 2008;68:392–408. doi: 10.1002/dneu.20596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thummel R, Bai S, Sarras MP, Song P, McDermott J, Brewer J, Perry M, Zhang X, Hyde DR, Godwin AR. Inhibition of zebrafish fin regeneration using in vivo electroporation of morpholinos against fgfr1 and msxb. Dev. Dyn. 2006;235:336–346. doi: 10.1002/dvdy.20630. [DOI] [PubMed] [Google Scholar]
- Matsuda T, Cepko CL. Electroporation and RNA interference in the rodent retina in vivo and in vitro. Proc. Natl. Acad. Sci U.S.A. 2004;101:16–22. doi: 10.1073/pnas.2235688100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kustermann S, Schmid S, Biehlmaier O, Kohler K. Survival, excitability, and transfection of retinal neurons in an organotypic culture of mature zebrafish retina. Cell Tissue Res. 2008;332:195–209. doi: 10.1007/s00441-008-0589-5. [DOI] [PubMed] [Google Scholar]


