Abstract
Platelet characteristics, such as platelet dose, platelet source (apheresis vs pooled), platelet donor-recipient ABO compatibility, and duration of platelet storage, can affect posttransfusion platelet increments, but it is unclear whether these factors impact platelet transfusion efficacy on clinical bleeding. We performed secondary analyses of platelet transfusions given in the prospective randomized Platelet Dose Study, which included 1272 platelet-transfused hematology-oncology patients who received 6031 prophylactic platelet transfusions. The primary outcome of these analyses was time from first transfusion to first World Health Organization ≥ grade 2 bleeding. Platelet transfusion increments were assessed at 0.25 to 4 hours and 16 to 32 hours after platelet transfusion. There were 778 patients evaluable for analysis of time to bleeding. Adjusted models showed that randomized dose strategy, platelet source, ABO compatibility, and duration of storage did not predict this outcome. Platelet increments were generally higher for transfusions of apheresis platelets, ABO-identical platelets, and platelets stored 3 days versus 4 to 5 days. Thus, although platelet source, ABO compatibility, and duration of storage exert a modest impact on both absolute and corrected posttransfusion platelet increments, they have no measurable impact on prevention of clinical bleeding. This trial was registered at www.clinicaltrials.gov as #NCT00128713.
Introduction
The effectiveness of a platelet transfusion can be variable as a result of differences in platelet dose, platelet source (apheresis [AP] vs whole blood platelet pools [WBP]), platelet donor-recipient ABO compatibility, and duration of platelet storage. Each of these characteristics can affect posttransfusion platelet increments, but it is unclear whether these factors impact the efficacy of platelet transfusions on clinical bleeding.
The Platelet Dose Study (PLADO) is a recently completed large multicenter trial that compared 3 different dosing strategies for prophylactic platelet transfusions in hematology-oncology patients: lower dose (LD), medium dose (MD), and higher dose (HD) per transfusion. The study used bleeding as the primary outcome and found no difference in the incidence of World Health Organization (WHO) ≥ grade 2 bleeding among the 3 study arms (LD, 71%; MD, 69%; and HD, 70%).1
The PLADO study involved 1272 platelet-transfused patients who received 8994 platelet transfusions, including 6031 prophylactic platelet transfusions, and patients were observed for 24 309 days. The PLADO database provides an opportunity to examine the impact of platelet characteristics, such as platelet source, platelet ABO compatibility, and duration of platelet storage, not only on platelet increments, but also on the more important clinical outcome of bleeding.
Methods
The PLADO study was a multicenter randomized controlled trial conducted by the National Heart, Lung, and Blood Institute Transfusion Medicine/Hemostasis Clinical Trials Network.1 Briefly, adult and pediatric patients undergoing hematologic stem cell transplantation or chemotherapy for hematologic or solid organ malignancy were randomized to 1 of 3 prophylactic platelet dosing strategies, 1.1 × 1011 (LD), 2.2 × 1011 (MD), or 4.4 × 1011 (HD) platelets per square meter of body surface area for each prophylactic platelet transfusion. The primary study end point was the percentage of patients with WHO ≥ grade 2 bleeding.2 Primary patient exclusions were ≥ grade 2 bleeding at presentation, platelet refractoriness within the last 30 days before study entry, known HLA antibody panel reactivity ≥ 20%, partial thromboplastin time > 1.3 times the upper limit of normal, fibrinogen < 100 mg/dL, or previous platelet transfusions for thrombocytopenia during the current hospitalization.
For each patient, the data coordinating center communicated to the blood bank the assigned platelet dose. Neither patient care nor research staff were informed of the study arm or assigned dose. All platelet and red cell components were leukoreduced. Sites could select either leukoreduced AP or leukoreduced WBP for transfusions but were asked to keep patients on the same type of platelet component while on study. A product platelet count was obtained at issue. Prophylactic platelet transfusions were given for morning counts of ≤ 10 000/μL. The patient's physician could change the platelet transfusion threshold and/or platelet dose based on clinical circumstances. After resolution of the clinical condition, the 10 000/μL threshold and/or the assigned dose were resumed.
Platelet ABO selection was based on local practice. In general, ABO-identical platelets were preferred. Platelet ABO matching categories were defined as follows: ABO-identical, platelet donor and recipient have the same ABO red cell/platelet antigens and plasma antibodies; ABO minor mismatch, donor's plasma ABO antibodies are incompatible with recipient's red cell/platelet ABO antigens (eg, O donor anti-A given to A recipient with A antigens); and ABO major mismatch, donor's red cell/platelet ABO antigens are incompatible with recipient's plasma ABO antibodies (eg, A donor A antigen given to O recipient with anti-A).
Platelets were stored under standard blood bank conditions for up to 5 days, except for a brief period during the study when the Food and Drug Administration allowed up to 7-day storage for AP only. Usually, the oldest platelets were issued first.
Patients were assessed daily by research staff for bleeding events using physical examination, patient interview, and medical record review, and the results were recorded on a standardized data collection form. Data forms were sent to the data coordinating center where a daily WHO bleeding grade was calculated by computer algorithm (Table 1). Platelet counts were measured daily, and a posttransfusion platelet count was obtained within 4 hours after each platelet transfusion. Study participation was completed as soon as the first of any of the following events occurred: hospital discharge, 10 days without a platelet transfusion, 30 days after the first platelet transfusion, death, or withdrawal from the study.
Table 1.
WHO bleeding grades
| Score | Type of bleeding |
|---|---|
| 0 | None |
| 1 | Petechiae, ecchymosis, occult blood in body secretions, mild vaginal spotting |
| 2 | Evidence of gross hemorrhage not requiring RBC transfusion over routine transfusion needs: epistaxis, hematuria, hematemesis |
| 3 | Hemorrhage requiring transfusion of 1 or more units of RBCs/day |
| 4 | Life-threatening hemorrhage, defined as either massive bleeding causing hemodynamic compromise or bleeding into a vital organ (eg, intracranial, pericardial, or pulmonary hemorrhage) |
WHO indicates World Health Organization; and RBC, red blood cell.
Statistical analyses
Bleeding outcome analyses.
Many patients received multiple platelet transfusions on study. The transfusions each patient received frequently differed in source, storage duration, and ABO matching. This was more likely to occur in patients who received multiple platelet transfusions. To include the maximum number of patients and minimize introducing a bias from excluding more heavily transfused patients, analyses of the associations between platelet characteristics and bleeding used a time-to-event approach, with the number of days from first platelet transfusion to first occurrence of ≥ grade 2 bleeding chosen as the primary bleeding outcome. Comparisons of platelet characteristics were made using frailty models.3,4 These models were adjusted for sex, age group (< 10 years, 10-19 years, 20-29 years, and 10-year increments through ≥ 70 years), and treatment stratum (allogeneic transplantation, autologous transplantation, chemotherapy without transplantation for either hematologic malignancy or solid tumors), and for site as a random effect. Patients were categorized by characteristics of their first platelet transfusion. Unless ≥ grade 2 bleeding had already occurred, time to bleeding was considered censored on the first date that any of the following occurred: (1) the patient received a transfusion that differed from the first transfusion on the characteristic(s) analyzed; (2) the patient received a transfusion that had mixed or missing data on the characteristic(s) analyzed; (3) the patient had missing data on whether ≥ grade 2 bleeding occurred; and (4) the patient ended the study.
That is, each patient could contribute data to the time-to-bleeding analysis until their first date with ≥ grade 2 bleeding or until their censoring date, whichever occurred first. Patients were excluded from the analysis of days to ≥ grade 2 bleeding if: (1) they experienced ≥ grade 2 bleeding before, or on the day of, the date of their first platelet transfusion; (2) their first platelet transfusion had mixed or missing data on the characteristic analyzed; (3) they received multiple transfusions on the date of their first platelet transfusion that differed on the characteristic analyzed; (4) they received any HLA-selected platelet units while on study; and (5) the platelets in their first transfusion were stored 6 to 7 days. Only 20 patients received such transfusions, and only 9 had a first platelet transfusion stored 6 to 7 days.
Similar analyses were done to examine the relationship between each of the platelet characteristics and days from first platelet transfusion to ≥ grade 3 bleeding.
Platelet increment analyses.
The “pre-transfusion” platelet count for both the “4-hour” and “24-hour” platelet increment, and corrected count increment (CCI) was defined as the closest platelet count before the start of the transfusion, but after completion of any previous platelet transfusion, granulocyte transfusion, or stem cell infusion. The 4-hour posttransfusion count was the first posttransfusion count taken within 4 hours after the end of the platelet transfusion, but before any subsequent transfusion, granulocyte transfusion, or stem cell infusion began. The 24-hour posttransfusion count was based on a platelet count taken between 16 and 32 hours after the end of the transfusion but before any subsequent transfusion. If there were multiple such counts, the count taken closest to 24 hours after the end of the platelet transfusion was used. Transfusions were excluded from the analyses of platelet responses if: (1) the transfusion was given for a reason other than prophylaxis (eg, active bleeding, or in association with an invasive procedure); (2) there was no usable pretransfusion or posttransfusion count; (3) there were missing data on the platelet contents of the transfusion; (4) the patient was 1 of the 41 patients who received HLA-selected platelets; (5) the platelets were stored for 6 to 7 days (only 23 prophylactic platelet transfusions were stored for 6 to 7 days); and (6) the transfusion had mixed or missing data on platelet source, storage duration, or ABO matching status.
CCIs were calculated as previously reported.1
Analyses of responses to platelet transfusion used mixed linear models.5 The individual platelet transfusion was the unit of analysis, but the analysis took into account that responses to multiple transfusions given at the same site, or received by the same patient, tended to be similar. The initial models for 4-hour and 24-hour platelet increment and 4 hour and 24 hour CCI included as predictors patient sex, patient age category (0-9, 10-19, …60-69, 70+), treatment stratum (allogeneic transplantation, autologous transplantation, chemotherapy without transplantation), randomized platelet dose strategy (LD, MD, HD), platelet source, ABO matching status, storage duration, and the platelet transfusion number (first transfusion on study, second, third, fourth, fifth, sixth to 10th, 11th or later), plus 6 pair-wise interactions: dose by source, dose by matching status, dose by storage duration, source by matching status, source by storage duration, and matching status by storage duration. Site and patient were treated as random effects. The platelet transfusion number was calculated based on all platelet transfusions the patient received on study, both prophylactic transfusions and those given for other reasons. The least significant interactions were then removed from each model, one by one, until all remaining interactions were significant at the .05 level, or until only the main effects remained in the model. Using the final model, least-square means were calculated for each category of each predictor, adjusted for all other variables in the model. The transfusion interval was defined as the number of hours from the start of a prophylactic platelet transfusion to the start of the next platelet transfusion given for any indication. Analyses of transfusion intervals were restricted to prophylactic platelet transfusions, which had no mixed or missing data on platelet source, storage duration, or ABO matching status, were not stored 6 to 7 days, and were given at 1 of the 23 sites that complied with the 10 000/μL prophylactic transfusion trigger on at least 90% of patient-days. Censoring occurred if a granulocyte transfusion or stem cell infusion took place, or the patient ended the study, before the next platelet transfusion. The analysis strategy was similar to that for platelet increments and CCIs, except that frailty models instead of mixed linear models were used.
In all analyses, patients were included in the dose group to which they were randomized, even if some or all of their prophylactic platelet transfusions were outside the patient's assigned dose range. All analyses were carried out using SAS Version 9.2,6 except for the frailty models, which were carried out using R Version 2.14.1.7 The research was approved by the participating institution's review boards, and all participants gave informed consent in accordance with the Declaration of Helsinki.
Results
Study subjects
The PLADO study enrolled 1351 patients at 26 sites between 2004 and 2007. Of these, 79 patients did not receive any platelet transfusions, and another 41 patients were excluded for receiving HLA-selected platelets, leaving 1231 patients potentially evaluable for these analyses. Baseline characteristics of these 1231 patients are shown in Table 2.
Table 2.
Baseline patient characteristics
| Baseline patient characteristics | Median (interquartile range) or no. (%) |
|---|---|
| Age, y | 48.6 (31.2-58.6) |
| Female sex | 477 (39) |
| Previous pregnancy, among female patients | 315 (66) |
| Previous platelet transfusion | 701 (57) |
| Previous RBC transfusion | 923 (75) |
| Primary diagnosis | |
| Acute leukemia | 544 (44) |
| Lymphoma | 259 (21) |
| Myeloma | 153 (12) |
| Chronic leukemia | 80 (7) |
| Myelodysplasia | 54 (4) |
| Solid tumor | 18 (1) |
| Other | 123 (10) |
| Treatment stratum | |
| Allogeneic transplantation | 505 (41) |
| Autologous/syngeneic transplantation | 426 (35) |
| Chemotherapy (nontransplantation therapy) for hematologic cancer | 293 (24) |
| Chemotherapy (nontransplantation therapy) for solid tumor | 7 (1) |
| Platelet count, × 103/μL | 38 (25-61) |
| Hemoglobin, g/dL | 9.8 (9.0-10.7) |
| Weight, kg | 78.1 (62.9-92.5) |
| Body surface area, m2 | 1.91 (1.69-2.08) |
| Platelet dose group | |
| LD | 396 (32) |
| MD | 412 (33) |
| HD | 423 (34) |
Baseline characteristics of the 1231 PLADO patients who received at least one platelet transfusion, but no HLA-selected platelet transfusions, while on study.
RBC indicates red blood cell; LD, low dose; MD, medium dose; and HD, high dose.
Bleeding outcomes:
Of the 1231 potential patients, 453 were excluded from the analyses of time to ≥ grade 2 bleeding; 254 (21%) because of experiencing ≥ grade 2 bleeding before the date of their first platelet transfusion; 174 (14%) because the first date with ≥ grade 2 bleeding was on the same date as their first platelet transfusion; 37 (3%) because they had 1 or more days of missing bleeding data on or before the date of their first platelet transfusion; and 8 (1%) because their first transfusion was stored for 6 to 7 days.
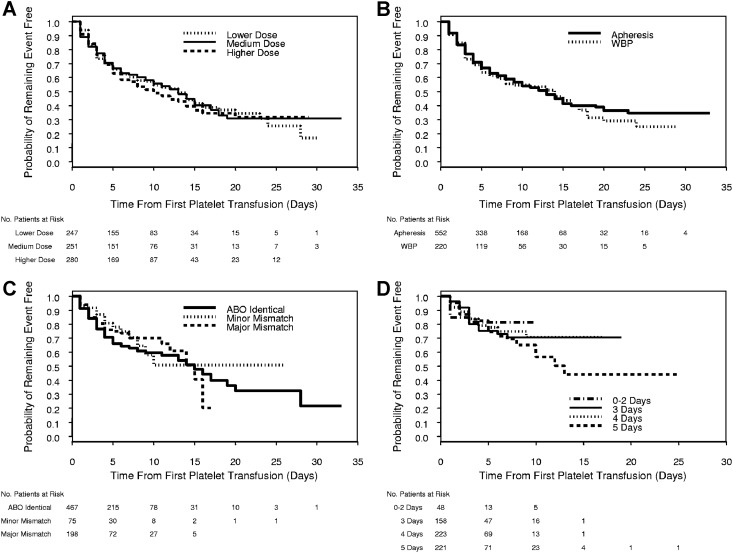
Some patients had more than one reason for exclusion, leaving 778 patients (63% of 1231) from 25 sites for analysis, of whom 247 (32%) were in the LD group, 251 (32% in the MD group, and 280 (36%) in the HD group. Overall, 383 patients (49.2% of 778) experienced a ≥ grade 2 bleeding episode before any censoring, including 121 (49.0%) in the LD group, 115 (45.8%) in the MD group, and 147 (52.5%) in the HD group. Figure 1A shows a Kaplan-Meier plot of time from first transfusion to first ≥ grade 2 bleeding by platelet dose group. In the adjusted model, platelet dose group did not affect the time to the first ≥ grade 2 bleeding (P = .77, Table 3).
Figure 1.
Kaplan-Meier plots of time from platelet transfusion to first ≥ grade 2 bleeding for each platelet characteristic. (A) Time from first platelet transfusion to first grade 2 or higher bleeding by platelet dose group. Time to bleeding was censored at the first date that any of the following occurred: missing data on whether ≥ grade 2 bleeding occurred; or end of study. Divergence of the curves after 24 days is probably the result of the small number of patients still at risk by that time. Platelet dose group was not a significant predictor of time to ≥ grade 2 bleeding (P = .77). (B) Time from first platelet transfusion to first grade 2 or higher bleeding by source of platelets. Time to bleeding was censored at the first date that any of the following occurred: transfusion of a platelet unit with a different source from the patient's initial platelet transfusion; missing data on whether ≥ grade 2 bleeding occurred; or end of study. Platelet source was not a significant predictor of time to ≥ grade 2 bleeding (P = .44). (C) Time from first platelet transfusion to first grade 2 or higher bleeding by ABO matching status. Time to bleeding was censored at the first date that any of the following occurred: transfusion of a platelet dose with a different ABO matching status from the patient's initial platelet transfusion or missing data on ABO matching status; missing data on whether ≥ grade 2 bleeding occurred; or end of study. Divergence of the curves after 15 days is probably the result of the small number of patients still at risk by that time. ABO matching status was not a significant predictor of time to ≥ grade 2 bleeding (P = .33). (D) Time from first platelet transfusion to first grade 2 or higher bleeding, by platelet storage duration, among patients whose first platelet transfusion was stored for 0 to 5 days. Time to bleeding was censored at the first date that any of the following occurred: transfusion of a platelet unit with a different storage duration from the patient's initial platelet transfusion; transfusion of a platelet unit with missing data on storage duration; transfusion of pooled WBP platelets of different storage durations; missing data on whether ≥ grade 2 bleeding occurred; or end of study. Divergence of the curves after 10 days is probably the result of the small number of patients still at risk by that time. Duration of platelet storage was not a significant predictor of time to ≥ grade 2 bleeding (P = .87).
Table 3.
Time to grade 2 or higher bleeding
| Characteristic | N | % censored | Hazard ratio | 95% CI | P |
|---|---|---|---|---|---|
| Randomized treatment group | .77 (2 df) | ||||
| LD | 247 | 51.0 | 1.00 | 0.77-1.30 | .99 |
| MD | 251 | 54.2 | Referent category | ||
| HD | 280 | 47.5 | 1.08 | 0.84-1.39 | .54 |
| Platelet source | .44 | ||||
| Apheresis | 552 | 54.5 | Referent category | ||
| WBP | 220 | 53.2 | 1.15 | 0.81-1.65 | .44 |
| ABO matching status | .33 (2 df) | ||||
| ABO-identical | 467 | 64.2 | Referent category | ||
| Minor mismatch | 75 | 76.0 | 0.85 | 0.52-1.40 | .52 |
| Major mismatch | 198 | 75.8 | 0.78 | 0.56-1.09 | .15 |
| Storage duration, d | .87 (3 df) | ||||
| 0-2 | 48 | 83.3 | 0.86 | 0.39-1.87 | .70 |
| 3 | 158 | 79.8 | Referent category | ||
| 4 | 223 | 81.2 | 1.03 | 0.64-1.64 | .91 |
| 5 | 221 | 77.8 | 1.13 | 0.72-1.79 | .59 |
Adjusted frailty models of days from first platelet transfusion to ≥ grade 2 bleeding. Hazard ratios > 1.00 indicate higher risk of bleeding in patients receiving platelets with the specified characteristic compared with patients receiving platelets with the reference characteristic. Each model includes one platelet characteristic (randomized dose assignment, source, matching status, or storage duration) and is also adjusted for patient sex, patient age group, patient treatment category, and site (with site treated as a random effect). Data on time to bleeding were censored if any of the following occurred before the patient experienced grade 2 or higher bleeding: a transfusion that differed from the patient's first transfusion in the characteristic being analyzed or had missing data on that characteristic; a day with missing data on whether grade 2 or higher bleeding had occurred; or end of study.
N indicates number of evaluable patients; LD, low dose; MD, medium dose; HD, high dose; and WBP, whole blood platelet pools.
Platelet source.
Of the 778 patients potentially available for analysis of platelet source as a predictor of time to grade 2 or higher bleeding, 6 (1%) had both AP and WBP on the first platelet transfusion date, leaving 772 patients for analysis. The source of the first platelet transfusion was AP in 552 patients (72%) of whom 251(45%) experienced ≥ grade 2 bleeding before censoring, and WBP in 220 patients (28%) of whom 103 (47%) experienced ≥ grade 2 bleeding before censoring. Figure 1B shows a Kaplan-Meier plot of time from first transfusion to first ≥ grade 2 bleeding by source of the first platelet transfusion. In the adjusted model, platelet source did not predict the time to the first ≥ grade 2 bleeding (P = .44, Table 3).
ABO matching status.
Of the 778 patients potentially available for the analysis of platelet ABO matching status as a predictor of time to ≥ grade 2 bleeding, 38 (5%) were excluded because they received platelets of > 1 ABO matching status (n = 37) or had missing data (n = 1) on their first transfusion date. Of the remaining 740 patients, the first platelet transfusion was ABO-identical in 467 patients (63%) of whom 167 (36%) experienced ≥ grade 2 bleeding before censoring, ABO minor mismatched in 75 patients (10%) of whom 18 (24%) experienced ≥ grade 2 bleeding before any censoring, and ABO major mismatched in 198 patients (27%) of whom 48 (24%) experienced ≥ grade 2 bleeding before censoring. Figure 1C shows a Kaplan-Meier plot of time from first transfusion to first ≥ grade 2 bleeding by ABO matching status. In the adjusted model, ABO matching status did not predict the time to ≥ grade 2 bleeding (P = .33, Table 3).
Duration of storage.
Of the 778 patients potentially available for the analysis of the duration of platelet storage as a predictor of time to ≥ grade 2 bleeding, 128 (16%) were excluded because they received platelets of > 1 storage time, or data on storage time were missing, for their first platelet transfusion. Of the 650 (84% of 778) remaining patients, the first transfused unit was stored 0 to 2 days for 48 patients (7%), 3 days for 158 patients (24%), 4 days for 223 patients (34%), and 5 days for 221 patients (34%). Overall, 131 of these 650 patients (20%) experienced ≥ grade 2 bleeding before censoring. Figure 1D shows a Kaplan-Meier plot of time from first transfusion to first ≥ grade 2 bleeding by duration of platelet storage of the first transfusion. In the adjusted model, platelet storage duration did not predict time to first ≥ grade 2 bleeding (P = .87, Table 3).
Multiparameter model of time to ≥ grade 2 bleeding.
To analyze whether any of these platelet transfusion characteristics was significantly associated with time from first transfusion to time of first ≥ grade 2 bleeding after adjusting for the other platelet characteristics, we fit an additional multiparameter model, including platelet dose, platelet source, ABO matching status, and storage duration as well as the 3 patient-level characteristics (age category, sex, and treatment stratum), and site as a random effect. Of the 778 potential patients, 141 had 1 or more exclusions, leaving 637 patients (82%) for analysis. Of these, 107 (17% of 637) experienced ≥ grade 2 bleeding before censoring. The multiparameter model showed that none of the platelet characteristics significantly predicted the time to ≥ grade 2 bleeding after adjusting for the other characteristics (Table 4).
Table 4.
Multiparameter model of days from first platelet transfusion to grade 2 or higher bleeding
| Characteristic | N | Hazard ratio | 95% CI | P |
|---|---|---|---|---|
| Platelet dose group | .28 (2 df) | |||
| LD | 220 | 0.75 | 0.45-1.25 | .27 |
| MD | 216 | Referent category | ||
| HD | 201 | 1.12 | 0.72-1.75 | .62 |
| Platelet source | .68 (1 df) | |||
| Apheresis | 507 | Referent category | ||
| WBP | 130 | 1.11 | 0.67-1.85 | .68 |
| ABO matching status | .36 (2 df) | |||
| ABO-identical | 395 | Referent category | ||
| Minor mismatch | 67 | 1.07 | 0.57-2.01 | .83 |
| Major mismatch | 175 | 0.72 | 0.44-1.18 | .19 |
| Storage duration, d | 1.00 (3 df) | |||
| 0-2 | 48 | 0.99 | 0.44-2.20 | .98 |
| 3 | 156 | Referent category | ||
| 4 | 216 | 1.06 | 0.64-1.76 | .83 |
| 5 | 217 | 1.02 | 0.62-1.70 | .93 |
The frailty model includes platelet dose, source, matching status, and storage duration, and is also adjusted for patient sex, patient age group, patient treatment category, and site (with site treated as a random effect). Data on time to bleeding were censored if any of the following occurred before the patient experienced grade 2 or higher bleeding: a transfusion that differed from the patient's first transfusion in source, matching status, or storage duration or had missing data on one of those characteristics; a day with missing data on whether grade 2 or higher bleeding had occurred; or end of study. Overall, 83.2% of patients had censored data.
N indicates number of evaluable patients; LD, low dose; MD, medium dose; HD, high dose; and WBP, whole blood platelet.
Models of time to ≥ grade 3 bleeding.
Only 64 patients (8.2%) experienced grade 3 or higher bleeding before any censoring. Similar analyses showed that none of the platelet characteristics was a statistically significant predictor of time to the first ≥ grade 3 bleeding event, although the power to detect an effect was low because of the infrequent observation of this event.
Posttransfusion platelet increments and CCI
Four-hour posttransfusion platelet increment and CCI.
Of the 5477 prophylactic platelet transfusions given to patients who received no HLA-selected transfusions, 4776 (87%) had the required platelet count information to calculate 4-hour CCI (ie, pretransfusion platelet count, posttransfusion platelet count, and total number of platelets transfused). However, of these 4776 transfusions, 705 (15%) had missing or mixed storage duration data, 22 (< 1%) were a mix of AP and WBP, 13 (< 1%) were stored 6 to 7 days, and 123 (3%) had unknown or mixed ABO status.
Some transfusions had more than one reason for exclusion, leaving 3993 transfusions (84% of 4776) to evaluate for the 4-hour posttransfusion platelet responses. There were 1019 (83% of 1231) patients, representing all 26 sites, with at least 1 evaluable 4-hour platelet transfusion and 704 patients with > 1 (range, 2-39 transfusions). The mean time from the pretransfusion platelet count to the platelet transfusion was 5.8 ± 3.2 hours and from the end of the transfusion to the posttransfusion platelet count was 1.3 ± 0.9 hours.
Four-hour absolute platelet increment.
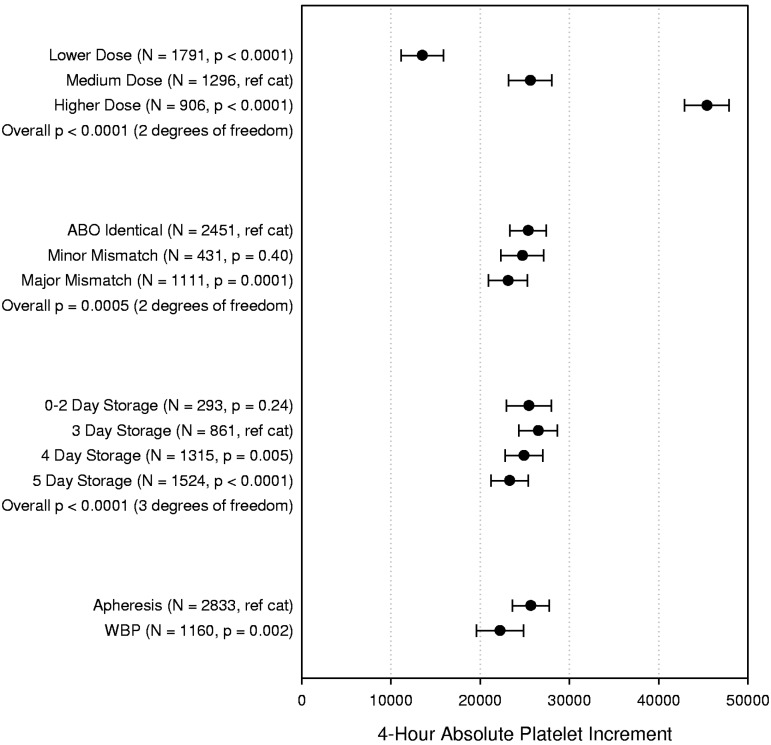
The multipredictor model for 4-hour absolute platelet increment is shown in Figure 2. No significant interactions were identified. As expected, the LD treatment group had a lower posttransfusion increment (on average, 12 092/μL lower) than the MD dose arm (P < .0001), and the MD arm had a lower increment (on average, 19 788/μL lower) than the HD arm (P < .0001). Major ABO mismatched platelet transfusions, but not minor, were associated with statistically smaller increments compared with ABO-identical platelet transfusions, but the magnitude of the difference was small (2249/μL, P = .0001). There is a decline in 4-hour increment for platelets stored 4 days (1602/μL lower, P = .005) or 5 days (3206/μL lower, P < .0001) versus 3 days. The absolute increments for WBP were on average 3455/μL lower than those for AP (P = .002).
Figure 2.
Multipredictor model of the effects of platelet dose, source, storage duration, and ABO matching status on 4-hour ABSOLUTE platelet count increment. The model also adjusts for transfusion number, patient sex, patient age group, patient treatment stratum, and within-site and within-person correlation. Data shown are the adjusted means and 95% confidence intervals. N = number of prophylactic platelet transfusions analyzed.
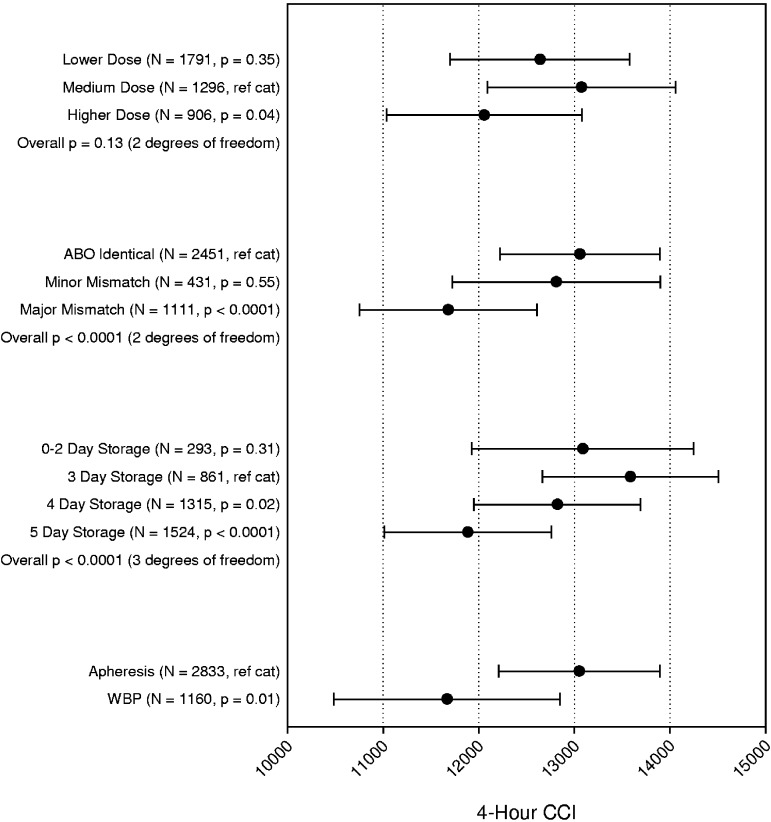
Four-hour CCI.
The 3993 transfusions analyzed for platelet increment were also analyzed for CCI, which corrects for the number of platelets transfused and size of the patient. The multiparameter model for 4-hour platelet CCI is shown in Figure 3. No significant interactions terms were identified. The overall test for an effect of dose was not statistically significant (P = .13), and there was no significant difference in the 4-hour CCI for the LD versus the MD arm. However, there was a small but statistically significantly lower CCI (1018 lower, P = .04) for the HD versus MD arm. Major ABO mismatched platelet transfusions, but not minor, were associated with a 1378 lower mean 4-hour CCI compared with ABO-identical platelet transfusions (P < .0001). There is a decline in 4-hour CCI for platelets stored 4 days (765 lower, P = .02) or 5 days (1700 lower, P < .0001) versus 3 days. The 4-hour CCI associated with WBP was significantly lower than for AP (11 667 vs 13 051, P = .01). Notably, for all platelet characteristics analyzed, the mean 4-hour CCI exceeds the generally accepted minimum value of 5000 to 7500 below which the ≤ 4-hour posttransfusion response is considered unsatisfactory.8
Figure 3.
Multipredictor model of the effects of platelet dose, source, storage duration, and ABO matching status on 4-hour CORRECTED platelet count increment. The model also adjusts for transfusion number, patient sex, patient age group, patient treatment stratum, and within-site and within-person correlation. Data shown are the adjusted means and 95% confidence intervals. N = number of prophylactic platelet transfusions analyzed.
Twenty-four-hour posttransfusion platelet increment and CCI
There were 2655 prophylactic platelet transfusions given to the 1231 patients who had the required information to calculate the 24-hour platelet increment and CCI. Of these 2655 transfusions, 301 (11%) had missing data or were of mixed storage duration, 11 (< 1%) were a mixture of AP and WBP, 12 (< 1%) were stored for 6 to 7 days, and 58 (2%) had unknown or were mixtures of ABO types.
Some transfusions had more than one reason for exclusion, leaving 2309 transfusions (87% of 2655) given to 838 patients to evaluate for the 24-hour posttransfusion platelet responses. The mean time from the last platelet count to the platelet transfusion was 4.9 ± 2.8 hours, and the mean time from the end of the transfusion to the posttransfusion platelet count was 19.9 ± 3.5 hours.
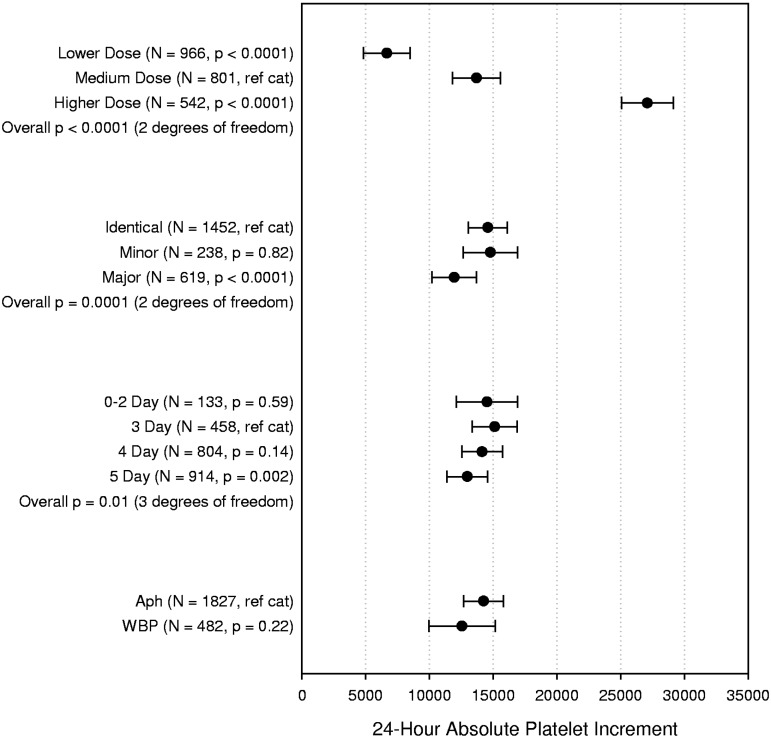
Twenty-four-hour absolute platelet increment
There were no statistically significant interaction terms for the final model of this analysis (Figure 4). As expected, the 24-hour increment for the LD arm was lower (on average, 7030/μL lower, P < .0001) compared with the increment observed in the MD arm. Similarly, the MD arm had a lower 24-hour increment (on average, 13 402/μL lower, P < .0001) compared with the HD arm. Major ABO mismatched platelet transfusions, but not minor, were associated with 2635/μL lower 24-hour increments on average compared with ABO-identical platelet transfusions (P < .0001). Platelets stored 0 to 2 days had similar 24-hour increment compared with 3-day platelets, after which there was a small decline, reaching statistical significance for platelets stored 5 days versus 3 days (2152/μL lower, P = .002). WBP were not associated with a significantly lower increment at 24 hours compared with AP (P = .22).
Figure 4.
Multipredictor model of the effects of platelet dose, source, storage duration, and ABO matching status on 24-hour ABSOLUTE platelet count increment. The model also adjusts for transfusion number, patient sex, patient age group, patient treatment stratum, and within-site and within-person correlation. Data shown are the adjusted means and 95% confidence intervals. N = number of prophylactic platelet transfusions analyzed.
Twenty-four-hour CCI
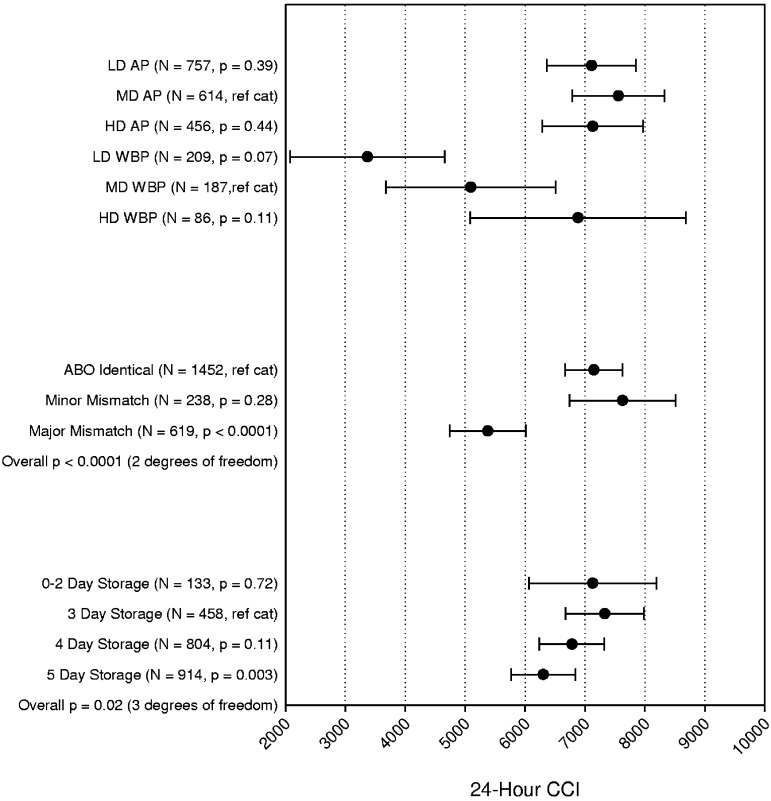
The 24-hour platelet CCI multiparameter model is shown in Figure 5. There was a significant interaction between platelet dose arm and platelet source (P = .015), so those data are shown together. For AP, there was no difference in 24-hour CCI for any of the dose arms. For WBP, however, the LD arm was associated with a trend toward lower 24-hour CCI (1725 lower, P = .07) compared with the MD, and the MD arm had a trend toward lower 24-hour CCI (1791 lower, P = .11) than the HD arm. Major ABO mismatched platelet transfusions, but not minor, were associated with a 1770 reduction in mean 24-hour CCI compared with ABO-identical platelet transfusions (P < .0001). Platelets stored 0 to 2 days had similar 24-hour CCI compared with 3-day platelets, after which there was a small decline, reaching statistical significance for platelets stored 5 days versus 3 days (1028 lower, P = .003).
Figure 5.
Multipredictor model of the effects of platelet dose, source, storage duration, and ABO matching status on 24-hour CORRECTED platelet count increment. The model also adjusts for transfusion number, patient sex, patient age group, patient treatment stratum, and within-site and within-person correlation. Data shown are the adjusted means and 95% confidence intervals. N = number of prophylactic platelet transfusions analyzed.
Transfusion intervals
There were 3447 transfusions evaluable for analysis of the time to next transfusion. As was reported in the previous PLADO analysis,1 dose had an expected major effect on transfusion interval. Our analysis, however, found no effect of storage duration on transfusion interval. ABO matching had a small effect (3.9 hours shorter median transfusion interval with ABO major mismatched platelets vs ABO-identical platelets, P < .0001). Platelet source had a small effect only among LD platelets (3.2 hours shorter median transfusion interval with LD WBP vs LD AP, P = .02).
Discussion
The PLADO study provided a large database of platelet transfusions in hematology oncology patients, who were carefully followed for bleeding and posttransfusion platelet responses. The key finding from our analysis is that, although platelet characteristics, such as platelet source, ABO compatibility, and duration of storage, had modest effects on platelet increments, these characteristics did not affect time to occurrence of ≥ grade 2 bleeding. Because patients often received transfusions from different sources, ABO matching statuses, or storage durations while on study, it was necessary to use time to occurrence of ≥ grade 2 bleeding as the bleeding end point. Our findings were similar when each of the characteristics was analyzed separately as a predictor of bleeding, adjusting for patient characteristics and site, and also, when they were analyzed in a larger model including treatment arm and other platelet characteristics.
In agreement with prior studies, PLADO data confirm that platelet increments and CCI are generally higher with AP versus WBP, ABO-identical platelets, and platelets stored for 3 days versus those stored longer. The lack of any association between platelet characteristics and the occurrence of bleeding is not surprising for several reasons. First, the mean 4-hour absolute platelet increments observed for any of the platelet characteristics is substantially more than the 13 519/μL mean increment observed with the LD strategy (Figure 2). Because the LD strategy was as effective as the other dose strategies for bleeding prophylaxis, as shown in our analyses and by Slichter et al,1 then it logically follows that the higher mean increments observed with platelets exhibiting any of the characteristics studied will be similarly effective. Second, even when comparison of platelet characteristics other than dose indicated statistically significant differences in either platelet increments or CCI, the magnitude of the differences was generally modest and, thus, unlikely to be of clinical significance. This is supported by our observation that, except for dose, none of the platelet characteristics studied had a substantial effect on the transfusion interval. All of the mean 4-hour CCI values exceeded the commonly accepted minimum of 5000 to 7500, below which the response is considered unsatisfactory.8 Similarly, all of the mean absolute platelet increments at 24 hours were more than 5200/μL higher than the increment observed with the LD arm (Figure 4).
Third, the PLADO study showed that, using a 10 000/μL trigger for platelet transfusion, patients had a 25% probability of ≥ grade 2 bleeding on days with a morning platelet count ≤ 5000/μL, compared with a relatively constant probability of approximately 17% for morning counts from 6000 to 80 000/μL. These data are consistent with previous reports9,10 indicating that a platelet count of 5000/μL or higher is sufficient to maintain endothelial integrity. All of the platelet characteristics studied had mean 4-hour platelet increments that would result in posttransfusion platelet counts above this threshold (Figure 2).
There is wide variation in the platelet components transfused in the United States. A recent survey11 reported that, of the approximately 2 million doses of platelets transfused in 2008, 87.1% were AP and 12.9% were WBP. It has been a continuing controversy whether one type of product is more efficacious than the other. A recent systematic review and meta-analysis of the efficacy of AP versus WBP identified 10 randomized controlled trials.12 Five of the trials included data on 1-hour CCI, with point estimates of the difference ranging from 1550 to 3120 in favor of AP. The combined result was a statistically significant weighted mean difference of 2490 in favor of AP. Four of the trials included data on 18 to 24-hour CCI, with point estimates of the difference ranging from 1080 in favor of WBP to 2240 in favor of AP. The combined result was a statistically significant weighted mean difference of 1640 in favor of AP. However, none of the 10 trials compared AP versus WBP with respect to bleeding outcomes. We confirmed the effect on platelet increments but showed that the source of platelets does not affect the risk of bleeding. It is not clear why the 24-hour CCI for LD and MD WBP tended to be lower than the other groups. If the WBP collection method resulted in platelet injury that affected survival of the transfused platelets at 24 hours, it would be expected to be observed at all doses. There may be an unrecognized bias in which platelet transfusions were evaluable for 24-hour CCI.
It is well established that platelets express ABO antigens on surface glycoproteins.13 There is substantial interdonor variation and variation related to donor ABO type, which appears to be a function of megakaryocyte expression and the plasma glycosyltransferase activity.14 The clinical significance of this variation has been long debated. A recent systematic review and meta-analysis identified 16 observational studies and 3 RCTs assessing the laboratory and clinical impact of ABO matching for platelet transfusions primarily in hematology oncology patients.15 The authors reported that transfusion of ABO-identical platelets was associated with slightly higher CCI than transfusion of nonidentical platelets, but there were insufficient data to make conclusions about bleeding events. The PLADO data confirm that ABO major mismatched platelet transfusions were associated with lower increments than ABO-identical transfusions but did not show a statistically significant difference between ABO minor mismatched platelet transfusions and ABO-identical transfusions. This is the first large study, however, to demonstrate that platelet ABO compatibility does not affect the risk of bleeding. Observational studies and small randomized studies have suggested that ABO-compatible platelets may confer other clinical advantages in terms of patient survival,16–18 platelet refractoriness,19,20 or transfusion reactions.19 Shehata et al found that the data describing these effects were inconclusive, suggesting that these observations must be confirmed in adequately powered randomized controlled trials.15
The duration of platelet storage is another factor that has been associated with the efficacy of platelet transfusion. Current Food and Drug Administration guidelines allow storage of platelets for up to 5 days. An analysis of platelet increments observed in the TRAP study found that platelets stored ≤ 48 hours were associated with significantly higher increments (1900/μL more) and longer intervals between transfusions.21 An adverse effect of platelet storage on CCI was reported by others to be more prominent for WBP platelets22 compared with AP,23 although this was not found by Slichter et al21 or in our data. These studies of platelet storage duration did not address whether there is an effect of longer storage on bleeding outcomes. The PLADO data confirm an effect of storage duration on platelet increments. The 4- and 24-hour CCI progressively declined with platelet storage duration greater than 3 days. Platelets stored 0 to 2 days were not associated with better increments compared with 3-day or 4-day-old platelets, suggesting that there is little functional advantage to “fresh” platelets. Most importantly, storage duration did not affect the risk of bleeding.
There are important limitations to our findings. First, patients in the PLADO study were randomized to a particular dosing strategy. Platelet characteristics, such as source, ABO matching status, and storage duration, were not assigned randomly. It is possible that there were biases in selecting platelet units with certain characteristics for specific patients. Study sites were requested to keep patients on the same platelet source for the patient's entire time on study, but this sometimes did not occur. Platelet selection based on ABO compatibility and duration of storage were determined per local practices. Second, the fact that patients often received transfusions with different characteristics necessitated the use of time to occurrence of ≥ grade 2 bleeding as the end point, with censoring if a patient received a transfusion that differed from the first transfusion in the characteristic(s) being analyzed. The large number of censored observations for the bleeding analysis reduced the statistical power for all variables in the model. However, this approach allowed inclusion of at least some patient days for the maximum number of patients. Patients who required a greater numbers of platelet transfusions were more likely to have at least 1 change in transfusion characteristics. Therefore, restricting the analyses to patients with no changes in transfusion characteristics would have resulted in a bias. For these same reasons, we could not analyze the effect of each platelet characteristic on the total number of platelet transfusions or total number of platelets transfused during the admission.
Third, the majority of platelet transfusions in the PLADO study were for prophylaxis rather than therapeutic for bleeding. Our analyses of platelet increments, CCI, and time to next transfusion were restricted to prophylactic transfusions, so that the calculated responses would not be affected by the bleeding event being treated or by bleeding during invasive procedures. It is possible that our results regarding responses to platelet transfusion may not apply to therapeutic platelet transfusions. Lastly, the study was restricted to hematology oncology patients with or without stem cell transplantations and thus may not apply to platelet transfusions in other patient populations.
In conclusion, although the platelet source, ABO compatibility, and duration of storage have a modest impact on posttransfusion platelet increments, they have no measurable impact on prevention of clinical bleeding. These data suggest that a strategy for routine platelet support for hematology oncology patients, in general, does not require specific selection policies for these characteristics.
Acknowledgments
The authors thank the Transfusion Medicine Hemostasis Clinical Trials Network investigators, study coordinators, research staff, and patients who participated in this study, and Dr Carrie Greene Wager for assistance with programming the frailty models.
This work was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health to the Data Coordinating Center at New England Research Institutes (HL072268), Case Western Reserve University (HL072033), Children's Hospital Boston (HL072291), Cornell University (HL072196), Duke University (HL072289), Emory University (HL072248), Johns Hopkins University (HL072191), Massachusetts General Hospital (HL072299), Puget Sound Blood Center (HL072305), Tulane University (HL072274), University of Iowa (HL072028), University of Maryland (HL072359), University of Minnesota (HL072072), University of North Carolina (HL072355), University of Oklahoma (Hl072283), University of Pennsylvania (HL072346), University of Pittsburgh (HL072331), and the BloodCenter of Wisconsin (HL072290).
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: D.J.T. and S.F.A. wrote the manuscript; D.J.T., S.F.A., R.G.S., P.M.N., J.R.H., R.M.K., and S.J.S. participated in trial design and conducted the clinical trial; S.F.A. and S.G. performed statistical analysis; D.J.T., S.F.A., R.G.S., P.M.N., J.R.H., R.M.K., and S.J.S. provided critical insight into the interpretation of the data; and all authors gave critical review and edits and final approval of the manuscript.
Conflict-of-interest disclosure: D.J.T. received consulting fees from Fenwal, Cerus, Carmell, and Novartis. R.G.S. received consulting fees from Terumo-BCT and Cerus & Baxter (in the past) and travel funds from Fenwal (recently). P.M.N. received consulting fees from Fenwal and Terumo-BCT. J.R.H received consulting fees from Hemerus Medical. S.J.S. received grant support from the Department of Defense, Navigant Biotechnologies (Terumo-BCT), and Pall Medical. The remaining authors declare no competing financial interests.
Correspondence: Darrell J. Triulzi, Institute for Transfusion Medicine, 3636 Boulevard of the Allies, Pittsburgh, PA 15213; e-mail: dtriulzi@itxm.org.
References
- 1.Slichter SJ, Kaufman RM, Assmann SF, et al. Dose of prophylactic platelet transfusions and prevention of hemorrhage. N Engl J Med. 2010;362(7):600–613. doi: 10.1056/NEJMoa0904084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47(1):207–214. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 3.Ripatti S, Palmgren J. Estimation of multivariate frailty models using penalized partial likelihood. Biometrics. 2000;56(4):1016–1022. doi: 10.1111/j.0006-341x.2000.01016.x. [DOI] [PubMed] [Google Scholar]
- 4.Therneau T, Grambsch P, Pankratz VS. Penalized survival models and frailty. J Comput Graph Stat. 2003;12(1):156–175. [Google Scholar]
- 5.Lindstrom ML, Bates DM. Newton-Raphson and EM algorithms for linear mixed-effects models for repeated-measures data. J Am Stat Assoc. 1988;83(404):1014–1021. [Google Scholar]
- 6.SAS Institute. SAS/STAT Software Version 9.2. Cary, NC: SAS Institute; 2000–2008. [Google Scholar]
- 7.R Foundation for Statistical Computing. R Version 2.14.1. Vienna, Austria: R Foundation for Statistical Computing; 2011. pp. 12–22. [Google Scholar]
- 8.Trial to Reduce Alloimmunization to Platelets Study Group. Leukocyte reduction and ultraviolet B irradiation of platelets to prevent alloimmunization and refractoriness to platelet transfusions. N Engl J Med. 1997;337(26):1861–1869. doi: 10.1056/NEJM199712253372601. [DOI] [PubMed] [Google Scholar]
- 9.Slichter SJ. Relationship between platelet count and bleeding risk in thrombocytopenic patients. Transfus Med Rev. 2004;18(2):153–167. doi: 10.1016/j.tmrv.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Heddle NM, Cook RJ, Sigouin C, Slichter SJ, Murphy M, Rebulla P. A descriptive analysis of international transfusion practice and bleeding outcomes in patients with acute leukemia. Transfusion. 2006;46(6):903–911. doi: 10.1111/j.1537-2995.2006.00822.x. [DOI] [PubMed] [Google Scholar]
- 11.U.S. Department of Health and Human Services. The 2009 National Blood Collection and Utilization Survey. Washington, DC: US Department of Health and Human Services; 2011. [Google Scholar]
- 12.Heddle NM, Arnold DM, Boye D, Webert KE, Resz I, Dumont LJ. Comparing the efficacy and safety of apheresis and whole blood-derived platelet transfusions: a systematic review. Transfusion. 2008;48(7):1447–1458. doi: 10.1111/j.1537-2995.2008.01731.x. [DOI] [PubMed] [Google Scholar]
- 13.Ogasawara K, Ueki J, Takenaka M, Furihata K. Study on the expression of ABH antigens on platelets. Blood. 1993;82(2):993–999. [PubMed] [Google Scholar]
- 14.Cooling LL, Kelly K, Barton J, Hwand D, Koerner TA, Olson JD. Determinants of ABH expression on human blood platelets. Blood. 2005;105(8):3356–3364. doi: 10.1182/blood-2004-08-3080. [DOI] [PubMed] [Google Scholar]
- 15.Shehata NS, Tinmouth A, Naglie G, Freedman J, Wilson K. ABO-identical versus nonidentical platelet transfusion: a systematic review. Transfusion. 2009;49(11):2442–2453. doi: 10.1111/j.1537-2995.2009.02273.x. [DOI] [PubMed] [Google Scholar]
- 16.Blumberg N, Heal JM, Liesveld JL, Phillips GL, Rowe JM. Platelet transfusions and survival in adults with acute leukemia. Leukemia. 2008;22(3):631–635. doi: 10.1038/sj.leu.2404920. [DOI] [PubMed] [Google Scholar]
- 17.Heal JM, Blumberg N, Kirkley SA, DiPersion JF, Rapoport AP, Rowe JM. Leukocyte reduced transfusions of ABO-identical platelets and clinical outcomes in autologous bone marrow transplantation for lymphoma. Bone Marrow Transplant. 1994;14(6):943–948. [PubMed] [Google Scholar]
- 18.Heal JM, Kenmotsu N, Rowe JM, Blumberg N. A possible survival advantage in adults with acute leukemia receiving ABO-identical platelet transfusions. Am J Hematol. 1994;45(2):189–190. doi: 10.1002/ajh.2830450219. [DOI] [PubMed] [Google Scholar]
- 19.Carr R, Hutton JL, Jenkins JA, Lucas GF, Amphlett NW. Transfusion of ABO-mismatched platelets leads to early platelet refractoriness. Br J Haematol. 1990;75(3):408–413. doi: 10.1111/j.1365-2141.1990.tb04356.x. [DOI] [PubMed] [Google Scholar]
- 20.Klumpp TR, Herman JH, Innis S, et al. Factors associated with response to platelet transfusion following hematopoietic stem cell transplantation. Bone Marrow Transplant. 1996;17(6):1035–1041. [PubMed] [Google Scholar]
- 21.Slichter SJ, Davis K, Enright H, et al. Factors affecting post-transfusion platelet increments, platelet refractoriness, and platelet transfusion intervals in thrombocytopenic patients. Blood. 2005;105(10):4106–4114. doi: 10.1182/blood-2003-08-2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schiffer CA, Lee EJ, Ness PM, Reilly J. Clinical evaluation of platelet concentrates stored for 1 to 5 days. Blood. 1986;67(6):1591–1594. [PubMed] [Google Scholar]
- 23.Leach MF, AuBuchon JP. Effect of storage time on clinical efficacy of single-donor platelets. Transfusion. 1993;33(8):661–664. doi: 10.1046/j.1537-2995.1993.33893342748.x. [DOI] [PubMed] [Google Scholar]