Abstract
Transport mechanisms that mediate the movements of anions must be coordinated tightly in order to respond appropriately to physiological stimuli. This process is of paramount importance in the function of diverse epithelial tissues of the body, such as, for example, the exocrine pancreatic duct and the airway epithelia. Disruption of any of the finely tuned components underlying the transport of anions such as Cl−, HCO3 −, SCN−, and I− may contribute to a plethora of disease conditions. In many anion-secreting epithelia, the interactions between the cystic fibrosis transmembrane conductance regulator (CFTR) and solute carrier family 26 (SLC26) transporters determine the final exit of anions across the apical membrane and into the luminal compartment. The molecular identification of CFTR and many SLC26 members has enabled the acquisition of progressively more detailed structural information about these transport molecules. Studies employing a vast array of increasingly sophisticated approaches have culminated in a current working model which places these key players within an interactive complex, thereby setting the stage for future work.
Keywords: Cl−, HCO3−, CFTR, SLC26, R domain, STAS domain, PDZ
Introduction
Epithelial tissues mediate the directional, or vectorial, movements of ions, solutes and water, all processes that rely on the polarized distribution of specialized transport proteins, operationally classified as either channels or transporters. Differential expression and localization of these key molecules determine whether an epithelium absorbs, secretes or transports bidirectionally. In addition to their polarized distribution, the intrinsic structural and functional integrity of transport proteins is essential for ensuring that a given epithelium produces the appropriate and sufficient fluxes.
Studies lending insights into such basic physiological processes often arise from the need to understand disease states. Disease mechanisms effectively provide object lessons that form a fundamental basis of molecular medicine. One well-studied example of this concept in action is cystic fibrosis (CF), an inherited disease that results from the mutation of the cystic fibrosis transmembrane conductance regulator gene (CFTR; Riordan et al. 1989). CFTR encodes a cAMP-regulated Cl− channel that is essential for the function of diverse systems. Over 1,500 mutations in CFTR (http://www.genet.sickkids.on.ca/cftr/) result in either the complete loss of the gene product or, alternatively, defective CFTR which manifest distinct classes of functional impairment. These include defects in folding/stability/trafficking, regulation/gating and conduction and reduced synthesis levels, manifesting in a wide spectrum of disease severity (Choo-Kang and Zeitlin 2000). The availability of structural information allows the investigator to scrutinize, by deploying in silico modeling and simulations, the consequences of mutations of each class, thereby enabling insights into each disrupted process—folding, gating, conduction and synthesis—at atomic resolution.
Strategies utilizing correlations between CFTR genotype and CF phenotype provide a window into understanding molecular structure and function. In the case of CFTR and CF, this approach can furnish a means of combating other diseases in which wild-type CFTR function becomes disrupted by pathogens, as in the case of secretory diarrhea. In this case, cholera toxin stimulates Cl− secretion via CFTR, suggesting the potential usefulness of CFTR inhibitors in the treatment for cholera. Detailed structures, together with functional models, provide critical tools that facilitate the design of potent inhibitory modulators, possibly also by aiding refinement of compounds identified by high-throughput screening (Ma et al. 2002; Muanprasat et al. 2004).
The collective knowledge of CFTR function in health and disease now includes important considerations of mechanisms involving intermolecular interactions. Thus, CFTR regulation of transporters residing apically in epithelial cells also bears physiological and clinical relevance. Solute carrier family 26 (SLC26) family members couple the exchange of Cl− with the transport of a host of physiologically important anions, including HCO3 −, I− and SCN− . 1 CFTR (and other Cl− channels) may furnish the Cl− required for SLC26 transporter activity. Importantly, the disruption of genes encoding SLC26 transporters produces a spectrum of unique and important diseases which, in their own right, profoundly affect the human condition. Thus, elucidation of the molecular details of functional coupling between SLC26 transporters and CFTR likely will enable the identification of potential treatment strategies for conditions resulting from bona fide SLC26 disruption as well.
The present review aims to summarize current knowledge on how CFTR and SLC26 transporters function in a concerted manner in epithelial HCO3 − secretion via intermolecular domain interactions. Substantial background information discussing this process is provided to lend the physiological context. Included is a brief description of post-synaptic density 95/discs large/zona occludens 1 (PDZ) domains, which are functional protein–protein interaction motifs found in many adaptor molecules. Such molecules act to scaffold both CFTR and SLC26, thereby promoting intermolecular interactions. The structural information available for the relevant and critical functional domains in both CFTR and SLC26 is reviewed. Although knowledge to date indicates that many challenges must be overcome, this also provides ample opportunities to decipher intermolecular interactions that are critical to human health and disease.
CFTR and SLC26 transporters critically determine anion secretion by diverse epithelia
The need for CFTR in HCO3− secretion
This important function is best illustrated by first considering transport in the pancreas. HCO3 − buffers many epithelial secretions and nowhere is this more evident than in the ducts of the exocrine pancreas, where the concentration of HCO3 − can achieve levels as high as 140 mM (Steward et al. 2005) and the alkalinity of secreted fluid can exceed pH 8.0. Such high pH and HCO3 − concentrations are essential for mucin solubilization as well as controlled activation of zymogens released by the pancreatic acini (Palade 1975). In CF, impaired HCO3 − secretion results in mucin precipitation and blockage of the ductal tree (Choi et al. 2001). Moreover, the resultant increased acidity promotes premature zymogen activation. The subsequent, progressive cycles of destruction lead ultimately to tissue fibrosis (Durie and Forstner 1989). But how does loss of a Cl− channel, CFTR, lead to defective HCO3 − secretion?
Parallel universes—CFTR mediates anion/HCO3- transport by diverse epithelia
The answer to this question applies not only to pancreas, but also to many other CFTR-expressing, anion/HCO3 − -transporting epithelia. Of note are the salivary ducts (Shcheynikov et al. 2008), epithelia lining both the male (Carlin et al. 2006; Pierucci-Alves et al. 2011) and female (Muchekehu and Quinton 2010; Wang et al. 2003) reproductive tracts and the airway submucosal glands (Ballard and Inglis 2004). One can regard the complex mixture of mucins and anti-microbial peptides in airway submucosal gland secretions as analogous to the mucins and zymogens comprising pancreatic gland secretions. The two are, essentially, parallel universes. In these tissues, as in the pancreas, compromised CFTR function associates with impaired HCO3 − secretion—with tissue-specific consequences.
How? Two possibilities immediately come to mind (Fig. 1). First, single channel studies of CFTR-overexpressing mammalian cell lines demonstrate permeation of other anions, including HCO3 − (Linsdell et al. 1997; Poulsen et al. 1994). These early findings suggested that HCO3 − secretion can result from direct efflux through CFTR; importantly, this has been measured in intact, native guinea pig pancreatic duct preparations (Ishiguro et al. 2009). Alternatively, HCO3 − might exit into the lumen via distinct transport molecules that are coupled functionally to cAMP-activated (i.e. CFTR-mediated) Cl− efflux (Garnett et al. 2011; Gray et al. 1989; Steward et al. 2005). In addition, both modes may co-exist in a given epithelium.
Fig. 1.
Possible means of transepithelial HCO3 − secretion. Top Schema outlining cystic fibrosis transmembrane conductance regulator (CFTR)-dependent transport of HCO3 − by either direct or indirect pathways. For simplicity, basolateral Cl− and HCO3 − uptake are shown with a single generic co-transport graphic element, but it should be noted that the Na+/K+/2Cl−and Na+/HCO3 − cotransporters are distinct molecular entities. Not shown is basolateral K+ conductance that enables the recycling of K+ taken up by Na+/K+ ATPase and the Na+/K+/2Cl− cotransporter. Left HCO3 − enters the cell basolaterally by Na+-coupled cotransport and exits into the lumen directly through CFTR. Anions transported by solute carrier family 26 (SLC26) transporters are indicated by A −. Other ions are indicated by standard nomenclature. Right Alternatively, Cl− secretion mediated by CFTR provides a counterion for HCO3 − exit via SLC26-mediated exchange pathways. Dotted arrow indicates functional interactions between CFTR and SLC26 transporters. Bottom In the absence of CFTR, both the direct (left) and the indirect (right) pathways are disabled
SLC26 transporters have diverse functional modes
Important candidates for CFTR regulation are members of the solute carrier family, SLC26, comprising 11 members having low amino acid sequence identity, cytoplasmically localized amino and carboxyl termini, and between 8 and 14 transmembrane domains. Mammalian SLC26 proteins bear homology to prokaryotic SO4 − transporters (SulP). Collectively, they can be referred to as SLC26/SulP proteins. Many SLC26A/SulP family members transport anions (Dorwart et al. 2008b; Mount and Romero 2004). Of the mammalian members, SLC26A1 and -A2 transport SO4 −2, whereas SLC26A3, -A4, -A6 exchange Cl− for a wide range of anions, including HCO3 −. Interestingly, SLC26A3 and -A6 can also function as conductances (Shcheynikov et al. 2006). Recent mutagenesis studies based on predictions of in silico modeling localized a single glutamate residue critical to switching between their different functional modes: E367 and E357 in SLC26A3 and SLC26A6, respectively (Ohana et al. 2011). Initial expression studies showed that SLC26A7 and SLC26A9 behave as anion channels (Dorwart et al. 2007; Kim et al. 2005). However, SLC26A9 can function in anion exchanger mode and furthermore can transport Na+ (Chang et al. 2009). To date, the protein products of SLC26A8 and SLC26A11 have poorly understood transport capabilities, and SLC26A10 is a pseudogene. SLC26A5, which encodes a motor protein in the outer hair cells of the cochlea, also is capable of electrogenic SCN− transport (Schaenzler and Fahlke 2011).
SLC26 transporters are relevant to human disease
Mutations in SLC26A2, SLC26A3 and SLC26A4 result in human diseases, namely diastrophic dysplasia (DTDST2) (Hastbacka et al., 1994; Superti-Furga et al. 1996), congenital chloride-losing diarrhea (CLD) (Holmberg 1986) and Pendred syndrome (Everett et al. 1997; Pendred 1896), respectively. Both CLD and Pendred syndrome highlight the roles of SLC26 transporters in epithelial function. The loss of SLC26A3 function causes CLD by abolishing the major pathway for colonic Cl− reabsorption (Melvin et al. 1999). Pendred syndrome is associated with mutations in SLC26A4. It manifests primarily as profound sensorineural deafness which results from disruption of cochlear endolymphatic fluid HCO3 − buffering, loss of the endocochlear potential and subsequent oxidative stress (Jabba et al. 2006; Singh and Wangemann 2008). Pendred syndrome also occasionally presents with euthyroid goiter, and indeed SLC26A4 normally localizes to the thyroid. This, and its ability to exchange I− for Cl−, led to the notion that SLC26A4 comprises the long-sought-after apical I− pathway in the thyroid. This conclusion is unsatisfactory on several levels (discussed in (Fong 2011; van den Hove et al. 2006)). Alternative conduits for I− exit therefore must exist and, pertinent to the present discussion, CFTR is a candidate (Devuyst et al. 1997; Fong 2011; Li et al. 2010; Li et al. 2012). As for HCO3 −, CFTR may directly carry I− or indirectly regulate its passage via SLC26A4 in the thyroid.
Domains in CFTR and SLC26 transporters interact to regulate their function
An early study provided evidence for the functional interaction between CFTR and select SLC26 transporters (SLC26A3, -A4 and -A6) (Ko et al. 2002). Using cell systems heterologously expressing these proteins, Ko et al. measured membrane potential, current and ΔpH, an obligatory consequence of SLC26 activity, in response to CFTR stimulation. Full responses were observed for cells expressing the wild type—but not mutants—of either the CFTR or SLC26 transporters. Later work from the same group (Ko et al. 2004) localized the relevant interacting regions to the R domain of CFTR and the carboxyl terminus [sulfate transporter and anti-sigma factor antagonist (STAS) domain] of SLC26 transporters (Aravind and Koonin 2000). Interestingly, disease-associated mutations in SLC26 transporters can localize to the STAS domain, as will be discussed.
CFTR and SLC26 transporters interact with scaffolding proteins
Specific carboxyl terminus residues in SLC26A3 and -A6 (-TKF and -TRL, respectively) interact with the PDZ domain protein, sodium–hydrogen exchange regulatory factor 1 (NHE-RF1) (Lamprecht et al. 2002; Lohi et al. 2003). PDZ domains mediate protein–protein interactions. Available structures show that the roughly 80–90 amino acids comprising PDZ domains assume an anti-parallel organization of six β strands (βA –βF), together with two intervening α helices (αA and αB). Figure 2a (top) summarizes the order of these secondary elements and some of the functionally pertinent residues, particularly those intervening between βA and βB and forming a carboxyl terminal-binding, connecting loop. A high-resolution structure (1.8 Å) of the PDZ-3 of postsynaptic density protein-95 (PSD-95) (Fig. 2a, bottom) shows the αB helix and the βB strand cradling its peptide binding partner. This interaction is promoted via main-chain interactions of the peptide with βB (Doyle et al. 1996). PDZ binding domains can be classified according to their preference for specific peptide sequences. Class I domains recognize a consensus motif containing T/S-X-ϕ, where X indicates any amino acid and ϕ denotes a hydrophobic residue (Sheng and Sala 2001; Songyang et al. 1997).
Fig. 2.
a. Depictions of post-synaptic density 95/discs large/zona occludens 1 (PDZ) domain organization and structure. Top Linear cartoon representation showing the predicted secondary structural elements above the relevant sequence (amino acid residues 309–393) from an archetypal PDZ domain, rat PSD-95 PDZ-3. Blue arrows β sheets, purple rectangles α helices. Residues shown in boldface contact the peptide binding partner. Note that the binding loop rests in the region intervening between βA and βB. Bottom Three-dimensional structure of the PDZ-3 of postsynaptic density protein-95 (PSD-95) showing the interacting peptide (orange arrow) wedged between the groove formed by the βB sheet and the αB helix; the carboxylate binding loop hovers above. This image is modified from Doyle et al. (1996), with permission from Elsevier. b Cartoon showing hypothesized apical macromolecular signaling complexes promoted by PDZ protein-facilitated interactions between the CFTR and SLC26 transporters. PDZ domain binding motif of SLC26A3, TKF, is shown in this figure; the counterpart for SLC26A6 is TRL. For CFTR, the motif is TRL as shown. This motif is lacking in SLC26A4, but the potential for regulation by sulfate transporter and anti-sigma factor antagonist (STAS) domain interactions with the R domain of CFTR is preserved
The carboxyl terminus tri-peptide of CFTR is identical to that of SLC26A6 and also binds PDZ proteins (Sheng and Sala 2001; Wang et al. 1998). Particularly important in CFTR function are sodium–hydrogen exchange regulatory factor 1, NHE-RF1 (or ezrin binding protein 50, EBP-50) and NHE-RF2 (exchanger type 3 kinase A regulatory protein, E3KARP) (Hall et al. 1998; Sun et al. 2000; Wang et al. 1998), both of which contain two PDZ interaction domains. Thus, PDZ proteins link CFTR and SLC26A3 (or -A6) together, behaving as associated adaptor molecules that thereby can promote interactions between the R and STAS domains (Ko et al. 2004) (Fig. 2b).
Although interactions with diverse PDZ proteins have been shown to regulate CFTR gating via phosphorylation, membrane trafficking, stability and turnover, an exhaustive treatment of these aspects is beyond the scope of the present discussion. Several excellent reviews highlight the scaffolding role of PDZ proteins in CFTR function (Li and Naren 2005; Seidler et al. 2009a, b). PDZ interactions also mediate associations between the lysophosphatidic acid receptor 2 (LPA2) and NHE-RF2. The binding of lysophosphatidic acid thereby can inhibit CFTR phosphorylation (Li et al. 2005; Zhang et al. 2011), as reviewed elegantly by Eckford and Bear (2011). Small-molecule disruption of the interactions of LPA2 with NHE-RF2 therefore enhances CFTR activity (Zhang et al. 2011).
It should be noted that PDZ associations regulating similar aspects of SLC26 transporter cell biology are not as extensively studied as those of CFTR. Such questions therefore represent potentially interesting and important areas for further investigations.
The R domain: a key structural element that is essential to the regulated function of CFTR
Cystic fibrosis transmembrane conductance regulator comprises 1,480 amino acids organized in functional domains typical of ATP-binding cassette (ABC) family proteins. Briefly, these include two membrane-spanning domains (MSD1, MSD2), each made up of six membrane-spanning α-helices forming the pore for Cl− passage. Each is followed by one of two cytosolic, nucleotide binding folds, NBD1 and NBD2, respectively. Unique to CFTR is a regulatory R) domain, a region that intervenes between NBD1 and MSD2. The R domain contains multiple sites for phosphorylation by protein kinase A (PKA) as well as a number of other kinases, notably adenosine monophosphate kinase (AMPK) and protein kinase C (PKC). Inhibitory stretches are also contained in the R domain (Baldursson et al. 2001; Rich et al. 1993; Wilkinson et al. 1997; Xie et al. 2002). Phosphorylation of the R domain relieves inhibition and allows binding of two ATP molecules between the NBDs, vastly increasing the probability of channel opening (“gating”). ATP binding opens CFTR and permits Cl− flux; the subsequent hydrolysis of one ATP molecule then closes the gate (Gadsby et al. 2006).
The CFTR R domain is disordered
As described previously, overexpression studies indicate functional interaction between CFTR’s phosphorylated R domain with the STAS domain of SLC26 transporters. What is known about the structure of the R domain? An important early study analyzing recombinant R domain protein provided a crucial clue (Ostedgaard et al. 2000): spectra obtained using circular dichroism (CD) characterized it as primarily random coil (disordered) having low helicity. The phosphorylated recombinant protein exhibited a slightly lower propensity to fold. Subsequent nuclear magnetic resonance (NMR) studies confirmed the intrinsic disorder of the R domain (Baker et al. 2007; Kanelis et al. 2011). Phosphorylation reduced the helical content, in agreement with the earlier CD measurements. Low residue relaxation rates confirmed its disordered nature and suggested transitional structural contacts and conformations. Computer simulations utilizing discrete molecular dynamics simulation (DMD) generated low-energy, R domain conformers with low helical content (Fig. 3), and subsequent comparisons of disorder patterns generated for different phosphorylated conformers yielded similar conclusions (Hegedus et al. 2008). Independent approaches thus have built a convincing case for disorder within the R domain. The notion that intrinsic disorder may prove advantageous is steadily gaining support. Notably, key, interactive “hub proteins” contain regions of high disorder which may underlie rapid and reversible interactions between multiple binding partners (Dosztanyi et al. 2006; Patil and Nakamura 2006; Radivojac et al. 2007).
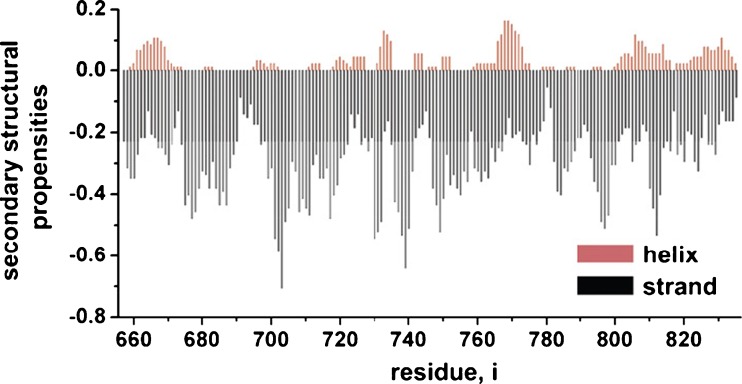
Fig. 3.
Secondary structural propensity plot generated by molecular dynamics simulation (DMD) simulations. The plot shows population-weighted averages for residues within the R domain. The positive ordinate values (red; helix) correspond to α-helical probabilities, whereas negative values (black; strand) reflect likelihood for β-sheets. This image is modified from Hegedus et al. (2008), with permission from Elsevier
Implications for intermolecular interactions
The paucity of secondary structural elements in the R domain therefore presents unique challenges in evaluating how it might interact not only with other domains within CFTR (Chappe et al. 2005) but also with the STAS domain of SLC26 transporters. A given phosphorylation event will determine the structure that sets the stage for the subsequent phosphorylation event. In disease-associated R-domain mutants, this sequence may be disrupted. Taking into account nine potential PKA sites in the R domain itself, the maximum number of possible ordered sequences for phosphorylation is nine! or 362,880. The likelihood of following any given conformer sequence will be determined by folding and energetic barriers at any partially phosphorylated step, thereby limiting the actual number of possible conformers. Still, these likely will lend substantial flexibility to any intermolecular interactions as well. Disease-associated R domain mutants are expected to produce an altered sequence of intermediates which in turn would affect interactions with SLC26 transporters. It is worth noting that recent structural models of R domain regulation by the binding of the oppositely acting kinases, AMPK and PKA, highlight the functional importance of disorder-associated structural flexibility (Siwiak et al. 2012).
STAS domains: important, interactive regions in SLC26 transporters
Aravind and Koonin first identified STAS domains in comparisons of the carboxyl-termini of SLC26 transporters with bacterial anti-sigma factor antagonists—in particular, the sporulation regulatory factor, SpoIIAA (Aravind and Koonin 2000). The NMR solution structure of unphosphorylated SpoIIAA showed four β-sheet regions and four α-helices arranged into a binding fold, presumably mediating its interaction with the kinase SpoIIAB (Kovacs et al. 1998); alternatively, five α-helices were assigned in predictive alignments with SLC26 family members (Aravind and Koonin 2000). In secondary structural predictions, the loop between the third β-sheet and the second α-helix was conserved in SpoIIAA and mammalian STAS domains (Aravind and Koonin 2000). In SpoIIAA, the STAS domain has a mild NTPase activity, and this conserved loop may coordinate phosphate binding. Phosphorylation of a serine residue (S57) immediately proximal to the second α-helix acts as a switch for SpoIIAA.
Interestingly, a point mutation in SLC26A4, F667C, localizes to this region of the conserved loop and moreover associates with the sensorineural deafness and goiter characteristic of Pendred syndrome (Aravind and Koonin 2000; Everett et al. 1997). Furthermore, mutation G678V, located near the end of SLC26A2’s second α-helix, is associated with DTDST (Superti-Furga et al. 1996). These findings underscore the importance of the conserved loop to the overall regulatory and interactive functions of STAS domains in SLC26A transport proteins.
Information from non-mammalian STAS-type structures
Available structural information on bacterial and plant STAS domains can offer insights into understanding important aspects of mammalian SLC26 protein function (Babu et al. 2010; Compton et al. 2011; Price and Howitt 2011; Rouached et al. 2005; Sharma et al. 2011b; Shibagaki and Grossman 2004; Shibagaki and Grossman 2006). Recent studies of a bacterial SLC26 protein (Yersinia enterocolitica Slc26A2) yielded a low-resolution structure indicating dimeric transporter stoichiometry, with the cytosolically localized STAS domains projecting away from the associated transmembrane domains (Compton et al. 2011). The dimeric stoichiometry agrees with previous functional measurements of bacterial, zebrafish and mammalian SLC26 transport (Detro-Dassen et al. 2008).
Earlier studies entailing large STAS domain deletions of the Arabadopsis thaliana SO4 − transporters, SULTR1.1 and SULTR1.2A, suggested a role in SO4 − accumulation by mediating either bona fide substrate transport or, alternatively, by enabling SULTR membrane localization (Shibagaki and Grossman 2004). A three-dimensional homology model of the STAS domain of SULTR1.2 aligned well with the known SpoIIAA crystal and NMR structures (Kovacs et al. 1998; Rouached et al. 2005; Seavers et al. 2001). The homology model provided a navigation tool with which deletions and mutations within the STAS domain were targeted. Transport by STAS-region SULTR1.2 mutants was quantified by growth rescue of a yeast strain deficient in SO4 − transport, defining regions critical to SO4 − transport but dispensable for proper membrane localization (Rouached et al. 2005). Using an extensive random mutagenesis approach, regions necessary for trafficking as well as intermolecular binding were identified by a similar growth rescue assay (Shibagaki and Grossman 2006).
Understanding mammalian STAS domain structure elucidates disease mechanisms
The availability of bacterial structures thus can provide an important framework with which to generate testable hypotheses aimed at discerning STAS domain functions in mammalian SLC26 proteins, but their usefulness is also limited. A systematic and elegant analysis of four SLC26A3 STAS mutants (I675/6ins, G702Tins, ΔY526/7 and I544N) associated with CLD (Makela et al. 2002) demonstrated structural and functional disruption by either of two distinct mechanisms: (1) domain misfolding, with retention at the level of the endoplasmic reticulum or (2) impaired intramolecular associations leading to diminished membrane trafficking (Dorwart et al. 2008a). Interestingly, the two insertion mutants exert their effects via the first mechanism, whereas the deletion and point mutations traffic inadequately. In the case of ΔY526/7, growth at lowered temperature (30°C) restored membrane targeting and function.
An exciting breakthrough recently emerged with the attainment of a high-resolution (1.57 Å) structure for rat Slc26a5 (Pasqualetto et al. 2010) (PDB 3IIoA). The structure of the crystallized protein indicated six β-sheets and five α-helices (Fig. 4a). Notably, the amino and carboxyl terminal boundaries extend beyond those inferred from SpoIIAA, suggesting that hurdles encountered in earlier attempts to analyze SLC26A3 STAS domains (Dorwart et al., 2008a) might be overcome by extending these boundaries. The structure predicts a surface for homologous (e.g. with intracellular loops linking membrane-spanning SLC26 domains) as well as heterologous (e.g. the R domain of CFTR?) interactions. In addition to the amino-terminal proline, the interactive surface contains a highly conserved phenylalanine within the intervening loop (conserved loop) between β-strand 3 and α-helix 2 (Fig. 4b).
Fig. 4.
Information derived from the rat Slc26a5 high-resolution structure. a Representation of the structure of the rat Slc26a5 STAS domain; green connecting loops joining 5 yellow β-strands and 5 red α-helices. The three-dimensional (3D) image is reprinted from Pasqualetto et al. (2010), with permission from Elsevier. A linear map showing sequential organization of the secondary structural elements is shown beneath the 3D representation. C.L. Conserved loop. b Residues in human SLC26A3-A6 STAS domains that putatively interact with the cytosolic interface of the apical membrane were determined by alignment with the structure rat Slc26a5 (Pasqualetto et al. 2010). Residues are identical in numbering for both rat Slc26a5 and human SLC26A5. For the four human SLC26 transporters compared, note the conservation of the proline residue within the extended N-terminus region (N), as well as the phenylalanine at the end of the C.L. between β-sheet 3 and α-helix 2
Modeling CLD-associated STAS mutations in SLC26A3 to the rat Slc26a5 STAS coordinates, Pasqualetto and coworkers revisited and confirmed previously proposed disease mechanisms (Dorwart et al. 2008a; Pasqualetto et al. 2010). These studies established proof-of-principle that the structure of rat Slc26a5 STAS may be used as a template for examining other mammalian SLC26 STAS domains. Indeed, Sharma and colleagues recently reported a model for SLC26A4 based on the published Slc26a5 structure (Sharma et al. 2011a). Future work directed at modeling—and empirically testing—wild-type and other deleterious, disease-associated STAS domain interactions with the R domain of CFTR likely will benefit tremendously from the availability of the rat Slc26a STAS domain template structure.
Conclusion
The last decade has witnessed an explosion of multidisciplinary studies focused on the inter-woven roles of CFTR and SLC26 transporters in vectorial HCO3 − transport, a process critical to the normal functioning of numerous organ systems. Several devastating human diseases result from impaired HCO3 − transport, and the strides made in understanding the basic properties of CFTR and SLC26 proteins pave the way for therapies and cures for these conditions. As the result of melding the disciplines of biophysics, biochemistry, cell biology, physiology and molecular medicine, the key role of intermolecular domain interactions in epithelial HCO3 − secretion is now recognized. These include direct interactions between CFTR and SLC26 transporters as well as those mediated by PDZ scaffolding proteins. Armed with the wealth of structural information now available for both CFTR and SLC26, the field is poised to accelerate toward a higher level of understanding these physiologically essential interactions. Multi-disciplinary approaches have facilitated—and will continue to facilitate—critical advances in this exciting field.
Acknowledgments
Research in the Fong lab is currently funded by the National Institutes of Health (COBRE NIH-P20-RR017686; Project 2) from the IDEA Program of the National Center for Research Resources, as well as an Innovative Research Award from the Kansas State University/Johnson Center for Basic Cancer Research (RFEES1). The contents of this work are solely the responsibility of the author and do not necessarily represent the official views of the Center of Biomedical Research Excellence for Epithelial Function in Health and Disease or NIH.
Conflict of interest
None.
Footnotes
HCO3 − is appreciated as an important buffer of not only the intracellular milieu, but also of fluid secretions. Thyroid hormone synthesis requires the incorporation, and hence the transport of I−. SCN− secretion by airway epithelia is necessary for the generation of OSCN− by interaction with peroxidase-generated H2O2. OSCN− has potent anti-microbial action, thereby playing a critical role in innate host airway defense.
DTDST is characterized by skeletal malformations, underscoring the role of SLC26A2 in transporting the substrate (SO4 -2) necessary for the sulfation of cartilage proteoglycans.
References
- Aravind L, Koonin EV. The STAS domain—a link between anion transporters and antisigma-factor antagonists. Curr Biol. 2000;10:R53–R55. doi: 10.1016/S0960-9822(00)00335-3. [DOI] [PubMed] [Google Scholar]
- Babu M, Greenblatt JF, Emili A, Strynadka NC, Reithmeier RA, Moraes TF. Structure of a SLC26 anion transporter STAS domain in complex with acyl carrier protein: implications for E. coli YchM in fatty acid metabolism. Structure. 2010;18:1450–1462. doi: 10.1016/j.str.2010.08.015. [DOI] [PubMed] [Google Scholar]
- Baker JM, Hudson RP, Kanelis V, Choy WY, Thibodeau PH, Thomas PJ, Forman-Kay JD. CFTR regulatory region interacts with NBD1 predominantly via multiple transient helices. Nat Struct Mol Biol. 2007;14:738–745. doi: 10.1038/nsmb1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldursson O, Ostedgaard LS, Rokhlina T, Cotten JF, Welsh MJ. Cystic fibrosis transmembrane conductance regulator Cl- channels with R domain deletions and translocations show phosphorylation-dependent and -independent activity. J Biol Chem. 2001;276:1904–1910. doi: 10.1074/jbc.M006934200. [DOI] [PubMed] [Google Scholar]
- Ballard ST, Inglis SK. Liquid secretion properties of airway submucosal glands. J Physiol. 2004;556:1–10. doi: 10.1113/jphysiol.2003.052779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin RW, Sedlacek RL, Quesnell RR, Pierucci-Alves F, Grieger DM, Schultz BD. PVD9902, a porcine vas deferens epithelial cell line that exhibits neurotransmitter-stimulated anion secretion and expresses numerous HCO3(-) transporters. Am J Physiol Cell Physiol. 2006;290:C1560–C1571. doi: 10.1152/ajpcell.00468.2005. [DOI] [PubMed] [Google Scholar]
- Chang MH, Plata C, Zandi-Nejad K, Sindic A, Sussman CR, Mercado A, Broumand V, Raghuram V, Mount DB, Romero MF. Slc26a9—anion exchanger, channel and Na+ transporter. J Membr Biol. 2009;228:125–140. doi: 10.1007/s00232-009-9165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappe V, Irvine T, Liao J, Evagelidis A, Hanrahan JW. Phosphorylation of CFTR by PKA promotes binding of the regulatory domain. EMBO J. 2005;24:2730–2740. doi: 10.1038/sj.emboj.7600747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JY, Muallem D, Kiselyov K, Lee MG, Thomas PJ, Muallem S. Aberrant CFTR-dependent HCO(3-) transport in mutations associated with cystic fibrosis. Nature. 2001;410:94–97. doi: 10.1038/35065099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo-Kang LR, Zeitlin PL. Type I, II, III, IV, and V cystic fibrosis transmembrane conductance regulator defects and opportunities for therapy. Curr Opin Pulm Med. 2000;6:521–529. doi: 10.1097/00063198-200011000-00011. [DOI] [PubMed] [Google Scholar]
- Compton EL, Karinou E, Naismith JH, Gabel F, Javelle A. Low resolution structure of a bacterial SLC26 transporter reveals dimeric stoichiometry and mobile intracellular domains. J Biol Chem. 2011;286:27058–27067. doi: 10.1074/jbc.M111.244533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detro-Dassen S, Schanzler M, Lauks H, Martin I, zu Berstenhorst SM, Nothmann D, Torres-Salazar D, Hidalgo P, Schmalzing G, Fahlke C. Conserved dimeric subunit stoichiometry of SLC26 multifunctional anion exchangers. J Biol Chem. 2008;283:4177–4188. doi: 10.1074/jbc.M704924200. [DOI] [PubMed] [Google Scholar]
- Devuyst O, Golstein PE, Sanches MV, Piontek K, Wilson PD, Guggino WB, Dumont JE, Beauwens R. Expression of CFTR in human and bovine thyroid epithelium. Am J Physiol. 1997;272:C1299–C1308. doi: 10.1152/ajpcell.1997.272.4.C1299. [DOI] [PubMed] [Google Scholar]
- Dorwart MR, Shcheynikov N, Wang Y, Stippec S, Muallem S. SLC26A9 is a Cl(-) channel regulated by the WNK kinases. J Physiol. 2007;584:333–345. doi: 10.1113/jphysiol.2007.135855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorwart MR, Shcheynikov N, Baker JM, Forman-Kay JD, Muallem S, Thomas PJ. Congenital chloride-losing diarrhea causing mutations in the STAS domain result in misfolding and mistrafficking of SLC26A3. J Biol Chem. 2008;283:8711–8722. doi: 10.1074/jbc.M704328200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorwart MR, Shcheynikov N, Yang D, Muallem S. The solute carrier 26 family of proteins in epithelial ion transport. Physiol Bethesda. 2008;23:104–114. doi: 10.1152/physiol.00037.2007. [DOI] [PubMed] [Google Scholar]
- Dosztanyi Z, Chen J, Dunker AK, Simon I, Tompa P. Disorder and sequence repeats in hub proteins and their implications for network evolution. J Proteome Res. 2006;5:2985–2995. doi: 10.1021/pr060171o. [DOI] [PubMed] [Google Scholar]
- Doyle DA, Lee A, Lewis J, Kim E, Sheng M, MacKinnon R. Crystal structures of a complexed and peptide-free membrane protein-binding domain: molecular basis of peptide recognition by PDZ. Cell. 1996;85:1067–1076. doi: 10.1016/S0092-8674(00)81307-0. [DOI] [PubMed] [Google Scholar]
- Durie PR, Forstner GG. Pathophysiology of the exocrine pancreas in cystic fibrosis. J R Soc Med. 1989;82(Suppl 16):2–10. [PMC free article] [PubMed] [Google Scholar]
- Eckford PD, Bear CE. Targeting the regulation of CFTR channels. Biochem J. 2011;435:e1–e4. doi: 10.1042/BJ20110461. [DOI] [PubMed] [Google Scholar]
- Everett LA, Glaser B, Beck JC, Idol JR, Buchs A, Heyman M, Adawi F, Hazani E, Nassir E, Baxevanis AD, Sheffield VC, Green ED. Pendred syndrome is caused by mutations in a putative sulphate transporter gene (PDS) Nat Genet. 1997;17:411–422. doi: 10.1038/ng1297-411. [DOI] [PubMed] [Google Scholar]
- Fong P. Thyroid iodide efflux: a team effort? J Physiol. 2011;589:5929–5939. doi: 10.1113/jphysiol.2011.218594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadsby DC, Vergani P, Csanady L. The ABC protein turned chloride channel whose failure causes cystic fibrosis. Nature. 2006;440:477–483. doi: 10.1038/nature04712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnett, JP, Hickman, E, Burrows, R, Hegyi, P, Tiszlavicz, L, Cuthbert, AW, Fong, P, Gray, MA (2011) Novel role for pendrin in orchestrating bicarbonate secretion in cystic fibrosis transmembrane conductance regulator (CFTR)-expressing airway serous cells. J Biol Chem 286:41069–41082 [DOI] [PMC free article] [PubMed]
- Gray MA, Harris A, Coleman L, Greenwell JR, Argent BE. Two types of chloride channel on duct cells cultured from human fetal pancreas. Am J Physiol. 1989;257:C240–C251. doi: 10.1152/ajpcell.1989.257.2.C240. [DOI] [PubMed] [Google Scholar]
- Hall RA, Ostedgaard LS, Premont RT, Blitzer JT, Rahman N, Welsh MJ, Lefkowitz RJ. A C-terminal motif found in the beta2-adrenergic receptor, P2Y1 receptor and cystic fibrosis transmembrane conductance regulator determines binding to the Na+/H+ exchanger regulatory factor family of PDZ proteins. Proc Natl Acad Sci USA. 1998;95:8496–8501. doi: 10.1073/pnas.95.15.8496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastbacka J, de la Chapelle A, Mahtani MM, Clines G, Reeve-Daly MP, Daly M, Hamilton BA, Kusumi K, Trivedi B, Weaver A, et al. The diastrophic dysplasia gene encodes a novel sulfate transporter: positional cloning by fine-structure linkage disequilibrium mapping. Cell. 1994;78:1073–1087. doi: 10.1016/0092-8674(94)90281-X. [DOI] [PubMed] [Google Scholar]
- Hegedus T, Serohijos AW, Dokholyan NV, He L, Riordan JR. Computational studies reveal phosphorylation-dependent changes in the unstructured R domain of CFTR. J Mol Biol. 2008;378:1052–1063. doi: 10.1016/j.jmb.2008.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg C. Congenital chloride diarrhoea. Clin Gastroenterol. 1986;15:583–602. [PubMed] [Google Scholar]
- Ishiguro H, Steward MC, Naruse S, Ko SB, Goto H, Case RM, Kondo T, Yamamoto A. CFTR functions as a bicarbonate channel in pancreatic duct cells. J Gen Physiol. 2009;133:315–326. doi: 10.1085/jgp.200810122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabba SV, Oelke A, Singh R, Maganti RJ, Fleming S, Wall SM, Everett LA, Green ED, Wangemann P. Macrophage invasion contributes to degeneration of stria vascularis in Pendred syndrome mouse model. BMC Med. 2006;4:37. doi: 10.1186/1741-7015-4-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanelis V, Chong PA, Forman-Kay JD. NMR spectroscopy to study the dynamics and interactions of CFTR. Methods Mol Biol. 2011;741:377–403. doi: 10.1007/978-1-61779-117-8_25. [DOI] [PubMed] [Google Scholar]
- Kim KH, Shcheynikov N, Wang Y, Muallem S. SLC26A7 is a Cl- channel regulated by intracellular pH. J Biol Chem. 2005;280:6463–6470. doi: 10.1074/jbc.M409162200. [DOI] [PubMed] [Google Scholar]
- Ko SB, Shcheynikov N, Choi JY, Luo X, Ishibashi K, Thomas PJ, Kim JY, Kim KH, Lee MG, Naruse S, Muallem S. A molecular mechanism for aberrant CFTR-dependent HCO3− transport in cystic fibrosis. EMBO J. 2002;21:5662–5672. doi: 10.1093/emboj/cdf580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko SB, Zeng W, Dorwart MR, Luo X, Kim KH, Millen L, Goto H, Naruse S, Soyombo A, Thomas PJ, Muallem S. Gating of CFTR by the STAS domain of SLC26 transporters. Nat Cell Biol. 2004;6:343–350. doi: 10.1038/ncb1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs H, Comfort D, Lord M, Campbell ID, Yudkin MD. Solution structure of SpoIIAA, a phosphorylatable component of the system that regulates transcription factor sigmaF of Bacillus subtilis. Proc Natl Acad Sci USA. 1998;95:5067–5071. doi: 10.1073/pnas.95.9.5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamprecht G, Heil A, Baisch S, Lin-Wu E, Yun CC, Kalbacher H, Gregor M, Seidler U. The down regulated in adenoma (dra) gene product binds to the second PDZ domain of the NHE3 kinase A regulatory protein (E3KARP), potentially linking intestinal Cl+/HCO3- exchange to Na+/H+ exchange. Biochemistry. 2002;41:12336–12342. doi: 10.1021/bi0259103. [DOI] [PubMed] [Google Scholar]
- Li C, Naren AP. Macromolecular complexes of cystic fibrosis transmembrane conductance regulator and its interacting partners. Pharmacol Ther. 2005;108:208–223. doi: 10.1016/j.pharmthera.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Li C, Dandridge KS, Di A, Marrs KL, Harris EL, Roy K, Jackson JS, Makarova NV, Fujiwara Y, Farrar PL, Nelson DJ, Tigyi GJ, Naren AP. Lysophosphatidic acid inhibits cholera toxin-induced secretory diarrhea through CFTR-dependent protein interactions. J Exp Med. 2005;202:975–986. doi: 10.1084/jem.20050421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Ganta S, Fong P. Altered ion transport by thyroid epithelia from CFTR(-/-) pigs suggests mechanisms for hypothyroidism in cystic fibrosis. Exp Physiol. 2010;95:1132–1144. doi: 10.1113/expphysiol.2010.054700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Ganta S, Fong P. Endogenous surface expression of F508-CFTR mediates cAMP-stimulated Cl- current in CFTRF508/F508 pig thyroid epithelial cells. Exp Physiol. 2012;97:115–124. doi: 10.1113/expphysiol.2011.060756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsdell P, Tabcharani JA, Rommens JM, Hou YX, Chang XB, Tsui LC, Riordan JR, Hanrahan JW. Permeability of wild-type and mutant cystic fibrosis transmembrane conductance regulator chloride channels to polyatomic anions. J Gen Physiol. 1997;110:355–364. doi: 10.1085/jgp.110.4.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohi H, Lamprecht G, Markovich D, Heil A, Kujala M, Seidler U, Kere J. Isoforms of SLC26A6 mediate anion transport and have functional PDZ interaction domains. Am J Physiol Cell Physiol. 2003;284:C769–C779. doi: 10.1152/ajpcell.00270.2002. [DOI] [PubMed] [Google Scholar]
- Ma T, Thiagarajah JR, Yang H, Sonawane ND, Folli C, Galietta LJ, Verkman AS. Thiazolidinone CFTR inhibitor identified by high-throughput screening blocks cholera toxin-induced intestinal fluid secretion. J Clin Invest. 2002;110:1651–1658. doi: 10.1172/JCI16112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makela S, Kere J, Holmberg C, Hoglund P. SLC26A3 mutations in congenital chloride diarrhea. Hum Mutat. 2002;20:425–438. doi: 10.1002/humu.10139. [DOI] [PubMed] [Google Scholar]
- Melvin JE, Park K, Richardson L, Schultheis PJ, Shull GE. Mouse down-regulated in adenoma (DRA) is an intestinal Cl(-)/HCO(3)(-) exchanger and is up-regulated in colon of mice lacking the NHE3 Na(+)/H(+) exchanger. J Biol Chem. 1999;274:22855–22861. doi: 10.1074/jbc.274.32.22855. [DOI] [PubMed] [Google Scholar]
- Mount DB, Romero MF. The SLC26 gene family of multifunctional anion exchangers. Pflugers Arch. 2004;447:710–721. doi: 10.1007/s00424-003-1090-3. [DOI] [PubMed] [Google Scholar]
- Muanprasat C, Sonawane ND, Salinas D, Taddei A, Galietta LJ, Verkman AS. Discovery of glycine hydrazide pore-occluding CFTR inhibitors: mechanism, structure-activity analysis, and in vivo efficacy. J Gen Physiol. 2004;124:125–137. doi: 10.1085/jgp.200409059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchekehu RW, Quinton PM. A new role for bicarbonate secretion in cervico-uterine mucus release. J Physiol. 2010;588:2329–2342. doi: 10.1113/jphysiol.2010.187237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohana E, Shcheynikov N, Yang D, So I, Muallem S. Determinants of coupled transport and uncoupled current by the electrogenic SLC26 transporters. J Gen Physiol. 2011;137:239–251. doi: 10.1085/jgp.201010531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostedgaard LS, Baldursson O, Vermeer DW, Welsh MJ, Robertson AD. A functional R domain from cystic fibrosis transmembrane conductance regulator is predominantly unstructured in solution. Proc Natl Acad Sci USA. 2000;97:5657–5662. doi: 10.1073/pnas.100588797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975;189:867. doi: 10.1126/science.189.4206.867-b. [DOI] [PubMed] [Google Scholar]
- Pasqualetto E, Aiello R, Gesiot L, Bonetto G, Bellanda M, Battistutta R. Structure of the cytosolic portion of the motor protein prestin and functional role of the STAS domain in SLC26/SulP anion transporters. J Mol Biol. 2010;400:448–462. doi: 10.1016/j.jmb.2010.05.013. [DOI] [PubMed] [Google Scholar]
- Patil A, Nakamura H. Disordered domains and high surface charge confer hubs with the ability to interact with multiple proteins in interaction networks. FEBS Lett. 2006;580:2041–2045. doi: 10.1016/j.febslet.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Pendred V (1896). Deaf-mutism and goitre. Lancet 2:532
- Pierucci-Alves F, Akoyev V, Stewart JC, 3rd, Wang LH, Janardhan KS, Schultz BD. Swine models of cystic fibrosis reveal male reproductive tract phenotype at birth. Biol Reprod. 2011;85:442–451. doi: 10.1095/biolreprod.111.090860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen JH, Fischer H, Illek B, Machen TE. Bicarbonate conductance and pH regulatory capability of cystic fibrosis transmembrane conductance regulator. Proc Natl Acad Sci USA. 1994;91:5340–5344. doi: 10.1073/pnas.91.12.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price GD, Howitt SM. The cyanobacterial bicarbonate transporter BicA: its physiological role and the implications of structural similarities with human SLC26 transporters. Biochem Cell Biol. 2011;89:178–188. doi: 10.1139/O10-136. [DOI] [PubMed] [Google Scholar]
- Radivojac P, Iakoucheva LM, Oldfield CJ, Obradovic Z, Uversky VN, Dunker AK. Intrinsic disorder and functional proteomics. Biophys J. 2007;92:1439–1456. doi: 10.1529/biophysj.106.094045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich DP, Berger HA, Cheng SH, Travis SM, Saxena M, Smith AE, Welsh MJ. Regulation of the cystic fibrosis transmembrane conductance regulator Cl- channel by negative charge in the R domain. J Biol Chem. 1993;268:20259–20267. [PubMed] [Google Scholar]
- Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- Rouached H, Berthomieu P, El Kassis E, Cathala N, Catherinot V, Labesse G, Davidian JC, Fourcroy P. Structural and functional analysis of the C-terminal STAS (sulfate transporter and anti-sigma antagonist) domain of the Arabidopsis thaliana sulfate transporter SULTR1.2. J Biol Chem. 2005;280:15976–15983. doi: 10.1074/jbc.M501635200. [DOI] [PubMed] [Google Scholar]
- Schaenzler M, Fahlke C (2011) Anion transport by the cochlear motor protein prestin. J Physiol. doi: 10.1113/jphysiol.2011.209577 [DOI] [PMC free article] [PubMed]
- Seavers PR, Lewis RJ, Brannigan JA, Verschueren KH, Murshudov GN, Wilkinson AJ. Structure of the Bacillus cell fate determinant SpoIIAA in phosphorylated and unphosphorylated forms. Structure. 2001;9:605–614. doi: 10.1016/S0969-2126(01)00623-2. [DOI] [PubMed] [Google Scholar]
- Seidler U, Singh A, Chen M, Cinar A, Bachmann O, Zheng W, Wang J, Yeruva S, Riederer B. Knockout mouse models for intestinal electrolyte transporters and regulatory PDZ adaptors: new insights into cystic fibrosis, secretory diarrhoea and fructose-induced hypertension. Exp Physiol. 2009;94:175–179. doi: 10.1113/expphysiol.2008.043018. [DOI] [PubMed] [Google Scholar]
- Seidler U, Singh AK, Cinar A, Chen M, Hillesheim J, Hogema B, Riederer B. The role of the NHERF family of PDZ scaffolding proteins in the regulation of salt and water transport. Ann N Y Acad Sci. 2009;1165:249–260. doi: 10.1111/j.1749-6632.2009.04046.x. [DOI] [PubMed] [Google Scholar]
- Sharma AK, Rigby AC, Alper SL. STAS domain structure and function. Cell Physiol Biochem. 2011;28:407–422. doi: 10.1159/000335104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma AK, Ye L, Baer CE, Shanmugasundaram K, Alber T, Alper SL, Rigby AC. Solution structure of the guanine nucleotide-binding STAS domain of SLC26-related SulP protein Rv1739c from Mycobacterium tuberculosis. J Biol Chem. 2011;286:8534–8544. doi: 10.1074/jbc.M110.165449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shcheynikov N, Wang Y, Park M, Ko SB, Dorwart M, Naruse S, Thomas PJ, Muallem S. Coupling modes and stoichiometry of Cl-/HCO3- exchange by slc26a3 and slc26a6. J Gen Physiol. 2006;127:511–524. doi: 10.1085/jgp.200509392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shcheynikov N, Yang D, Wang Y, Zeng W, Karniski LP, So I, Wall SM, Muallem S. The Slc26a4 transporter functions as an electroneutral Cl-/I-/HCO3- exchanger: role of Slc26a4 and Slc26a6 in I- and HCO3- secretion and in regulation of CFTR in the parotid duct. J Physiol. 2008;586:3813–3824. doi: 10.1113/jphysiol.2008.154468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Sala C. PDZ domains and the organization of supramolecular complexes. Annu Rev Neurosci. 2001;24:1–29. doi: 10.1146/annurev.neuro.24.1.1. [DOI] [PubMed] [Google Scholar]
- Shibagaki N, Grossman AR. Probing the function of STAS domains of the Arabidopsis sulfate transporters. J Biol Chem. 2004;279:30791–30799. doi: 10.1074/jbc.M403248200. [DOI] [PubMed] [Google Scholar]
- Shibagaki N, Grossman AR. The role of the STAS domain in the function and biogenesis of a sulfate transporter as probed by random mutagenesis. J Biol Chem. 2006;281:22964–22973. doi: 10.1074/jbc.M603462200. [DOI] [PubMed] [Google Scholar]
- Singh R, Wangemann P. Free radical stress-mediated loss of Kcnj10 protein expression in stria vascularis contributes to deafness in Pendred syndrome mouse model. Am J Physiol Renal Physiol. 2008;294:F139–F148. doi: 10.1152/ajprenal.00433.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siwiak M, Edelman A, Zielenkiewicz P. Structural models of CFTR-AMPK and CFTR-PKA interactions: R-domain flexibility is a key factor in CFTR regulation. J Mol Model. 2012;188:83–90. doi: 10.1007/s00894-011-1029-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songyang Z, Fanning AS, Fu C, Xu J, Marfatia SM, Chishti AH, Crompton A, Chan AC, Anderson JM, Cantley LC. Recognition of unique carboxyl-terminal motifs by distinct PDZ domains. Science. 1997;275:73–77. doi: 10.1126/science.275.5296.73. [DOI] [PubMed] [Google Scholar]
- Steward MC, Ishiguro H, Case RM. Mechanisms of bicarbonate secretion in the pancreatic duct. Annu Rev Physiol. 2005;67:377–409. doi: 10.1146/annurev.physiol.67.031103.153247. [DOI] [PubMed] [Google Scholar]
- Sun F, Hug MJ, Lewarchik CM, Yun CH, Bradbury NA, Frizzell RA. E3KARP mediates the association of ezrin and protein kinase A with the cystic fibrosis transmembrane conductance regulator in airway cells. J Biol Chem. 2000;275:29539–29546. doi: 10.1074/jbc.M004961200. [DOI] [PubMed] [Google Scholar]
- Superti-Furga A, Hastbacka J, Wilcox WR, Cohn DH, van der Harten HJ, Rossi A, Blau N, Rimoin DL, Steinmann B, Lander ES, Gitzelmann R. Achondrogenesis type IB is caused by mutations in the diastrophic dysplasia sulphate transporter gene. Nat Genet. 1996;12:100–102. doi: 10.1038/ng0196-100. [DOI] [PubMed] [Google Scholar]
- van den Hove MF, Croizet-Berger K, Jouret F, Guggino SE, Guggino WB, Devuyst O, Courtoy PJ. The loss of the chloride channel, ClC-5, delays apical iodide efflux and induces a euthyroid goiter in the mouse thyroid gland. Endocrinology. 2006;147:1287–1296. doi: 10.1210/en.2005-1149. [DOI] [PubMed] [Google Scholar]
- Wang S, Raab RW, Schatz PJ, Guggino WB, Li M. Peptide binding consensus of the NHE-RF-PDZ1 domain matches the C-terminal sequence of cystic fibrosis transmembrane conductance regulator (CFTR) FEBS Lett. 1998;427:103–108. doi: 10.1016/S0014-5793(98)00402-5. [DOI] [PubMed] [Google Scholar]
- Wang XF, Zhou CX, Shi QX, Yuan YY, Yu MK, Ajonuma LC, Ho LS, Lo PS, Tsang LL, Liu Y, Lam SY, Chan LN, Zhao WC, Chung YW, Chan HC. Involvement of CFTR in uterine bicarbonate secretion and the fertilizing capacity of sperm. Nat Cell Biol. 2003;5:902–906. doi: 10.1038/ncb1047. [DOI] [PubMed] [Google Scholar]
- Wilkinson DJ, Strong TV, Mansoura MK, Wood DL, Smith SS, Collins FS, Dawson DC. CFTR activation: additive effects of stimulatory and inhibitory phosphorylation sites in the R domain. Am J Physiol. 1997;273:L127–L133. doi: 10.1152/ajplung.1997.273.1.L127. [DOI] [PubMed] [Google Scholar]
- Xie J, Adams LM, Zhao J, Gerken TA, Davis PB, Ma J. A short segment of the R domain of cystic fibrosis transmembrane conductance regulator contains channel stimulatory and inhibitory activities that are separable by sequence modification. J Biol Chem. 2002;277:23019–23027. doi: 10.1074/jbc.M201661200. [DOI] [PubMed] [Google Scholar]
- Zhang W, Penmatsa H, Ren A, Punchihewa C, Lemoff A, Yan B, Fujii N, Naren AP. Functional regulation of cystic fibrosis transmembrane conductance regulator-containing macromolecular complexes: a small-molecule inhibitor approach. Biochem J. 2011;435:451–462. doi: 10.1042/BJ20101725. [DOI] [PMC free article] [PubMed] [Google Scholar]