Abstract
OBJECTIVE
The purpose of this article is to review cancer- and therapy-specific tumor response assessment criteria used in clinical trials and in practice, with illustrative case examples, and to discuss future directions toward “personalized” tumor response assessment.
CONCLUSION
Although Response Evaluation Criteria in Solid Tumors will remain as the primary generalized criteria for response assessment, newer cancer- and therapy-specific criteria will play an important role in providing state-of-the-art response assessment of tumor following molecular targeted therapy and will contribute to personalized cancer care in the era of molecular medicine.
Keywords: CT, personalized medicine, RECIST, response assessment
Recent advances in molecular biology have elucidated the different molecular mechanisms of cancer development and progression, which are specific to certain types of cancer. New anticancer therapeutic agents have been developed to target the specific genomic abnormalities and have been used to treat genomically characterized subsets of patients with specific types of cancer. One of the representative examples is imatinib, a tyrosine kinase inhibitor that inhibits the product of the C-KIT protooncogene and that has been shown to be effective in patients with advanced gastrointestinal stromal tumor (GIST) driven by a mutated KIT receptor tyrosine kinase (CD117) [1, 2]. Other examples include gefitinib and erlotinib, epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors that are associated with dramatic clinical response in patients with non–small cell lung cancer (NSCLC) whose tumors harbor somatic activating mutations of the EGFR tyrosine kinase domain [3–5]. The discovery of genomic abnormalities specific to certain types of cancer and the clinical application of such discoveries for the selection of therapy have transformed the way oncologists approach cancer and plan treatment. Genomic characterization of tumors from either surgical or biopsy specimens allows oncologists to select the therapeutic regimen best suited for individual patients to target the specific underlying pathways driving their tumors, enabling truly personalized cancer treatment in the era of molecular medicine [6, 7].
Imaging plays a major role in objectively assessing response to therapy during anticancer treatment. In 1979, the World Health Organization (WHO) proposed uniform criteria, known as the WHO criteria, to report the results of cancer treatment [8, 9]. Subsequently, the Response Evaluation Criteria in Solid Tumors (RECIST) guidelines were introduced to unify the various modifications of the WHO criteria and to provide standardized and simplified criteria that allow meaningful comparison among studies [10]. The RECIST guideline, published in 2000 and revised in 2009, has become the most widely accepted criteria for response evaluation for clinical trials and practice in most solid tumors [10–12], with the exception of malignant lymphoma. For malignant lymphoma, the International Working Group response criteria (also known as the Cheson criteria), introduced in 1999 and revised in 2007, have been widely adopted [13, 14].
Although RECIST provides a standardized and practical method to assess response and define progression in solid tumors in general, pitfalls and limitations of RECIST have been noted in various clinical scenarios. Some of the pitfalls and limitations are cancer and therapy specific and are observed in patients with specific genomic mutations treated with specific targeted therapies [15, 16]. Such clinical observations indicate that traditional RECIST-based criteria, originally developed to assess response to cytotoxic chemo-therapeutic agents, may not be sufficient to fully characterize tumor response and progression in genomically defined subsets of patients treated with specific targeted therapies. To complement such pitfalls and limitations of RECIST, several newer response criteria are being proposed for patients with specific types of cancer treated with specific therapeutic agents, and some of these criteria have been applied in oncology trials and practice. In this article, we review cancer- and therapy-specific limitations of RECIST, as well as new criteria proposed and used in clinical oncology practice with illustrative case examples, to provide a summary guide of these criteria for radiologists. We also discuss future directions toward “personalized” tumor response assessment, by which radiologists can meaningfully contribute to cancer patient care in the era of molecular medicine.
The Conventional Criteria for Tumor Response to Therapy: WHO, RECIST, and Cheson Criteria
Before the discussion of the newer criteria, familiarity with the conventional response assessment methods, including RECIST, WHO, and Cheson criteria, is important. The outline of these three criteria are summarized in Tables 1 and 2. The key features of the original RECIST (RECIST 1.0) included definitions of minimum size of measurable lesions, instructions on the number of lesions to follow, and the use of unidimensional measurement of tumor size to provide a simple and practical way to assess response to therapy [10, 11] (Table 1). In 2009, the revised RECIST (RECIST 1.1) introduced several changes, including the reduction of number of target lesions, assessment of pathologic lymph nodes, clarification of disease progression, and inclusion of FDG PET scan in detection of new lesions, to further simplify, optimize, and standardize the assessment of tumor burden [11] (Tables 1 and 2).
TABLE 1.
Outline of Response Evaluation Criteria in Solid Tumors (RECIST), World Health Organization (WHO), and Cheson Criteria: Imaging Modality and Guidelines for Measurement and Response Assessment
| Criterion | RECISTa | WHO | Cheson (Revised in 2007) |
|---|---|---|---|
| Imaging modality | CT, MRI, and chest radiography are recommended modalities. FDG PET scan is included in detection of new lesions by RECIST 1.1. | No particular mention of imaging modality. | CT scan is used to assess lymph node size. PET is strongly recommended before treatment of patients with routinely FDG-avid potentially curable lymphomas in revised Cheson criteria. Gallium scan, initially encouraged in 1999, is not considered state-of-the-art by revised Cheson criteria. |
| Measurable lesions | A longest diameter of ≥ 10 mm on CT with a slice thickness of ≤ 5 mm; a longest diameter of ≥ 20 mm on nonhelical CT with a slice thickness of > 10 mm; a longest diameter of ≥ 20 mm on chest radiography; short axis ≥ 15 mm for lymph nodes by RECIST 1.1. | No mention of minimal size of the lesion. | Abnormal lymph nodes or nodal masses or hepatic or splenic nodules that are clearly measurable in at least 2 perpendicular dimensions, selected from disparate regions of the body, including mediastinal and retroperitoneal areas if these sites are involved. |
| Measurement and response assessment | Target lesions include all measurable lesions up to 5 per organ and 10 in total by RECIST 1.0; up to 2 per organ and 5 in total by RECIST 1.1. All other lesions or site of disease are recorded as nontarget lesions.b A sum of the longest diameter for all target lesions is used for assessment. | No mention of the number of lesions to be selected. Bidimensional measurement (product of the longest diameter and the greatest perpendicular diameter) is used for assessment. | Up to 6 lesions representing abnormal lymph nodes or nodal masses or hepatic or splenic nodules are included in measurement. The sum of the products of the greatest diameters is used for response assessment. |
The original RECIST (RECIST 1.0) was published in 2000 [10], and the revised RECIST (RECIST 1.1) was published in 2009 [11].
Nonmeasurable lesions by RECIST include other lesions that do not meet the criteria as measurable lesions, such as small lesions with a longest diameter of < 10 mm, skeletal metastases without a soft-tissue component, ascites, pleural effusion, lymphangitic spread of tumor, leptomeningeal disease, inflammatory breast disease, cystic or necrotic lesions, lesions in an irradiated area, and an abdominal mass not confirmed by imaging, are recorded as “non-target lesions” [10]. Lymph node measuring ≥ 10 mm but < 15 mm in short axis is considered “non-measureable” and therefore recorded as “non-target lesions” by RECIST 1.1 [11].
TABLE 2.
Response Category by Response Evaluation Criteria in Solid Tumors (RECIST), World Health Organization (WHO), and Cheson Criteria
| Response Category | RECISTa | WHO | Cheson (Revised in 2007) |
|---|---|---|---|
| Complete response | Disappearance of all target and nontarget lesions. All lymph nodes must be < 10 mm short axis by RECIST 1.1. | Disappearance of all known disease. | Disappearance of all evidence of disease. Nodes: For FDG-avid lymphoma, mass of any size permitted if PET negative; for variably FDG-avid lymphoma or FDG avidity unknown, regression to normal size on CT.b Spleen and liver: Not palpable, nodules disappeared. Bone marrow: Infiltrate cleared on repeat biopsy; if indeterminate by morphology, immunohistochemistry should be negative. |
| Partial response | ≥ 30% decrease in the sum of the longest diameters of target lesions compared with baseline. | ≥ 50% decrease in target lesions, without a 25% increase in any one target lesion or the appearance. | Regression of measurable disease and no new sites. Nodes: ≥ 50% decrease in sum of the products of the greatest diameters of up to 6 largest dominant masses with no increase in size of other nodes. For FDG-avid lymphoma, PET positive in one or more at previously involved site. For variably FDG-avid lymphoma or FDG avidity unknown, ≥ 50% decrease in sum of the products of the greatest diameters on CT is used. Liver and spleen: ≥ 50% decrease in sum of the products of the greatest diameters of nodules with no increase in size of liver or spleen. Bone marrow: Irrelevant if positive before therapy. |
| Stable disease | Neither partial response nor progressive disease. | Neither partial response nor progressive disease.c | Failure to attain complete or partial response or progressive disease. |
| Progressive disease | ≥ 20% increase in the sum of the longest diameter of target lesions compared with the smallest sum of longest diameter recorded (5 mm absolute increase in size is also required by RECIST 1.1), or the appearance of one or more new lesions, or unequivocal progression of nontarget lesions. | ≥ 25% increase in the size of measurable lesions, appearance of new lesions, or unequivocal progression of nontarget lesions. | Any new lesion or increase by ≥ 50% in sum of the products of the greatest diameters of previously involved sites from nadir. Nodes: Appearance of a new lesion > 1.5 cm in any axis, ≥ 50% increase in sum of the products of the greatest diameters of more than one node, or ≥ 50% increase in longest diameter of a previously identified node > 1 cm in short axis; PET-positive lesions for FDG-avid lymphoma. Liver and spleen: ≥ 50% increase from nadir in the sum of the products of the greatest diameters of any previous lesions. Bone marrow: New or recurrent involvement. |
The original RECIST (RECIST 1.0) was published in 2000 [10], and the revised RECIST (RECIST 1.1) was published in 2009 [11].
Normal size on Cheson criteria: ≤ 1.5 cm in the greatest transverse diameter for nodes > 1.5 cm before therapy; previously involved nodes that were 1.1–1.5 cm in their long axis and more than 1.0 cm in their short axis before treatment must have decreased to ≤ 1.0 cm in their short axis after treatment [13, 14].
The category was called “No change (NC)” in the WHO criteria [8].
Choi Response Criteria for GIST Treated With Imatinib
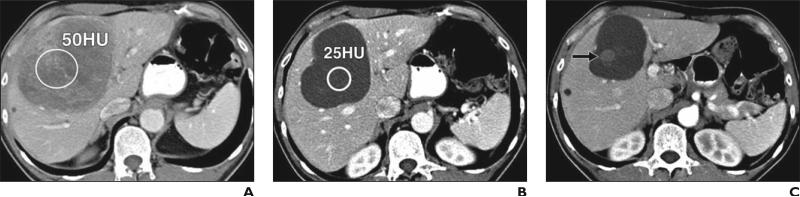
The Choi response criteria, which incorporate tumor density and use small changes in tumor size on CT [15, 16], have been proposed and used in assessing the response of metastatic GIST treated with imatinib and represent one of the first and most representative examples of cancer- and therapy-specific criteria for response assessment in solid tumors that has replaced RECIST. In the Choi criteria, response is defined as a 10% decrease in unidimensional tumor size or a 15% decrease in CT attenuation, as opposed to a 30% decrease in unidimensional tumor size defined by RECIST [15, 16] (Figs. 1A and 1B). The Choi criteria also define tumor progression on the basis of CT findings, including the appearance of new lesions or metastasis, the appearance of new intratumoral tumor nodules or an increase in the size of existing intratumoral tumor nodules, or an increase in overall tumor size by more than 20%, in the absence of posttreatment hypo-dense change [15, 16] (Figs. 1C and 1D).
Fig. 1.
58-year-old man with advanced gastrointestinal stromal tumor with liver metastasis, treated with imatinib mesylate.
A, Baseline contrast-enhanced CT of abdomen before therapy shows heterogeneously enhancing mass in liver representing metastasis, measuring 10 cm in longest diameter and 50 HU in CT attenuation (circle).
B, Follow-up CT scan obtained 8 weeks after initiation of imatinib mesylate therapy shows significant decrease in CT attenuation of tumor (circle; 25 HU), meeting criteria for response by Choi criteria, with minimal decrease in size (9.5 cm in longest diameter).
C, Patient continued receiving imatinib mesylate therapy. Follow-up CT scan at 2 years revealed new intratumoral tumor nodule (arrow), meeting criteria for progression by Choi criteria. Note measurement of longest diameter alone by Response Evaluation Criteria in Solid Tumors (7.5 cm) fails to detect progression. Adjacent small lesion in anterior segment of liver remained unchanged since baseline, most likely representing benign lesion.
The development of these new criteria dedicated for GIST treated with imatinib originated with the clinical observations that the most dramatic changes in responding GISTs are seen within individual tumor masses, which becomes homogeneous and hypodense on CT, and that decreases in tumor size are minimal at an early posttreatment time point in many tumors, therefore failing to meet the conventional response criteria of 30% decrease in size by RECIST [15–17] (Fig. 1). On the basis of these observations, Choi et al. [16] studied 172 lesions in 40 patients with metastatic GIST treated with imatinib, who had pretreatment and 2-month follow-up CT and FDG PET examinations, with multivariate analysis using tumor size and density (Hounsfield units) on CT as well as maximum standardized uptake value on FDG PET. The study found that a decrease in tumor size of more than 10% or a decrease in tumor density of more than 15% on CT (now known as Choi response criteria) had a higher sensitivity in identifying PET responders than did standard RECIST (97% by Choi criteria vs 52% by RECIST) [16].
Subsequently, Benjamin et al. [18] showed the prognostic value of the Choi response criteria by studying 58 patients with imatinib-treated GIST who were evaluated by contrast-enhanced CT before treatment and at a 2-month follow-up examination, and who were followed up for 60 months for survival analysis. In that study, responders by Choi criteria on CT after 2 months of imatinib therapy had significantly longer time to progression than those who did not (p = 0.0002), whereas responders by RECIST did not show significant correlation with time to progression (p = 0.74). In addition, disease-specific survival was also significantly correlated with responders by Choi criteria (p = 0.04), but not with responders by RECIST (p = 0.45) [18]. Since the publication of these findings in 2007, the Choi response criteria have been widely used as a method for response assessment in GISTs and have been used in clinical trials and practice [19, 20]. The use of Choi criteria or similar modified criteria incorporating CT attenuation changes have also been studied and proposed in other solid tumors treated with targeted therapies, including other sarcomas, as well as renal cell carcinomas and hepatocellular carcinomas treated with antiangiogenic therapy [21–23].
The introduction of the Choi response criteria in GISTs treated with imatinib was groundbreaking in tumor response assessment in many ways: first, the criteria dedicated for a specific type of tumor treated with a specific targeted therapy were proposed for the first time; second, the criteria were based on clinical observations in the specific subset of patients for whom conventional RECIST failed to provide adequate assessment; third, the criteria included an additional CT parameter, tumor density or CT attenuation, in addition to tumor size; and fourth, the criteria have been shown to be a better prognostic indicator than conventional RECIST on the basis of patient survival data. The introduction and successful application of the Choi criteria opened a door to a new era of response assessment in personalized cancer treatment by providing a strategy to overcome the limitations of RECIST in a subset of patients receiving molecular targeting therapy. As a consequence, several newer criteria have been proposed and investigated in tumors other than GIST, to optimize response assessment and treatment course and to predict prolonged survival in specifically defined patient populations.
Morphology, Attenuation, Size, and Structure Criteria for Renal Cell Carcinoma Treated With Antiangiogenic Targeted Therapy
Recent advances in the understanding of genomic abnormalities in metastatic clear cell renal cell carcinoma (RCC), which accounts for 85% of all RCC, have led to the development and approval of molecular targeted anti-angiogenic therapies using tyrosine kinase inhibitors, such as sunitinib and sorafenib [24, 25]. Clear cell RCC has a high frequency of mutations in the von Hippel–Lindau gene, which results in up-regulation of receptor and cellular tyrosine kinases responsible for tumor proliferation and angiogenesis [26]. Therapy using multitargeted tyrosine kinase inhibitors, including sunitinib and sorafenib, has been shown to improve progression-free survival in patients with metastatic RCC [27–31]. Given the advances in therapeutic strategy based on the molecular background in metastatic RCC, several investigations have been performed to optimize the assessment of therapeutic response to antiangiogenic targeted therapy in patients with metastatic RCC [31–34]. Observations of patients with metastatic RCC treated with antiangiogenic tyrosine kinase inhibitors suggested that RECIST-based size criteria alone may not be adequate, because these therapies may not always produce tumor shrinkage [29]. Moreover, similar to GIST treated with imatinib, metastatic RCC treated with antiangiogenic tyrosine kinase inhibitors has decreased attenuation of the tumor on contrast-enhanced CT, which has been correlated with pathologic evidence of necrosis on resection of the mass, with minimal size change [31–34].
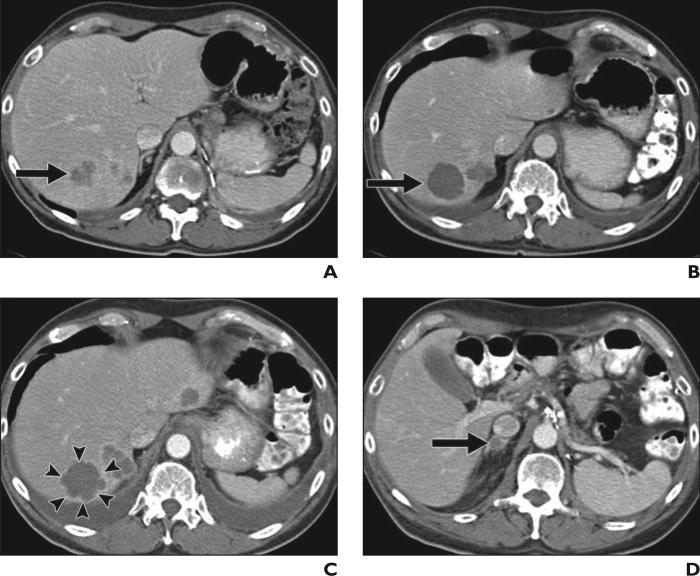
In January 2010, Smith et al. [35] proposed new imaging criteria, the size and attenuation CT criteria, which include long-axis measurements and volumetric mean tumor attenuation of target lesions on contrast-enhanced CT images for metastatic RCC treated with antiangiogenic targeted therapy. Soon after, the same investigators proposed further modified criteria—morphology, attenuation, size, and structure (MASS) criteria—to overcome limitations of size and attenuation CT and include specific morphologic or structural changes in treated metastases [36]. Although they use the size measurements described in RECIST (i.e., the sum of the longest diameters of the target lesions) and CT attenuation, MASS criteria also use morphologic or structural changes, including “marked central necrosis,” which is defined as greater than 50% of the enhancing central portion of a predominantly solid enhancing mass subjectively changing to near-fluid attenuation (necrosis) after treatment (Figs. 2A and 2B), and “marked central fill-in,” which is defined as a subjective change from marked central necrosis to complete or nearly complete central intratumoral enhancement on contrast-enhanced CT [36].
Fig. 2.
57-year-old man with metastatic renal cell carcinoma treated with antiangiogenic therapy using multitargeted receptor tyrosine kinase inhibitor, sunitinib malate.
A, Baseline contrast-enhanced CT of abdomen before therapy shows heterogeneously enhancing mass (arrow) in posterior segment of liver with similar adjacent lesions, representing vascularized metastasis.
B, Follow-up CT scan 12 weeks after initiation of sunitinib malate therapy shows significant decrease in CT attenuation of mass (arrow) to near fluid attenuation due to necrosis, showing “marked central necrosis” by morphology, attenuation, size, and structure (MASS) criteria and therefore “favorable response.”
C and D, Follow-up CT scans at 20 weeks of sunitinib malate therapy show new enhancement in peripheral rim of mass (arrowheads, C), as well as new adrenal lesion (arrow, D), representing “unfavorable response” by MASS criteria.
MASS criteria classify objective response into three categories, which include “favorable response,” “indeterminate response,” and “unfavorable response” [36]. Favorable response is defined as a decrease in tumor size of 20% or more, or as one or more predominantly solid enhancing lesions with marked central necrosis or marked decreased attenuation (≥ 40 HU), without new lesions (Figs. 2A and 2B). Unfavorable response is defined as an increase in tumor size of 20% or more in the absence of marked central necrosis or marked decreased attenuation or as new metastases, marked central fill-in, or new enhancement of a previously homogeneously hypoattenuating nonenhancing mass (Figs. 2C and 2D). Indeterminate response is used when the changes do not fit criteria for favorable response or unfavorable response [36]. In a retrospective review of 84 patients with metastatic clear cell RCC treated with first-line sunitinib or sorafenib therapy (n = 84), a favorable response defined by MASS criteria had a sensitivity of 86% and specificity of 100% in identifying patients with a good clinical outcome (progression-free survival of > 250 days) versus 17% and 100%, respectively, for partial response defined by RECIST [36].
Although the MASS criteria remain to be validated in a larger prospective cohort of patients, an important aspect of imaging-based response assessment is illustrated in the criteria. Because “criteria” for response assessment aim for “objective” evaluation of tumor response to therapy, they tend to have clear-cut numeric definitions for response and progression (i.e., > 20% increase in the sum of the longest diameters of target lesions for progression by RECIST). However, imaging provides significantly more information than the numeric parameters, and some of the information can only be qualitatively defined and therefore subjectively evaluated. Although it is often difficult to include the qualitative findings into response assessment criteria, such findings may truly reflect the changes of tumor in response to therapy that cannot be captured with numeric parameters. By defining the morphologic and structural changes as objectively as possible, MASS criteria present one of the solutions for this dilemma that is inherent in imaging-based response assessment.
Immune-Related Response Criteria for Advanced Melanoma Treated With Immunotherapeutic Agents
Metastatic melanoma has limited options for effective treatment, and the median survival of patients with melanoma with distant metastases is less than 1 year [37, 38]. Until recently, no therapy has been shown in a phase 3 randomized controlled trial to improve overall survival in patients with metastatic melanoma [38–41]. Increasing understanding of regulatory pathways that limit the immune response to cancer has led to the development and application of immunotherapeutic agents. Ipilimumab is a fully human monoclonal antibody (IgG1) that promotes antitumor immunity by blocking cytotoxic T lymphocyte–associated antigen 4, an immune checkpoint molecule that down-regulates pathways of T cell activation [42, 43]. Ipilimumab has shown activity in patients with metastatic melanoma as monotherapy in phase 2 studies and has recently been shown to significantly improve overall survival of patients with metastatic melanoma in a randomized double-blind phase 3 trial that involved 125 centers in 13 countries [43–46]. On the basis of those results, the Food and Drug Administration approved ipilimumab for the treatment of advanced melanoma as a second-line therapy in March 2011.
In patients with metastatic melanoma treated with immunotherapeutic agents such as ipilimumab, which work by enhancing antitumor immune responses rather than directly inducing cytotoxicity to tumor cells, clinical observations suggested that “stable disease” by RECIST or WHO criteria may be an indicator of meaningful therapeutic effect [43]. Furthermore, response to immunotherapy was noted to occur after an increase in tumor burden characterized as progressive disease by RECIST or WHO criteria [43]. These observations raised concern for relying solely on these conventional criteria in patients treated with immunotherapy [47]. Given this background, approximately 200 oncologists, immunotherapists, and regulatory experts held a series of workshops in 2004–2005 to discuss their experience with immunotherapeutic agents in patients with cancer [43]. As a result, a novel set of criteria were developed to capture additional response patterns observed in advanced melanoma treated with immunotherapy that are not described by RECIST or WHO criteria and were designated as “immune-related response criteria” [47]. The immune-related response criteria were evaluated in a series of large multinational studies, representing a clinical trial program of 487 patients with advanced melanoma who received ipilimumab [47].
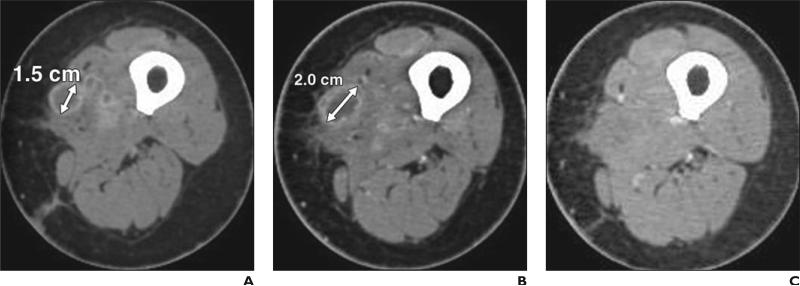
The immune-related response criteria describe four distinct patterns of tumor response to ipilimumab observed in patients with advanced melanoma treated in the trial: response in baseline lesions evident by 12 weeks since the initiation of therapy, with no new lesions (pattern A); “stable disease,” which in some patients was followed by a slow steady decline in total tumor burden (pattern B); responses after an initial increase in total tumor burden (pattern C) (Fig. 3); and a reduction in total tumor burden during or after the appearance of new lesions at time points later than 12 weeks since the initiation of therapy (pattern D) [47] (Fig. 4). Although patterns A and B would be captured by conventional RECIST or WHO criteria, patterns C and D would be classified as progression by conventional criteria, instead of response.
Fig. 3.
56-year-old woman with metastatic melanoma treated with ipilimumab.
A, Baseline contrast-enhanced CT scan before ipilimumab therapy shows metastatic nodule (double-ended arrow) in left upper thigh, measuring 1.5 cm in longest diameter.
B, Follow-up CT scan after 12 weeks of ipilimumab therapy reveals increase in size of nodule (double-ended arrow), measuring 2.0 cm in longest diameter.
C, Follow-up CT scan after 24 weeks of ipilimumab therapy shows complete disappearance of nodule, representing response pattern C in immune-related response criteria.
Fig. 4.
56-year-old woman with metastatic melanoma treated with ipilimumab.
A and B, Contrast-enhanced CT scans of abdomen before (A) and 12 weeks after (B) initiation of ipilimumab therapy reveal new subcutaneous nodule (arrow, B) suspicious for new site of metastasis at 12 weeks.
C, Follow-up CT scan at 24 weeks reveals resolution of nodule. Overall tumor burden also decreased, showing response pattern D in immune-related response criteria
To systematically characterize these additional response patterns, the immune-related response criteria use the sum of the products of the two largest perpendicular diameters of all index lesions at the baseline assessment. At each subsequent follow-up assessment, the sum of the products of the two largest perpendicular diameters of the index lesions as well as that for new measurable lesions (≥ 5 × 5 mm) are included to reflect the total tumor burden, in distinct contrast to WHO or RECIST criteria, which do not require measurement of new lesions and which score progression if a new lesion is present [10–12, 47]. The threshold of response for the immune-related response criteria remains the same as for the WHO criteria (Table 2). Overall response assessment by the immune-related response criteria includes complete response, which requires complete disappearance of all lesions, no new lesions, and confirmation by a repeat consecutive assessment within 4 weeks; partial response, which requires the decrease in tumor burden 50% or higher relative to baseline, confirmed by a consecutive assessment within 4 weeks; stable disease, not meeting criteria for a complete response or partial response in the absence of progression; and progressive disease, which requires the increase in tumor burden 25% or greater relative to the minimum recorded tumor burden, as confirmed by a repeat consecutive assessment no less than 4 weeks from the date first documented [47]. The immune-related response criteria are distinct from the conventional criteria in that patients are considered to have partial response or stable disease even if new lesions are present, if they meet the thresholds of response. In addition, patients are not assessed as having progression even if new lesions are present, as long as the tumor burden of all lesions does not increase by 25% or more [47]. The criteria also require confirmation of progressive disease by a second scan in the absence of rapid clinical deterioration, to capture late-responding patients with a trend toward response within 4 weeks after initial progressive disease (pattern C).
The analysis of a total of 227 patients with advanced melanoma treated with ipilimumab in the phase 2 randomized clinical trial identified 22 patients (9.7%) who were initially classified as progression by WHO criteria but who showed evidence of efficacy consistent with response to ipilimumab by the immune-related response criteria (partial response or stable disease) [47]. More important, the Kaplan-Meier analysis for overall survival indicated that these patients have survival comparable to that for patients with complete response, partial response, or stable disease by WHO criteria, suggesting that the immune-related response criteria can identify at least an additional 10% of patients who will benefit from ipilimumab therapy in survival prolongation [47].
The immune-related response criteria present a novel way of evaluating the response to immune therapy in patients with advanced melanoma and enable capturing patients who show response patterns that are not detected in the conventional criteria but still benefit from therapy. The additional patterns described in the immune-related response criteria are also supported by histologic findings. The initial apparent increase in tumor burden preceding response can be due to either tumor growth until a sufficient immune response develops, or transient immune cell infiltrate with or without edema on tumor biopsies [48, 49]. In addition, apparent new lesions may be due to T cell infiltration into radiographically undetectable tumor deposits that are already present at baseline [47]. Although the development of the immune-related response criteria are based on patients with advanced melanoma treated with ipilimumab, it is expected that the criteria will have broad applicability to assessing response to immunotherapeutic agents. The criteria are currently being prospectively evaluated in phase 3 clinical trials with ipilimumab to determine their association with the survival [47].
Alternate Method Incorporating Cavitation in Response Assessment for NSCLC Treated With Antiangiogenic Therapy
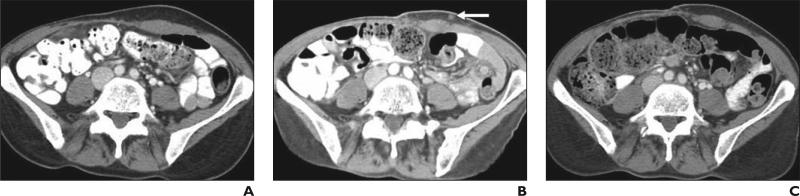
Lung cancer is the leading cause of cancer death in the United States and worldwide, accounting for nearly 160,000 deaths per year in the United States [50, 51]. Recent investigations of the molecular basis of lung cancer have enabled clinical applications of targeted therapeutic agents, including the EGFR tyrosine kinase inhibitors and antiangiogenic agents, such as vascular EGFR inhibitors [3–5, 52]. Tumor cavitation of pulmonary lesions is commonly observed in NSCLC treated with vascular EGFR inhibitors [53, 54]. Because the cavitary portion of the tumor filled with air does not contribute to the tumor volume, the assessment of tumor burden may be improved by incorporating the cavitation into the measurement. On the basis of these observations, Crabb et al. [54] proposed an alternate method incorporating cavitation in response assessment for NSCLC treated with vascular EGFR inhibitors. In this method, the central cavity diameter is subtracted from the overall longest diameter of the lesion (Fig. 5). All other details for response assessment are identical to RECIST [54]. In a retrospective review of 33 patients treated with vascular EGFR inhibitor combined with platinum-based chemotherapy, tumor cavitation was observed in 24% of the patients. However, the alternate method for response assessment resulted in an alteration of response assessment, time to best response, duration of response, and time of disease progression in only a minority of patients compared with RECIST [54].
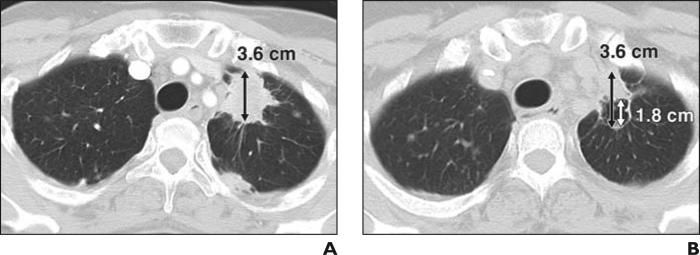
Fig. 5.
53-year-old woman with stage IV adenocarcinoma of lung treated with paclitaxel, carboplatin, and concurrent vascular epidermal growth factor receptor inhibitor, bevacizumab.
A, Baseline CT scan of chest before bevacizumab therapy shows spiculated mass (double-ended arrow) in left upper lobe, which measured 3.6 cm in longest diameter.
B, Follow-up CT after 6 weeks of therapy reveals development of tumor cavitation. Measurement of lesion by Response Evaluation Criteria in Solid Tumors would be 3.6 cm (black double-ended arrow), which is not different compared with baseline, even though decrease of tumor volume is evident after bevacizumab therapy. Using alternate method incorporating cavitation, measurement of lesion would be 1.8 cm (white double-ended arrow) because diameter of cavity (1.8 cm) should be extracted from longest diameter of entire lesion (3.6 cm). Measurement by alternate method shows 50% decrease compared with baseline, meeting criteria for partial response.
More recently, Lee et al. [55] proposed another set of criteria for NSCLC treated with EGFR tyrosine kinase inhibitors, which include tumor constituents such as solid and ground-glass opacity components, tumor cavitation, and CT attenuation changes. In their analysis of 75 patients with NSCLC treated with EGFR tyrosine kinase inhibitors, the criteria had a statistically significant association with overall survival [55]. Both criteria remain to be prospectively tested in a larger patient population.
Clinical Scenario Requiring New Criteria: Case of Clinical Benefit After Progressive Disease
One clinical scenario for which criteria have not yet been established is the case of clinical benefit even after progressive disease is documented. This is illustrated in the use of crizotinib, an inhibitor of the anaplastic lymphoma kinase tyrosine kinase, which has shown striking activity against non–small cell lung adenocarcinoma harboring EML4-ALK translocation [56]. In the crizotinib development plan, patients with RECIST progressive disease may continue taking crizotinib if they are judged by the investigator to still be receiving clinical benefit. Among 116 evaluable patients in an expanded cohort of the original phase 1 study, 16 have continued taking crizotinib after a designation of disease progression. The time taking crizotinib after progressive disease has ranged from 22 to more than 447 days, suggesting that substantial clinical benefit can be maintained in some patients [57]. Some of the events determining disease progression were in the brain, suggesting that CNS progression may need to be considered separately from systemic progression in some instances. In other cases, assessment of the overall rate of change of tumor burden may be important in determining who may still continue to benefit from a kinase inhibitor. These data are provocative and suggest other instances in which new criteria beyond RECIST will be needed to guide clinical practice.
Summary and Future Directions
Because the response assessment to anticancer therapy is performed in oncology clinical trials and practice worldwide, the tumor response criteria have to be easily performed in a standardized fashion across multiple institutions over the world. Therefore, RECIST will remain the primary generalized criteria for response assessment in solid tumors. Recently, newer or modified criteria for response assessment have been proposed and used to complement pitfalls of RECIST in a cancer- and therapy-specific fashion. Although many of these criteria have been developed for specific cancers and therapies, they may be found to be applicable to different cancers that depend on similar genomic drivers, or to other anticancer agents that target similar molecular pathways. Ultimately, prospective validation of these types of criteria will be necessary for patients with specific types of cancer treated with specific targeted agents.
The concept of personalized medicine has been well applied in therapeutic decision making and patient management in clinical oncology, enabling personalized cancer treatment in individual patients. Tumor response assessment, which should evolve hand-in-hand with the advances in cancer treatment, should also incorporate a similar concept to provide up-to-date response evaluation that meets the needs of oncologists delivering state-of-the-art cancer care. We propose future directions toward “personalized” tumor response assessment by applying these cancer- and therapy-specific criteria to correct pitfalls of the conventional criteria. Therefore, these criteria represent a critically important contribution to the assessment of efficacy by novel targeted therapies, allowing the radiology community to be part of personalized cancer care in the era of molecular medicine.
Acknowledgments
The investigators were supported by 2009–2011 Agfa HealthCare/RSNA Research Scholar Grant (to M. Nishino) and the National Institutes of Health grant 1K23CA157631-01A1 (grant 5 R21 CA11627-02 to H. Hatabu).
References
- 1.Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–480. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 2.Blanke CD, Rankin C, Demetri GD, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol. 2008;26:626–632. doi: 10.1200/JCO.2007.13.4452. [DOI] [PubMed] [Google Scholar]
- 3.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 4.Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 5.Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci USA. 2004;101:13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDermott U, Settleman J. Personalized cancer therapy with selective kinase inhibitors: an emerging paradigm in medical oncology. J Clin Oncol. 2009;27:5650–5659. doi: 10.1200/JCO.2009.22.9054. [DOI] [PubMed] [Google Scholar]
- 7.Macconaill LE, Garraway LA. Clinical implications of the cancer genome. J Clin Oncol. 2010;28:5219–5228. doi: 10.1200/JCO.2009.27.4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization . WHO handbook for reporting results of cancer treatment: offset publication no. 48. World Health Organization; Geneva, Switzerland: 1979. [Google Scholar]
- 9.Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207–214. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 10.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors (RECIST Guidelines). J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 11.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumors: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki C, Jacobsson H, Hatschek T, et al. Radiologic measurements of tumor response to treatment: practical approaches and limitations. RadioGraphics. 2008;28:329–344. doi: 10.1148/rg.282075068. [DOI] [PubMed] [Google Scholar]
- 13.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. J Clin Oncol. 1999;17:1244–1253. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 14.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 15.Choi H, Charnsangavej C, Faria SC, et al. CT evaluation of the response of gastrointestinal stromal tumors after imatinib mesylate treatment: a quantitative analysis correlated with FDG-PET findings. AJR. 2004;183:1619–1628. doi: 10.2214/ajr.183.6.01831619. [DOI] [PubMed] [Google Scholar]
- 16.Choi H, Charnsangavej C, Faria SC, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007;25:1753–1759. doi: 10.1200/JCO.2006.07.3049. [DOI] [PubMed] [Google Scholar]
- 17.Shankar S, vanSonnenberg E, Desai J, Dipiro PJ, Van Den Abbeele A, Demetri GD. Gastrointestinal stromal tumor: new nodule-within-a-mass pattern of recurrence after partial response to imatinib mesylate. Radiology. 2005;235:892–898. doi: 10.1148/radiol.2353040332. [DOI] [PubMed] [Google Scholar]
- 18.Benjamin RS, Choi H, Macapinlac HA, et al. We should desist using RECIST, at least in GIST. J Clin Oncol. 2007;25:1760–1764. doi: 10.1200/JCO.2006.07.3411. [DOI] [PubMed] [Google Scholar]
- 19.Casali PG, Blay JY, ESMO/CONTICANET/EUROBONET Consensus Panel of Experts Gastrointestinal stromal tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(suppl 5):v98–v102. doi: 10.1093/annonc/mdq208. [DOI] [PubMed] [Google Scholar]
- 20.Benjamin RS, Schöffski P, Hartmann JT, et al. Efficacy and safety of motesanib, an oral inhibitor of VEGF, PDGF, and Kit receptors, in patients with imatinib-resistant gastrointestinal stromal tumors. Cancer Chemother Pharmacol. 2011;68:69–77. doi: 10.1007/s00280-010-1431-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi H. Response evaluation of gastrointestinal stromal tumors. Oncologist. 2008;13(suppl 2):4–7. doi: 10.1634/theoncologist.13-S2-4. [DOI] [PubMed] [Google Scholar]
- 22.Stacchiotti S, Collini P, Messina A, et al. High-grade soft-tissue sarcomas: tumor response assessment—pilot study to assess the correlation between radiologic and pathologic response by using RECIST and Choi criteria. Radiology. 2009;251:447–456. doi: 10.1148/radiol.2512081403. [DOI] [PubMed] [Google Scholar]
- 23.Faivre S, Zappa M, Vilgrain V, et al. Changes in tumor density in patients with advanced hepato-cellular carcinoma treated with sunitinib. Clin Cancer Res. 2011;17:4504–4512. doi: 10.1158/1078-0432.CCR-10-1708. [DOI] [PubMed] [Google Scholar]
- 24.Hutson TE. Targeted therapies for the treatment of metastatic renal cell carcinoma: clinical evidence. Oncologist. 2011;16(suppl 2):14–22. doi: 10.1634/theoncologist.2011-S2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finley DS, Pantuck AJ, Belldegrun AS. Tumor biology and prognostic factors in renal cell carcinoma. Oncologist. 2011;16(suppl 2):4–13. doi: 10.1634/theoncologist.2011-S2-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karumanchi SA, Merchan J, Sukhatme VP. Renal cancer: molecular mechanisms and newer therapeutic options. Curr Opin Nephrol Hypertens. 2002;11:37–42. doi: 10.1097/00041552-200201000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Hiles JJ, Kolesar JM. Role of sunitinib and sorafenib in the treatment of metastatic renal cell carcinoma. Am J Health Syst Pharm. 2008;65:123–131. doi: 10.2146/ajhp060661. [DOI] [PubMed] [Google Scholar]
- 28.Michaelson MD. ASCO 2006 highlights: targeted therapy for renal cell carcinoma. Cancer Treat Rev. 2007;33:381–390. doi: 10.1016/j.ctrv.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 30.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 31.Motzer RJ, Michaelson MD, Redman BG, et al. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24:16–24. doi: 10.1200/JCO.2005.02.2574. [DOI] [PubMed] [Google Scholar]
- 32.Motzer RJ, Michaelson MD, Rosenberg J, et al. Sunitinib efficacy against advanced renal cell carcinoma. J Urol. 2007;178:1883–1887. doi: 10.1016/j.juro.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 33.Baccala A, Jr, Hedgepeth R, Kaouk J, Magi-Galluzzi C, Gilligan T, Fergany A. Pathological evidence of necrosis in recurrent renal mass following treatment with sunitinib. Int J Urol. 2007;14:1095–1097. doi: 10.1111/j.1442-2042.2007.01902.x. [DOI] [PubMed] [Google Scholar]
- 34.Griffin N, Gore ME, Sohaib SA. Imaging in metastatic renal cell carcinoma. AJR. 2007;189:360–370. doi: 10.2214/AJR.07.2077. [DOI] [PubMed] [Google Scholar]
- 35.Smith AD, Lieber ML, Shah SN. Assessing tumor response and detecting recurrence in metastatic renal cell carcinoma on targeted therapy: importance of size and attenuation on contrast-enhanced CT. AJR. 2010;194:157–165. doi: 10.2214/AJR.09.2941. [DOI] [PubMed] [Google Scholar]
- 36.Smith AD, Shah SN, Rini BI, Lieber ML, Remer EM. Morphology, attenuation, size, and structure (MASS) criteria: assessing response and predicting clinical outcome in metastatic renal cell carcinoma on antiangiogenic targeted therapy. AJR. 2010;194:1470–1478. doi: 10.2214/AJR.09.3456. [DOI] [PubMed] [Google Scholar]
- 37.Tsao H, Atkins MB, Sober AJ. Management of cutaneous melanoma. N Engl J Med. 2004;351:998–1012. doi: 10.1056/NEJMra041245. [DOI] [PubMed] [Google Scholar]
- 38.Agarwala SS. Current systemic therapy for metastatic melanoma. Expert Rev Anticancer Ther. 2009;9:587–595. doi: 10.1586/era.09.25. [DOI] [PubMed] [Google Scholar]
- 39.Eggermont AM, Kirkwood JM. Re-evaluating the role of dacarbazine in metastatic melanoma: what have we learned in 30 years? Eur J Cancer. 2004;40:1825–1836. doi: 10.1016/j.ejca.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 40.Petrella T, Quirt I, Verma S, Haynes AE, Charette M, Bak K. Single-agent interleukin-2 in the treatment of metastatic melanoma: a systematic review. Cancer Treat Rev. 2007;33:484–496. doi: 10.1016/j.ctrv.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 41.Trinh VA. Current management of metastatic melanoma. Am J Health Syst Pharm. 2008;65(suppl 9):S3–S8. doi: 10.2146/ajhp080460. [DOI] [PubMed] [Google Scholar]
- 42.Melero I, Hervas-Stubbs S, Glennie M, Pardoll DM, Chen L. Immunostimulatory monoclonal antibodies for cancer therapy. Nat Rev Cancer. 2007;7:95–106. doi: 10.1038/nrc2051. [DOI] [PubMed] [Google Scholar]
- 43.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weber J, Thompson JA, Hamid O, et al. A randomized, double-blind, placebo controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res. 2009;15:5591–5598. doi: 10.1158/1078-0432.CCR-09-1024. [DOI] [PubMed] [Google Scholar]
- 45.Wolchok JD, Neyns B, Linette G, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11:155–164. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- 46.O'Day SJ, Maio M, Ciarion-Sileni V, et al. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: a multicenter single-arm phase II study. Ann Oncol. 2010;21:1712–1717. doi: 10.1093/annonc/mdq013. [DOI] [PubMed] [Google Scholar]
- 47.Wolchok JD, Hoos A, O'Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 48.Hodi FS, Butler M, Oble DA, et al. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci USA. 2008;105:3005–3010. doi: 10.1073/pnas.0712237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hodi FS, Oble DA, Drappatz J, et al. CTLA-4 blockade with ipilimumab induces significant clinical benefit in a female with melanoma metastases to the CNS. Nat Clin Pract Oncol. 2008;5:557–561. doi: 10.1038/ncponc1183. [DOI] [PubMed] [Google Scholar]
- 50.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 51.American Cancer Society . Cancer facts and figures 2010. American Cancer Society; [December 19, 2011]. Website. www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-026238.pdf. Published 2010. [Google Scholar]
- 52.Sandler A, Gray R, Perry MC, et al. Paclitaxelcarboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 53.Marom EM, Martinez CH, Truong MT, et al. Tumor cavitation during therapy with antiangiogenesis agents in patients with lung cancer. J Thorac Oncol. 2008;3:351–357. doi: 10.1097/JTO.0b013e318168c7e9. [DOI] [PubMed] [Google Scholar]
- 54.Crabb SJ, Patsios D, Sauerbrei E, et al. Tumor cavitation: impact on objective response evaluation in trials of angiogenesis inhibitors in non-small-cell lung cancer. J Clin Oncol. 2009;27:404–410. doi: 10.1200/JCO.2008.16.2545. [DOI] [PubMed] [Google Scholar]
- 55.Lee HY, Lee KS, Ahn MJ, et al. New CT response criteria in non-small cell lung cancer: proposal and application in EGFR tyrosine kinase inhibitor therapy. Lung Cancer. 2011;73:63–69. doi: 10.1016/j.lungcan.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 56.Kwak EL, Bang Y-J, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non–small-cell lung cancer. N Engl J Med. 2010;363:1693–1703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Camidge DR. Progression-free survival (PFS) from a phase I study of crizotinib(PF-02341066) in patients with ALK-positive non-small cell lung cancer (NSCLC). J Clin Oncol. 2011;29(suppl) abstr 2501. [Google Scholar]