Abstract
This Review summarizes and updates the work on adenosine A2A receptor antagonists for Parkinson’s disease from 2006 to the present. There have been numerous publications, patent applications, and press releases within this time frame that highlight new medicinal chemistry approaches to this attractive and promising target to treat Parkinson’s disease. The Review is broken down by scaffold type and will discuss the efforts to optimize particular scaffolds for activity, pharmacokinetics, and other drug discovery parameters. The majority of approaches focus on preparing selective A2A antagonists, but a few approaches to dual A2A/A1 antagonists will also be highlighted. The in vivo profiles of compounds will be highlighted and discussed to compare activities across different chemical series. A clinical report and update will be given on compounds that have entered clinical trials.
Keywords: Adenosine, A2A receptor antagonist, A1 receptor antagonist, Parkinson’s disease, catalepsy, 6-OHDA, MPTP
Parkinson’s disease (PD) is a chronic, progressive neurological disease that affects ∼1% of the population over the age of 65.1 It is characterized by progressive impairment in motor function that is often accompanied by disturbances in mood and cognitive function. The majority of motor impairments of PD are caused by a gradual loss of dopamine (DA) producing neurons in the ventral midbrain and concomitant loss of DA input to forebrain (striatal) motor structures.2,3 The loss of DA input to the neostriatum leads to dysregulation of striatal function and the classic motor symptoms of PD, such as resting tremor, muscular rigidity, and bradykinesia.
The majority of treatments aim to restore dopamine signaling and thereby reduce the severity of the motor symptoms. Dopamine replacement therapy using L-DOPA, the precursor to dopamine, remains the gold-standard treatment for PD. Other approaches include inhibition of DA turnover using monoamine oxidase type B (MAO-B) inhibitors,4 catechol O-methyl-transferase (COMT) inhibitors,5 and inhibition of dopamine reuptake6 or direct agonists7 of postsynaptic dopamine receptors. Although the dopamine targeted therapies work well to address the PD related motor disturbances, they all produce undesirable side effects (dyskinesia, hallucinations, on–off effects) that become more severe and problematic with continued treatment. Also, the aforementioned therapies typically show reduced efficacy as motor functions deteriorate and the disease progresses. Moreover, these treatments do not alter disease progression and do not address the mood, postural instability, or cognitive disturbances that frequently accompany PD.
The dopamine replacement agents have significant limitations which influenced researchers to find nondopamine based treatments for PD. One nondopaminergic approach that has received considerable attention is modulation of adenosine receptors which is the topic highlighted in this Review.8 Adenosine is a neuromodulator that coordinates responses to dopamine and other neurotransmitters in areas of the brain that are responsible for motor function, mood, and learning and memory.9 Adenosine comprises four distinct receptor subtypes designated A1, A2A, A2B, and A3 belonging to the G protein-coupled receptor superfamily.10 Adenosine A1 and A3 receptors are coupled to inhibitory G proteins, while A2A and A2B receptors are coupled to stimulatory G proteins. Autoradiography studies in rodents showed that the greatest densities of A2A receptors are found in the striatum11 which closely matches the distribution in humans based on PET imaging.12
As described above, loss of dopamine input into the neostriatum is a hallmark of PD and causes many of the cardinal motor symptoms of this disorder. In the striatum adenosine A2A receptors colocalize and physically associate with dopamine D2 receptors.2,3 A2A and D2 receptors have opposing effects on adenylate cyclase and cAMP production in cells, such that activation of A2A receptors inhibits dopamine D2 receptor signaling. Conversely, A2A receptor antagonists enhance D2 dependent signaling as shown by induction of immediate early gene c-fos expression in the striatopallidal pathway13 and facilitate other D2 mediated responses. Of importance to PD, pharmacological blockade of A2A receptors has shown dramatic beneficial effects in preclinical animal models of PD, showing potentiation of dopamine-mediated responses in dopamine depleted (6-OHDA-treated)14 animals and dramatic relief of parkinsonian symptoms in MPTP-treated15 nonhuman primates.16 A2A antagonists facilitate dopamine receptor signaling and thereby normalize motor function in animal models of dopamine dysregulation. As a result of these findings, the adenosine A2A receptor has become a sought after target for treating PD. Blockade of A2A signaling by selective A2A receptor antagonists17 was shown to be beneficial for not only enhancing the therapeutic effects of L-DOPA but also reducing dyskinesia from long-term L-DOPA treatment.18
The adenosine A1 receptor is also expressed in the striatum; based on anatomical and in vivo microdialysis studies, A1 receptors appear to be localized presynaptically of dopamine axon terminals where they inhibit dopamine release.19 A1 receptor antagonists facilitate dopamine release in the striatum, and like A2A receptors potentiate dopamine mediated responses. Antagonism of both the A2A and A1 would be synergistic: inhibition of the A1 receptor will facilitate dopamine release, while inhibition of the A2A receptor will enhance postsynaptic responses to dopamine. Interestingly, the A1 receptor is also concentrated in neocortical and limbic system structures that are important for cognitive function. Pharmacological inhibition of A1 receptors enhances neurotransmitter release in the hippocampus20 and enhances performance in animal models of learning and memory.21 Antagonism of A1 receptors could present a potential liability, as it is known that some A1 antagonists have cardiovascular22 and diuretic23 liabilities.
The major unmet medical needs of PD are as follows: improved symptomatic treatment without inducing adverse effects (primarily dyskinesia) associated with long-term L-DOPA or dopamine agonist therapy; opportunity to slow disease progression by protecting midbrain dopamine and other neurons from degeneration; treatment of disease comorbidities, including cognitive dysfunction, anxiety, and depression. Based on published preclinical and human clinical data, the majority of approaches focus on selective antagonism of A2A receptors while a few approaches suggest that a dual A2A/A1 receptor antagonist may be better suited to address many of these unmet needs. There have been a number of excellent reviews on A2A receptor antagonists reported in the literature,24 but the following Review will highlight the work done on this target in the past five years. The Ki values represented in the figures refer to binding affinities unless noted otherwise. Updates on key compounds that have entered the clinic will also be highlighted.
Xanthine Based A2A Antagonists
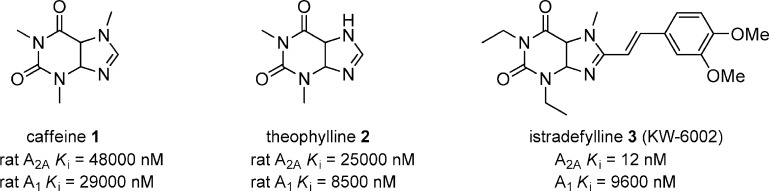
Adenosine A2A receptor antagonists have been traditionally categorized as xanthine based or nonxanthine based derivatives. Caffeine (1) and theophylline (2) are two of the oldest known adenosine antagonists, and both have weak affinity for A2A and A1 (Figure 1).25 Numerous efforts to identify xanthine based derivatives have been explored but will not be discussed in this Review.26 However, istradefylline (3), KW-6002, is a xanthine based, selective A2A antagonist that showed efficacy in numerous preclinical models of PD27 and was investigated in several clinical trials for PD.28 Istradefylline completed phase III clinical trials in 2009, but it was not approved by the FDA due to lack of efficacy.29 There are two active phase III studies using istradefylline (clinicaltrials.gov identifier: NCT00955526 and NCT00957203). More recent efforts have focused on nonxanthine based scaffolds to identify viable selective A2A and dual A2A/A1 receptor antagonists.
Figure 1.
Xanthine based A2A antagonists.
Tricyclic A2A Antagonists
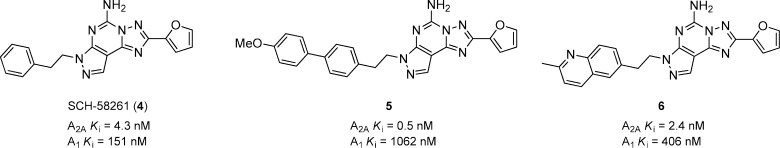
Schering-Plough identified a new series of nonxanthine based derivatives that led to SCH-58261 (4) as a potent A2A antagonist with moderate 35-fold selectivity over the A1 receptor (Figure 2).30 SCH-58261 showed efficacy in rodent models of PD after intraperitoneal (i.p.) administration, but it suffers from poor solubility and is not active when dosed orally. There have been numerous efforts to increase the selectivity and pharmacokinetic (PK) properties of this particular scaffold. A series of biaryl substituted pyrazolo[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidines was also prepared.31 The methoxy biaryl compound 5 had very potent affinity for A2A and is significantly more selective (2124-fold) selective against the A1 receptor compared to 4. This compound showed 30 and 5% inhibition of rat catalepsy at 1 and 4 h, respectively, at 1 mg/kg, and exhibited very poor plasma exposure. Efforts to replace the methoxyaryl substituent with heterocycles such as pyridines and pyrazines resulted in compounds that retained potency and selectivity, but generally suffered from poor solubility and poor exposures in rat after oral dosing. Contracting the biaryl substituent into a quinoline, represented in 6, gave potent compounds with good selectivity versus A1. More importantly, 6 exhibited a superior PK profile in rats, compared to 4 and 5, and reversed catalepsy in rats by 86% (1 h) and 38% (4 h) after 3 mg/kg, p.o.
Figure 2.
Pyrazolo[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidines.
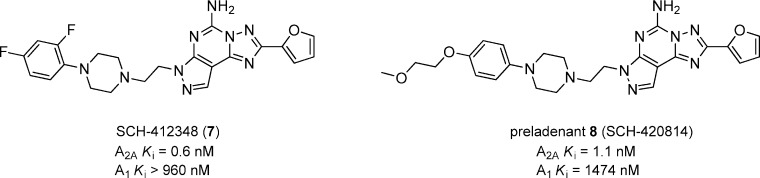
The most successful strategy replaced the phenethyl side chain (compounds 4–6) with an aryl-piperazine side chain represented in 7 (Figure 3).32 A very promising compound, SCH-412348 (7), showed very potent A2A affinity and impressive selectivity (>1600-fold) versus A1. Although SCH-412348 showed excellent ability to reverse haloperidol-induced catalepsy (75 and 80% inhibition at 1 and 4 h, respectively) in rats after a 1 mg/kg dose, po, the compound was not progressed further due to poor aqueous solubility. Further optimization led to the methoxyethoxy analogue 8 (SCH-420814, preladenant), which also had excellent binding affinity for A2A with good selectivity (1340-fold) over A1 and improved solubility. Preladenant (1 mg/kg) reversed haloperidol-induced catalepsy (77 and 70% inhibition at 1 and 4 h, respectively) in rats and was further characterized in multiple preclinical animal models of PD.33 Preladenant showed significant activity when dosed orally in the rat hypolocomotion model (minimum effective dose 0.1 mg/kg, p.o.), 6-OHDA-lesioned rat model (minimum effective dose 0.03 mg/kg, p.o.), and MPTP-treated monkey model.24 Preladenant has completed phase II clinical trials for PD, and Merck (Schering-Plough) is currently recruiting for several phase III trials (clinicaltrials.gov identifier: NCT01155479, NCT01227265, NCT01155466, NCT01294800, NCT01215227, NCT01323855).34
Figure 3.
Aryl piperazine substituted pyrazolo[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidines.
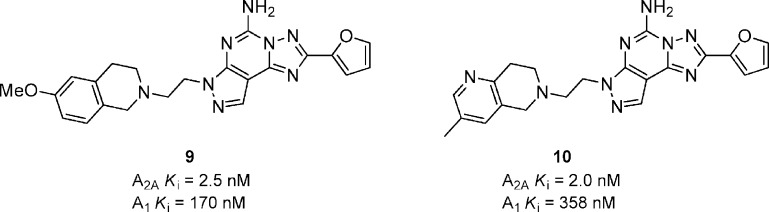
More recently a variety of fused heterocyclic derivatives of the tricyclic scaffold were prepared that had comparable A2A binding affinities with respect to the aryl piperazines 7 and 8 but were generally much less selective against the A1 receptor.35 The tetrahydyroisoquinoline derivative 9 had excellent binding affinity and was 68-fold selective versus A1 (Figure 4). The compound had moderate plasma exposure in rats and reversed catalepsy in rat by 50 and 58% at 1 and 4 h, respectively, after a 3 mg/kg dose, p.o. A related azaisoquinoline 10 had a > 5-fold higher exposure in rat compared to 9 and reversed catalepsy in rat (3 mg/kg) by 85 and 30% at 1 and 4 h, respectively. A variety of substituted isoindolines and benzazepines were also prepared.
Figure 4.
Fused heterocyclic substituted pyrazolo[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidines.
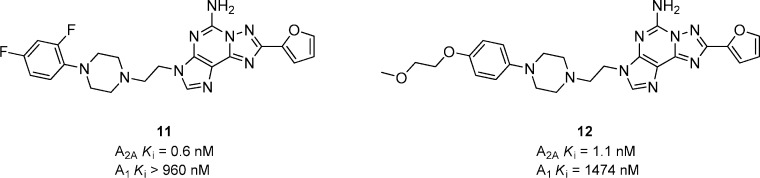
Replacing the pyrazole ring, present in the previously described scaffolds, with an imidazole ring gave a series of 3H-[1,2,4]-triazolo[5,1-i]purin-5-amines (Figure 5).36 Compounds 11 and 12 contain the optimal aryl piperazine substituents present in the pyrazolopyrimidines 7 and 8. Both 11 and 12 have good affinities for A2A while maintaining good selectivity versus A1 (88- and 669-fold, respectively), albeit considerably lower than the selectivities obtained from 7 and 8. Compound 12 (1 mg/kg, p.o.) showed 55 and 50% inhibition of catalepsy in rat at 1 and 4 h, respectively, but in general the compounds from this series were inferior to the corresponding compounds from the pyrazolopyrimidine scaffold. Efforts to replace the furan substituent with substituted aryls resulted in compounds having good A2A potency but were much less selective versus A1 receptors.
Figure 5.
Aryl piperazine substituted 3H-[1,2,4]-triazolo[5,1-i]purin-5-amines.
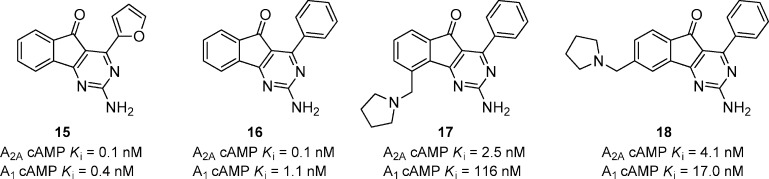
A series of methylene amine substituted arylindenopyrimidines was reported as dual A2A/A1 receptor antagonists (Figure 6).37 Compound 15 was the original lead compound that was potent in both A2A and A1 functional assays and had an ED50 of 5.0 mg/kg, p.o. in the haloperidol-induced catalepsy model in mice. Compound 15 suffered from poor solubility, and it was found to be Ames positive. A variety of heterocyclic furan replacements were prepared that generally maintained good in vitro potency but lost in vivo activity. Replacing the furan with phenyl gave 16 that had good in vitro potency and comparable in vivo activity (ED50 = 8.0 mg/kg, p.o.) reversing catalepsy in mice. Compound 16 was negative in the Ames screen, but it still suffered from poor solubility. A variety of amines were incorporated at the 9-position of the scaffold to increase solubility and resulted in the synthesis of 17 that had good in vitro potency and had an ED50 of 3.8 mg/kg, p.o. in the mouse catalepsy model. Moving the pyrrolidine to the 8-position gave compound 18 that was equipotent at A2A, more potent at A1, and had significantly increased potency in vivo (ED50 = 0.2 mg/kg, p.o.) in the mouse catalepsy model. Further characterization of 18 showed that it was active in rat catalepsy (ED50 = 0.5 mg/kg, p.o.), and had minimum effective doses of 1.0, 1.0, and 10 mg/kg for reserpine-induced akinesia model in mice, 6-OHDA lesion model in rats, and reversing motor disability in MPTP-treated marmosets, respectively.38
Figure 6.
Arylindenopyrimidines.
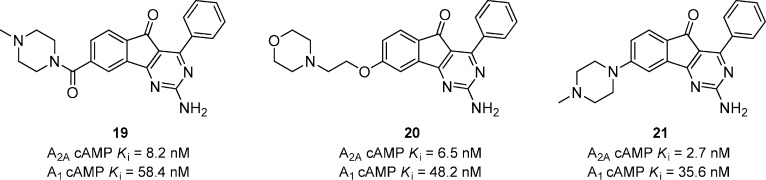
Further evaluation revealed that metabolism of 18 resulted in the formation of reactive metabolites that were attributed to some adverse events seen in the 28-day GLP toxicology studies in nonhuman primates.39 The development of 18 was discontinued based on these findings, and the focus was to identify a suitable back up compound devoid of the metabolic liabilities. Oxidative metabolism was occurring on the pyrrolidine ring and at the benzylic methylene, so a number of molecules were synthesized to address this issue (Figure 7).39 Compound 19 is a representative amide that had good functional activity at both A2A and A1 receptors and reversed haloperidol-induced catalepsy in mice (ED50 = 0.4 mg/kg, p.o.). The ether linked compound 20 was very potent in vivo with an ED50 < 0.1 mg/kg, p.o. in the mouse catalepsy model. A tissue distribution study in rats (10 mg/kg, p.o.) showed that 20 had a brain Cmax of 4.1 μM and a brain to plasma ratio of 3:1. The amino linked compound 21 was potent in vitro and reversed catalepsy in mice with an ED50 < 1.0 mg/kg, p.o. All of the compounds were devoid of the metabolic liabilities associated with compound 18.
Figure 7.
Substituted arylindenopyrimidines.
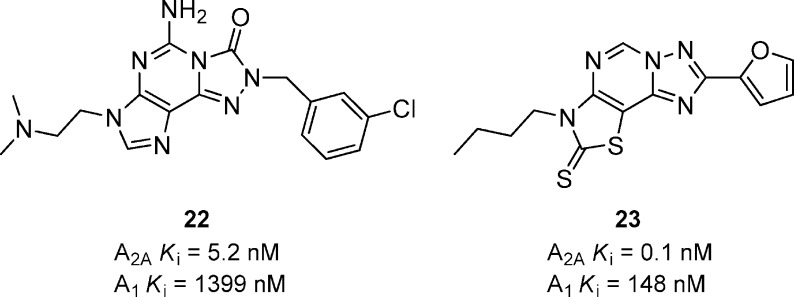
A variety of ethylamino derivatives were prepared in a pyrazolo[4,3-e]-1,2,4-trizolo[4,3-c]pyrimidon-3-one series (Figure 8).40 The ethylenedimethylamino analogue 22 has good binding affinity for A2A and is 269-fold selective versus A1 receptors. In general, analogues had good binding affinities but were not able to reverse haloperidol-induced catalepsy in rat at 10 mg/kg, p.o., and showed very low exposures in rat brain. A series of thiazolotriazolopyrimidines was developed with multiple compounds having sub-nanomolar binding affinities.41 Compound 23 had potent A2A binding affinity and exhibited good selectivity against A1 receptors. Compound 23 also showed very potent functional (cAMP) activity (Ki = 0.1 nM) and was able to reverse both haloperidol-induced catalepsy and akinesia in mice after a 10 mg/kg dose, p.o.
Figure 8.
Pyrazolo[4,3-e]-1,2,4-trizolo[4,3-c]pyrimidon-3-one and thiazolotriazolopyrimidines.
Bicyclic A2A Antagonists
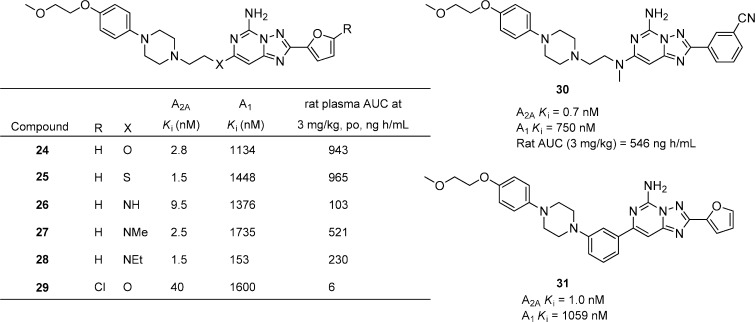
Substituted 1,2,4-triazolo[1,5-c]pyrimidines are a bicyclic system related to preladenant without the fused pyrazole ring (Figure 9).42 A variety of linkers were explored including O, S, NH, NMe, and NEt corresponding to compounds 24–28 while keeping the optimized aryl piperazine, present in preladenant, fixed.39 In general, the binding affinities for A2A were good for all of the linkers with good selectivity versus A1, but the O and S linkages gave the best plasma exposures in rats compared to any of the nitrogen linked analogues. Interestingly, a chlorine substituent (29) on the furan significantly reduced A2A activity and dramatically reduced plasma exposure compared to the des-chloro analogue 24. Replacement of the furan in 24 with 3-cyanophenyl afforded compound 30 that had excellent binding affinity for A2A, good selectivity versus A1, and nearly identical exposure in rats. The compounds were also evaluated in haloperidol-induced catalepsy model in rats. Compound 25 gave the best results with 65% inhibition of catalepsy at both 1 and 4 h after 3 mg/kg, p.o. A related series of substituted 7-phenyl-[1,2,4]-triazolo[1,5-c]pyrimidine-5-amines was also reported.43 Compound 31 was identified as having potent affinity for A2A and good selectivity versus A1. Further evaluation showed that 31 significantly reversed haloperidol-induced catalepsy at 3 mg/kg, p.o.
Figure 9.
Substituted 1,2,4-triazolo[1,5-c]pyrimidines.
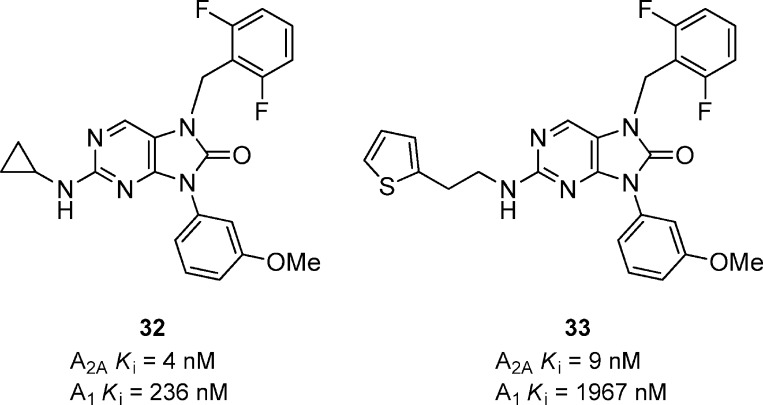
A series of trisubstituted purinones was discovered and elucidated from a high throughput screen (Figure 10).44 An initial structure–activitiy relationship (SAR) was developed by substituting N-7 and N-9 with various aryl and benzyl substituents while keeping the cyclopropyl amine substitution at the 2-position constant. Compound 32 was the most potent compound for A2A and was 59-fold selective versus A1 receptors. Replacement of the cyclopropyl amine with 2-(thiophen-2-yl)ethanamine afforded compound 33 that had comparable activity for A2A but was significantly more selective (218-fold) versus A1 compared to compound 32. The thiophene analogue also had good functional activity (cAMP) in rat (Ki = 18 nM), but no in vivo results were reported for any compounds within this series.
Figure 10.
Substituted purinones.
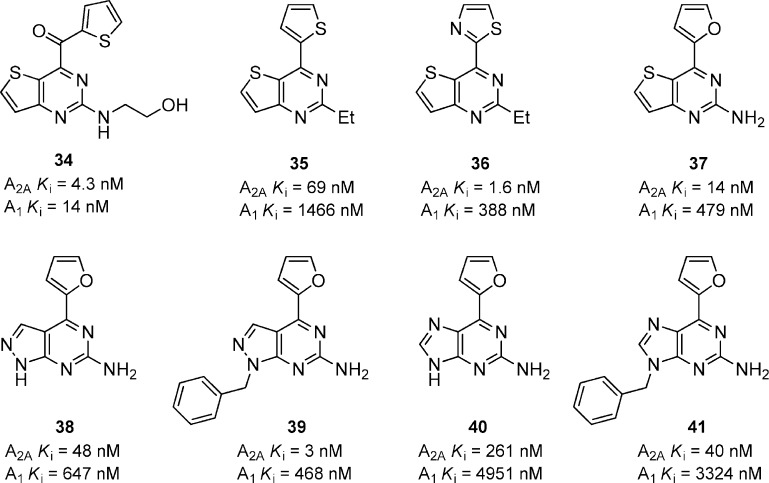
A series of thieno[3,2-d]pyrimidine-4-methanone derivatives was prepared as adenosine A2A receptor antagonists with modest selectivity over A1 (Figure 11).45 The most potent compound 34 was potent at both A2A and A1, and was found to be active in haloperidol-induced hypolocomotion model in mice when dose at 30 mg/kg, i.p. Removing the ketone linker and directly attaching the heterocycle to the pyrimidine core afforded a series of 4-arylthieno[3,2-d]pyrimidines having good affinity for A2A receptors.46 Compound 35 was moderately potent for A2A (Ki = 69 nM), but it did not reverse haloperidol-induced hypolocomotion in mice following i.p. administration of 30 mg/kg. Interestingly, replacing the 2-thienyl substituent with 2-thiazolyl resulted in a significant increases in A2A binding affinity (Ki = 1.6 nM) and selectivity over A1. Compound 36 was also able to reverse hypolocomotion in mice at 30 mg/kg, i.p. A similar aminopyrimidine 37 was identified that had good receptor binding for A2A but was moderately selective versus A1. Efforts to identify other bicyclic templates identified pyrazolo[3,4-d]pyrimidines 38 and 39 and 6-arylpurines 40 and 41.47 The pyrazolo analogues 38 and 39 both show good affinity for A2A and were both able to reverse haloperidol-induced hypolocomotion in mice at 30 mg/kg, i.p. None of the analogues within this series, however, were active when given orally. Interestingly, the 6-arylpurine 40 had much weaker affinity for A2A but was effective in reversing hypolocomotion in mice when dosed at 10 mg/kg, i.p., and 1 mg/kg, p.o. No plasma or brain exposures were reported to explain this unexpected result. The benzyl analogue 41 was also active in vivo at 30 mg/kg, i.p, but not active when dosed orally.
Figure 11.
Thieno[3,2-d]pyrimidines, pyrazolo[3,4-d]pyrimidines, and 6-arylpurines.
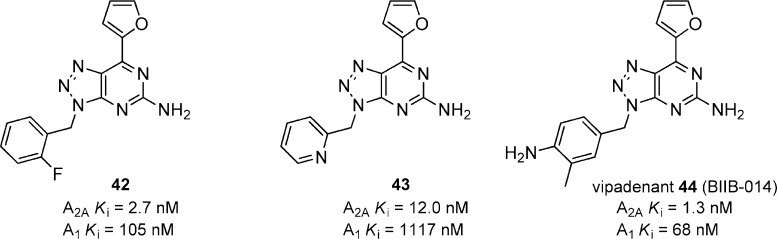
Related benzyl substituted triazolo[4,5-d]pyrimidines like 42 were synthesized and evaluated (Figure 12).48 Compound 42 had potent A2A activity and exhibited moderate selectivity versus A1. A variety of substituted benzyl analogues were prepared that had good A2A activity but were not able reverse haloperidol-induced hypolocomotion when dosed orally. Inactivity was attributed to low solubility and poor PK profiles and led to the synthesis of heterocyclic compounds such as the 2-pyridyl analogue 43. Despite good in vitro activity and increased solubility, 43 was not active in vivo as 30 mg/kg, p.o. Further optimization of this scaffold led to the discovery of vipadenant (44) (V2006/BIIB-014) which was evaluated in phase II clinical trials.49 Vipadenant showed excellent binding affinity for A2A and had modest selectivity against A1 and A2B (Ki = 63 nM), while it showed good selectivity against A3 (Ki = 1005 nM). When tested in vivo in the mouse and rat haloperidol-induced hypolocomotion models, 44 had a minimum effective dose of 0.1 and 1 mg/kg, respectively. Vipadenant was able to increase contralateral rotations in 6-OHDA lesioned rats when dosed orally in combination with apomorphine at 3 and 10 mg/kg.24a The compound also showed reversal of motor disability in MPTP-treated marmosets without dyskinesias at a minimum effective dose of <5 mg/kg, p.o.24a The excellent in vivo data and a favorable safety profile helped progress this compound through phase II clinical trials. Despite positive phase II clinical results, in July 2010 Vernalis and Biogen have discontinued the development of vipadenant due to some preclinical toxicology findings that showed adverse side effects.50
Figure 12.
Benzyl substituted triazolo[4,5-d]pyrimidines.
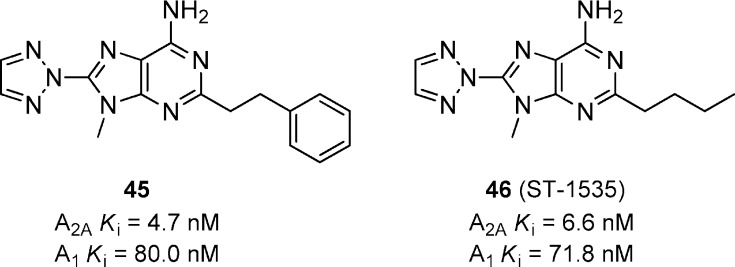
A series of triazolo-9H-purines was reported as potent A2A antagonists having modest selectivity versus A1 (Figure 13).51 Compounds 45 and 46 (ST-1535) are representative examples of the series developed by Sigma Tau. ST-1535 has been characterized extensively in various animal models of PD. ST-1535 has minimum effective doses of 5.0 and 1.25 mg/kg in hypolocomotion and haloperidol-induced catalepsy models in mice, respectively.52 Further studies show that ST-1535 potentiated L-DOPA activity in 6-OHDA lesioned rats.53 In the MPTP-treated marmosets, ST-1535 dosed alone at 40 mg/kg significantly increased locomotor activity but did not improve motor disability.54 However, when dosed at 20 mg/kg in combination with L-DOPA (2.5 mg/kg), a significant reversal of motor disability was seen compared to 20 mg/kg alone of ST-1535. The significance was not reported with respect to L-DOPA (2.5 mg/kg) alone. ST-1535 is currently under active development in phase I clinical trials by Sigma Tau.
Figure 13.
Triazolo-9H-purines.
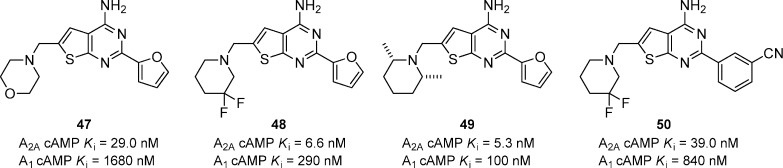
A series of aminomethyl substituted thieno[2,3-d]pyrimidines has been reported as A2A antagonists having moderate selectivity versus A1 (Figure 14).55 The in vitro activities for A2A and A1 are reported as functional activity, not binding affinity. The unsubstituted furan proved to be the optimal substituent for A2A potency and selectivity versus A1. The basicity of amine substituent was the key to obtain in vivo activity in the haloperidol-induced catalepsy model in mice. Very basic amines such as pyrrolidines and piperidines had good in vitro potency but were not able to reverse catalepsy in vivo at 10 mg/kg, p.o. The morpholine compound 47 was much less basic, based on calculated pKa values, than the corresponding pyrrolidines and piperidines and had a reported oral ED50 of 1.3 mg/kg in the mouse catalepsy model. A variety of less basic amines were explored to determine the scope and to identify if the basicity was indeed a real trend. Compounds 48 and 49 were even more potent in vitro than 47 and significantly reversed catalepsy at 10 mg/kg, p.o. Further characterization of 48 showed that it had an ED50 of <1 mg/kg, p.o., but suffered from a suboptimal PK profile that was attributed to the furan. Efforts to substitute the 5-position of the furan with methyl, ethyl, isopropyl, cyclopropyl, −CHF2, chlorine, and bromine generally resulted in slight decreases of in vitro potency, but dramatic decreases of in vivo activity. Replacing the furan with various 5-membered heterocycles resulted in a very large decrease of in vitro potency. Interestingly, the furan could be replaced with a 3-cyanophenyl group and maintain good A2A activity. Compound 50 was ∼6-fold less potent than the corresponding furan analogue, but maintained good activity in vivo (ED50 < 1 mg/kg) and had a superior PK profile.
Figure 14.
Aminomethyl substituted thieno[2,3-d]pyrimidines.
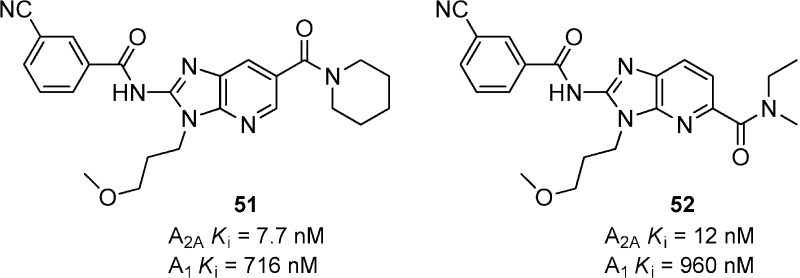
A series of 2-aminoimidazopyridines 51 and 52 resulted from the optimization of a high throughput screen hit (Figure 15).56 Compound 51 showed good binding affinity for A2A with 93-fold selectivity over A1. A variety of amides were explored in the 6-position but generally suffered from poor microsomal stability. Moving the amide substituent to the 5-position resulted in compounds with similar binding affinities, but in general led to better microsomal stability. Compound 52 was a potent binder for A2A and showed moderate plasma and brain exposure when dosed orally, but it was not evaluated in animal models of PD.
Figure 15.
2-Aminoimidazopyridines.
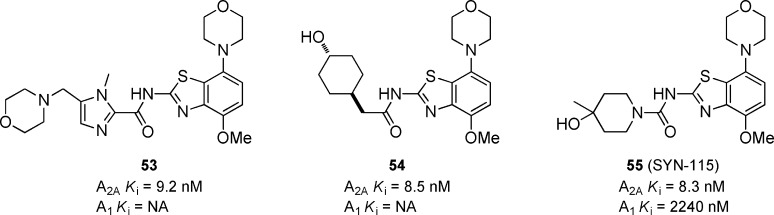
A 4-morpholino-benzothiazole based series of compounds has been reported in the patent literature by Hoffmann-La Roche (Figure 16).57 The imidazole 53(58) and the cyclohexane 54(59) are representative examples that each show good binding affiinites for A2A, but the A1 activity was not reported. Further optimization of this scaffold led to the urea compound 55 which had good potency for A2A and was 270-fold and >4000-fold selective versus A1 in binding and functional assays, respectively. Compound 55 had an ED50 of 0.5 mg/kg when dosed orally in the APEC-induced hypolocomotion model in rats.53,60 In January 2007, Synosia acquired the rights from Roche to develop 55 (SYN-115) for the treatment of PD.61 SYN-115 successfully completed a phase II clinical trial and is currently recruiting for additional phase II/III clinical trials for PD (clinicaltrials.gov identifier: NCT01283594) and for a phase 0 clinical trial for cocaine dependence (clinicaltrials.gov identifier: NCT00783276). In February 2011, Synosia was acquired by Biotie Therapeutics.
Figure 16.
Substituted 4-morpholino-benzothiazoles.
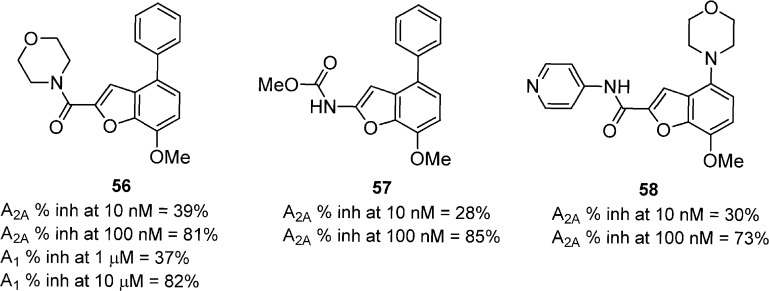
A series of 4-aryl substituted benzofurans was identified, and the 4-phenyl-substituent was found to be the most consistent group for A2A activity (Figure 17).62 The morpholine amide 56 showed good affinity for A2A and significantly reversed (78%) CGS-induced catalepsy in mice at 10 mg/kg, p.o. Further exploration of this scaffold showed that the methyl carbamate 57 also had good A2A activity and reversed catalepsy by 86% at 10 mg/kg, p.o. A variety of amides were prepared from a related 4-morpholinobenzofuran series and were evaluated in vitro and in vivo.63 The analogues in general exhibited similar in vitro potencies with respect to the 4-phenylbenzofurans, but they also had increased aqueous solubility and improved PK properties. Compound 58 was identified having good in vitro activity for A2A and excellent activity reversing (76% at 10 mg/kg, p.o.) CGS-induced catalepsy in mice. The compound also showed significant reduction in motor disabilities and increased locomotor activity in MPTP-treated marmosets for 5 h after a oral 10 mg/kg dose.
Figure 17.
4-Aryl and 4-morpholino substituted benzofurans.
Monocyclic A2A Antagonists
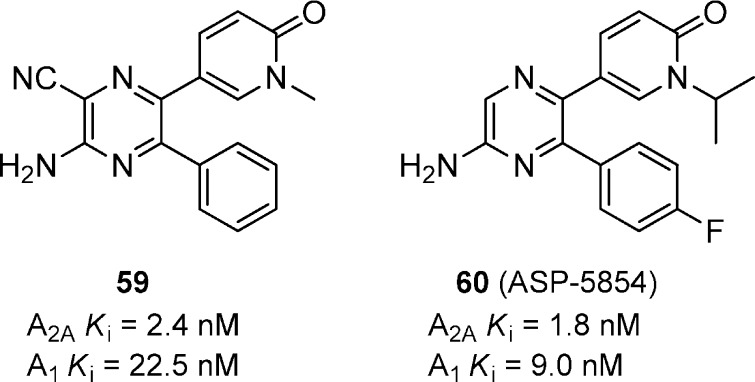
An aminopyrazine based series of compounds was reported as dual A2A/A1 receptor antagonists (Figure 18).64 Compound 59 had potent affinity for both A2A and A1 and significantly reversed haloperidol-induced catalepsy in mice at 3.2 mg/kg, p.o. Further optimization of the pyrazine scaffold led to the identification of 60 (ASP-5854) that has been characterized extensively in numerous animal models of PD and cognition.65 ASP-5854 reversed CGS21680-induced and haloperidol-induced catalepsy in mice with ED50 values of 0.15 and 0.07 mg/kg, p.o., respectively, and had a minimum effective dose of 0.1 mg/kg to reverse haloperidol-induced catalepsy in rats. In rhesus monkeys, ASP-5854 gave >85% occupancy of A2A receptors in the striatum and reversed haloperidol-induced catalepsy with an ED50 of 0.1 mg/kg, p.o.66 ASP-5854 effectively potentiated L-DOPA-induced rotation in 6-OHDA lesioned rats with a minimum effective dose of 0.03 mg/kg, p.o. Statistical significance was reached in MPTP-treated mice and MPTP-treated marmoset models at 0.1 and 1 mg/kg, p.o, respectively.61,67 ASP-5854 produced positive results in scopolamine-induced memory deficits in the mouse Y-maze test and rat passive avoidance test, both models of cognition, with minimum effective doses of 0.3 and 0.1 mg/kg, p.o., respectively.61 The selective A2A antagonist KW-6002 (3) was also tested in parallel for comparison in these models and was significantly less effective having a minimum effective dose of 10 mg/kg, p.o. in the mouse Y-maze test and no effect in the rat passive avoidance test up to 10 mg/kg, p.o. These results suggest that a dual A2A/A1 may provide added benefit to PD patients to address the cognitive impairments associated with the disease.
Figure 18.
Pyridone substituted pyrazines.
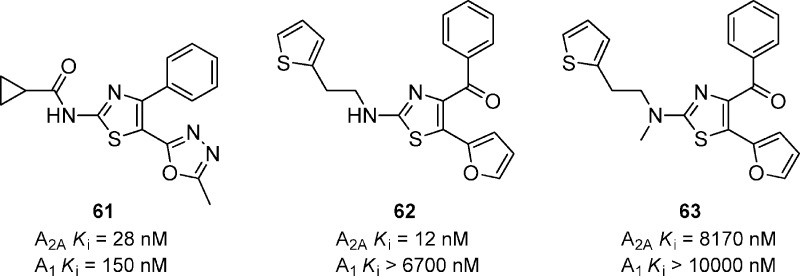
A variety of carboxamides of 2-amino-4-phenyl-thiazoles were prepared as adenosine A2A receptor antagonists (Figure 19).68 A lead optimization effort identified that the cyclopropyl amide was the optimal amide substituent. Further evaluation into the substitution at the 5-position of the thiazole led to the 5-methyl-[1,2,4]-oxadiazole compound 61 which had good affinity for A2A and A1. Compound 61 also showed good activity in the haloperidol-induced hypolocomotion model in mice with an ED50 of 7 mg/kg. A series of related substituted 2-amino-5-benzoyl-4-(2-furyl)thiazoles was designed from a high throughput screening hit.69 Lead optimization led to compound 62 which had good affinity for A2A and excellent selectivity versus A1. Interestingly, a simple methyl substitution (63) dramatically decreases the binding affinity for A2A. No in vivo evaluation of 62 was reported.
Figure 19.
Heterocyclic substituted 2-amino-thiazoles.
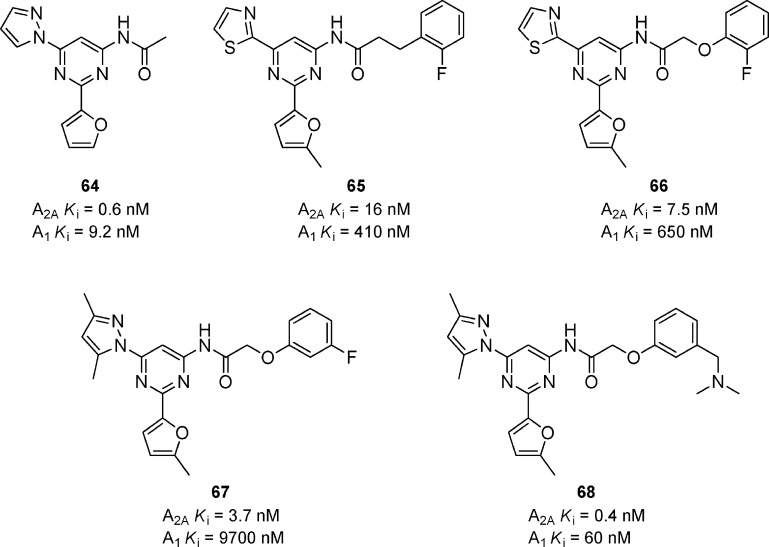
A series of trisubstituted pyrimidines was reported to have potent A2A activity (Figure 20).70 Compound 64 was a potent A2A antagonist, but it lacked the desired selectivity versus A1 and had poor metabolic stability that was attributed to the unsubstituted furan moiety. SAR studies resulted in the synthesis of the thiazole compounds 65 and 66 that maintained potency for A2A. Interestingly, replacing the benzylic methylene, present in 65, with an oxygen atom resulted in the selective compound 66. Compound 66 was further optimized by replacing the thiazole with the dimethylpyrazole to give compound 67 that was very potent for A2A and had excellent selectivity versus A1. Despite the increase in selectivity, 67 suffered from poor solubility, so basic amines were appended to the phenol ring to increase solubility and improve PK properties. A number of the amino alkyl compounds had good binding affinities for A2A and good selectivity versus A1, but had significant hERG liabilities. Compound 68 had the best hERG profile (patch clamp IC50 = 1.2 μM) and showed oral activity in the haloperidol-induced catalepsy in rats with a minimal effective dose of 10 mg/kg.
Figure 20.
Various trisubstituted pyrimidines.
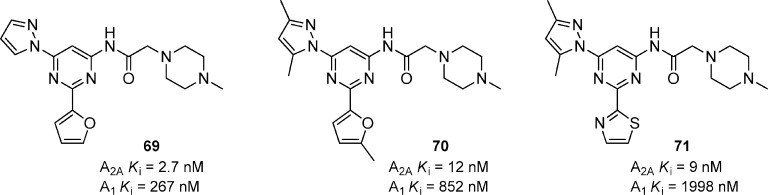
A series of 2-amino-N-pyrimidin-4-ylacetamides was prepared with compound 69 being the representative example (Figure 21).71 Compound 69 had a high affinity for A2A and was 99-fold selective versus A1. Tissue distribution studies showed very good exposure (2700 ng/g) in rat brain at 2 h after a 10 mg/kg oral dose. The compound effectively reversed haloperidol-induced catalepsy in rats at 10 mg/kg, p.o. Like 64, compound 69 had metabolic liabilities that were associated with the unsubstituted furan, so there was an effort to find a suitable furan replacement.72 A variety of combinations consisting of 5- and 6-membered heterocycles were made to replace the furan and pyrazole substituents. Compounds 70 and 71 were identified as the most promising compounds having good binding affinities and good selectivity versus A1. Further evaluation of 70 showed it to be a potent inhibitor of hERG with an IC50 of 950 nM (patch clamp). The basicity of the piperazine was enhancing the hERG activity, so various replacements were prepared in effort to decrease the hERG liability. Although the hERG inhibition could not be completely eliminated, removal of one of the basic amines decreased the hERG activity. Several compounds within the series showed activity in haloperidol-induced catalepsy in rats.
Figure 21.
Piperazine substituted pyrimidine acetamides.
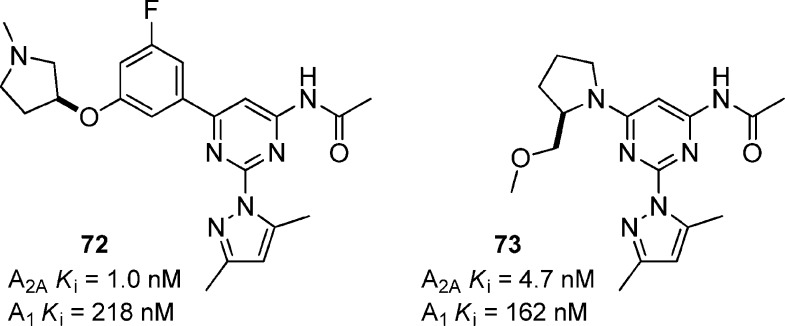
A related series of 2,6-diaryl-4-acylaminopyrimidines was developed with improved solubility and metabolic stability (Figure 22).73 Compound 72 was potent for A2A and was 218-fold selective against A1. More importantly, the addition of the fluorine atom significantly increased metabolic stability, decreased intrinsic clearance, and reduced CYP inhibition compared to the corresponding des-fluoro analogue (not shown). Further characterization of 72 showed that it significantly reversed haloperidol-induced catalepsy in rats at 30 mg/kg, p.o. Another related compound 73 was able to reverse catalepsy in rats at 10 mg/kg, p.o. Compound 73 also showed the ability to potentiate L-DOPA-induced rotational behavior in the 6-OHDA lesioned rat at 10 and 30 mg/kg oral doses.
Figure 22.
Acylaminopyrimidines.
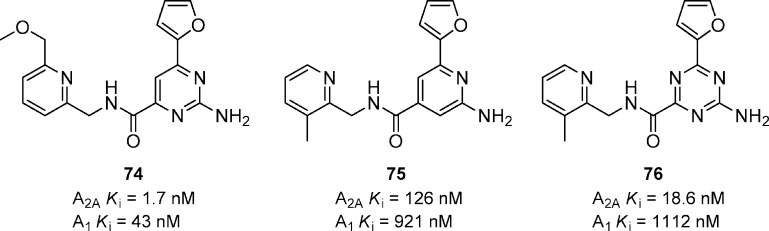
Analogues of pyrimidine-4-carboxamides have been reported to display good potency for A2A and with good selectivity over other adenosine receptor subtypes (Figure 23).74 Compound 74 is a representative example having binding affinities of 1.7, 43, 460, and 1740 nM for A2A, A1, A2B, and A3, respectively. Compound 75 also had a minimum effective dose of 0.1 mg/kg in the haloperidol-induced hypolocomotion model in mice. Related pyridine 75 and triazine 76 have also been reported, but they are significantly less potent than the corresponding pyrimidines.75
Figure 23.
Pyrimidine, pyridine, and triazine carboxamides.
Summary
Adenosine A2A receptor antagonists continue to be a sought after target for the treatment of PD. There have been a variety of chemical scaffolds reported with interesting SAR features that showed dramatic activity differences, both in vitro and in vivo, from subtle modifications. Despite the relentless research efforts to identify potent compounds, there still remains the challenge of achieving selectivity, solubility, and acceptable pharmacokinetic/pharmacodynamic properties to progress into the clinic. There have been a number of compounds that are in or have been in clinical trials including istradefylline (3), preladenant (8), vipadenant (44), ST-1535 (46), and SYN-115 (55). The progression through the clinic with positive trial results indicates the viability of A2A antagonists to treat PD patients with potential advantages over the current standard treatments. Two examples of efforts to identify dual A2A/A1 were also described as having the potential to provide added benefit to PD patients, via A1 antagonism, to address the cognitive impairments that worsen as the disease progresses. Indenopyrimidine 18 and ASP-5854 (60) are potent A2A/A1 receptor antagonists that showed very potent activity across a number of PD models. ASP-5854 also showed potent and effective activity in a number of rodent cognition models, while the selective A2A antagonist istradefylline showed no or marginal effects in these models. The continued research to identify selective A2A and dual A2A/A1 antagonists should provide some new and interesting opportunities for PD patients for years to come.
Acknowledgments
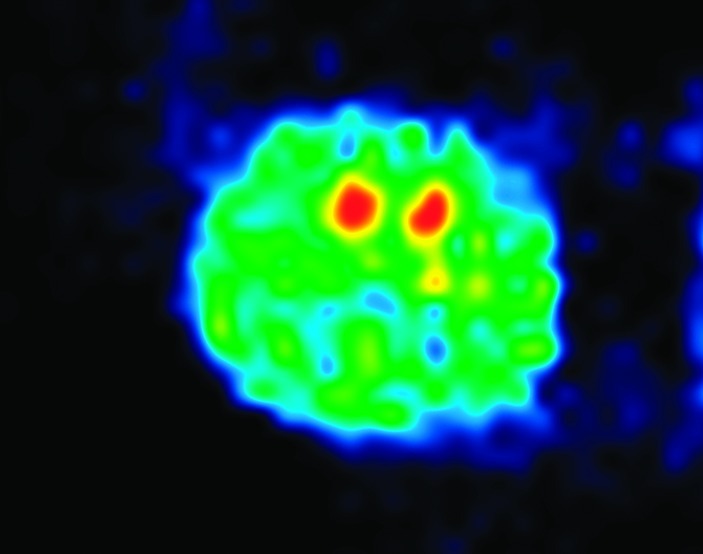
The table of contents graphic represents a positron emission tomography (PET) picture of adenosine A2A receptors in rat striatum using 11C-TMSX, an A2A receptor antagonist. We thank Dr. Paul Acton and Carlos Cotto for performing the PET experiments.
Glossary
Abbreviations
- 6-OHDA
6-hydroxydopamine
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- PD
Parkinson’s disease
- DA
dopamine
- L-DOPA
levodopamine
- MAO-B
monoamine oxidase type B
- COMT
catchol O-methyl-transferase
- cAMP
cyclic adenosine monophosphate
- PK
pharmacokinetics
References
- Lozano A. M.; Lang A. E.; Hutchison W. D.; Dostrovsky J. O. (1998) New developments in understanding the etiology of Parkinson’s disease and in its treatment. Curr. Opin. Neurobiol. 8, 783–790. [DOI] [PubMed] [Google Scholar]
- Fink J. S.; Weaver D. R.; Rivkees S. A.; Peterfreund R. A.; Pollack A. E.; Adler E. M.; Reppert S. M. (1992) Molecular cloning of the rat A2 adenosine receptor: selective co-expression with D2 dopamine receptors in rat striatum. Mol. Brain Res. 14, 186–195. [DOI] [PubMed] [Google Scholar]
- Schiffmann S. N.; Lipert F.; Vassart G.; Vanderhaeghen J. J. (1991) Distribution of adenosine A2receptor mRNA in the human brain. Neurosci. Lett. 130, 177–181. [DOI] [PubMed] [Google Scholar]
- Olanow C. W. (1993) MAO-B inhibitors in Parkinson’s disease. Adv. Neurol. 60, 666–671. [PubMed] [Google Scholar]
- a Gordin A.; Brooks D. J. (2007) Clinical pharmacology and therapeutic use of COMT inhibition in Parkinson’s disease. J. Neurol. 254, IV/37–IV/48. [Google Scholar]; b Olanow C. W.; Stocchi F. (2004) COMT inhibitors in Parkinson’s disease. Can they prevent and/or reverse levodopa-induced motor complications?. Neurology 62, S72–S81. [DOI] [PubMed] [Google Scholar]
- a Antonini A.; Clilia R. (2009) Behavioural adverse effects of dopaminergic treatments in Parkinson’s disease: incidence, neurobiological basis, management and prevention. Drug Safety 32, 475–488. [DOI] [PubMed] [Google Scholar]; b Hansard M. J.; Smith L. A.; Jackson M. J.; Cheetham S. C.; Jenner P. (2002) Dopamine, but not norepinephrine or serotonin reuptake inhibition reverses motor deficits in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated primates. J. Pharmacol. Exp. Ther. 303, 952–958. [DOI] [PubMed] [Google Scholar]
- a Antonini A.; Tolosa E.; Mizuno Y.; Yamamoto M.; Poewe W. H. (2009) A reassessment of risks and benefits of dopamine agonists in Parkinson’s disease. Lancet Neurol. 8, 929–937. [DOI] [PubMed] [Google Scholar]; b Yamamoto M.; Schapira A. H. V. (2008) Dopamine agonists in Parkinson’s disease. Expert Rev. Neurother. 8, 671–677. [DOI] [PubMed] [Google Scholar]
- a Szaboo N.; Kincses Z. T.; Veecsei L. (2011) Novel therapy in Parkinson’s disease: adenosine A2A receptor antagonists. Expert Opin. Drug Metab. Toxicol. 7, 441–455. [DOI] [PubMed] [Google Scholar]; b Salamone J. D. (2010) Facing dyskinesia in Parkinson’s disease: nondopaminergic approaches. Drugs Future 35, 567–573. [Google Scholar]
- a Latini S.; Pedata F. (2001) Adenosine in the central nervous system: release mechanisms and extracellular concentrations. J. Neurochem. 79, 463–484. [DOI] [PubMed] [Google Scholar]; b Dunwiddie T. V.; Masino S. A. (2001) The role and regulation of adenosine in the central nervous system. Annu. Rev. Neurosci. 24, 31–55. [DOI] [PubMed] [Google Scholar]
- Stiles G. L. (1992) Adenosine receptors. J. Biol. Chem. 267, 6451–6454. [PubMed] [Google Scholar]
- a Rosin D. L.; Robeva A.; Woodard R. L.; Guyenet P. G.; Linden J. (1998) Immunohistochemical localization of adenosine A2A receptors in rat central nervous system. J. Comp. Neurol. 401, 163–186. [PubMed] [Google Scholar]; b Fredholm B. B.; Svenningsson P. (1998) Striatal adenosine A2A receptors-where are they? What do they do? Comments. Trends Pharmacol. Sci. 19, 46–47. [DOI] [PubMed] [Google Scholar]
- a Ishiwata K.; Mishina M.; Kimura Y.; Oda K.; Sasaki T.; Ishii K. (2005) First visualization of adenosine A2A receptors in the human brain by positron emission tomography with [11C]TMSX. Synapse 55, 133–136. [DOI] [PubMed] [Google Scholar]; b Svenningsson P.; Hall H.; Sedvall G.; Fredholm B. B. (1997) Distribution of adenosine receptors in the postmortem human brain: an extended autoradiographic study. Synapse 27, 322–335. [DOI] [PubMed] [Google Scholar]
- Pollack A. E.; Fink J. S. (1995) Adenosine antagonists potentiate D2 dopamine dependent activation of Fos in the striatopallidal pathway. Neuroscience 68, 721–728. [DOI] [PubMed] [Google Scholar]
- a Ungerstedt U. (1968) 6-Hydroxydopamine-induced generation of central monoamine neurons. Eur. J. Pharmacol. 5, 107–110. [DOI] [PubMed] [Google Scholar]; b Ungerstedt U.; Arbuthnott G. W. (1970) Quantitative recording of rotational behavior in rats after 6-hydroxy-dopamine lesions of the nigrostriatal dopamine system. Brain Res. 24, 485–493. [DOI] [PubMed] [Google Scholar]; c Luthman J.; Fredriksson A.; Sundstroem E.; Jonsson G.; Archer T. (1989) Selective lesion of central dopamine or moradenaline neuron systems in the neonatal rat: motor behavior and monoamine alteration at adult stage. Behav. Brain Res. 33, 267–277. [DOI] [PubMed] [Google Scholar]
- a Bankiewicz K. S. (1991) MPTP-induced parkinsonism in nonhuman primates. Methods Neurosci. 7, 168–182. [Google Scholar]; b Jakowec M. W.; Petzinger G. M. (2004) 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced lesion model of Parkinson’s disease, with emphasis on mice and nonhuman primates. Comp. Med. 54, 497–513. [PubMed] [Google Scholar]; c Campos-Romo A.; Ojeda-Flores R.; Moreno-Briseno P.; Fernandez-Ruiz J. (2009) Quantitative evaluation of MPTP-treated nonhuman parkinsonian primates in the hallway task. J. Neurosci. Methods 177, 361–368. [DOI] [PubMed] [Google Scholar]
- a Chen J. F.; Xu K.; Petzer J. P.; Staal R.; Xu Y. H.; Beilstein M.; Sonsalla P. K.; Castagnoli K.; Castagnoli N.; Schwarzchild M. A. (2001) Neuroprotection by caffeine and A2A adenosine receptor inactivation in a model of Parkinson’s disease. J. Neurosci. 21, RC143/1–RC143/6. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Grondin R.; Bedard P. J.; Tahar A. J.; Gregoire L.; Mori A.; Kase H. (1999) Antiparkinsonian effect of a new adenosine A2A receptor antagonist in MPTP-treated monkeys. Neurology 52, 1673–1677. [DOI] [PubMed] [Google Scholar]; c Ongini E.; Monopoli A.; Impagnatiello F.; Fredduzzi S.; Schwarzchild M.; Chen J. F. (2001) Dual actions of A2A adenosine receptor antagonists on motor dysfunction and neurodegenerative processes. Drug Dev. Res. 52, 379–386. [Google Scholar]; d Ikeda K.; Kurokawa M.; Aoyama S.; Kuwana Y. (2002) Neuroprotection by adenosine A2A receptor blockade in experimental models of Parkinson’s disease. J. Neurochem. 80, 262–270. [DOI] [PubMed] [Google Scholar]
- a Kanda T.; Jackson M. J.; Smith L. A.; Pearce R. K. B.; Nakamura J.; Kase H.; Kuwana Y.; Jenner P. (1998) Adenosine A2A antagonist: a novel antiparkinsonian agent that does not provoke dyskinesia in parkinsonian monkeys. Ann. Neurol. 43, 507–513. [DOI] [PubMed] [Google Scholar]; b Koga K.; Kurokawa M.; Ochi M.; Nakamura J.; Kuwana Y. (2000) Adenosine A2A receptor antagonists KF17837 and KW-6002 potentiate rotation induced by dopaminergic drugs in hemi-Parkinsonian rats. Eur. J. Pharmacol. 408, 249–255. [DOI] [PubMed] [Google Scholar]
- a Kanda T.; Jackson M. J.; Smith L. A.; Pearce R. K.; Nakamura J.; Kase H.; Kuwana Y.; Jenner P. (2000) Combined use of the adenosine A2A antagonist KW-6002 with L-DOPA or with selective D1 or D2 dopamine agonists increases antiparkinsonian activity but not dyskinesia in MPTP-treated monkeys. Exp. Neurol. 162, 321–327. [DOI] [PubMed] [Google Scholar]; b Hauser R. A.; Hubble J. P.; Truong D. D. (2003) Randomized trial of adenosine A2A receptor antagonist istradefylline in advanced PD. Neurology 61, 297–303. [DOI] [PubMed] [Google Scholar]; c Bara-Jimenez W.; Sherzai A.; Dimitrova T.; Favit A.; Bibbiani F.; Gillespie M.; Morris M. J.; Mouradian M. M.; Chase T. N. (2003) Adenosine A2A receptor antagonist treatment of Parkinson’s disease. Neurology 61, 293–296. [DOI] [PubMed] [Google Scholar]
- Ballarin M.; Reiriz J.; Ambrosio S.; Mahy N. (1995) Effect of locally infused 2-cholroadenosine, an A1 receptor agonist, on spontaneous and evoked dopamine release in rat neostriatum. Neurosci. Lett. 185, 29–32. [DOI] [PubMed] [Google Scholar]
- a Moore K. A.; Nicoll R. A.; Schmitz D. (2003) Adenosine gates synaptic plasticity at hippocampal mossy fiber synapses. Proc. Natl. Acad. Sci. U.S.A. 100, 14397–14402. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Rebola N.; Pinheiro P. C.; Oliveira C. R.; Malva J. O.; Cunha R. A. (2003) Subcellular localization of adenosine A1 receptors in nerve terminal and synapses of the rat hippocampus. Brain Res. 987, 49–58. [DOI] [PubMed] [Google Scholar]
- Maemoto T.; Miho T.; Takuma M.; Noriko U.; Hideaki M.; Katsuya H.; Takayuki Y.; Kiyoharu S.; Satoru K.; Atsushi A.; Akinori I.; Nobuya M.; Seitaro M. (2004) Pharmacological characterization of FR194921, a new potent, selective, orally active antagonist for central adenosine A1 receptors. J. Pharmacol. Sci. 96, 42–52. [DOI] [PubMed] [Google Scholar]
- Gottlieb S. S.; Skettino S. L.; Wolff W.; Beckman E.; Fisher M. L.; Freudenberger R.; Gladwell T.; Marshall J.; Cines M.; Bennett D.; Liittschwager E. B. (2000) Effects of BG9719 (CVT-124), an A1-adenosine receptor antagonist, and furosemide on glomerular filtration rate and natriuresis in patients with congestive heart failure. J. Am. Coll. Cardiol. 35, 56–59. [DOI] [PubMed] [Google Scholar]
- Givertz M . M.; Massie B. M.; Fields T. K.; Pearson L. L.; Dittrich H. C. (2007) The effects of KW-3902, an adenosine A1-receptor antagonist,on diuresis and renal function in patients with acute decompensated heart failure and renal impairment or diuretic resistance. J. Am. Coll. Cardiol. 50, 1551–1560. [DOI] [PubMed] [Google Scholar]
- a Shah U.; Hodgson R. (2010) Recent progress in the discovery of adenosine A2A receptor antagonists for the treatment of Parkinson’s disease. Curr. Opin. Drug Discovery Dev. 13, 466–480. [PubMed] [Google Scholar]; b Pinna A. (2009) Novel investigational adenosine A2A receptor antagonists for Parkinson’s disease. Expert Opin. Invest. Drugs 18, 1619–1631. [DOI] [PubMed] [Google Scholar]; c Baraldi P. G.; Tabrizi M. A.; Gessi S.; Borea P. A. (2008) Adenosine Receptor Antagonists: Translating Medicinal Chemistry and Pharmacology into Clinical Utility. Chem. Rev. 108, 238–263. [DOI] [PubMed] [Google Scholar]
- a Yeung S. H.; Green R. D. (1984) [3H]5′-N-ethylcarboxamido adenosine binds to both Ra and Ri adenosine receptors in rat striatum. Naunyn-Schmiedeberg's Arch. Pharmacol. 325, 218–225. [DOI] [PubMed] [Google Scholar]; b Cronstein B. N.; Levin R. I.; Belanoff J.; Weissmann G.; Hirschhorn R. (1986) Adenosine: an endogenous inhibitor of neutrophil-mediated injury to endothelial cells. J. Clin. Invest. 78, 760–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller C. E.; Ferre S. (2007) Blocking striatal adenosine A2A receptors: A new strategy for basal ganglia disorders. Recent Pat. CNS Drug Discovery 2, 1–21. [DOI] [PubMed] [Google Scholar]
- a Kanda T.; Tashiro T.; Kuwana Y.; Jenner P. (1998) Adenosine A2A receptors modify motor function in MPTP-treated common marmosets. NeuroReport 9, 2857–2860. [DOI] [PubMed] [Google Scholar]; b Grondin R.; Bedard P. J.; Hadj T. A.; Gregoire L.; Mori A.; Kase H. (1999) Antiparkinsonian effect of a new selective adenosine A2A receptor antagonist in MPTP-treated monkeys. Neurology 52, 1673–1677. [DOI] [PubMed] [Google Scholar]
- a Bara-Jimenez W.; Sherzai A.; Dimitrova T.; Favit A.; Bibbiani F.; Gillespie M.; Morris M. J.; Mouradian M. M.; Chase T. N. (2003) Adenosine A2A receptor antagonist treatment of Parkinson’s disease. Neurology 61, 293–296. [DOI] [PubMed] [Google Scholar]; b Hauser R. A.; Hubble J. P.; Truong D. D. (2003) Randomized trial of the adenosine A2A receptor antagonist istradefylline in advanced PD. Neurology 61, 297–303. [DOI] [PubMed] [Google Scholar]
- Kyowa Hakko Kogyo Co. Ltd. (2008) Kyowa Hakko receives not approvable letter from FDA for istradefylline (KW-6002). Press Release, February 28.
- a Gatta F.; Del Giudice M. R.; Borioni A.; Borea P. A.; Dionisotti S.; Ongini E. (1993) Synthesis of imidazo[1,2-c]pyrazolo[4,3-e]pyrimidines, pyrazolo[4,3-e]1,2,4-triazolo[1,5-c]pyrimidines and 1,2,4-triazolo[5,1-i]purines: New potent adenosine A2 receptor antagonists. Eur. J. Med. Chem. 28, 569–576. [Google Scholar]; b Ongini E. (1997) SCH 58261: A selective A2A adenosine receptor antagonist. Drug Dev. Res. 42, 63–70. [Google Scholar]
- Shah U.; Boyle C. D.; Chackalamannil S.; Neustadt B. R.; Lindo N.; Greenlee W. J.; Foster C.; Arik L.; Zhai Y.; Ng K.; Wang S.; Monopoli A.; Lachowicz J. E. (2008) Biaryl and heteroaryl derivatives of SCH 58261 as potent and selective adenosine A2A receptor antagonists. Bioorg. Med. Chem. Lett. 18, 4199–4203. [DOI] [PubMed] [Google Scholar]
- Neustadt B. R.; Hao J.; Lindo N.; Greenlee W. J.; Stamford A. W.; Tulshian D.; Ongini E.; Hunter J.; Monopoli A.; Bertorelli R.; Foster C.; Arik L.; Lachowicz J.; Ng K.; Feng K. I. (2007) Potent, selective, and orally active adenosine A2A receptor antagonists: arylpiperazine derivatives of pyrazolo[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidines. Bioorg. Med. Chem. Lett. 17, 1376–1380. [DOI] [PubMed] [Google Scholar]
- Hodgson R. A.; Bertorelli R.; Varty G. B.; Lachowicz J. E.; Forlani A.; Fredduzzi S.; Cohen-Williams M. E.; Higgins G. A.; Impagnatiello F.; Nicolussi E.; Parra L. E.; Foster C.; Zhai Y.; Neustadt B. R.; Stamford A. W.; Parker E. M.; Reggiani A.; Hunter J. C. (2009) Characterization of the potent and highly selective 2A receptor antagonists preladenant and SCH 412348 [7-[2-[4–2,4-difluorophenyl]-1-piperazinyl]ethyl]-2-(2-furanyl)-7H-pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidin-5-amine] in rodent models of movement disorders and depression. J. Pharmacol. Exp. Ther. 330, 294–303. [DOI] [PubMed] [Google Scholar]
- Schering-Plough Corp. (2008) Schering-Plough reports preladenant meets primary endpoint in phase II dose-finding trial for Parkinson’s disease. Press Release, November 24.
- Shah U.; Lankin C. M.; Boyle C. D.; Chackalamannil S.; Greenlee W. J.; Neustadt B. R.; Cohen-Williams M. E.; Higgins G. A.; Ng K.; Varty G. B.; Zhang H.; Lachowicz J. E. (2008) Design, synthesis, and evaluation of fused heterocyclic analogs of SCH 58261 as adenosine A2A receptor antagonists. Bioorg. Med. Chem. Lett. 18, 4204–4209. [DOI] [PubMed] [Google Scholar]
- Silverman L. S.; Caldwell J. P.; Greenlee W. J.; Kiselgof E.; Matasi J. J.; Tulshian D. B.; Arik L.; Foster C.; Bertorelli R.; Monopoli A.; Ongini E. (2007) 3H-[1,2,4]-Triazolo[5,1-i]purin-5-amine derivatives as adenosine A2A antagonists. Bioorg. Med. Chem. Lett. 17, 1659–1662. [DOI] [PubMed] [Google Scholar]
- Shook B. C.; Rassnick S.; Hall D.; Rupert K. C.; Heintzelman G. R.; Hansen K.; Chakravarty D.; Bullington J. L.; Scannevin R. H.; Magliaro B.; Westover L.; Carroll K.; Lampron L.; Russell R.; Branum S.; Wells K.; Damon S.; Youells S.; Li X.; Osbourne M.; Demarest K.; Tang Y.; Rhodes K.; Jackson P. F. (2010) Methylene amine substituted arylindenopyrimidines as potent adenosine A2A/A1 antagonists. Bioorg. Med. Chem. Lett. 20, 2864–2867. [DOI] [PubMed] [Google Scholar]
- Shook B. C.; Rassnick S.; Osborne M. C.; Davis S.; Westover L.; Boulet J.; Hall D.; Rupert K. C.; Heintzelman G. R.; Hansen K.; Chakravarty D.; Bullington J. L.; Russell R.; Branum S.; Wells K. M.; Damon S.; Youells S.; Li X.; Beauchamp D. A.; Palmer D.; Reyes M.; Demarest K.; Tang Y. -T.; Rhodes K.; Jackson P. F. (2010) In Vivo Characterization of a Dual Adenosine A2A/A1 Receptor Antagonist in Animal Models of Parkinson’s Disease. J. Med. Chem. 53, 8104–8115. [DOI] [PubMed] [Google Scholar]
- Shook B. C.; Rassnick S.; Chakravarty D.; Wallace N.; Ault M.; Crooke J.; Barbay J. K.; Wang A.; Leonard K.; Powell M. T.; Alford V.; Hall D.; Rupert K. C.; Heintzelman G. R.; Hansen K.; Bullington J. L.; Scannevin R. H.; Carroll K.; Lampron L.; Westover L.; Russell R.; Branum S.; Wells K.; Damon S.; Youells S.; Beauchamp D.; Li X.; Rhodes K.; Jackson P. F. (2010) Optimization of arylindenopyrimidines as potent adenosine A2A/A1 antagonists. Bioorg. Med. Chem. Lett. 20, 2868–2871. [DOI] [PubMed] [Google Scholar]
- Harris J. M.; Neustadt B. R.; Zhang H.; Lachowicz J.; Cohen-Williams M.; Varty G.; Hao J.; Stamford A. W. (2011) Potent and selective adenosine A2A receptor antagonists: [1,2,4]-triazolo[4,3-c]pyrimidin-3-ones. Bioorg. Med. Chem. Lett. 21, 2497–2501. [DOI] [PubMed] [Google Scholar]
- Mishra C. B.; Barodia S. K.; Prakash A.; Senthil Kumar J. B.; Luthra P. M. (2010) Novel 8-(furan-2-yl)-3-substituted thiazolo [5,4-e][1,2,4] triazolo[1,5-c] pyrimidine-2(3H)-thione derivatives as potential adenosine A2A receptor antagonists. Bioorg. Med. Chem. 18, 2491–2500. [DOI] [PubMed] [Google Scholar]
- Neustadt B. R.; Liu H.; Hao J.; Greenlee W. J.; Stamford A. W.; Foster C.; Arik L.; Lachowicz J.; Zhang H.; Bertorelli R.; Fredduzzi S.; Varty G.; Cohen-Williams M.; Ng K. (2009) Potent and selective adenosine A2A receptor antagonists: 1,2,4-Triazolo[1,5-c]pyrimidines. Bioorg. Med. Chem. Lett. 19, 967–971. [DOI] [PubMed] [Google Scholar]
- Matasi J. J.; Caldwell J. P.; Zhang H.; Fawzi A.; Higgins G. A.; Cohen-Williams M. E.; Varty G. B.; Tulshian D. B. (2005) 2-(2-Furanyl)-7-phenyl[1,2,4]triazolo[1,5-c]pyrimidin-5-amine analogs as adenosine A2A antagonists: The successful reduction of hERG activity. Bioorg. Med. Chem. Lett. 15, 3675–3678. [DOI] [PubMed] [Google Scholar]
- Shao Y.; Cole A. G.; Brescia M.-R.; Qin L.-Y.; Duo J.; Stauffer T. M.; Rokosz L. L.; McGuinness B. F.; Henderson I. (2009) Synthesis and SAR studies of trisubstituted purinones as potent and selective adenosine A2A receptor antagonists. Bioorg. Med. Chem. Lett. 19, 1399–1402. [DOI] [PubMed] [Google Scholar]
- Gillespie R. J.; Adams D. R.; Bebbington D.; Benwell K.; Cliffe I. A.; Dawson C. E.; Dourish C. T.; Fletcher A.; Gaur S.; Giles P. R.; Jordan A. M.; Knight A. R.; Knutsen L. J. S.; Lawrence A.; Lerpiniere J.; Misra A.; Porter R. H. P.; Pratt R. M.; Shepherd R.; Upton R.; Ward S. E.; Weiss S. M.; Williamson D. S. (2008) Antagonists of the human adenosine A2A receptor. Part 1: Discovery and synthesis of thieno[3,2-d]pyrimidine-4-methanone derivatives. Bioorg. Med. Chem. Lett. 18, 2916–2919. [DOI] [PubMed] [Google Scholar]
- Gillespie R. J.; Cliffe I. A.; Dawson C. E.; Dourish C. T.; Gaur S.; Giles P. R.; Jordan A. M.; Knight A. R.; Lawrence A.; Lerpiniere J.; Misra A.; Pratt R. M.; Todd R. S.; Upton R.; Weiss S. M.; Williamson D. S. (2008) Antagonists of the human adenosine A2A receptor. Part 2: Design and synthesis of 4-arylthieno[3,2-d]pyrimidine derivatives. Bioorg. Med. Chem. Lett. 18, 2920–2923. [DOI] [PubMed] [Google Scholar]
- Gillespi R. J.; Cliffe I. A.; Dawson C. E.; Dourish C. T.; Gaur S.; Jordan A. M.; Knight A. R.; Lerpiniere J.; Misra A.; Pratt R. M.; Roffey J.; Stratton G. C.; Upton R.; Weiss S. M.; Williamson D. S. (2008) Antagonists of the human adenosine A2A receptor. Part 3: Design and synthesis of pyrazolo[3,4-d]pyrimidines, pyrrolo[2,3-d]pyrimidines and 6-arylpurines. Bioorg. Med. Chem. Lett. 18, 2924–2929. [DOI] [PubMed] [Google Scholar]
- Gillespie R. J.; Bamford S. J.; Botting R.; Comer M.; Denny S.; Gaur S.; Griffin M.; Jordan A. M.; Knight A. R.; Lerpiniere J.; Leonardi S.; Lightowler S.; McAteer S.; Merrett A.; Misra A.; Padfield A.; Reece M.; Saadi M.; Selwood D. L.; Stratton G. C.; Surry D.; Todd R.; Tong X.; Ruston V.; Upton R.; Weiss S. M. (2009) Antagonists of the Human A2A Adenosine Receptor. 4. Design, Synthesis, and Preclinical Evaluation of 7-Aryltriazolo[4,5-d]pyrimidines. J. Med. Chem. 52, 33–47. [DOI] [PubMed] [Google Scholar]
- Vernalis. (2004) Vernalis and Biogen Idec to collaborate on research for Parkinson’s disease. Press Release, June 24.
- Vernalis. (2010) Vernalis announces A2A receptor antagonist programme for Parkinson’s disease with next generation compound. Press Release, July 16.
- Minetti P.; Tinti M. O.; Carminati P.; Castorina M.; Di Cesare M. A.; Di Serio S.; Gallo G.; Ghirardi O.; Giorgi F.; Giorgi L.; Piersanti G.; Bartoccini F.; Tarzia G. (2005) 2-n-Butyl-9-methyl-8-[1,2,3]triazol-2-yl-9H-purin-6-ylamine and Analogues as A2A Adenosine Receptor Antagonists. Design, Synthesis, and Pharmacological Characterization. J. Med. Chem. 48, 6887–6896. [DOI] [PubMed] [Google Scholar]
- Stasi M. A.; Borsini F.; Varani K.; Vincenzi F.; Di Cesare M. A.; Minetti P.; Ghirardi O.; Carminati P. (2006) ST 1535: a preferential A2A adenosine receptor antagonist. Int. J. Neuropsychopharmacology 9, 575–584. [DOI] [PubMed] [Google Scholar]
- a Rose S.; Ramsay Croft N.; Jenner P. (2007) The novel adenosine A2a antagonist ST1535 potentiates the effects of a threshold dose of L-dopa in unilaterally 6-OHDA-lesioned rats. Brain Res. 1133, 110–114. [DOI] [PubMed] [Google Scholar]; b Tronci E.; Simola N.; Borsini F.; Schintu N.; Frau L.; Carminati P.; Morelli M. (2007) Characterization of the antiparkinsonian effects of the new adenosine A2A receptor antagonist ST1535: Acute and subchronic studies in rats. Eur. J. Pharmacol. 566, 94–102. [DOI] [PubMed] [Google Scholar]
- Rose S.; Jackson M. J.; Smith L. A.; Stockwell K.; Johnson L.; Carminati P.; Jenner P. (2006) The novel adenosine A2a receptor antagonist ST1535 potentiates the effects of a threshold dose of L-DOPA in MPTP treated common marmosets. Eur. J. Pharmacol. 546, 82–87. [DOI] [PubMed] [Google Scholar]
- Shook B. C., Chakravarty D., Barbay J. K., Wang W., Leonard K., Alford V., Powell M., Beauchamp D. A., Rassnick S., Scannevin R., Carroll K., Wallace N., Crooke J., Ault M., Lampron L., Westover L., Rhodes K., and Jackson P. F. (2011) Aminomethyl substituted thieno[2,3-d]pyrimidines as adenosine A2A receptor antagonists. Med. Chem. Commun. published online May 19, 2011. DOI: 10.1039/C1MD00082A. [DOI] [PubMed] [Google Scholar]
- McGuinness B. F.; Cole A. G.; Dong G.; Brescia M.-R.; Shao Y.; Henderson I.; Rokosz L. L.; Stauffer T. M.; Mannava N.; Kimble E. F.; Hicks C.; White N.; Wines P. G.; Quadros E. (2010) Discovery of 2-aminoimidazopyridine adenosine A2A receptor antagonists. Bioorg. Med. Chem. Lett. 20, 6845–6849. [DOI] [PubMed] [Google Scholar]
- Flohr A., Moreau J. L., Poli S. M., Riemer C., and Steward L. (2005) 4-Hydroxy-4-methyl-piperidine-1-carboxylic acid (4-methoxy-7-morpholin-4-yl-benzothiazol-2-yl)-amide. US 20050261289.
- Flohr A., and Riemer C. (2006) Benzothiazole derivatives. WO 2006008041.
- Flohr A., and Riemer C. (2006) Substituted benzothiazoles. WO 2006008040.
- Woiwode T., and Noran M. (2000) 4-Hydroxy-4-methyl-piperidine-1-carboxylic acid (4-methoxy-7-morpholin-4-yl-benzothiazol-2-yl)-amide for the treatment of post-traumatic stress disorder. PCT Int. Appl. WO 2009015236.
- Synosis Therapeutics. (2007) Roche and Synosis Therapeutics announce partnership to explore potential of five compounds targeting the central nervous system. Press Release, January 4.
- Saku O.; Saki M.; Kurokawa M.; Ikeda K.; Takizawa T.; Uesaka N. (2010) Synthetic studies on selective adenosine A2A receptor antagonists: Synthesis and structure–activity relationships of novel benzofuran derivatives. Bioorg. Med. Chem. Lett. 20, 1090–1093. [DOI] [PubMed] [Google Scholar]
- Saku O.; Saki M.; Kurokawa M.; Ikeda K.; Uchida S.-I.; Takizawa T.; Uesaka N. (2010) Synthetic studies on selective adenosine A2A receptor antagonists. Part II: Synthesis and structure-activity relationships of novel benzofuran derivatives. Bioorg. Med. Chem. Lett. 20, 3768–3771. [DOI] [PubMed] [Google Scholar]
- Yonishi S., Aoki S., Matsushima Y., Akahane A. (2005) Preparation of pyrazines as adenosine A1 and A2A receptor antagonists and their pharmaceutical compositions. PCT Int. Appl. WO 2005040151.
- Mihara T.; Mihara K.; Yarimizu J.; Mitani Y.; Matsuda R.; Yamamoto H.; Aoki S.; Akahane A.; Iwashita A.; Matsuoka N. (2007) Pharmacological characterization of a novel, potent adenosine A1 and A2A receptor dual antagonist, 5-[5-amino-3-(4-fluorophenyl)pyrazin-2-yl]-1-isopropylpyridine-2(1H)-one (ASP5854), in models of Parkinson’s disease and cognition. J. Pharmacol. Exp. Ther. 323, 708–719. [DOI] [PubMed] [Google Scholar]
- Mihara T.; Noda A.; Arai H.; Mihara K.; Iwashita A.; Murakami Y.; Matsuya T.; Miyoshi S.; Nishimura S.; Matsuoka N. (2008) Brain adenosine A2A receptor occupancy by a novel A1/A2A receptor antagonist, ASP5854, in rhesus monkeys: relationship to anticataleptic effect. J. Nucl. Med. 49, 1183–1188. [DOI] [PubMed] [Google Scholar]
- Mihara T.; Iwashita A.; Matsuoka N. (2008) A novel adenosine A1 and A2A receptor antagonist ASP5854 ameliorates motor impairment in MPTP-treated marmosets: Comparison with existing anti-Parkinson’s disease drugs. Behav. Brain Res. 194, 152–161. [DOI] [PubMed] [Google Scholar]
- Sams A. G.; Mikkelsen G. K.; Larsen M.; Torup L.; Brennum L. T.; Schroder T. J.; Bang-Andersen B. (2010) Hit-to-lead optimization of a series of carboxamides of ethyl 2-amino-4-phenylthiazole-5-carboxylates as novel adenosine A2A receptor antagonists. Bioorg. Med. Chem. Lett. 20, 5241–5244. [DOI] [PubMed] [Google Scholar]
- Cole A. G.; Stauffer T. M.; Rokosz L. L.; Metzger A.; Dillard L. W.; Zeng W.; Henderson I. (2009) Synthesis of 2-amino-5-benzoyl-4-(2-furyl)thiazoles as adenosine A2A receptor antagonists. Bioorg. Med. Chem. Lett. 19, 378–381. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Rueter J. K.; Chen Y.; Moorjani M.; Lanier M. C.; Lin E.; Gross R. S.; Tellew J. E.; Williams J. P.; Lechner S. M.; Markison S.; Joswig T.; Malany S.; Santos M.; Castro-Palomino J. C.; Crespo M. I.; Prat M.; Gual S.; Diaz J.-L.; Saunders J.; Slee D. H. (2008) Synthesis of N-pyrimidinyl-2-phenoxyacetamides as adenosine A2A receptor antagonists. Bioorg. Med. Chem. Lett. 18, 1778–1783. [DOI] [PubMed] [Google Scholar]
- Slee D. H.; Zhang X.; Moorjani M.; Lin E.; Lanier M. C.; Chen Y.; Rueter J. K.; Lechner S. M.; Markison S.; Malany S.; Joswig T.; Santos M.; Gross R. S.; Williams J. P.; Castro-Palomino J. C.; Crespo M. I.; Prat M.; Gual S.; Diaz J.-L.; Wen J.; O’Brien Z.; Saunders J. (2008) Identification of Novel, Water-Soluble, 2-Amino-N-pyrimidin-4-yl Acetamides as A2A Receptor Antagonists with In Vivo Efficacy. J. Med. Chem. 51, 400–406. [DOI] [PubMed] [Google Scholar]
- Slee D. H.; Chen Y.; Zhang X.; Moorjani M.; Lanier M. C.; Lin E.; Rueter J. K.; Williams J. P.; Lechner S. M.; Markison S.; Malany S.; Santos M.; Gross R. S.; Jalali K.; Sai Y.; Zuo Z.; Yang C.; Castro-Palomino J. C.; Crespo M. I.; Prat M.; Gual S.; Diaz J.-L.; Saunders J. (2008) 2-Amino-N-pyrimidin-4-ylacetamides as A2A Receptor Antagonists: 1. Structure-Activity Relationships and Optimization of Heterocyclic Substituents. J. Med. Chem. 51, 1719–1729. [DOI] [PubMed] [Google Scholar]
- Moorjani M.; Luo Z.; Lin E.; Vong B. G.; Chen Y.; Zhang X.; Rueter J. K.; Gross R. S.; Lanier M. C.; Tellew J. E.; Williams J. P.; Lechner S. M.; Malany S.; Santos M.; Crespo M. I.; Diaz J.-L.; Saunders J.; Slee D. H. (2008) 2,6-Diaryl-4-acylaminopyrimidines as potent and selective adenosine A2A antagonists with improved solubility and metabolic stability. Bioorg. Med. Chem. Lett. 18, 5402–5405. [DOI] [PubMed] [Google Scholar]
- Gillespie R. J.; Bamford S. J.; Gaur S.; Jordan A. M.; Lerpiniere J.; Mansell H. L.; Stratton G. C. (2009) Antagonists of the human A2A receptor. Part 5: Highly bio-available pyrimidine-4-carboxamides. Bioorg. Med. Chem. Lett. 19, 2664–2667. [DOI] [PubMed] [Google Scholar]
- Gillespie R. J.; Bamford S. J.; Clay A.; Gaur S.; Haymes T.; Jackson P. S.; Jordan A. M.; Klenke B.; Leonardi S.; Liu J.; Mansell H. L.; Ng S.; Saadi M.; Simmonite H.; Stratton G. C.; Todd R. S.; Williamson D. S.; Yule I. A. (2009) Antagonists of the human A2A receptor. Part 6: Further optimization of pyrimidine-4-carboxamides. Bioorg. Med. Chem. 17, 6590–6605. [DOI] [PubMed] [Google Scholar]