Abstract
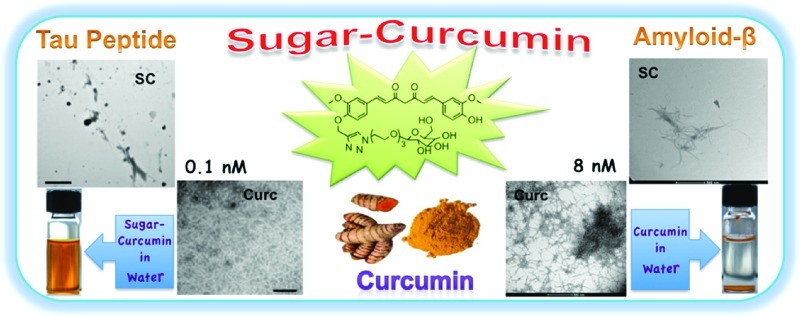
The synthesis of a water/plasma soluble, noncytotoxic, “clicked” sugar-derivative of curcumin with amplified bioefficacy in modulating amyloid-β and tau peptide aggregation is presented. Curcumin inhibits amyloid-β and tau peptide aggregation at micromolar concentrations; the sugar–curcumin conjugate inhibits Aβ and tau peptide aggregation at concentrations as low as 8 nM and 0.1 nM, respectively. In comparison to curcumin, this conveniently synthesized Alzheimer’s drug candidate is a more powerful antioxidant.
Keywords: Alzheimer’s disease (AD), amyloid-β, tau peptide, curcumin, “click” reaction, antioxidant potential
There is a compelling need to find drugs for the treatment of Alzheimer’s disease (AD). AD presently affects 18 million people worldwide and is expected to increase to 25 million by 2025.1 Medications approved by the United States Food and Drug Administration (FDA) treat the symptoms of AD, but do not alter progression of the disease.2,3 The concept that small aggregates of proteins are pathological entities in many amyloid diseases including AD is an emerging paradigm finding increasing support from recent studies on proteins such as tau, Aβ, α-synuclein, and prion protein whose aggregation is associated with neurodegenerative diseases.4 The aggregation process can be triggered by different factors: redox active copper(II) ion can expedite amyloid aggregation via binding to Aβ peptides, and it can also generate reactive oxygen species (ROS), that initiate lipid peroxidation which induces oxidative stress.5 The major target for drug discovery for AD has been Aβ that forms insoluble senile plaques, and recently, with the failure of the trials based only on amyloid-β, there is an increased interest in incorporating tau aggregates in the therapeutic approach. Binding to and altering the distribution of tau and amyloid aggregates is likely to disrupt their toxic action on neurons. Drug targets that are limited to a single target have proven to have disappointing results: Antibodies that directly target monomeric or oligomeric Aβ have been developed, and they have however failed in clinical trials.6 Several small molecules which have been shown to inhibit Aβ aggregation7 have had limited success in clinical studies. Several Cu(II) ion chelators have been considered as drug candidates, for example, Clioquinol and Apocyclen. Therapeutic approaches for reduction of phosphorylated tau include pharmacologic manipulation of cellular protein refolding/degradation pathways8 and immunotherapy.9 It is evident from the above discussion that there are many pathways that contribute to AD. An ideal AD drug candidate should therefore be broad spectrum with activity against all the pathways that contribute to AD. An ideal AD drug should (a) disrupt amyloid aggregates, (b) depolymerize tau aggregates, (c) chelate and remove metal ions such a copper, (d) reduce oxidative stress in the brain, and (e) have other neuroprotective characteristics.
The active ingredient in turmeric (curcumin) is a potential safe bullet to simultaneously target the multifaceted features of AD. Epidemiological studies have correlated the consumption of turmeric by the southeast asian population with significantly reduced occurrence of AD compared to western countries.10 Curcumin [1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione], a FDA Generally Regarded as Safe (GRAS) polyphenolic component of turmeric, has been shown to possess activity against most of the biochemical pathways associated with the onset and progression of AD.11 Curcumin is an antioxidant,12 anti-inflammatory13 and has promising anti-AD activity.11b,11c,12 Curcumin has been shown to cross the blood brain barrier and reduce pathology in AD mouse models overproducing Aβ.14 We have recently shown that curcumin disrupts tau peptide aggregates.15 Curcumin appears to have multiple neuroprotective mechanisms including inhibition of inflammation, disruption of amyloid-β and tau peptide aggregation, reduction of reactive oxygen species by chelating metals, inhibition of stress pathways, and induction of heat shock proteins.11b,14c,16
Two crucial structural features have been associated with many small molecule amyloid inhibitors: the presence of phenolic rings capable of disrupting peptide aromatic π–π stacking and groups capable of competitive hydrogen bonding (β-sheet breakers).17 The curcumin pharmacophore has the same features present in the amyloid targeting Alzheimer’s drug candidate and β-sheet breaker, N,N- bis(3-hydroxyphenyl)pyridazine-3,6-diamine, which was found using high-throughput screening of 113 000 compounds (like curcumin, it inhibits amyloid fibril formation at micromolar concentrations).18 The phenolic group of curcumin can interact with the aromatic residues in amyloid fibrils disrupting peptide π–π stacking while the hydroxyl groups and β-diketone unit can act as β-sheet breakers via competitive hydrogen bonding. The β-diketone component is also capable of chelating copper ions.19 The mechanism of action of curcumin in inhibiting tau aggregates has not been correlated with its structure; one possibility is that it works via copper ion chelation.20 Despite its broad spectrum bioactivity, curcumin by itself is a poor therapeutic agent for AD. One of the major limitations of using curcumin as a drug is its poor water and plasma solubility and consequent low bioavailability.21 There have been some attempts at addressing this issue by producing micellar and solid lipid nanoparticle formulations of curcumin with improved water/plasma solubility and by evaluating CNB001, a derivative of curcumin,22 for safety and for the potential to treat AD. CNB001 is synthesized by covalent modification of one of the ketone groups in the β-diketone bridge of curcumin with phenyl hydrazine and is more hydrophobic than curcumin. CNB001 does not possess the vital β-diketone unit which is necessary for disruption of amyloid-β aggregation via competitive hydrogen bonding. The leaching of encapsulated curcumin/CNB001, the lack of transport of the micelles/nanoparticles across the gastrointestinal tract, slow release kinetics of the payloads from the nanoparticles, lack of in vivo safety of nanoparticles,23 and limited transport of the micelles/nanoparticles across the blood brain barrier (BBB)24 are some of the hurdles which have to be addressed with a noncovalent encapsulation (formulation) approach to improving the bioefficacy of curcumin. A recent report25 describes the synthesis of hydrophobic cholesterol-curcumin derivatives with activity which is approximately similar to curcumin (micromolar range) against amyloidal aggregates; it must be noted that amyloid fibrils are extracellular, curcumin can independently incorporate in lipid bilayers,26 and curcumin has been shown to cross the blood brain barrier,27 so attaching cholesterol to curcumin does not, in principle, enhance it to perform as a better AD drug candidate.
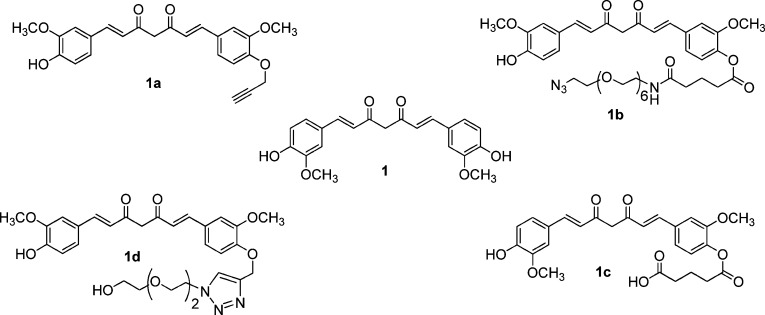
It is thus evident that the fundamental issue of curcumin bioavailability has not yet been solved and that there is a compelling need for better curcumin based amyloid-β and tau inhibitors that work at a much lower concentration, ideally in the nanomolar range. We synthesized monofunctional curcumin derivatives that possess an unique carboxylic acid/azide/alkyne group in accordance with our reported procedure11c and evaulated the molecules for anti-AD activity (Figure 1).
Figure 1.
Monofunctional curcumin derivatives.
Preliminary studies were carried out to assess whether the monofunctional curcumin derivatives retained the ability to bind and dissolve amyloid fibrils in vitro. Human heart tissue containing intercellular amyloid was stained with Congo Red according to the “Benhold’s” protocol (control sample) or with the reactive monofunctional curcumin derivatives and imaged using a polarized light microscope; the monofunctional derivatives label amyloid fibrils very effectively (see Supporting Information Figure S5a, b). Curcumin and its derivatives have the advantage that they can effectively label fibrils at a much lower concentration than Congo Red: 50 nM of 1-1d compared to 0.014 M for Congo Red. The ability of 1-1d to dissolve amyloid aggregates (fibrils) was also evaluated. In a typical experiment, amyloid fibrils were formed by incubating Aβ 1–40 peptide. Either the curcumin derivative or control buffer was added to the fibrils and incubated followed by visualization using transmission electron microscopy (TEM). A network of fibrils was observed in the TEM image of the control amyloid fibril sample (see Supporting Information Figure S5c, d), whereas the fibrils were absent in the sample treated with the curcumin derivatives (see Supporting Information Figure S5d). This observation was further supported by UV spectroscopy (see Supporting Information Figure S7). These experiments indicate the curcumin derivatives are as effective (function in the micromolar range) in inhibiting amyloid fibril formation as the parent molecule curcumin. It should be noted that the curcumin derivatives do not have significantly improved water/plasma solubility in comparison to curcumin.
Our goal was to synthesize a water/plasma soluble, nontoxic, biocompatible derivative of curcumin with bioactivity against both amyloid and tau aggregates at significantly lower concentrations compared to curcumin. We hypothesized that attaching a sugar moiety to curcumin would result in the desired construct which retains all the characteristics of the curcumin pharmacophore. In addition, the sugar would serve to significantly increase the water/plasma solubility of the molecule. The hydroxyl groups of sugar could participate in competitive hydrogen bonding. The loss in antioxidant potential caused by blocking one of the phenolic groups would be compensated by the vastly increased water/plasma solubility. The sugar–curcumin conjugate would retain other structural characteristics of the curcumin pharmacophore such as the ability to chelate metal ions. The desired molecule, a soluble “clicked” sugar conjugate of curcumin (SC), was synthesized in accordance with Scheme 1; SC inhibits amyloid-β peptide aggregation (Aβ fragments 25–35 and Aβ peptide 1–42) and tau aggregates at nanomolar concentrations. Preliminary studies indicate that SC is nontoxic to neurons and is a stronger antioxidant than curcumin.
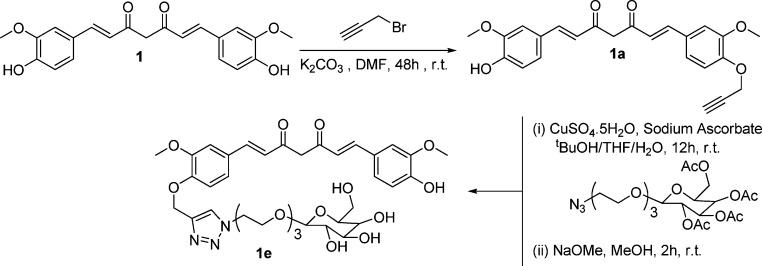
Scheme 1. Synthesis of Curcumin “Clicked” Mono-Galactose 1e.
Curcumin monoalkyne derivative of curcumin 1a was reacted with commercially available 2-[2-(2-azidoethoxy)ethoxy]ethyl-2,3,4,6-tetra-O-acetyl-d-galactopyranoside (acetyl-protected galactose azide) under “click” reaction conditions (Scheme 1).
The 8.1 ppm peak (triazole proton) in the 1H NMR and ESI-MS data m/z = 912.3 confirms the conjugation of curcumin with sugar. Acetyl-protected galactose was employed in the click reaction due to the solubility mismatch of sugars and 1a in organic solvents. Further, deprotection using NaOMe afforded the acetyl-deprotected sugar–curcumin conjugate 1e. The absence of acetyl-CH3 peaks at 2.22–2.38 ppm in 1H NMR and 20.48–20.65 ppm in 13C NMR along with the presence of a peak at m/z = 744.3 in ESI-MS confirms the complete deprotection of the galactose. The sugar–curcumin conjugate 1e (SC) is freely soluble in water.
A UV–vis experiment was conducted to evaluate the superior water solubility of sugar–curcumin compared to curcumin. The same molar quantities of both curcumin and sugar–curcumin were vortexed in water, the resulting solution was centrifuged to remove undissolved material. The supernatant liquid was diluted and the UV–vis spectrum was recorded (Figure 2). From the spectrum (using a previously determined molar extinction coefficient) it was estimated that SC is ∼1000 times more soluble than curcumin in water. It should be noted that the solubility study outlined above measures the direct solubility of the compounds in water and closely models “real life” conditions. This is in contrast to other experiments reported in literature in which the compounds are first dissolved in other solvents followed by dilution in water.
Figure 2.
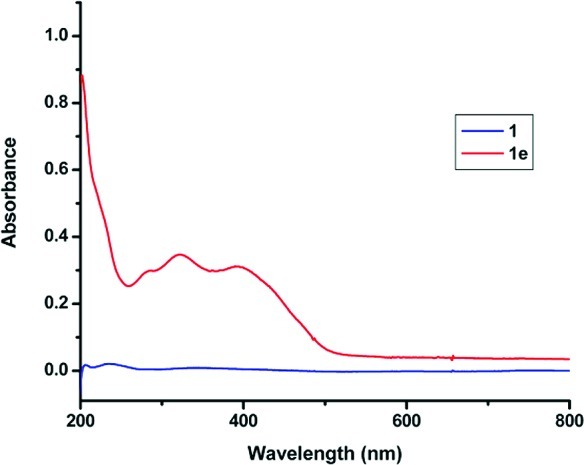
UV–vis spectrometric comparison of solubility of curcumin 1 and sugar–curcumin 1e in water: sugar–curcumin in water (5 mg/mL, red) and curcumin in water (5 mg/mL, blue).
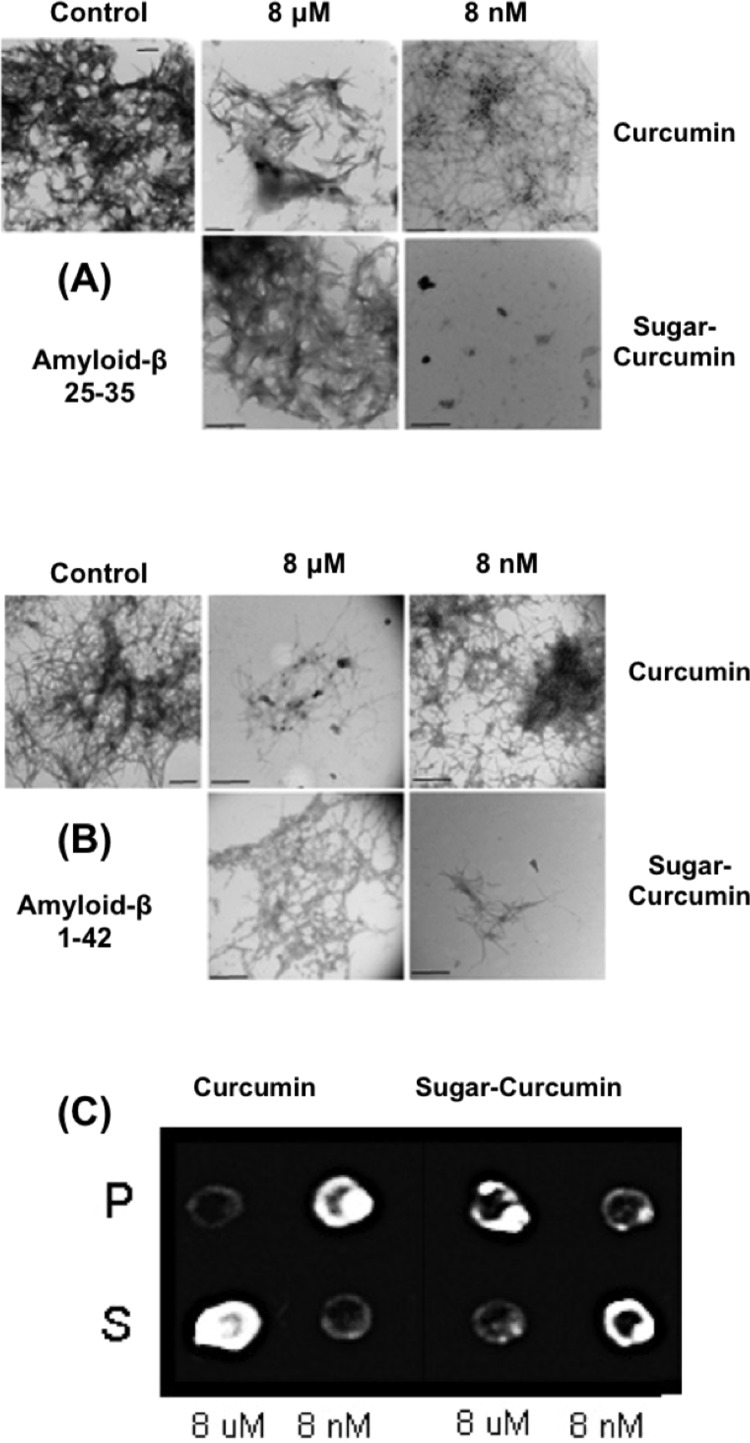
In order to evaluate SC’s ability to dissolve amyloid-β fibrils, Aβ fragments 25–35 and Aβ peptide 1–42 were incubated with different concentrations of either curcumin or SC at 37 °C for 5 days. The aggregation of Aβ to form fibrils was determined via TEM where the samples were negatively stained using freshly prepared and filtered 0.2% phosphotungstic acid. SC inhibits the Aβ aggregation at significantly lower concentrations compared to curcumin (Figure 3A, B). SC completely inhibits amyloid-β fiber formation at concentrations as low as 8 nM, but is not as effective at higher concentrations; the consistency of this result was confirmed by repeating the experiments multiple times. To further confirm this result, we performed a dot blot assay (Figure 3C). Aβ peptide 1–42 was incubated with varying concentrations of SC for 5 days at 37 °C and then centrifuged at 10 000 rpm; the resulting pellet and supernatant were employed in a dot blot assay and probed with a Aβ peptide 1–42 specific antibody. SC inhibited Aβ assembly at 8 nM, since no aggregate was detected in the pellet fraction whereas almost all of it was in the pellet fraction when regular curcumin was analyzed at the same concentration. At micromolar concentrations of SC, the inhibition of aggregation is not effective when compared to 8 nM.
Figure 3.
Sugar-curcumin inhibits amyloid-β assembly at lower concentrations than regular curcumin. Amyloid-β peptides 25–35 (A) and 1–42 (B) were incubated with 8 μM or 8 nM of SC or curcumin. It is shown that, at 8 nM concentration, SC is able to significantly inhibit aggregation. Curcumin is only able to inhibit aggregation at micromolar concentrations. The assembly is shown by negatively stained electron microscopy (all scale bars are 500 nm). (C) Dot blot assay of Aβ 1–42. TEM microscopy results were confirmed by immuno dot blot assay. Note: P, pellet; S, supernatant.
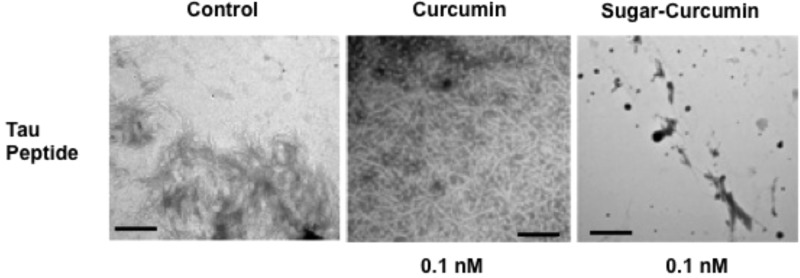
Preliminary experiments to evaluate the tau inhibitory activity of SC and curcumin were performed. The peptide VQIVYK has been identified as the sequence responsible for tau self-assembly.28 VQIVYK peptide sequence was incubated with different concentrations of either curcumin or SC at 37 °C for 2 h. The aggregation of tau to form filaments was determined via TEM. SC inhibits tau aggregation at significantly lower concentrations compared to curcumin. Where curcumin begins to have an inhibitory effect on the tau aggregation at a concentration of 8 μM, SC completely inhibits tau peptide aggregation at concentrations as low as 0.1 nM (Figure 4). However, the same trend observed in case of Aβ experiments was repeated in the tau study: at higher concentrations, SC is not a very effective tau inhibitor. The reduced efficacy of SC to inhibit Aβ and tau peptide aggregation at higher concentrations may be due to the possible formation of SC micelles (the molecule is amphiphilic in nature). The increased solubility of SC in water (1000-fold more) is reflected in its enhanced ability (∼1000 times more effective) to inhibit amyloid-β and tau aggregation. It should be noted that assays such as the thioflavin assay are not appropriate for testing anti-amyloidogenic properties of curcumin and its derivatives because the strong absorptive and fluorescent properties curcumin have been shown to significantly interfere with the thiofloflavin fluorescence readings.29
Figure 4.
TEM results from the inhibitory experiment of tau peptide aggregation. SC is far more effective than curcumin at 0.1 nM.
To assess whether the enhanced bioefficacy of SC can be correlated with the antioxidant potential (AOP), we measured the AOP of curcumin, curcumin monoalkyne, and sugar–curcumin via the linoleic acid peroxidation method in accordance with a literature procedure.30 SC showed higher AOP compared to both the curcumin and curcumin monoalkyne in water (see Supporting Information Figure S2). This could be attributed to the higher solubility of 1e in water than 1 and 1a. A preliminary experiment to investigate the neurotoxicity of the SC was performed via a MTT assay on cultured hippocampal slices of mouse brain. The results of the MTT assay (Supporting Information Figure S4) showed that at over a concentration range (8 nM and 80 μM) the viability of SC treated cells is similar to that of the control samples. This suggests that the sugar–curcumin conjugate did not have a harmful effect on the normal brain tissue with respect to cell viability.
In conclusion, we have successfully synthesized a “clicked” water-soluble sugar derivative of curcumin with amplified bioactivity (∼1000 times more potent against amyloid-β and tau peptide aggregation) in comparison to curcumin and its monofunctional derivatives 1-1d. SC is a more powerful antioxidant than curcumin. It is anticipated that by varying the sugar in our modular synthetic design we can optimize the uptake across the blood brain barrier via the glucose transporter mechanism.31 Further studies to exploit this conveniently synthesized Alzeimer’s drug candidate including novel approaches to deliver the molecule and in vivo experiments are in progress.
Methods
Synthesis of Protected Curcumin–“Clicked”-Monogalactose Conjugate
Curcumin monoalkyne (1a) (500 mg, 1.23 mmol) was dissolved in 2 mL of THF and added to 2 mL of t-BuOH containing commercially available acetyl-protected azido-galactose derivative [2-[2-(2-azidoethoxy)ethoxy]ethyl-2,3,4,6-tetra-O-acetyl-d-galactopyranoside] (625 mg, 1.23 mmol) in a round-bottom flask (r.b.). Fresh solutions of CuSO4·5H2O (76 mg, 0.3 mmol) and sodium ascorbate (90 mg, 0.45 mmol) were prepared separately in 1 mL of Millipore water. Sodium ascorbate solution was added to the r.b. followed by CuSO4 solution and stirred for 12 h. The reaction was stopped, and the solvent was removed by evaporation. The crude product was extracted from the water–chloroform mixture. The organic layer was dried over anhydrous NaSO4, and solvent was evaporated. Finally, the product was purified via column chromatography using a chloroform/ethyl acetate (90:10) mixture to yield orange solid. Yield: 785 mg (70%). 1H NMR (CDCl3, 600 MHz): δ (ppm) 2.22 (s, 3H), 2.27–2.29 (d, 6H), 2.38 (s, 3H), 3.81–3.85 (m, 6H), 3.95 (m, 1H), 4.11–4.17 (m, 10H), 4.34–4.40 (m, 2H), 4.76–4.80 (m, 3H), 5.24–5.27 (dd, 1H), 5.42–5.45 (m, 1H), 5.54–5.57 (m, 2H), 5.61–5.62 (m, 1H), 6.05 (s, 1H), 6.71–6.75 (m, 2H), 7.16–7.17 (d, 1H), 7.29–7.35 (m, 4H), 7.80–7.84 (m, 2H), 8.10 (s, 1H). 13C NMR (CDCl3, 150 MHz): δ (ppm) 20.48, 20.54, 20.56, 20.65, 50.23, 55.82, 55.84, 61.09, 62.77, 66.92, 68.69, 69.04, 69.28, 70.07, 70.45, 70.47, 70.53, 70.75, 101.18, 101.23, 109.61, 110.30, 113.57, 114.84, 121.59, 122.15, 122.24, 122.84, 124.25, 139.99, 140.61, 143.39, 146.82, 147.93, 149.50, 149.51, 169.33, 170.04, 170.13, 170.28, 182.73, 183.52. ESI-MS for C44H53N3O18: calculated, 911.33; observed, 912.3 [M + H]+.
Synthesis of Curcumin–“Clicked”-Monogalactose (Sugar-Curcumin) (1e)
Protected curcumin–“clicked”-galactose (90 mg, 0.098 mmol) was dissolved in 3 mL of 0.3 M NaOMe in anhydrous MeOH. The mixture was stirred at room temperature for 2 h. The pH of the solution was neutralized to pH 7 using Amberlyst15 ion-exchange resin, and the color of the solution became light yellow from dark orange. The solution was filtered, and the solvent was removed. Finally, the crude product was purified via column chromatography using CHCl3/MeOH (95:5) to yield 1e as a dark yellow solid. Yield: 55 mg (76%). 1H NMR (CD3OD, 600 MHz): δ (ppm) 3.30 (s, 4H), 3.39–3.46 (m, 2H), 3.50–3.53 (m, 2H), 3.57 (s, 4H), 3.59–3.62 (m, 2H), 3.65–3.75 (m, 3H), 3.79–3.80 (d, 2H), 3.85–3.89 (m, 6H), 3.94–3.96 (m, 1H), 4.18–4.20 (d, 1H), 4.57–4.58 (t, 2H), 5.21 (s, 2H), 6.59–6.65 (m, 2H), 6.80–6.81 (d, 1H), 7.07–7.21(m, 4H), 7.53–7.56 (m, 2H), 8.11 (s, 1H). 13C NMR (CDCl3, 150 MHz): δ (ppm) 49.84, 51.48, 56.48, 56.53, 62.54, 62.79, 63.34, 69.59, 70.30, 71.09, 71.31, 71.37, 71.41, 72.50, 74.89, 76.66, 105.03, 111.79, 112.08, 115.35, 116.59, 123.55, 124.19, 126.57, 128.54, 130.50, 131.21, 141.30, 142.37, 144.47, 149.40, 150.50, 151.06, 151.37, 183.92, 185.37. ESI-MS for C36H45N3O14: calculated, 743.29; observed, 744.3 [M + H]+.
Acknowledgments
We are thankful to Shawon Debnath and Amit Mogha in assisting us with AOP and MTT assay experiments and Robert Truzzolino for assistance with peptide aggregation experiments.
Supporting Information Available
Experimental procedures and characterization of synthesized compounds, experimental details concerning antioxidant potentials, Aβ and tau peptide inhibition, hippocampal slice culture, and MTT assay. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
The synthesis and characterization performed by S.D., W.S., S.A., and D.O. The bioassay were performed by C.S. and M.F. All the synthesis and characterization experiments including AOP determination were designed and supervised by K.R. The bioassay and mouse hippocampal slice experiments were supervised by A.A. and P.B.
This research was supported by a CUNY collaborative grant CIRG 1627 and PSC-CUNY.
Supplementary Material
References
- Abbott A. (2011) Dementia: a problem for our age. Nature 475(7355), S2–S4. [DOI] [PubMed] [Google Scholar]
- a Fowler D. M.; Kelly J. W. (2009) Aggregating knowledge about prions and amyloid. Cell 137(1), 20–22. [DOI] [PubMed] [Google Scholar]; b Rauk A. (2009) The chemistry of Alzheimer’s disease. Chem Soc Rev. 38(9), 2698–2715. [DOI] [PubMed] [Google Scholar]; c Rostagno A.; Holton J. L.; Lashley T.; Revesz T.; Ghiso J. (2010) Cerebral amyloidosis: amyloid subunits, mutants and phenotypes. Cell. Mol. Life Sci. 67(4), 581–600. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Smith T. J.; Stains C. I.; Meyer S. C.; Ghosh. I. (2006) Inhibition of beta-amyloid fibrillization by directed evolution of a beta-sheet presenting miniature protein. J. Am. Chem. Soc. 128(45), 14456–14457. [DOI] [PubMed] [Google Scholar]
- a Alonso A. C.; Li B.; Grundke-Iqbal I.; Iqbal K. (2008) Mechanism of tau-induced neurodegeneration in Alzheimer disease and related tauopathies. Curr. Alzheimer Res. 5(4), 375–384. [DOI] [PubMed] [Google Scholar]; b Alonso A. D.; Di-Clerico J.; Li B.; Corbo C. P.; Alaniz M. E.; Grundke-Iqbal I.; Iqbal. K. (2010) Phosphorylation of tau at Thr212, Thr231, and Ser262 combined causes neurodegeneration. J. Biol. Chem. 285(40), 30851–30860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayed R.; Head E.; Thompson J. L.; McIntire T. M.; Milton S. C.; Cotman C. W.; Glabe C. G (2003) Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 300(5618), 486–489. [DOI] [PubMed] [Google Scholar]
- Curtain C. C.; Ali F.; Volitakis I.; Cherny R. A.; Norton R. S.; Beyreuther K.; Barrow C. J.; Masters C. L.; Bush A. I.; Barnham K. J. (2001) Alzheimer’s Disease Amyloid-β Binds Copper and Zinc to Generate an Allosterically Ordered Membrane-penetrating Structure Containing Superoxide Dismutase-like Subunits. J. Biol. Chem. 276, 20466–20473. [DOI] [PubMed] [Google Scholar]
- Nitsch R. M.; Hock C. (2008) Targeting beta-amyloid pathology in Alzheimer’s disease with Abeta immunotherapy. Neurotherapeutics 5(3), 415–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a LeVine H. (2002) The challenge of inhibiting Abeta polymerization. Curr. Med. Chem. 9(11), 1121–1133. [DOI] [PubMed] [Google Scholar]; b Walsh D. M.; Townsend M.; Podlisny M. B.; Shankar G. M.; Fadeeva J. V.; El- Agnaf O.; Hartley D. M.; Selkoe D. J. (2005) Certain inhibitors of synthetic amyloid beta-peptide (Abeta) fibrillogenesis block oligomerization of natural Abeta and thereby rescue long-term potentiation. J. Neurosci. 25(10), 2455–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goryunov D.; Liem R. K. (2007) CHIP-ping away at tau. J. Clin. Invest. 117(3), 590–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asuni A. A.; Boutajangout A.; Quartermain D.; Sigurdsson E. M. (2007) Immunotherapy targeting pathological tau conformers in a tangle mouse model reduces brain pathology with associated functional improvements. J. Neurosci. 27(34), 9115–9129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringman J. M.; Frautschy S. A.; Cole G. M.; Masterman D. L.; Cummings J. L. (2005) A potential role of the curry spice curcumin in Alzheimer’s disease. Curr. Alzheimer Res. 2(2), 131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Raja K. S.; Balambika R.; Dolai S.; Shi W. (2009) The Concept of a Green Drug, Curcumin and its derivatives as a model-system. Mini-Rev. Org. Chem. 6(2), 152–158. [Google Scholar]; b Cole G. M.; Teter B.; Frautschy S. A. (2007) Neuroprotective effects of curcumin. Adv. Exp. Med. Biol. 595, 197–212. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Shi W.; Dolai S.; Rizk S.; Hussain A.; Tariq H.; Averick S.; L’Amoreaux W.; El ldrissi A.; Banerjee P.; Raja K. (2007) Synthesis of monofunctional curcumin derivatives, clicked curcumin dimer, and a PAMAM dendrimer curcumin conjugate for therapeutic applications. Org. Lett. 9(26), 5461–5464. [DOI] [PubMed] [Google Scholar]
- Strimpakos A. S.; Sharma R. A. (2008) Curcumin: preventive and therapeutic properties in laboratory studies and clinical trials. Antioxid. Redox Signaling 10(3), 511–545. [DOI] [PubMed] [Google Scholar]
- Jagetia G. C.; Aggarwal B. B. (2007) ”Spicing up” of the immune system by curcumin. J. Clin. Immunol. 27(1), 19–35. [DOI] [PubMed] [Google Scholar]
- a Lim G. P.; Chu T.; Yang F.; Beech W.; Frautschy S. A.; Cole G. M. (2001) The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J. Neurosci. 21(21), 8370–8377. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Yang F.; Lim G. P.; Begum A. N.; Ubeda O. J.; Simmons M. R.; Ambegaokar S. S.; Chen P.; Kayed R.; Glabe C. G.; Frautschy S. A.; Cole G. M. (2005) Curcumin Inhibits Formation of Amyloid β Oligomers and Fibrils, Binds Plaques, and Reduces Amyloid in Vivo. J. Biol. Chem. 280(7), 5892–5901. [DOI] [PubMed] [Google Scholar]; c Ma Q. L.; Yang F.; Calon F.; Ubeda O. J.; Hansen J. E.; Weisbart R. H.; Beech W.; Frautschy S. A.; Cole G. M. (2008) P21- activated kinase aberrant activation and translocation in Alzheimer’s disease pathogenesis. J. Biol. Chem. 283(20), 14132–14143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Rojas P. M., Shi W., Davidowitz E. J., Dolai S., Moe J. G., Averick S., and Raja K. S. (2008) Targeting tau oligomers with curcumin derivatives for developing therapeutics for Alzheimer’s disease and other tauopathies. Neuroscience, Abstract # 2008-S-114182- SfN. [Google Scholar]
- Narlawar R.; Pickhardt M.; Leuchtenberger S.; Baumann K.; Krause S.; Dyrks T.; Weggen S.; Mandelkow E.; Schmidt B. (2008) Curcumin-Derived Pyrazoles and Isoxazoles: Swiss Army Knives or Blunt Tools for Alzheimer’s Disease?. ChemMedChem 3(1), 165–172. [DOI] [PubMed] [Google Scholar]
- Porat Y.; Abramowitz A.; Gazit. E. (2006) Inhibition of amyloid fibril formation by polyphenols: structural similarity and aromatic interactions as a common inhibition mechanism. Chem. Biol. Drug Des. 67(1), 27–37. [DOI] [PubMed] [Google Scholar]
- Nakagami Y.; Nishimura S.; Murasugi T.; Kaneko I.; Meguro M.; Marumoto S.; Kogen H.; Koyama K.; Oda. T. (2002) A novel beta-sheet breaker, RS-0406, reverses amyloid beta-induced cytotoxicity and impairment of long-term potentiation in vitro. Br. J. Pharmacol. 137, 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barik A.; Mishra B.; Shen L.; Mohan H.; Kadam R. M.; Dutta S.; Zhang H. Y.; Priyadarsini K. I. (2005) Evaluation of a new copper(II)–curcumin complex as superoxide dismutase mimic and its free radical reactions. Free Radical Biol. Med. 39(6), 811–822. [DOI] [PubMed] [Google Scholar]
- Hung Y. H.; Bush A. I.; Cherny R. A. (2010) Copper in the brain and Alzheimer’s disease. J. Biol. Inorg. Chem. 15(1), 61–76. [DOI] [PubMed] [Google Scholar]
- a Cheng A. L.; Hsu C. H.; Lin J. K.; Hsu M. M.; Ho Y. F.; Shen T. S.; Ko J. Y.; Lin J. T.; Lin B. R.; Wu M. S.; Yu H. S.; Jee S. H.; Chen G. S.; Chen T. M.; Chen C. A.; Lai M. K.; Pu Y. S.; Pan M. H.; Wang Y. J; Tsai C. C.; Hsieh C. Y. (2001) Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 21, 2895–2900. [PubMed] [Google Scholar]; b Anand P.; Kunnumakkara A. B.; Newman R. A.; Aggarwal B. B. (2007) Bioavailability of curcumin: problems andn promises. Mol. Pharmaceutics 4(6), 807–818. [DOI] [PubMed] [Google Scholar]
- Lapchak P. A.; McKim J. M. Jr. (2011) CeeTox Analysis of CNB-001 a Novel Curcumin-Based Neurotrophic/Neuroprotective lead compound to Treat Stroke: Comparison with NXY-059 and Radicut. Transl. Stroke Res. 2(1), 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson K.; Seaton A. (2007) The Janus Faces of Nanoparticles. J. Nanosci. Nanotechnol. 7(12), 4607–4611. [PubMed] [Google Scholar]
- Koziara J. M.; Lockman P. R.; Allen D. D.; Mumper R. J. (2006) The Blood-Brain Barrier and Brain Drug Delivery. J. Nanosci. Nanotechnol. 6(9–10), 2712–2735. [DOI] [PubMed] [Google Scholar]
- Lenhart J. A.; Ling X.; Gandhi R.; Guo T. L.; Gerk P. M.; Brunzell D. H.; Zhang S. (2010) Clicked” Bivalent Ligands Containing Curcumin and Cholesterol As Multifunctional Aβ Oligomerization Inhibitors: Design, Synthesis, and Biological Characterization. J. Med. Chem. 53(16), 6198–6209. [DOI] [PubMed] [Google Scholar]
- Ingolfsson H. I.; Koeppe R. E. II; Andersen O. S. (2007) Curcumin is a Modulator of Bilayer Material Properties. Biochemistry 46(36), 10384–10391. [DOI] [PubMed] [Google Scholar]
- Purkayastha S.; Berliner A.; Fernando S. S.; Ranasinghe B.; Ray I.; Tariq H.; Banerjee P. (2009) Curcumin Blocks Brain Tumor Formation. Brain Res. 1266C, 130–138. [DOI] [PubMed] [Google Scholar]
- von Bergen M.; Friedhoff P.; Biernat J.; Heberle J.; Mandelkow E. M.; Mandelkow E. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 5129–5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson S. A.; Ecroyd H.; Kee T. W.; Carver J. A. (2009) FEBS J. 276, 5960–5972. [DOI] [PubMed] [Google Scholar]
- Jayaprakasha G. K.; Jaganmohan Rao L.; Sakariah K. K. (2006) Antioxidant activities of curcumin, demethoxycurcumin and bisdemethoxycurcumin. Food Chem. 98(4), 720–724. [Google Scholar]
- Pardridges W. M.; Boado R. J.; Farrell C. R. (1990) Brain-type glucose transporter (GLUT-1) is selectively localized to the blood-brain barrier. Studies with quantitative western blotting and in situ hybridization. J. Biol. Chem. 265(29), 18035–18040. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.