Abstract
The ultrastructural details of presynapses formed between artificial substrates of submicrometer silica beads and hippocampal neurons are visualized via cryo-electron microscopy (cryo-EM). The silica beads are derivatized by poly-d-lysine or lipid bilayers. Molecular features known to exist at presynapses are clearly present at these artificial synapses, as visualized by cryo-EM. Key synaptic features such as the membrane contact area at synaptic junctions, the presynaptic bouton containing presynaptic vesicles, as well as microtubular structures can be identified. This is the first report of the direct, label-free observation of ultrastructural details of artificial synapses.
Keywords: Artificial synapses, cryo-EM, electron microscopy, synaptic vesicles, hippocampal neurons
Synapses are specialized cell–cell junctions formed between neuronal cells or between neurons and muscle cells through which they communicate with one another.1 Neuronal synapses are generally formed between axons (presynaptic side) and dendrites (postsynaptic side). In chemical synapses, the presynaptic side releases low molecular weight neurotransmitters upon depolarization of a neuron. These released neurotransmitters are then received at the postsynaptic side via specific postsynaptic receptors. In healthy neurons, the synaptic cleft (the region between the pre- and postsynaptic membranes) is ca. 20–25 nm wide.2 The synaptic cleft allows for efficient diffusion of released neurotransmitters from the presynaptic side to the postsynaptic side of a synapse. Although optical microscopy techniques have been much-used to observe synapse formation and related studies in vitro,3 the high resolution capabilities of electron microscopy are required for observing the molecular details of such structures.4 It has been shown that cryo-electron tomography can be used for morphological characterization of native synapses formed in culture when primary neurons were grown directly on electron microscopy grids.5
Artificial neural network formation has been explored using a number of substrates.6−8 One approach to generate alternative methods to repair damaged nerve terminals is to create some form of functional artificial synapses. We have shown recently that functional presynapses can in fact be formed on surfaces interfaced with either poly-d-lysine (PDL) or cationic bilayer lipid-membranes (SS-BLMs) deposited on micrometer-sized spherical substrates.7,8 While studies to date have focused on the structural and functional properties of artificial synapses, their ultrastructural features are presently not known. Given that molecular level details are clearly interrelated with structural and functional characteristics, such a lack of ultrastructural details diminishes the range of experimental paths to follow.
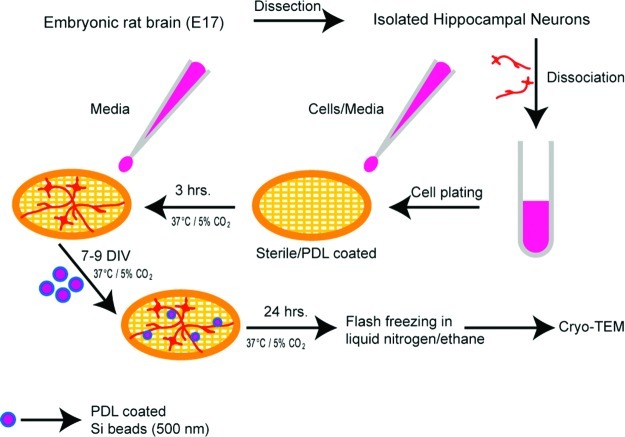
We demonstrate here that in vitro presynapses, when formed on submicrometer sized spherical substrates, can be visualized at nanometer length scales using cryo-electron microscopy (cryo-EM). Cryo-EM is a transmission electron microscopy (TEM) based technique that allows biological samples to be observed in a fully hydrated physiological environment without fixing or staining.9 Although the approach presented here requires some alternative methods in cell culture, the observation is direct and does not require further sample processing steps that can be both tedious and experimentally biasing. This is especially true in the case of artificial synapses formed on silica beads, where thin sectioning of samples using an ultramicrotome would be difficult to achieve. Scheme 1 shows the overall experimental steps involved. In a typical procedure, hippocampal neurons are dissected from embryonic day 17–18 Sprague–Dawley rat embryos and dissociated to a single cell suspension as described previously.10 Neurons were cultured on sterile, poly-l-lysine (PLL)-coated Au/Quantifoil EM grids in Neurobasal medium supplemented with l-glutamine and B27.
Scheme 1. Scheme (not to scale) Illustrating the Experimental Steps.
Isolated hippocampal neurons are plated onto sterile, PLL coated Au/Quantifoil EM grids. These cells are cultured for 7 days or more prior to cryo-EM imaging.
We initially observed that the small number of neurons grown on the EM grids were less healthy when grown alone in the absence of other neurons. Therefore, neurons were grown on separate coverslips in the same dish, along with the EM grids, in order to provide trophic support to the neurons on the grids. The hippocampal neurons were resuspended in Neurobasal culture medium (250 000 cells/mL), and a small drop of this cell suspension was added to the EM grids and coverslips (6 and 80 μL, respectively). Confining the neurons to a small drop of media on the EM grids during the initial plating allows the neurons to specifically attach to the grid surface. After 3–4 h at 37 °C/5% CO2, when the neurons have attached to the substrates, 3–4 mL of Neurobasal culture medium was added to the entire dish and left for 7 or more days in vitro (DIV). The cells are then vitrified by rapidly plunging the grid in ethane slush and kept under liquid nitrogen temperature (−180 °C) until they are ready to image.
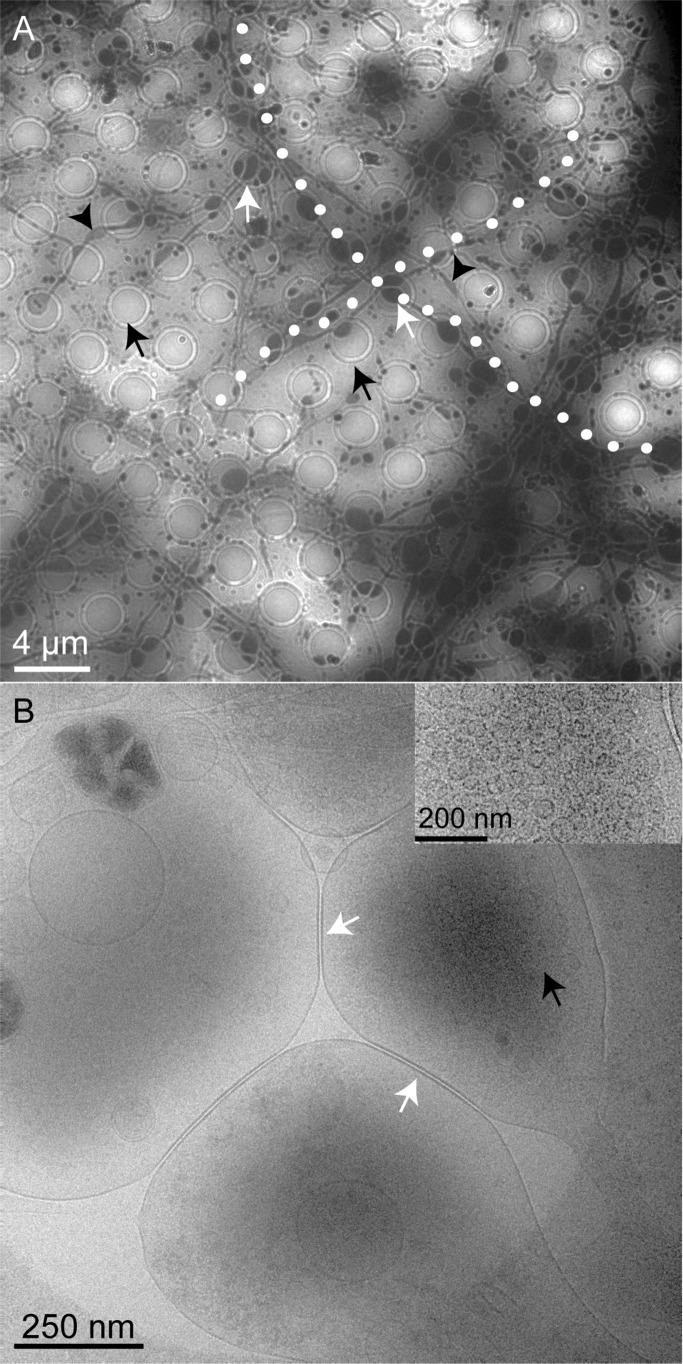
Figure 1A shows a representative low magnification cryo-EM image of a Au/Quantifoil grid containing hippocampal neurons grown for 8 DIV. The circles (black arrows) are 2 μm diameter holes in the EM grid that allow for the cells and the surrounding media to be vitrified. These vitreous films thus provide cells with an environment close to physiological conditions during EM sample preparation. The axons are observed across the grids, growing at very large length scales. The cell morphology appears to look slightly different from that observed on glass coverslips at physiological conditions.7,8 It is important to note that the axons are larger when they grow across a hole (white arrows). We believe that the axons in culture on the Au/Quantifoil support are growing wider mostly on the holes because (i) there is more space available for growth, or (ii) the axons find a more favorable substrate (plastic) underneath the grid while passing through the holes. A slight widening of axons is infrequently observed on the support as well, but this effect is more prominent in the holes as seen in Figure 1A. Figure 1B is a magnified image of one of the holes in the EM grid. Several neuronal processes can be visualized in this area, and the higher electron density represents the areas where synaptic vesicles (black arrows) are present. The inset in Figure 1B shows accumulated synaptic vesicles at a synaptic bouton. It is important to note that the ultrastructural details of the cellular entities are clearly visible in the absence of stain due to the difference in electron scattering of lipids, proteins, and water. Cell bodies or thicker bundles of neuronal processes are too thick to be transparent to the electrons and were not distinguishable from areas of the grids where the ice is too thick (data not shown). Additional images of neurons and neuronal synapses on grids are shown in the Supporting Information (Figures SI.1 and SI.2).
Figure 1.
Representative cryo-EM images showing hippocampal neurons (DIV 8) grown on a sterile, PLL-coated Au/Quantifoil grid. (A) Neuronal processes are visible along the grids, which when on the holes (black arrows) grow a little wider (white arrows) than on the support portion of the grid. The black arrowheads indicate the areas where the axons grow slightly wider outside the holes. Two axonal processes are highlighted (white dots) to indicate the large length scales they are grown on the grid. (B) Magnified view of one of the holes where synapses (white arrows) from closely associated neural processes are visible. High electron density is observed at the areas where synaptic vesicles have accumulated (black arrows). The inset in (B) shows synaptic vesicles observed at the presynaptic boutons.
To observe the artificial synapses using cryo-EM, hippocampal neurons were grown on Au/Quantifoil EM grids as described above. Between 7 to 9 DIV, PDL coated or lipid coated beads (500 nm) were added to the culture. We have tried both 1 μm and 500 nm beads in our cryo-EM experiments and have observed that 500 nm beads were optimal. In the case of 1 μm beads, the vitreous films formed were too thick, which resulted in poor quality images. After 24 h of incubation of beads with the cells (Scheme 1), the grids were flash frozen in ethane slush and kept under liquid nitrogen until ready to image.
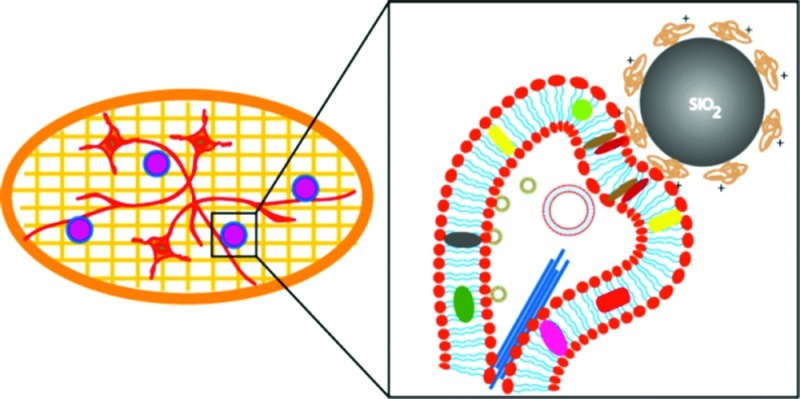
Figure 2A shows a representative cryo-EM image of a presynapse formed between hippocampal neurons and PDL coated beads. The presynaptic bouton seen in this image is formed when a growing axon extends onto a bead and forms a synapse, which resembles known structures of native synapses.4 In this case, the PDL coated bead serves as the postsynaptic side of a native synapse. The membrane contact area (white arrow) looks slightly thicker compared to the rest of the membrane around the presynaptic bouton (black arrows). This is consistent with the increased presence of adhesion molecules and specific protein accumulation on membranes at the synaptic junctions. This corresponds to features observed in native synapses when imaged using conventional TEM.4 Microtubules (white arrowheads) following the direction of axonal growth are clearly seen in this image. The image in Figure 2A, however, shows only a few presynaptic vesicles visible (black arrowheads). This could be attributed to the specific conditions used in cryo-EM imaging and/or the thickness of the sample. Small membrane structures such as synaptic vesicles are usually better visualized using different imaging parameters, as opposed to large membrane structures such as a synaptic bouton. This attributes to the difficulty in visualizing such entities simultaneously in a single image. The beads that are not in contact with any neuronal cells and thus did not take part in synapse formation are also seen in the same image. In general, we have observed an average of five to seven beads per square mesh (200 μm) on an EM grid that form synapses upon contact with axonal processes. One to two beads per 200 μm square mesh do not however form stable synapses. It is important to note that the concentration of beads that could be added to the culture is limiting, as higher concentrations of beads were found to be toxic to the cells. Figure 2B is a sketch depicting the cellular organization of different synaptic elements at the presynaptic bouton observed in Figure 2A.
Figure 2.
Representative cryo-EM image (A) showing an artificial synapse formed between hippocampal neurons (DIV 8) grown on a sterile, PLL coated Au/Quantifoil grid and poly-d-lysine coated 500 nm silica beads. Microtubular networks (white arrow heads) are also visible. Similarly coated beads that are not in contact with neurons are also visible in the image. Few synaptic vesicles (black arrowheads) are also visible. White arrow indicates the contact area at the synaptic junction, which in this case is between an axon and a bead. (B) Simplified sketch (not to scale) depicting the structural components of the artificial synapse seen in (A).
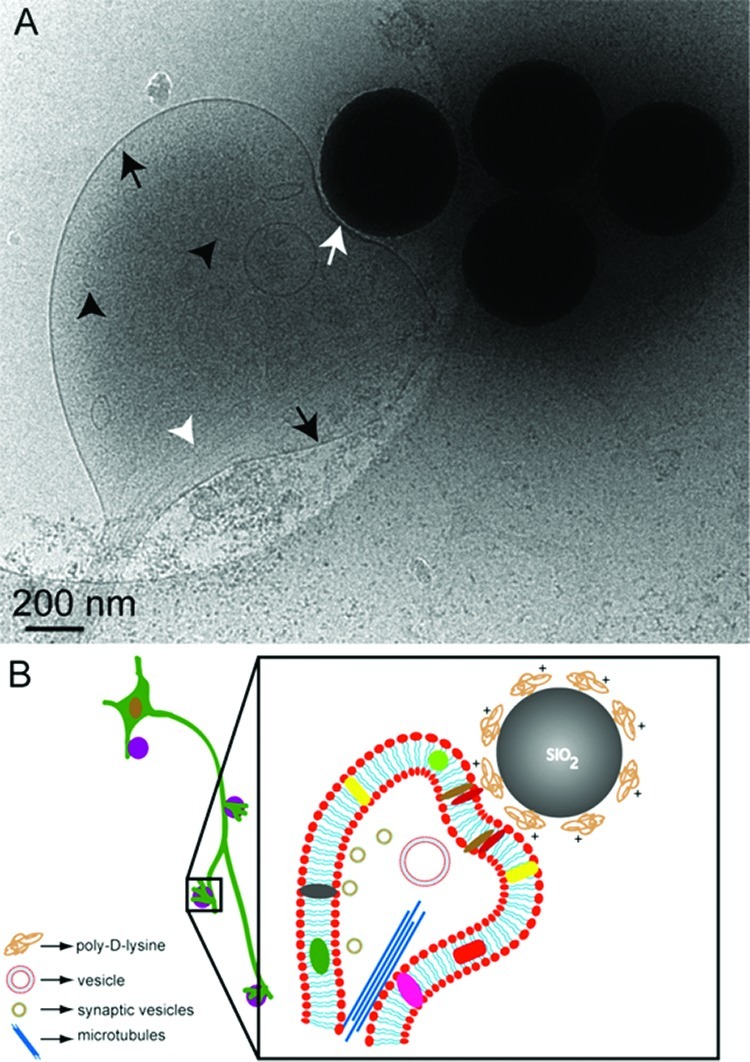
The presynaptic vesicle pool is clearly visible in Figure 3A (white arrow heads) compared to the synaptic bouton shown in Figure 2A. The majority of these vesicles are in the range of 30 nm, which is in good agreement with the size of synaptic vesicles. However, some larger vesicles (∼100 nm) are also seen (black arrowheads) that could be attributed to transport vesicles. The presence of a thicker membrane at the synaptic junction as well as microtubular networks is also evident in this image. Black arrows show the axon shaft of the neuronal process from which the synaptic bouton is extended onto the bead in culture. As seen in this image, microtubules seem to follow the path of the axonal shaft. Figure 3B is the corresponding sketch depicting the cellular level organization of the synaptic elements visible in Figure 3A. It is interesting to note that mitochondria are also clearly visible at the synaptic boutons as well as along the axons (Supporting Information Figure SI.3). Cryo-EM based studies might thus be very useful for research involving such intracellular organelles, for example, following morphological changes in mitochondria in dysfunctional mitochondrial related diseases.11
Figure 3.
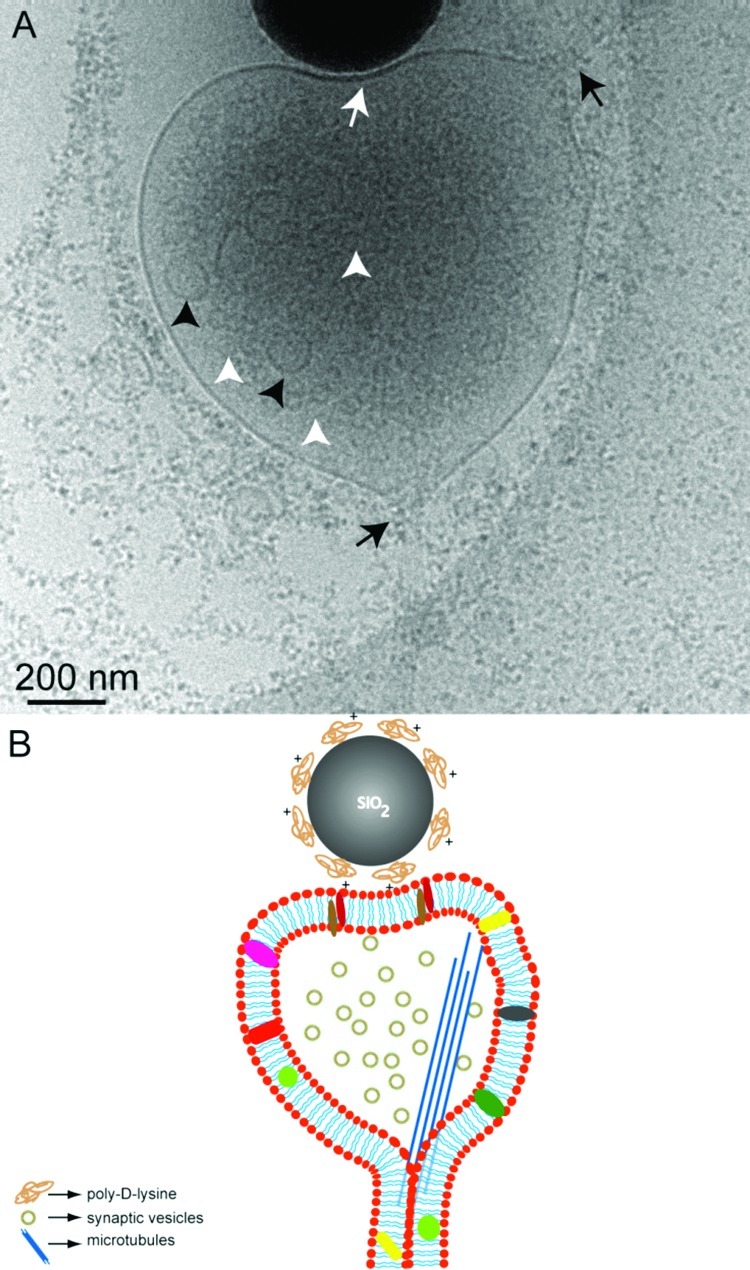
Representative cryo-EM image (A) showing an artificial synapse formed between hippocampal neurons (DIV 8) grown on a sterile, PLL coated Au/Quantifoil grid and poly-d-lysine coated 500 nm silica bead. Only part of the bead is seen in this image. The white arrow indicates the contact area at the synaptic junction. Synaptic vesicles are more clearly visualized (white arrowheads) in this image as compared to Figure 1. The larger vesicles (black arrowheads) could be the transport vesicles. Black arrows show the direction of the axonal shaft growth. (B) Simplified sketch (not to scale) depicting the structural components of the artificial synapse seen in (A).
We and others have previously demonstrated the formation of artificial synapses on different substrates. Both planar and spherical substrates have been employed in these studies.12,6−8 Spherical substrates are a powerful tool for interrogating and interfacing with cells. The formation of synapses on artificial substrates such as planar lipid bilayers requires that the cells either grow directly on these substrates or are grown separately in culture and added to the substrates at the appropriate time. This is extremely difficult to achieve with many adherent cells, in particular, neurons, which must be grown in culture for at least 1 week before they are capable of forming synapses. Also, once mature, neurons cannot be detached from their original growth substrate and placed onto another substrate. Thus, spherical substrates, which can be modified as desired and then added to the cells at a specific point in time, allow cells to interface with artificial substrates at any time, without disturbing the growth environment of the cells.7,8 Spherical substrates provide enhanced imaging possibilities as well as the opportunity for applying other characterization techniques. This is especially true when functionality studies were performed on artificial presynaptic boutons in culture and in isolated forms.7,13 Importantly, spherical substrates also provide the advantage of accessing different radii of curvature simply by using different size beads.14
The design of substrates for artificial synapse formation is of great interest both in applied and in fundamental aspects of in vitro studies involving synaptogenesis. However, studies to date have focused mainly on functional similarities between native and artificial synapses and thus often have not pursued the direct visualization of the structural details. Maintaining the surface chemistry constant on smaller size silica beads allows one to perform label-free visualization of artificial synapses using cryo-EM. The membrane contact area, synaptic vesicle pool, as well as microtubular networks that are essential components at a functional presynapse are clearly visible, showing the validity of our interpretation.
In summary, a cryo-EM approach has been utilized here for direct visualization of artificial synapses formed on different substrates. This is the first report to date that establishes the ultrastructural details of a chemically induced artificial synapse. Given the considerable interest in identifying structural and functional requirements for neuroregenerative approaches, this work confirms the structural details of artificial synapses.
Methods
Primary Cultures of Rat Hippocampal Neurons on EM grids
Hippocampal neurons were dissected from Sprague–Dawley rat embryos (embryonic day 17–18) and dissociated to a single cell suspension using a modification of a protocol described previously by Kaech and Banker.10 Neurons were cultured on sterile, PLL-coated Au/Quantifoil EM grids (Electron Microscopy Sciences, Hatfield, PA) together with sterile, PLL-coated coverslips in Neurobasal medium, as described below. Prior to use, the EM grids were immersed in ethanol for 15 min and washed several times using Milli-Q water. They were then cleaned with air plasma in a plasma cleaner (Harrick Plasma, Ithaca, NY) in order to remove any remaining contaminants and to make them more hydrophilic. The dissociated hippocampal neurons were resuspended in Neurobasal culture medium supplemented with l-glutamine and B27 at a density of 250 000 cells/mL, and a small drop of this cell suspension was added in a Petri dish containing EM grids and coverslips (6 and 80 μL, respectively). After 3–4 h in the incubator at 37 °C/5% CO2, 3–4 mL of Neurobasal culture medium was added to the entire dish and the dish was returned to the incubator and kept for 7 or more days in vitro (DIV) prior to imaging, while replacing one-third of the medium every 3–4 days. All culture media were purchased from Gibco (Invitrogen). All animal work was performed in accordance with the Canadian Council on Animal Care Guidelines.
Preparation of Poly-d-lysine Coated Beads
Silica beads of 500 nm (Bangs Laboratories) were diluted to a concentration of 3 × 106 particles/mL in PBS, washed twice in PBS by centrifugation, resuspended, and then incubated in 1 mL of PBS containing 0.05 mg/mL poly-d-lysine overnight at 4 °C. The poly-d-lysine treated beads were then washed several times in PBS by centrifugation.
Coculture with Silica Beads
A total of 5 μL of the poly-d-lysine coated silica bead solution (sterile PBS, pH 7.4) was added to the neural culture dish directly on top of the TEM grids. The bead/cell coculture was returned to the incubator and left at 37 °C/5% CO2 for 24 h prior to freezing of the sample.
Cryo-EM
The grids were taken directly from the incubator and were frozen immediately. A total of 5 μL solution of neural culture media was added to the EM grid held by tweezers, blotted, and then frozen hydrated by plunging into a bath of liquid ethane slush.9 The frozen TEM grids were stored at −180 °C until ready to image. A 626 Single Tilt Cryotransfer System (Gatan Inc.) was used to transfer the EM-grids, which were observed with a FEI G2 F20 cryo-STEM microscope operated at 200 kV (FEI, Inc.). Images were recorded under low dose conditions on a Gatan Ultrascan 4k × 4k digital (CCD) camera system camera.
Supporting Information Available
Additional cryo-EM images. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
G.G., D.R.C., and R.B.L. conceptually designed the study. G.G., P.T.Y., and I.R. designed and performed the experiments. C.M. and M.B. supported the experiments. G.G., P.T.Y., and R.B.L. wrote the manuscript, and all the authors contributed to the editing of the manuscript.
Author Status
⊥ Deceased June 1, 2011.
Dedication
This work is dedicated to the memory of Dr. David R. Colman (1949–2011).
The research was supported by a grant from the Regenerative Medicine and Nanomedicine Initiative of the Canadian Institutes of Health Research (CIHR) to D.R.C. and R.B.L. The NeuroEngineering Program of McGill University is also supported by grants from Rio Tinto Alcan and the Molson Foundation. This work was also supported by grants from NSERC (R.B.L.), FQRNT (R.B.L.), and CIHR #MOP-86693 (I.R.). I.R. is recipient of a CIHR New Investigator Award.
Supplementary Material
References
- a Cowan W. M., Südhof T. C., and Stevens C. F., Eds. (2001) Synapses, Johns Hopkins University Press: Baltimore. [Google Scholar]; b Dustin M. L.; Colman D. R. (2002) Neural and Immunological Synaptic Relations. Science 298, 785–789. [DOI] [PubMed] [Google Scholar]; c Bury L. A. D.; Sabo S. L. (2010) Coordinated trafficking of synaptic vesicle and active zone proteins prior to synapse formation. Mol. Interventions 10, 282–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Agnati L. F.; Zoli M.; Stromberg I.; Fuxe K. (1995) Intercellular communication in the brain: wiring versus volume transmission. Neuroscience 69, 711–726. [DOI] [PubMed] [Google Scholar]; b Clemens J. D. (1996) Transmitter timecourse in the synaptic cleft: Its role in central synaptic function. Trends Neurosci. 19, 163–171. [DOI] [PubMed] [Google Scholar]; c Savtchenko L. P.; Rusakov D. A. (2007) The optimal height of the synaptic cleft. Proc. Natl. Acad. Sci. U.S.A. 104, 1823–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Fletcher T. L.; Decamilli P.; Banker G. J. (1994) Synaptogenesis in hippocampal cultures: evidence indicating that axons and dendrites become competent to form synapses at different stages of neuronal development. Neurosci. 14, 6695–6706. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Jontes J. D.; Buchanan J.; Smith S. J. (2000) Growth cone and dendrite dynamics in zebrafish embryos: early events in synaptogenesis imaged in vivo. Nat. Neurosci. 3, 231–237. [DOI] [PubMed] [Google Scholar]; c Fuger P.; Behrends L. B.; Mertel S.; Sigrist S. J.; Rasse T. M. (2007) Live imaging of synapse development and measuring protein dynamics using two-color fluorescence recovery after photo-bleaching at Drosophila synapses. Nat. Protoc. 2, 3285–3298. [DOI] [PubMed] [Google Scholar]; d Di Biase V.; Obermair G. J.; Flucher B. E. (2009) Resolving sub-synaptic compartments with double immunofluorescence labeling in hippocampal neurons. J. Neurosci. Methods 176, 78–84. [DOI] [PubMed] [Google Scholar]
- a Rostaing P.; Real E.; Siksou L.; Lechaire J. P.; Boudier T.; Boeckers T. M.; Gertler F.; Gundelfinger E. D.; Triller A.; Marty S. (2006) Analysis of synaptic ultrastructure without fixative using high-pressure freezing and tomography. Eur. J. Neurosci. 24, 3463–3474. [DOI] [PubMed] [Google Scholar]; b Leitch B. (1992) Ultrastructure of electrical synapses: Review. Electron Microsc. Rev. 5, 311–339. [DOI] [PubMed] [Google Scholar]; c Staehelin L. (1974) Structure and function of intercel- lular junctions, A. Int. Rev. Cytol. 39, 191–283. [DOI] [PubMed] [Google Scholar]
- a Fernandez-Busnadiego R.; Zuber B.; Maurer U. E.; Cyrklaff M.; Baumeister W.; Lucic V. (2010) Quantitative analysis of the native presynaptic cytomatrix by cryoelectron tomography. J. Cell Biol. 188, 145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Lucic V.; Yang T.; Schweikert G.; Forster F.; Baumeister W. (2005) Morphological characterization of molecular complexes present in the synaptic cleft. Structure 13, 423–434. [DOI] [PubMed] [Google Scholar]
- a Burry R. W. (1986) Presynaptic elements on artificial surfaces. A model for the study of development and regeneration of synapses. Neurochem. Pathol. 5, 345–360. [DOI] [PubMed] [Google Scholar]; b Blau A.; Weini C.; Mack J.; Kienle S.; Jung G.; Ziegler C. (2001) Promotion of neural cell adhesion by electrochemically generated and functionalized polymer films. J. Neurosci. Methods 112, 65–73. [DOI] [PubMed] [Google Scholar]; c Xiong Y.; Zeng Y. S.; Zeng C. G.; Du B. L.; He L. M.; Quan D. P.; Zhang W.; Wang J. M.; Wu J. L.; Li Y.; Li J. (2009) Synaptic transmission of neural stem cells seeded in 3-dimensional PLGA scaffolds. J. Biomater. 30, 3711–3722. [DOI] [PubMed] [Google Scholar]
- Lucido A. L.; Sanchez F. S.; Thostrup P.; Kwiatkowski A. V.; Leal-Ortiz S.; Gopalakrishnan G.; Liazoghli D.; Belkaid W.; Lennox R. B.; Grutter P.; Garner C. C.; Colman D. R. (2009) Rapid assembly of functional presynaptic boutons triggered by adhesive contacts. J. Neurosci. 29, 12449–12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishnan G.; Thostrup P.; Lucido A. L.; Belkaid W.; Rouiller I.; Colman D. R.; Lennox R. B. (2010) Lipid bilayer membrane-triggered presynaptic vesicle assembly. ACS Chem. Neurosci. 1, 86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubochet J.; Adrian M.; Chang J.-J.; Homo J.-C.; Lepault J.; McDowall A. W.; Schultz P. (1988) Cryo-electron microscopy of vitrified specimens. Q. Rev. Biophys. 21, 129–228. [DOI] [PubMed] [Google Scholar]
- Kaech S.; Banker G. (2006) Culturing hippocampal neurons. Nat. Protoc. 1, 2406–2415. [DOI] [PubMed] [Google Scholar]
- a Zhao J.; Liu T.; Jin S.; Wang X.; Qu M.; Uhlén P.; Tomilin N.; Shupliakov O.; Lendahl U.; Nistér M. (2011) Human MIEF1 recruits Drp1 to mitochondrial outer membranes and promotes mitochondrial fusion rather than fission. EMBO J. 30, 2762–2778. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Song W.; Chen J.; Petrilli A.; Liot G.; Klinglmayr E.; Zhou Y.; Poquiz P.; Tjong J.; Pouladi M. A.; Hayden M. R.; Masliah E.; Ellisman M.; Rouiller I.; Schwarzenbacher R.; Bossy B.; Perkins G.; Bossy-Wetzel E. (2011) Mutant huntingtin binds the mitochondrial fission GTPase dynamin-related protein-1 and increase its enzymatic activity. Nat. Med. 17, 377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossman K.; Groves J. (2007) Chem. Soc. Rev. 36, 46–54. [DOI] [PubMed] [Google Scholar]; Pautot S.; Lee H.; Isacoff E. Y.; Groves J. T. (2005) Nat. Chem. Biol. 1, 283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]; Baksh M. M.; Dean C.; Pautot S.; DeMaria S.; Isacoff E.; Groves J. T. (2005) Langmuir 21, 10693–10698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucido A. L.; Gopalakrishnan G.; Yam P. T.; Colman D. R.; Lennox R. B. (2010) Isolation of functional presynaptic complexes from CNS neurons: A cell-free preparation for the study of presynaptic compartments in vitro. ACS Chem. Neurosci. 1, 535–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishnan G.; Rouiller I.; Colman D. R.; Lennox R. B. (2009) Supported bilayers formed from different phospholipids on spherical silica substrates. Langmuir 25, 5455–5458. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.