Abstract
The most known fullerenes are spherical carbon compounds composed of 60 carbon atoms. C60 fullerenes have shown biochemical and biomedical properties in the last years such as as blockade of apoptosis and neuroprotection. The nucleoside adenosine has a neuroprotective role mainly due to inhibition of glutamate release, which is a neurotransmitter related to excitotoxicity and cell death. In the present work, we have determined the presence of adenosine receptors in SK-N-MC cells, a neuroepithelioma human cell line, and analyzed the effect of fullerenes in these receptors by using radioligand binding, immunoblotting, and quantitative real time PCR assays. Results demonstrated that SK-N-MC cells endogenously express adenosine receptors. Fullerene exposure of these cells did not affect cell viability measured by MTT reduction assay. However, adenosine A1 and A2A receptors were both increased in SK-N-MC cells after treatment. These results suggest for the first time the modulation of adenosine receptors after C60 fullerenes exposure.
Keywords: Adenosine receptor, fullerene, SK-N-MC cells
From its discovery in 1985 by Curl, Kroto, Heath, O’Brien, and Smalley, C60 fullerenes have been extensively used in organic chemistry in the recent years, mainly due to their chemical properties and potential applications in many science fields.1 Furthermore, in the past decade, a lot of experimental evidence has supported the use of C60 for biomedical and biochemical purposes.2 Some biological properties of C60 derivatives have been investigated such as studies in photocleavage of DNA, neuroprotection of cells, blockade of programmed cell death,3,4 and also as radical scavenger in induced oxidative stress.5,6 In this sense, it has been shown that fullerenes acting as free radical scavengers7 reduce excitotoxic and apoptotic death of cultured cortical neurons exposed to glutamate, a neurotransmitter which can also acts as an excitotoxin.8 The greatest difficulty in testing the activity of C60 is its poor solubility in aqueous systems, due to apolar characteristics of its carbon cage;9 to overcome this inconvenience, a new series of hydrosoluble C60 bis adducts have been synthesized and tested on the HIV virus with promising results10 and their hemolysis properties were also tested.11
As extensively reported, there is much evidence about the role of adenosine receptors in neurodegenerative process of healthy neurons in the human brain12 (for a review, see ref (13)). Adenosine receptors are G-protein coupled receptors which have been classified in A1, A2A, A2B, and A3 receptors. A1 and A3 receptors inhibit adenylyl cyclase (AC) through Gi/o proteins, and A2A and A2B receptors activate AC through Gs proteins.14,15 Adenosine receptors participate in many physiological responses such as as inflammatory processes, ischemia, and hypoxia,16−18 in which they seem to have a protective role. These receptors are modulated in several neurodegenerative diseases, for instance, the up-regulation of A1 receptors in Pick’s disease19 and Alzheimer’s disease post-mortem brains,20 where metabotropic glutamate receptors are also impaired.21,22
The aim of the present work was to study the activity of the C60 fullerene hydrosoluble bis-adduct isomer trans-3 (T3SS) on the expression of adenosine receptors in an in vitro model of tumoral SK-N-MC cells derived from human neuroepithelioma and its toxic effect, if any, on these cells.
Results
Synthesis of C60 Fullerene Hydrosoluble bis-Adduct Isomer trans-3
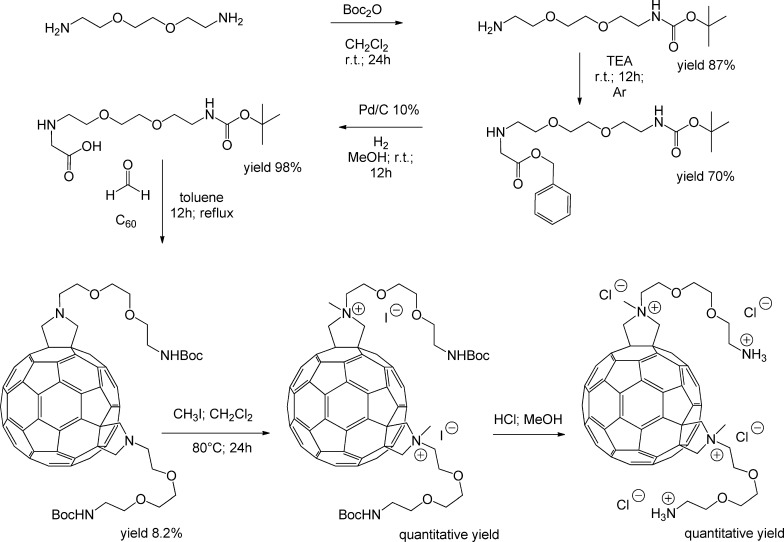
The trans-3 isomer C60 fullerene bis-adduct derivative (T3SS) was obtained as described previously.10 The diagram of the synthesis is represented in Figure 1.
Figure 1.
Scheme for the synthesis of trans-3 isomer [60]fullerene bis-adduct derivative (for more details and procedures, see ref (10)).
SK-N-MC Cells Endogenously Express Adenosine Receptors
We first evaluated which adenosine receptors are naturally expressed by these cells. Thus, Western blotting assays on SK-N-MC cells using isolated plasma membrane preparations revealed the presence of A1, A2A, and A2B subtypes of adenosine receptors (Figure 2A). The presence of adenosine A2A receptors was confirmed by immunofluorescence in intact cells (Figure 2B).
Figure 2.
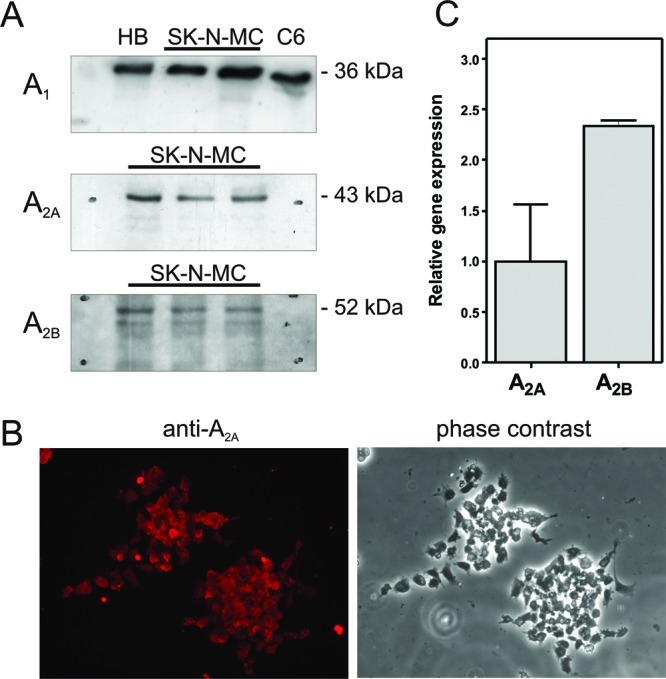
Adenosine receptors presence in SK-N-MC cells. (A) Adenosine receptors A1, A2A, and A2B were specifically detected by Western-blotting, as stated in the Methods section, in plasma membranes from human brain (HB), C6 glioma cells (C6), and SK-N-MC cells (SK-N-MC). (B) Representative image showing A2A receptors in intact cells by immunocytochemistry. (C) Relative expression levels of A2A and A2B receptors in SK-N-MC cells.
In order to determine the relative gene expression of these adenosine receptors, we analyzed the RNA obtained from these cells by using real time PCR technique, which revealed the presence of the specific gene coding for adenosine receptor types A2A and A2B (Figure 2C). However, we were unable to detect the expression of the gene coding for A1 receptors in the same conditions, even with different specific oligonucleotides for A1 mRNA (TaqMan assays Hs00181231_m1 and Hs00379752_m1).
Effect of Fullerene Treatment on SK-N-MC
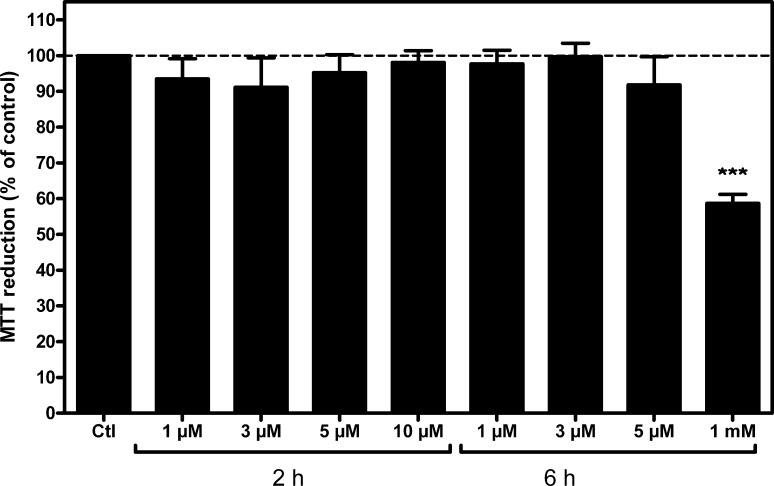
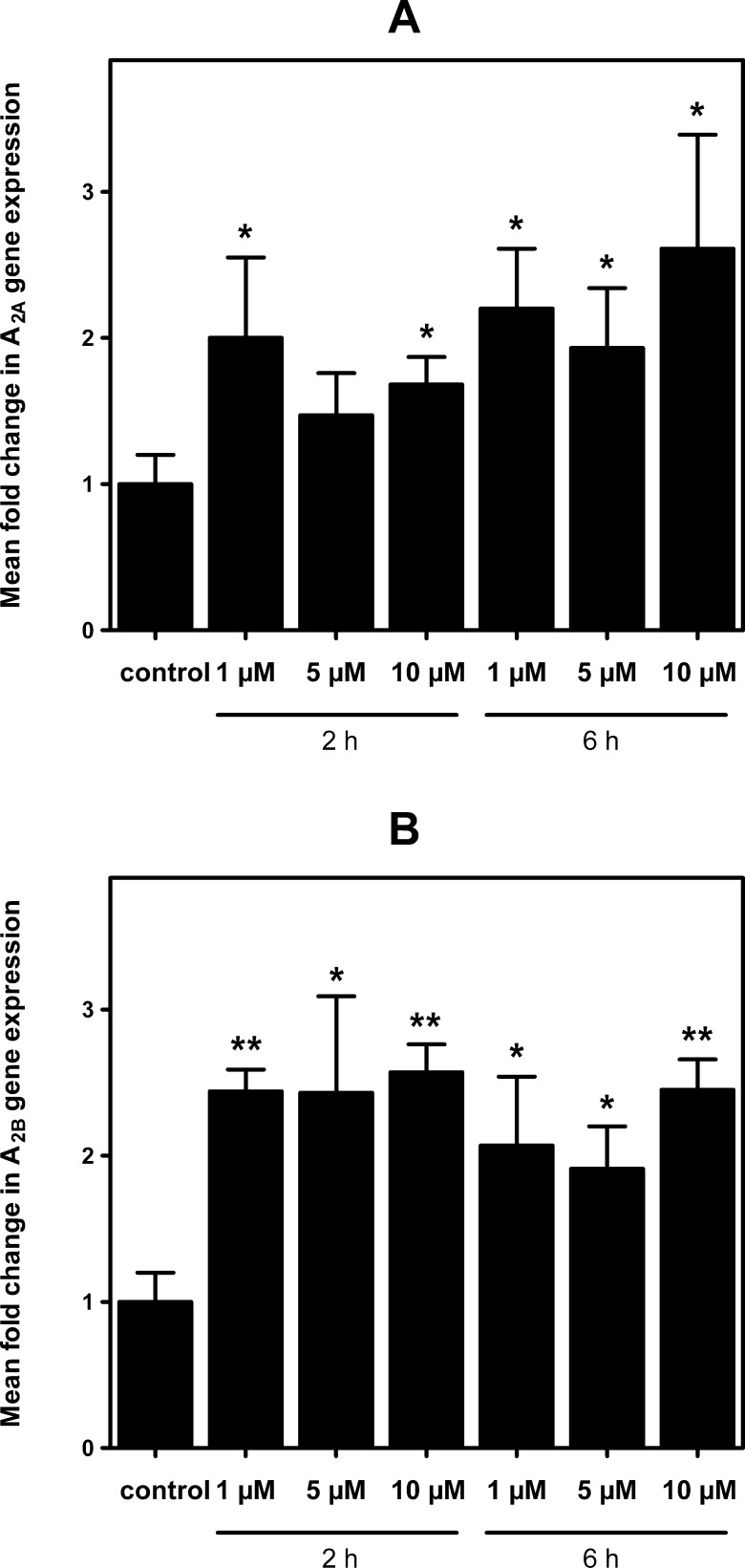
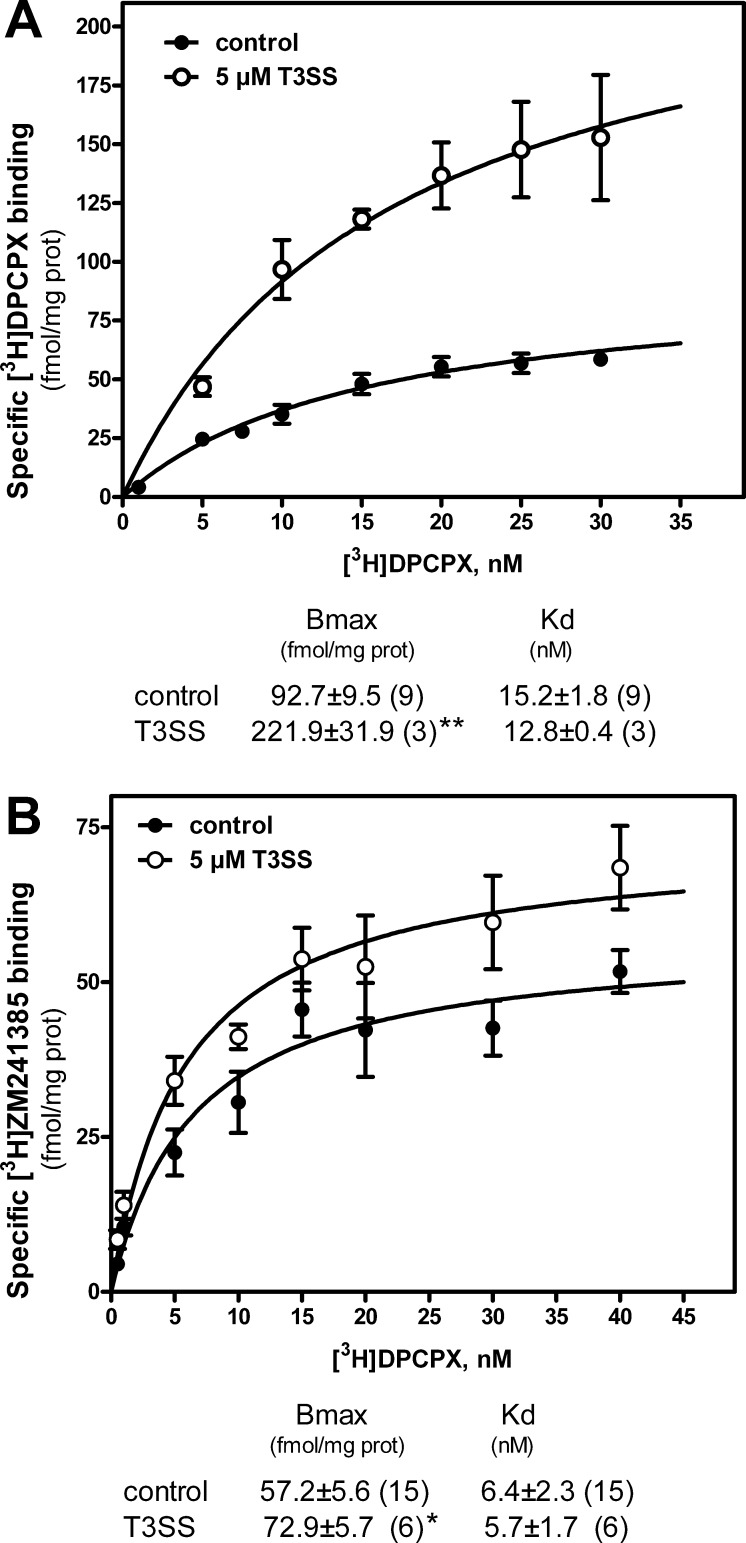
In order to study the effect that trans-3 derivative of C60 fullerene (T3SS) may have on these cells, we first evaluated T3SS effect on cell viability at different concentrations and time of exposure (Figure 3). Results show no toxicity for used concentration and observed times of exposure, except at 1 mM for 6 h; this concentration represents only a limit out of the range of acceptable concentrations usually used, and it has been tested only to establish the minimum toxic dose of T3SS for these cells. Subsequently, it was analyzed the possible variation in the expression of genes coding for adenosine receptors under different concentrations of T3SS (Figure 4). These results show a significant increase in adenosine A2A and A2B receptor gene expression in treated cells. Although we were unable to amplify A1 receptor gene transcripts in these cells by real time PCR assays, A1 receptor protein was measured by radioligand binding assay performed in intact cells. As Figure 5 shows, T3SS treatment, at the concentration of 5 μM, promotes an increase in the specific binding to both A1 (Figure 5A) and A2A (Figure 5B) receptors at the cell surface of intact cells after 6 h of 5 μM T3SS treatment.
Figure 3.
Evaluation of fullerene derivative effect on cell viability. SK-N-MC cells were exposed to fullerene derivative T3SS for the indicated time and dose, and cell viability was measured by the MTT reduction method, as stated in the Methods section. ***, p < 0.001 significantly different from control (Ctl) value.
Figure 4.
Effect of fullerene derivative on adenosine A2 gene expression. Real time PCR assays were performed at indicated times and dose of fullerene derivative treatment, using specific oligonucleotides for A2A (panel A) and A2B (panel B) mRNA. Mean fold change calculation as well as specific set of primers are indicated in the Methods section. Data are mean ± SEM values obtained from 3–5 independent experiments performed in duplicate. *, p < 0.05 and **, p < 0.01 significantly different from control values.
Figure 5.
Effect of T3SS treatment on adenosine receptor specific binding. After preincubation with adenosine deaminase in order to remove endogenous adenosine, control and 5 μM T3SS-treated (6 h) SK-N-MC intact cells were incubated with different concentrations of [3H]DPCPX or [3H]ZM241385 to measure specific binding to A1 (panel A) or A2A (panel B) receptors. Nonspecific binding was determined in the presence of CPA and theophylline, as stated in the Methods section. Data are mean ± SEM values obtained from n (in brackets) separate experiments carried out in triplicate. Inset shows Bmax and Kd values for control and treated cells. *, p < 0.05 and **, p < 0.01 significantly different from control values.
Discussion
In the last years, our group has focused on the study of mechanisms of neurodegenerative/neuroprotective processes, from the point of view of neurotransmitter receptors involved, mainly adenosine and glutamate receptors. The aim of the present work was to study the effect of the hydrosoluble T3SS fullerene derivative on cell viability and on adenosine receptors in SK-N-MC cells endogenously expressing these receptors.
A major concern in the potential use of fullerenes is their safety. Nanomaterial cytotoxicity in cells varies with chemical characteristics and surface properties of the molecule, including hydrophobicity, hydrophilicity, and surface area per molecule.11,23 Mori et al.24 could not find toxicity in rodents for C60 and C70 mixtures after oral administration of a dose of 2000 mg/kg BW and did not observe evidence of genotoxic or mutagenic potential in vitro. Moreover, the clearance kinetics of fullerene C60 nanoparticles from rat lungs, liver, and brain after intratracheal C60 instillation and inhalation C60 exposure has been analyzed. Pulmonary C60 burden decreased with time and depended on the C60 concentration administered, while the concentration of C60 in the liver and brain was below 8.9 ng/g tissue, the detection limit of the method employed.25 A comprehensive review on fullerene toxicity is given by Kolosnjaj et al.26 These authors review the works on fullerene toxicity and conclude that very little evidence gathered since the discovery of fullerenes indicates that C60 is toxic. Furthermore, it has been demonstrated that unmodified C60 fullerenes are not toxic to cells and therefore could be useful for several biological applications.27 However, the poor solubility of C60 fullerene in aqueous systems impedes its analysis for biochemical applications. The most versatile methodology to overcome this problem is based on the chemical modification of the sphere that leads to a wide variety of C60 derivatives, possessing different physical and chemical properties.28 Thus, various functionalizations have been utilized both to increase the hydrophilicity (e.g., −OH, −COOH, −NH2) and to prepare new compounds presenting biological and pharmacological activity; some examples are inhibition of HIV proteases and other enzymes, antimicrobial and antiapoptotic activities, DNA photocleavage, and its role as radical scavenger.3 A new series of hydrosoluble C60 bis-adducted derivatives have been synthesized and tested on HIV virus with promising results.10 Between them, trans-3 isomer (T3SS) has been used in the present work.
As mentioned above, a major concern in the potential use of fullerenes is their safety. Available data shows that pristine C60 has no acute or subacute toxicity in a large variety of living organisms, from bacterial and fungal to human leukocytes, and also in drosophila, mice, rats, and guinea pigs. However, some C60 derivatives can be highly toxic.26 No significant cell viability loss has been detected in our SK-N-MC cell cultures at doses equal or less than 10 μM T3SS; even at 1 mM, which largely exceeds any therapeutic dose, viability loss was 40% (p < 0.001).
Results reported herein suggest a new approach in the study of biological properties of [60] fullerene derivatives and the investigation of their possible neuroprotective activity. It has been previously described that hydrosoluble fullerenes, such as fullerenols, inhibit ionotropic glutamate receptor binding, lowering the intracellular calcium, acting therefore as neuronal protective molecules.29 Adenosine A1 and A2 receptors modulate neuronal and synaptic function in a range of ways that may make them relevant to the occurrence, development, and treatment of brain ischemic damage and degenerative disorder receptors.30 These receptors are endogenously expressed in SK-N-MC cells, as here reported, and other proliferating cell lines such as as C6 glioma,31 28A,32 and DDT1MF-2.33 SK-N-MC cells present RNA transcripts of genes coding for adenosine A2A and A2B receptors subtypes. However, the corresponding mRNA for A1 was undetectable in our conditions. A very recent study demonstrates that real-time polymerase chain reaction of the plant gene heat shock transcription factor was fully inhibited in the presence of a fullerene derivative C60(OH)20 possibly by the clear tendency for hydrogen bonding between the nanoparticle and both the dNTPs and ssDNA components of the polymerase chain reaction.34 As experimental conditions were the same for both A2 and A1 receptor mRNA analysis, a direct effect of nanoparticle presence during PCR reaction can be ruled out. Then one possible explanation could be a very low rate of A1 gene expression in these cells. In fact, this is, to our knowledge, the first study reporting adenosine receptor presence in SK-N-MC cells. Adenosine A1 receptors were, at least, detected by radioligand binding assay and Western blotting and found to be abundant on the cell surface, even higher than A2A levels, as Bmax values from saturation data suggest.
Treatment of SK-N-MC cells using a single nontoxic concentration of C60 bis-adduct hydrosoluble derivative (T3SS) led to an increase in A2A and A2B receptor mRNA expression and higher A1 and A2A protein levels. It is usually considered that prolonged agonist exposure of G-protein coupled receptors results in a progressive loss of receptor (down-regulation), whereas antagonist exposure causes increased receptor level (up-regulation35). Most studies of the effects of antagonist treatment report an increase in total receptor number as an adaptive response to high antagonist exposure of receptor. Hydrosoluble fullerenes, such as fullerenols, inhibit binding to ionotropic glutamate receptors. Furthermore, AMPA receptors were found to be more sensitive to fullerenols than NMDA and KA receptors.29 This antagonist-like role of fullerene could affect the adenosine receptor level in a similar way to that detected after antagonist treatment. In the case of adenosine A1 receptors, up-regulation after chronic caffeine (36,37) and theophylline38,39 treatment has been reported. However, previous experiments (data not shown) demonstrated that T3SS is unable to act as an A1 receptor antagonist in radioligand binding assays, even at a range of high concentrations, including around 10 mM; T3SS cannot substitute CPA in the unspecific binding determination. This result excludes that T3SS may occupy the specific binding site of A1, suggesting another way to regulate these receptors. In line with this, other C60 fullerene reported effects are a reduced contractile response for acetylcholine, histamine, and 5-HT in muscle of guinea pig and rat which may be due to a change in postreceptor processes.40 Recent studies on the mechanisms involved in the regulation of adenosine receptor expression have shed light on the participation of transcription factors, such as nuclear factor-kappa B (NFκB), hypoxic-inducible factor 1, and cAMP-responsive element binding protein (CREB; for a review see ref (41)). Thus, reactive oxygen species (ROS) can increase the expression of the A1 receptor by activating NF kappa B regulatory site(s) on this gene and thereby enhance the cytoprotective role of adenosine.42 Interestingly, the protective effect elicited by the hydrophilic fullerene derivative C60(C(COOH)2)2 against H2O2 toxicity in cerebral microvessel endothelial cells (CMECs) seems to be mediated by a decreased phosphorylation of JNK and inhibition of ROS production. Consequently, C60(C(COOH)2)2 treatment could regulate several downstream signaling events, including reduced activation of transcription factor c-Jun and caspase-3 and inhibition of PARP cleavage and mitochondrial cytochrome c release.43
Effector mechanisms other than the classical adenylate cyclase and phospholipase C mediated pathways are associated with the stimulation of adenosine receptors. For example, adenosine action can activate phosphoinositide 3-kinase (PI3K), mitogen-activated protein kinases (MAPKs), and extracellular receptor signal-induced kinase (ERK).44 It is well-known the cross-talking between the two types of adenosine receptors studied here, including the formation of heterodimers complexes between A1 and A2A receptors.45,46 We have previously reported the modulation of A1 and A2A receptors during hypoxia in an in vitro model of C6 glioma cells culture, where tonic activation of A1 by endogenous released adenosine promotes modulation on A1 receptors but on A2A levels as well.47 A similar mechanism could take place after fullerene derivative treatment, promoting changes on receptor densities through postreceptor processes.
In summary, the present study provides clear and innovative evidence about the effect of this fullerene derivative on the adenosine receptors, by using SK-N-MC neuroepithelioma cells as a neurodegenerative model. The data show that these cells natively express both A1 and A2 receptors, which can be modulated by fullerene derivative exposure without significantly affecting cell viability. This low toxicity in cells even at the high concentration used confirms a good biocompatibility of this fullerene derivative in our in vitro model.
Methods
Materials
The hydrosoluble [60]fullerene bis-adduct trans-3 (T3SS) was synthesized as previously described.10,11 Cyclopentyl-1,3-dypropylxanthine 8-[dipropy-2,3-3H(N)] ([3H] DPCPX 120 Ci/mmol) was purchased from Amersham. [2-3H](4-(2-[7-Amino-2-(2-fury1)[1,2,4]triazolo[2,3-a][1,3,5]triazin-5-ylamino]ethyl)phenol ([3H]ZM241385 27.4 Ci/mmol) was from Tocris. N6-Cyclopentyladenosine (CPA), N6-cyclohexyladenosine (CHA), and calf intestine adenosine deaminase (ADA) were obtained from Sigma. All other products were of analytical grade.
Cell Culture
SK-N-MC neuroepithelioma cells (purchased from ATCC, HTB-10)48 were grown in modified Eagle’s medium (MEM, Gibco) supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 2 mM pyruvate, 1% nonessential amino acids, and antibiotics in a humidified atmosphere with 5% CO2 at 37 °C, as previously reported.49 Cells were subcultured on 10 mL Petri dishes and when at confluence were detached by using 4 mL of Trypsin (Tryple Express, Gibco) and centrifuged, and the pellet was resuspended in complete medium and cells seeded in 24- or 96-well plates and allowed to grow for 24 h before experiment, to have a final density per well of 2 × 105 cells and 3 × 104 cells, respectively.
Detection of Adenosine A1 and A2A Receptors in Intact Cells by Radioligand Binding Assay
Radioligand binding assays using intact cells were performed in 24-well plates at 80–90% of confluence (2 × 105 cells/well) as previously described50 with some modifications. Briefly, cells were washed with serum-free MEM pH 7.4 and preincubated with 2 U/mL adenosine deaminase at 37 °C for 30 min to remove endogenous adenosine. After incubation, in order to measure adenosine A1 receptors, the indicated concentrations of [3H]DPCPX (1–30 nM) were added in the absence or the presence of 1 mM nonlabeled CPA to obtain nonspecific binding. Adenosine A2A receptors were quantified by using the radioligand [3H]ZM241385 (0.1–40 nM) and 3 mM theophylline to obtain nonspecific binding. After incubation at 25 °C for 2 h in a final volume of 250 μL, cells were washed with 500 μL of ice-cold medium and disrupted with 0.1% sodium dodecyl sulfate (SDS). Well contents were then transferred to vials and scintillation liquid mixture was added in order to measure radioactivity. At least two wells from each plate were reserved for protein concentration measurement.
MTT Reduction Assay
Cell viability was determined using an in vitro toxicology assay kit based on the reduction of 3-[4,5-dimetylthiazol-2-yl]-2,5-dipheniltetrazolium bromide (MTT) purchased from Sigma, according to Mosmann.51 Briefly, SK-N-MC neuroepithelioma cells were seeded at 3 × 104 cells per well in 96-well plates. At the end of fullerene derivative treatment, SK-N-MC cells were incubated in culture medium with MTT solution (5 mg/mL) at 37 °C for 3 h. After incubation, MTT solubilization solution (10% Triton X-100 plus 0.1 N HCl in anhydrous isopropanol) was added to the wells to dissolve formazan crystals. The plates were thoroughly shaken, and the absorbance of each well was measured at 570 nm.
Plasma Membrane Isolation
The isolation of plasma membranes was performed as previously described.52 Cells were homogenized in isolation buffer (50 mM Tris-HCl pH 7.4, containing 10 mM MgCl2 and protease inhibitors) and centrifuged for 5 min at 1000g in a Beckman JA 21 centrifuge. The supernatant was centrifuged for 20 min at 27 000g, and the pellet was resuspended in isolation buffer. The concentration of protein was measured by the method of Lowry, using bovine serum albumin as the standard.
Western Blotting Assay
Western blotting assay was performed as previously described53 by using 50 μg of protein. After transfer to nitrocellulose membranes, proteins were incubated with rabbit polyclonal anti-A1R (Calbiochem), anti-A2AR (Upstate), and anti-A2BR (Santa Cruz Biotechnologies) antibodies, all at dilution of 1:1000. The incubation with the secondary anti-rabbit IgG antibody (Dako) was carried out at a dilution of 1:5000. The monoclonal anti-β-actin antibody (Sigma) was used as a gel loading control (1:5000).
Total RNA isolation and Preparation of cDNA
Total RNA was extracted using an ABI 6100 Nucleic Acid PrepStation according to the manufacturer’s protocol. All chemicals for the ABI 6100 were purchased from Applied Biosystems. Total RNA from cells was isolated and stored at −80 °C. The ratio of A260/A280 (purity of RNA) was in the range 1.9–2.1. RNA concentrations were determined from A260. One microgram of total RNA was reverse transcribed using the Applied Biosystems High-Capacity cDNA Archive Kit according to manufacturer’s protocol.
Quantitative Real Time RT-PCR Analysis
To assess relative gene expression in SK-N-MC neuroepithelioma cells quantitative real time RT-PCR analysis54 was performed with an Applied Biosystems Prism 7500 Fast Sequence Detection System, using TaqMan universal PCR master mix according to the manufacturer’s specifications (Applied Biosystems Inc.) for the A1, A2A, A2B, and β-actin genes for which validated TaqMan gene expression assays are available. The TaqMan probes and primers for A1 (assay ID: Hs 00181231), A2A (assay ID: Hs 00386497), A2B (assay ID: Hs 00169123), and β-actin (assay ID: Hs 99999903) were assay-on-demand gene expression products (AppliedBiosystems). The TaqMan primer and probe sequences are packaged together in a 20× solution. The sequences are proprietary, so they are not available. The gene-specific probes were labeled using reporter dye FAM. A nonfluorescent quencher and the minor groove binder were linked at the 3′ end of probe as quenchers. The thermal cycler conditions were as follows: hold for 20 s at 95 °C, followed by two step PCR for 40 cycles of 95 °C for 3 s, followed by 60 °C for 30 s. Levels of RNA expression were determined using the 7500 Fast System SDS software version 1.3.1 (Applied Biosystems) according to the 2–ΔΔCt method. Briefly, expression results of a gene were normalized to internal control β-actin relative to a calibrator, consisting of the mean expression level of the receptor gene as follows: 2–ΔΔCt = 2 – ((Ct receptor gene–Ct actin)sample–(Ct receptor gene–Ct actin)calibrator). The results from 4–5 independent repeat assays, performed in different plates each using different cDNAs from the cultures analyzed, were averaged to produce a single mean quantity value for each mRNA.
Fluorescence Microscopy
Images for labeled fluorescent antibody of receptors A2A have been obtained with a digital camera Leica DFC350FX, attached to a Leica DMI6000B (Leica Microsystem) fluorescent inverted microscope. A 20× HCX PL FLUOTAR objective and the LAS AF Lite software were used. The protocol for the fluorescent immune assay was performed as follows: SK-N-MC cells were grown in complete medium for 24 h before assay. After this time, complete medium was replaced with phosphate saline buffer (PBS), allowed to sit for 5 min, and repeated for three times, and the cells were fixed in 4% paraformaldehyde in phosphate buffer for 15 min. Cells were then incubated with bovine serum albumin (3% BSA) and Triton TX-100 (0.1% in PBS) at room temperature for 10 min and then incubated with primary antibody goat anti-mouse A2A (Santa Cruz) diluted 1:100 in PBS/3% BSA for 3 h at 37 °C. Cells were then washed three times with PBS at 37 °C and incubated with secondary IgG anti-goat antibody labeled with fluorescein isothiocyanate (FITC, Invitrogen)) at dilution of 1:500 in PBS/% BSA for 30 min. Cells were then washed to remove nonfixed fluorescent antibody.
Protein Determination
Protein concentration for radioligand binding assay and Western blotting was measured by the method of Lowry, using bovine serum albumin as standard.
Statistical and Data Analysis
Data statistical analysis was performed using the Student’s t test. Differences between mean values were considered statistically significant at p < 0.05. Saturation (Bmax, Kd) binding curves were analyzed by performing Scatchard and nonlinear regression analysis of binding data with the GraphPad Prism 5 program (GraphPad Software).
This study was funded by grants from the European Union through the Marie-Curie Research Training Network PRAIRIES (Contract MRTN-CT-2006-035810), the Fundació La Marató de TV3 (090331), and the Ministerio de Ciencia e Innovación (BFU2008-00138). D.G. is an early stage researcher (ESR) fellow involved in PRAIRIES project.
Author Contributions
D.G. performed experimental procedures here reported and wrote sections. D.L. and I.B.-Y. researched the scientific literature. T.R. performed synthetic chemistry work. J.L.A. and M.M. oversaw and designed all experiments. J.L.A. compiled, reviewed, and edited the collated manuscript.
There is no conflict of interest including any financial, personal, or other relationships with other people or organizations.
References
- Kroto H. W.; Heath J. R.; O'Brien S. C.; Curl R. F.; Smalley S. E. (1985) C60: Buckminsterfullerene. Nature 318, 162–163. [Google Scholar]
- Partha R.; Conyers J. L. (2009) Biomedical applications of functionalized fullerene-based nanomaterials. Int. J. Nanomed. 4, 261–275. [PMC free article] [PubMed] [Google Scholar]
- Bosi S.; Da Ros T.; Spalluto G.; Prato M. (2003) Fullerene derivatives: an attractive tool for biological applications. Eur. J. Med. Chem. 38, 913–923. [DOI] [PubMed] [Google Scholar]
- Djordjevic A.; Bogdanovic G.; Dobric S. (2006) Fullerenes in biomedicine. J. BUON 11, 391–404. [PubMed] [Google Scholar]
- Tsai M. C.; Chen Y. H.; Chiang L. Y. (1997) Polyhydroxylated C60, fullerenol, a novel free-radical trapper, prevented hydrogen peroxide- and cumene hydroperoxide-elicited changes in rat hippocampus in-vitro. J. Pharm. Pharmacol. 49, 438–445. [DOI] [PubMed] [Google Scholar]
- Takada H.; Kokubo K.; Matsubayashi K.; Oshima T. (2006) Antioxidant activity of supramolecular water-soluble fullerenes evaluated by beta-carotene bleaching assay. Biosci., Biotechnol., Biochem. 70, 3088–3093. [DOI] [PubMed] [Google Scholar]
- Markovic Z.; Trajkovic V. (2008) Biomedical potential of the reactive oxygen species generation and quenching by fullerenes (C60). Biomaterials 29, 3561–3573. [DOI] [PubMed] [Google Scholar]
- Dugan L. L.; Gabrielsen J. K.; Yu S. P.; Lin T. S.; Choi D. W. (1996) Buckminsterfullerenol free radical scavengers reduce excitotoxic and apoptotic death of cultured cortical neurons. Neurobiol. Dis. 3, 129–135. [DOI] [PubMed] [Google Scholar]
- Torres V. M.; Posa M.; Srdjenovic B.; Simplicio A. L. (2011) Solubilization of fullerene C60 in micellar solutions of different solubilizers. Colloids Surf., B 82, 46–53. [DOI] [PubMed] [Google Scholar]
- Bosi S.; Da Ros T.; Spalluto G.; Balzarini J.; Prato M. (2003) Synthesis and anti-HIV properties of new water-soluble bis-functionalized[60]fullerene derivatives. Bioorg. Med. Chem. Lett. 13, 4437–4440. [DOI] [PubMed] [Google Scholar]
- Bosi S.; Feruglio L.; Da Ros T.; Spalluto G.; Gregoretti B.; Terdoslavich M.; Decorti G.; Passamonti S.; Moro S.; Prato M. (2004) Hemolytic effects of water-soluble fullerene derivatives. J. Med. Chem. 47, 6711–6715. [DOI] [PubMed] [Google Scholar]
- Ongini E.; Adami M.; Ferri C.; Bertorelli R. (1997) Adenosine A2A receptors and neuroprotection. Ann. N.Y. Acad. Sci. 825, 30–48. [DOI] [PubMed] [Google Scholar]
- Cunha R. A. (2005) Neuroprotection by adenosine in the brain: From A(1) receptor activation to A (2A) receptor blockade. Purinergic Signalling 1, 111–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralevic V.; Burnstock G. (1998) Receptors for purines and pyrimidines. Pharmacol. Rev. 50, 413–492. [PubMed] [Google Scholar]
- Fredholm B. B.; Ijzerman A. P.; Jacobson K. A.; Klotz K. N.; Linden J. (2001) International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol. Rev. 53, 527–552. [PMC free article] [PubMed] [Google Scholar]
- Pedata F.; Corsi C.; Melani A.; Bordoni F.; Latini S. (2001) Adenosine extracellular brain concentrations and role of A2A receptors in ischemia. Ann. N.Y. Acad. Sci. 939, 74–84. [DOI] [PubMed] [Google Scholar]
- Ham J.; Rees D. A. (2008) The adenosine a2b receptor: its role in inflammation. Endocr., Metab. Immune Disord.: Drug Targets 8, 244–254. [DOI] [PubMed] [Google Scholar]
- Bjorklund O.; Shang M.; Tonazzini I.; Dare E.; Fredholm B. B. (2008) Adenosine A1 and A3 receptors protect astrocytes from hypoxic damage. Eur. J. Pharmacol. 596, 6–13. [DOI] [PubMed] [Google Scholar]
- Albasanz J. L.; Rodriguez A.; Ferrer I.; Martin M. (2007) Up-regulation of adenosine A1 receptors in frontal cortex from Pick’s disease cases. Eur. J. Neurosci. 26, 3501–3508. [DOI] [PubMed] [Google Scholar]
- Albasanz J. L.; Perez S.; Barrachina M.; Ferrer I.; Martin M. (2008) Up-regulation of adenosine receptors in the frontal cortex in Alzheimer’s disease. Brain Pathol. 18, 211–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalfo E.; Albasanz J. L.; Rodriguez A.; Martin M.; Ferrer I. (2005) Abnormal group I metabotropic glutamate receptor expression and signaling in the frontal cortex in Pick disease. J. Neuropathol. Exp. Neurol. 64, 638–647. [DOI] [PubMed] [Google Scholar]
- Albasanz J. L.; Dalfo E.; Ferrer I.; Martin M. (2005) Impaired metabotropic glutamate receptor/phospholipase C signaling pathway in the cerebral cortex in Alzheimer’s disease and dementia with Lewy bodies correlates with stage of Alzheimer’s-disease-related changes. Neurobiol. Dis. 20, 685–693. [DOI] [PubMed] [Google Scholar]
- Colvin V. L. (2003) The potential environmental impact of engineered nanomaterials. Nat. Biotechnol. 21, 1166–1170. [DOI] [PubMed] [Google Scholar]
- Mori T.; Takada H.; Ito S.; Matsubayashi K.; Miwa N.; Sawaguchi T. (2006) Preclinical studies on safety of fullerene upon acute oral administration and evaluation for no mutagenesis. Toxicology 225, 48–54. [DOI] [PubMed] [Google Scholar]
- Shinohara N.; Nakazato T.; Tamura M.; Endoh S.; Fukui H.; Morimoto Y.; Myojo T.; Shimada M.; Yamamoto K.; Tao H.; Yoshida Y.; Nakanishi J. (2011) Clearance kinetics of fullerene C nanoparticles from rat lungs after intratracheal C instillation and inhalation C exposure. Toxicol. Sci. 118, 564–573. [DOI] [PubMed] [Google Scholar]
- Kolosnjaj J.; Szwarc H.; Moussa F. (2007) Toxicity studies of fullerenes and derivatives. Adv. Exp. Med. Biol. 620, 168–180. [DOI] [PubMed] [Google Scholar]
- Levi N.; Hantgan R. R.; Lively M. O.; Carroll D. L.; Prasad G. L. (2006) C60-fullerenes: detection of intracellular photoluminescence and lack of cytotoxic effects. J. Nanobiotechnol. 4, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch A. (1994) The chemistry of the fullerenes, Georg Thieme Verlag, Stuttgart. [Google Scholar]
- Jin H.; Chen W. Q.; Tang X. W.; Chiang L. Y.; Yang C. Y.; Schloss J. V.; Wu J. Y. (2000) Polyhydroxylated C(60), fullerenols, as glutamate receptor antagonists and neuroprotective agents. J. Neurosci. Res. 62, 600–607. [DOI] [PubMed] [Google Scholar]
- Stone T. W.; Ceruti S.; Abbracchio M. P. (2009) Adenosine receptors and neurological disease: neuroprotection and neurodegeneration. Handb. Exp. Pharmacol. 535–587. [DOI] [PubMed] [Google Scholar]
- Castillo C. A.; Albasanz J. L.; Fernandez M.; Martin M. (2007) Endogenous expression of adenosine A1, A2 and A3 receptors in rat C6 glioma cells. Neurochem. Res. 32, 1056–1070. [DOI] [PubMed] [Google Scholar]
- Spielman W. S.; Klotz K. N.; Arend L. J.; Olson B. A.; LeVier D. G.; Schwabe U. (1992) Characterization of adenosine A1 receptor in a cell line (28A) derived from rabbit collecting tubule. Am. J. Physiol. 263, C502–C508. [DOI] [PubMed] [Google Scholar]
- Gerwins P.; Nordstedt C.; Fredholm B. B. (1990) Characterization of adenosine A1 receptors in intact DDT1MF-2 smooth muscle cells. Mol. Pharmacol. 38, 660–666. [PubMed] [Google Scholar]
- Shang J.; Ratnikova T. A.; Anttalainen S.; Salonen E.; Ke P. C.; Knap H. T. (2009) Experimental and simulation studies of a real-time polymerase chain reaction in the presence of a fullerene derivative. Nanotechnology 20, 415101. [DOI] [PubMed] [Google Scholar]
- Bohm S. K.; Grady E. F.; Bunnett N. W. (1997) Regulatory mechanisms that modulate signalling by G-protein-coupled receptors. Biochem. J. 322(Pt 1), 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettinger-Smith B. D.; Leid M.; Murray T. F. (1996) Chronic exposure to adenosine receptor agonists and antagonists reciprocally regulates the A1 adenosine receptor-adenylyl cyclase system in cerebellar granule cells. J. Neurochem. 67, 1921–1930. [DOI] [PubMed] [Google Scholar]
- Johansson B.; Georgiev V.; Lindstrom K.; Fredholm B. B. (1997) A1 and A2A adenosine receptors and A1 mRNA in mouse brain: effect of long-term caffeine treatment. Brain Res. 762, 153–164. [DOI] [PubMed] [Google Scholar]
- Murray T. F. (1982) Up-regulation of rat cortical adenosine receptors following chronic administration of theophylline. Eur. J. Pharmacol. 82, 113–114. [DOI] [PubMed] [Google Scholar]
- Lupica C. R.; Berman R. F.; Jarvis M. F. (1991) Chronic theophylline treatment increases adenosine A1, but not A2, receptor binding in the rat brain: an autoradiographic study. Synapse 9, 95–102. [DOI] [PubMed] [Google Scholar]
- Satoh M.; Matsuo K.; Takanashi Y.; Takayanagi I. (1995) Effects of acute and short-term repeated application of fullerene C60 on agonist-induced responses in various tissues of guinea pig and rat. Gen. Pharmacol. 26, 1533–1538. [DOI] [PubMed] [Google Scholar]
- St Hilaire C.; Carroll S. H.; Chen H.; Ravid K. (2009) Mechanisms of induction of adenosine receptor genes and its functional significance. J. Cell. Physiol. 218, 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie Z.; Mei Y.; Ford M.; Rybak L.; Marcuzzi A.; Ren H.; Stiles G. L.; Ramkumar V. (1998) Oxidative stress increases A1 adenosine receptor expression by activating nuclear factor kappa B. Mol. Pharmacol. 53, 663–669. [DOI] [PubMed] [Google Scholar]
- Lao F.; Chen L.; Li W.; Ge C.; Qu Y.; Sun Q.; Zhao Y.; Han D.; Chen C. (2009) Fullerene nanoparticles selectively enter oxidation-damaged cerebral microvessel endothelial cells and inhibit JNK-related apoptosis. ACS Nano 3, 3358–3368. [DOI] [PubMed] [Google Scholar]
- Schulte G.; Fredholm B. B. (2003) Signalling from adenosine receptors to mitogen-activated protein kinases. Cell. Signalling 15, 813–827. [DOI] [PubMed] [Google Scholar]
- Ferre S.; Ciruela F.; Quiroz C.; Lujan R.; Popoli P.; Cunha R. A.; Agnati L. F.; Fuxe K.; Woods A. S.; Lluis C.; Franco R. (2007) Adenosine receptor heteromers and their integrative role in striatal function. ScientificWorldJournal 7, 74–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferre S.; Ciruela F.; Borycz J.; Solinas M.; Quarta D.; Antoniou K.; Quiroz C.; Justinova Z.; Lluis C.; Franco R.; Goldberg S. R. (2008) Adenosine A1-A2A receptor heteromers: new targets for caffeine in the brain. Front. Biosci. 13, 2391–2399. [DOI] [PubMed] [Google Scholar]
- Castillo C. A.; Leon D.; Ruiz M. A.; Albasanz J. L.; Martin M. (2008) Modulation of adenosine A(1) and A(2A) receptors in C6 glioma cells during hypoxia: involvement of endogenous adenosine. J. Neurochem. 105, 2315–2329. [DOI] [PubMed] [Google Scholar]
- Biedler J. L.; Helson L.; Spengler B. A. (1973) Morphology and growth, tumorigenicity, and cytogenetics of human neuroblastoma cells in continuous culture. Cancer Res. 33, 2643–2652. [PubMed] [Google Scholar]
- Seeger R. C.; Rayner S. A.; Banerjee A.; Chung H.; Laug W. E.; Neustein H. B.; Benedict W. F. (1977) Morphology, growth, chromosomal pattern and fibrinolytic activity of two new human neuroblastoma cell lines. Cancer Res. 37, 1364–1371. [PubMed] [Google Scholar]
- Ruiz M. A.; Escriche M.; Lluis C.; Franco R.; Martin M.; Andres A.; Ros M. (2000) Adenosine A(1) receptor in cultured neurons from rat cerebral cortex: colocalization with adenosine deaminase. J. Neurochem. 75, 656–664. [DOI] [PubMed] [Google Scholar]
- Mosmann T. (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 65, 55–63. [DOI] [PubMed] [Google Scholar]
- Albasanz J. L.; Leon D.; Ruiz M. A.; Fernandez M.; Martin M. (2002) Adenosine A1 receptor agonist treatment up-regulates rat brain metabotropic glutamate receptors. Biochim. Biophys. Acta 1593, 69–75. [DOI] [PubMed] [Google Scholar]
- Albasanz J. L.; Rodriguez A.; Ferrer I.; Martin M. (2006) Adenosine A2A receptors are up-regulated in Pick’s disease frontal cortex. Brain Pathol. 16, 249–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi R.; Fockler C.; Dollinger G.; Watson R. (1993) Kinetic PCR analysis: real-time monitoring of DNA amplification reactions. Biotechnology (N.Y.) 11, 1026–1030. [DOI] [PubMed] [Google Scholar]