Abstract
This Review summarizes the medicinal chemistry found in publications on both orthosteric and allosteric modulators of the metabotropic glutamate receptor 1 (mGlu1) from 2005 to the present. The time period covered by the scope of this current review has been particularly rich in mGlu1-related publications with numbers quadrupling when compared to the preceding five year period of 2000−2005. Publications in the field peaked in 2007 with over 35 articles appearing in the peer reviewed literature in the course of that year. Given that glutamate is one of the primary excitatory neurotransmitters in the mammalian central nervous system (CNS), it is unsurprising that it acts upon several receptors that are considered to be of potential therapeutic interest for many indications. Orthosteric and allosteric modulation of the receptor is possible, with a logical extrapolation to the chemotypes used for each strategy. The last five years of publications have yielded many mGlu1 selective antagonist chemotypyes, most of which have shown efficacy in pain in vivo models. However, the primary impact of these compounds has been to highlight the mechanistic safety risks of mGlu1 antagonism, independent of chemotype. As a review in medicinal chemistry, the primary focus of this paper will be on the design and, to a lesser degree, synthetic strategies for the delivery of subtype selective, CNS penetrant, druglike compounds through a “medchem” program, targeting modulators of the mGlu1 receptor.
Keywords: mGlu1 review, metabotropic glutamate receptor type 1, modulator, allosteric, orthosteric, antagonist
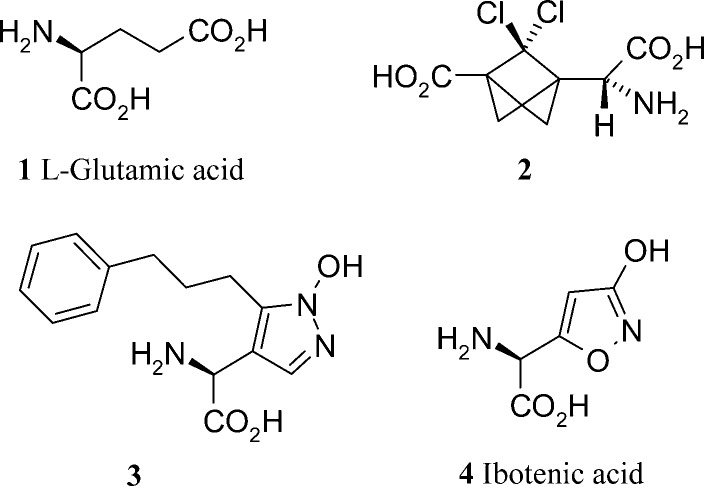
Having established in the late 1980s that glutamate (1, Figure 1) acted upon metabotropic glutamate recpeptors (and not just on the previously identified ionotropic glutamate-gated ion channels such as the N-methyl-d-aspartate (NMDA), α-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA), and kainic acid receptors), this new class of glutamate targeted GPCRs became increasingly better characterized and of potential therapeutic interest.1 The subtype nomenclature of mGlu1 through to mGlu8 simply reflects the order in which they were first cloned. mGlu1 and mGlu5 are further subclassified among the mGlu receptors as being Group I, based on a high degree of sequence similarity and receptor homology. This Group I class of mGlu receptors is further differentiated from groups II and III through its signaling pathway, in that it leads to the downsteam release of intracellular calcium (via the activation of phospholipase C).2 In contrast, mGlu receptors from Groups II and III are (negatively) coupled to adenylyl cyclase activity.2 Within the Group I receptors, selective mGlu5 antagonists continue to be an area of significant therpeutic interest.
Figure 1.
Orthosteric mGluR1 modulators.
mGlu1 shares the common mGlu receptor characteristic of a large extracellular domain where the enodgenous agonist glutamate can bind. The functional receptor is an mGlu1 homodimer with the bound glutamate agonist in the orthosteric binding site.3 This functional receptor serves to control the release of key neurotransmitters such as GABA and glutamate at the synapse and also interacts with ionotropic glutamate receptors such as NMDA. No genetic link to human disease has correlated to mutations of any of the glutamate receptor subunit genes, but molecular antagonist intervention at the mGlu1 receptor has shown promise in vivo through models of pain and many other neurological disorders (stroke, epilepsy, drug addiction, Huntingdon’s disease, anxiety, and neurodegeneration).4 Equally, mGlu1 knockout mice have revealed a negative impact on motor function and capabilities in learning and memory.5,6 Receptor distibution is particularly compelling for pain indications with the mGlu1 receptors found to be localized such that they can act specifically on postsynaptic processes. When combined with their known presence in the primary afferent nerve terminals, that register nociception, and their distribution within spinal cord, thalamus, and brain cortex, the case for the mGlu1 receptor as a viable target for pain therapy appears compelling.7 The mouse mGlu1 knockout data does point to some mechanistic safety concerns that would need to be evaluated and quantified.
Glutamate Derived Selective Competitive Antagonists
Publications on mGlu1 antagonists designed to bind at the orthosteric (glutamate) binding site constitute less than 10% of the total papers published over the five year period under review.8 In general, competitive antagonists are unsurprisingly analogues of glutamate. A natural consequence of this structural similarity to the endogenous agonist is the difficulty in making highly subtype selective glutamate antagonists. Physicochemically, the compounds are likely to be hydrophilic, doubly acidic amino acid analogues. The publications from Pelliciari et al.9 and Madsen et al.10 both fall into this catagory. Pelliciari has extended his work on carboxybicyclo-[1,1,1]-pentyl glycine analogues to investigate the effect of chlorination on the propellane core. The three compounds synthesized were micromolar mGlu1 antagonists with a maximum 6-fold selectivity over the fellow Group I subtype, mGlu5. All compounds were inactive against the mGlu receptors of Groups II and III. This profile was of sufficient interest to progress in vivo where compound 2 (Figure 1) blocked NMDA induced convulsions when administered in an intracerebroventricular fashion. Madsen’s analogues (3, Figure 1) of ibotenic acid show structure−activity relationships (SARs) related to lipophilic substituents at the pyrazole 5-position. These changes seem to faciliate differing affinities for both iGluR and mGluR receptor subtypes. The compounds are relatively weak with EC50 and Ki values >5 μM (generally in the 100s of μM), but small structural changes offer some insight into receptor selectivity derived from compounds designed from the nonselective, glutamate starting point. From a general compound design point of view, the use of an N1-hydroxylated pyrazole (pyrazolol) in the analogues, along with the 3-isoxoazolol found the natural product starting point (4, Figure 1) as carboxylic acid isosteres, is of medicinal chemistry design interest. A table of measured pKa’s in the paper show these to be weak acids that could find utility in carboxylic acid based pharmacophores elsewhere.
Negative Noncompetitive mGlu1 Modulators: Allosteric Antagonists
Layton’s review of five years ago8 clearly describes the roles of compounds such as CPCCOEt, BAY36-7620, and EM-TBPC as the tools that established the presence and location of allosteric binding sites in mGlu1. The compounds also helped identify key residue changes between human and rat mGlu1, some of which are in the allosteric binding site. The possibility of inverse agonist behavior for certain compounds at high concentrations was also established by these molecules. Access to these molecular tools and the receptor pharmacology understanding that they bring has meant that many of the publications from the last five years have concentrated on the identification of druglike mGlu1 antagonist chemotypes by pharmaceutical companies. Perhaps most importantly, the identification of a nonglutamate binding, allosteric site11 improves the chances of identifying mGluR subtype selective molecules with better overall druglike properties. The odds of delivering a selective, oral, central nervous system (CNS) penetrant compound for the clinic, having taken nonselective glutamate and its associated physicochemistry as the lead matter for an antagonist program, look rather slim.
Pfizer
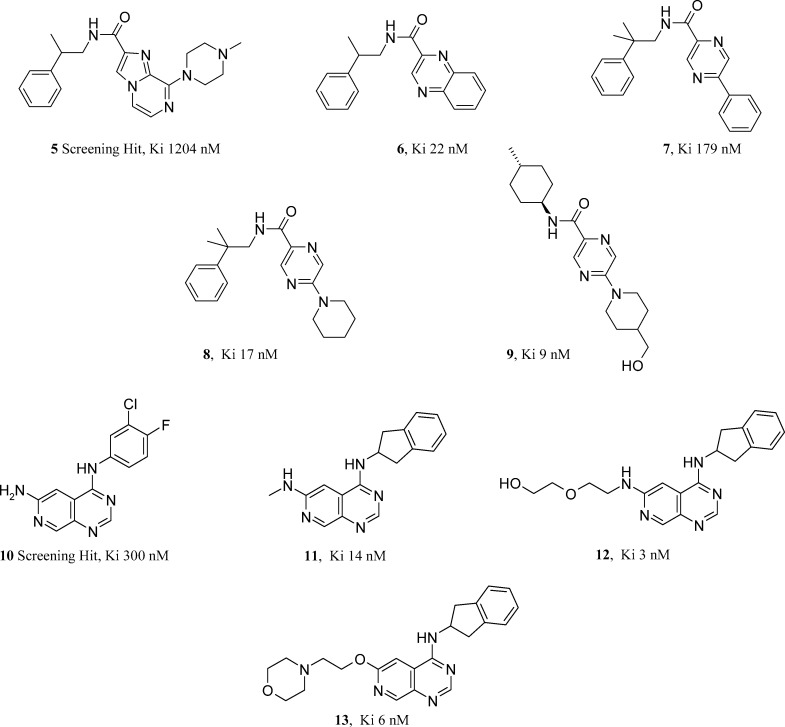
Pfizer has published on two chemical series that have proved to be noncompetitive, selective mGlu1 antagonists. In 2007, a hit to lead program that yielded a series of 2,5-disubstituted pyrazines was described by Owen et al.12 Weak (<1 μM) imidazopyridine mGlu1 leads (such as 5, Figure 2) were identified through non-High Throughput Screening (HTS) file picks. Splitting these amidic compounds into their constiuent amine and acid components allowed further small arrays to be designed using untried acid or amine monomers in combination (taken from the company monomer collection). Phenethylamine monomers related to the initial leads were very active in combination with a quinoxaline acid monomer (6, Figure 2). In an effort to extend the scope of the chemical space related to the quinoxaline amides, branching a substituent from a pyrazine was considered as an alternative to the fused bicyclic system. These biaryls featuring pyrazine were moderately active alternatives to the quinoxaline (such as 7, Figure 2), but more importantly they served as a pointer to an established parallel synthesis protocol based on chloroheterocycle amine substitution chemistry.
Figure 2.
Pfizer mGlu1 antagonists.
This opportunity was identified using a computational tool known as a Bayesian Idea Generator, BIG (devised and now published upon by Van Hoorn of Pfizer).13 BIG is a method that applies Bayesian staistics to identify the nearest validated parallel synthesis-enabled chemical space related to your single input molecule. BIG identified a two step library protocol based on using the amine monomer set twice (in a substitution reaction followed by an amide coupling sequence). The input molecule 7 was clearly derived from a cross coupling reaction and an amide coupling. Application of this technique not only changed the synthetic strategy and design scope, but also delivered a 20-fold increase in mGlu1 antagonism with the piperidine analogue 8 (Figure 2) of <20 nM. With a protocol capable of delivering thousands of compounds, designs were limited and triaged with a view to optimizing physiochemistry for pharmacokinetic, toxicity, and CNS penetration reasons. In two cycles of library design, compound 9 (Figure 2) was identified as a 9 nM mGlu1 antagonist, stable in human liver microsomes and of attractive lipophilicity. Mantell et al. published on a more extensively profiled Pfizer series of azaquinazolines in 2009, sharing both in vivo pharmacokinetic and pain efficacy data.14 Mantell describes how a moderately active screening hit (10, Figure 2) was initially tackled with a view to replacing the unattractive aniline functionality. The indanamine in 11 (Figure 2) proved to be a suitable aniline replacement. The heterocylic core changes attempted proved costly, leaving the 4-substituent as the remaining variable to explore. The NH2 at this position in the 300 nM hit was homologated to present pharmacologically tolerated, solublizing functionality such as amines and alcohols. Compound 12 (Figure 2) was a highly selective mGlu1 antagonist, >1000-fold selective over mGlu5. It is orally bioavailable in rat but has a relatively short half-life. Efficacy was demonstarted in a pain EMG-pinch model using compound 12, but this appeared to require high doses and many multiples of the binding Ki in free plasma concentration. The three H-bond donors found in 12 make it an unlikely candidate for good CNS penetration. It was measured at 2% in the cerebrospinal fluid (CSF) as part of the in vivo efficacy study. An oxygen linker in combination with a morpholine side chain reduced the H-bond donor count to one and combined with a log D value of 3.4 gave compound 13 (Figure 2) and significantly improved the CNS penetration (50% in CSF). This compound further established the need to achieve 10× the mGlu1 binding Ki in the CSF to see efficacy equivalent to morphine in the pinch-EMG model. Enabling synthetic chemistry that looked at N, O, and C linked analogues in the azaquinazoline 4-position was published by Harbottle et al. of Pfizer in 2007.15
Abbott
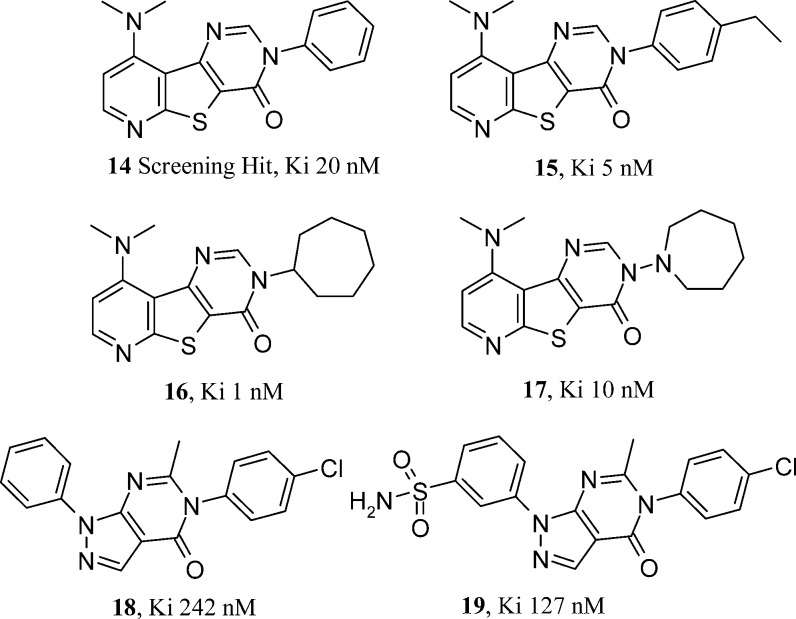
Abbott, through the authorship of Zheng and co-workers and Wang et al., have published three papers describing a well characterized lead series16,17 and hit to lead efforts in a second.18 A highly selective 20 nM mGlu1 triazafluorenone HTS hit 14 (Figure 3) prompted considerable synthetic follow up from the Abbott team. Hypotheses centered around some synthetically hard-earned tricyclic heterocycle core changes that involved nitrogen shuffling, insertion, and deletion. In certain templates, attractive use of synthetic strategy used the abundant amine monomer set twice, late on in a nine-step sequence. The most extensive SAR explorations studied the N-substituent of the pyrimidinone ring of the tricyclic core. Directly bonded aromatic and heteroaromatic rings were all tolerated with only N-benzylation causing a noticeable drop off in potency. The effect of para-substitution on the ring was further explored. The tolerated size of this group was finite with ethyl analogue 15 (Figure 3) proving to be optimal and the likes of t-butyl more than 1000-fold less active. It was also clear from the SAR that the N-aryl group could be replaced by cycloalkyl alternatives. The very potent 1 nM N-cycloheptyl analogue 16 (Figure 3) shows an aliphatic, cyclic substituent also found in competitor mGlu1 series. This prompts thoughts around potential overlays and common binding modes for these chemotypes. The hypotheses behind the heterocyclic core changes explored synthetically were not explained in the publication, but they did serve to rule out areas of mGlu1 activity. The chemotype looks as if it may have solubility concerns from the flat tricyclic nature of the core. The potential for an increased toxicity risk through DNA interchelation from such planar compounds is also a possibility. With only in vitro mGluR pharmacology available in the publication, it has to be assumed that potency was the primary design concern. The SAR indicates that the original core found by HTS was optimal and that para-ethylation of the N-phenyl group gained 7-fold in mGlu1 potency over the original HTS hit. This ethylated analogue 15 of the HTS lead 14 was progressed to in vivo studies, showing efficacy in Complete Freund’s Adjuvent and formalin induced pain models. No side effects were seen in a rat rotorod model at doses 20-fold higher than those required in the efficacy studies. The pharmacokinetics in rat were suitable for these studies with a useful 2 h half-life for compound 15. This compound featured in Abbott’s follow up communication around the effect of compound brain distribution on mGlu1 antagonist mediated efficacy in the Cheung model of neuropathic pain. Having shown efficacy in multiple general pain models, compound efficacy was less predictable in those models specific to neuropathic pain. Abbott in vivo data on five compounds draws a correlation between brain/plasma ratios and efficacy in the Cheung model. Efficacy was not related to total or free brain levels. Compound 17 (Figure 3), with a supraproportional amount in the brain versus plasma, showed complete efficacy in the Cheung model. The Abbott group highlight a sweet spot in calculated lipophilicity for compound 17, when compounds of both higher and lower cLogP proved to be only partially efficacious. Efficacy could be rationalized in possession of measured brain to plasma ratios. Compound 17 looks to be a very useful tool given its excellent CNS penetration, pharmacokinetics, and pharmacology. Abbott’s second series publication shows some follow up of a second HTS hit that shared some structural features from the first. Retaining the N-arylated pyrimidinone from compound 15, the second series pyrazolopyrimidinone hit was a weaker mGlu1 starting point at 242 nM (18, Figure 3).
Figure 3.
Abbott mGlu1 antagonists.
In terms of structure (solubility/toxicity risk) and synthetic opportunity, this series appears to have more potential. As before, the hit compound seemed hard to improve on with no changes gaining more than 3-fold in potency. The fused pyrazole did represent a new region of space to explore, although once again no improvements in mGlu1 potency were made in the designs published. The compound highlighted by Wang (19, Figure 3) is a tolerated primary sulfonamide addition to the second series lead compound 14. In line with its cLogP, the more lipophilic chlorinated HTS hit has higher in vitro metabolism than the designed sulfonamide. This did not compromise permeability and improved solubility. It would seem that the second series was designed and developed with a more multiparameter view on compound profiles. This approach delivered compounds with useful rat phramacokinetics and excellent oral bioavailability.
Glaxo SmithKline
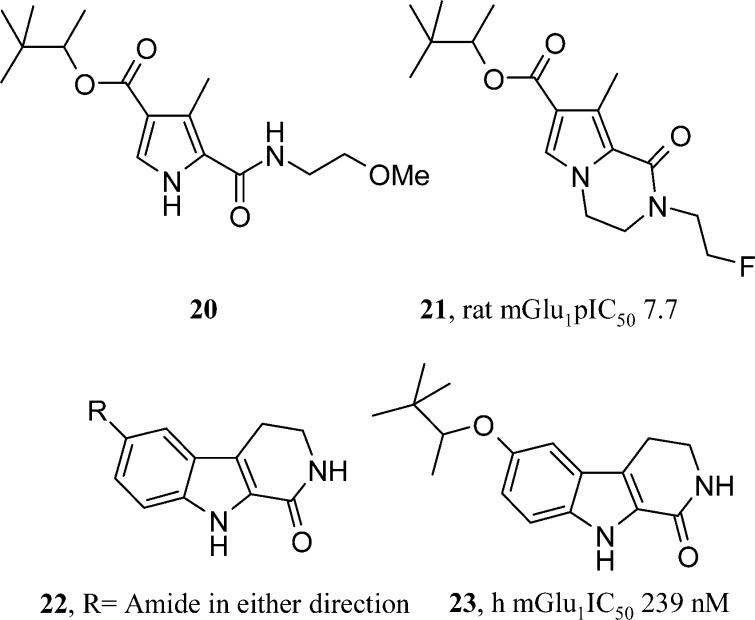
Micheli et al. and Di Fabio et al. have continued a line of publications in the mGlu1 field from Glaxo SmithKline (GSK).8 Two further papers have appeared post 2005 where the previously reviewed 2,4-dicarboxypyrroles have been used as starting points for new bi- and tricyclic templates. Studies in the earlier pyrrole-based series identified sub-10 nM mGlu1 antagonists (such as 20, Figure 4). However, few examples had been identified with sufficient metabolic stability to have useful pharmacokinetics. The monocyclic pyrrole core did present substituents containing multiple rotatable bonds or groups that could be seen as metabolic soft spots, such as benzylic methyl groups. The GSK group used their knowledge of tolerated substitution points on the pyrrole to cyclize open chain substituents to form bicyclic templates. Pyrrolo[1,2-a]pyrazinones are a novel mGlu1 antagonist template (such as 21, Figure 4).19 An N-alkylation of resulting pyrazinone was vital for activity. pIC50 values of 6.5−7.5 were recorded in SARs featuring mainly short aliphatic chains introduced through alkylation chemistry. Despite trailing the possibility of improved metabolic stability from this cyclization strategy, no data is presented on the outcome for this hypothesis. Having succeeded with one cyclization strategy, a second paper from GSK follows another possible cyclization of substituents, this time in two directions off the pyrrole.20 Once again, starting from their pyrrole template, positions 2 and 3 are cyclized to a lactam and 4 and 5 become a fused phenyl group. Unlike the earlier paper, the indole NH remains unfunctionalized. The resulting tricyclic template is a reduced β-carboline. SAR studies concentrated on an amide substituent constructed in both directions (22, Figure 4). All compounds were at least 5-fold less potent than the monocyclic pyrrole prototype. Four compounds (each >70 nM mGlu1 antagonists) were progressed to oral pharmacokinetic studies. The best compound (23, Figure 4) gave an oral bioavailability of 36%, while others suffered solubility problems and low exposure. Compound 23 had excellent CNS penetration and was fully efficacious at 30 mg/kg (p.o.) in both phases of the formalin pain model. In conclusion, good knowledge of the monocyclic pyrrole SAR allowed cyclization strategies to improve pharmacokinetics and CNS penetration. Further changes are still required to produce a sub-10 nM mGlu1 antagonist in this series.
Figure 4.
Glaxo SmithKline mGlu1 antagonists.
Merck-Banyu
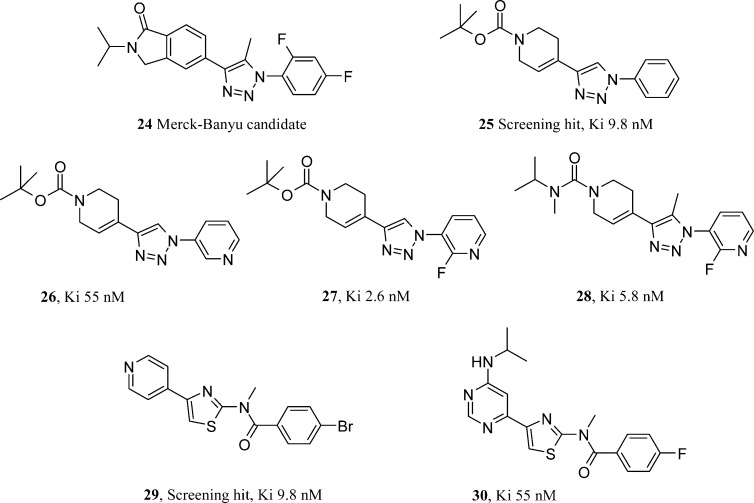
The Merck-Banyu group have published discovery and process chemistry papers within the last three years on mGlu1 antagonists. The compound selected for process chemistry discussion21 (24, Figure 5) was not mentioned in the discovery paper and most likely predates the discovery work published. All compounds are from the same 1,2,3-triazole series. In contrast to the lipophillic development candidate, the follow-up on the Banyu discovery publication22 addresses physicochemical property optimization from the outset. They initially identified a very potent mGlu1 lead compound 25 (Figure 5) from high throughput screening, 9.8 nM in their human mGlu1 assay. However, it did have a log D of >4. The modular nature of the synthesis allows variation of both halves of the molecule on either side of the triazole core. Despite some very potent analogues that retained a log D greater than 4, the Banyu group pursued the weaker 55 nM pyridine (26, Figure 5) due to its improved polarity and prospects. Suspecting the negative effect of the pyridine basicity on mGlu1 antagonism, a pKa modulating fluorine was added to the pyridine 2-position (27, Figure 5). This gave the most potent compound yet at 2.6 nM and rendered the compound neutral. The capping the BOC group on the tetrahydropyridine was deemed to be too acid labile, but as yet the group had been an integral part of the pharmacophore. Other carbamates and urea alternatives to BOC were weaker until a methyl group was added at the triazole 5-position (28, Figure 5). The authors were unable to explain this substituent cross talk, but it allowed the installation of a more attractive urea functionality to replace the previously required BOC group. Compound 28 had a very clean profile in broad ligand profiling, a log D of 2.1, and much improved solubility. This allowed it to be used in an efficacy study where good CNS penetration was achieved. Unfortunately, the urea was relatively rapidly demethylated through oxidative metabolism, making it an unlikely clinical candidate. Much like the Pfizer and Abbott groups, Banyu also ran a second series program in support of their lead compounds.23 They took a weaker (210 nM), nontriazole containing hit compound (29, Figure 5) with a view to appropriately balancing potency and physicochemical properties. Two thirds of the HTS hit was effectively retained throughout the program; however, the Banyu team developed the 4-pyridyl group into a more polar and synthetically versatile pyrimidine system. This also allayed fears over P450 inhibition from the 4-pyridyl group. This design proved not only to make the compound more active but also won the series the room to bring in a small pyrimidine substiuent without compromising cLogP. With any small amine derived substituent giving sub-5 nM compounds at the pyrimidine 6-position, N-isopropylamine analogue 30 (Figure 5) was unique in conferring both solubility and metabolic stability on the compounds as well. Solubility differences were most striking with 30 being 40-fold more soluble than its ethyl equivalent. Compound 30 was extensively profiled and found to be orally active at low doses as an antipsychotic in mouse in vivo models. Perhaps the most useful aspect of the compound comes back to its synthetic preparation. The N-methyl group on the amide is installed in the final step by an alkylation. This allows for a relatively straightforward synthesis of a labeled compound for use as a PET ligand.
Figure 5.
Merck-Banyu mGlu1 antagonists.
Schering-Plough
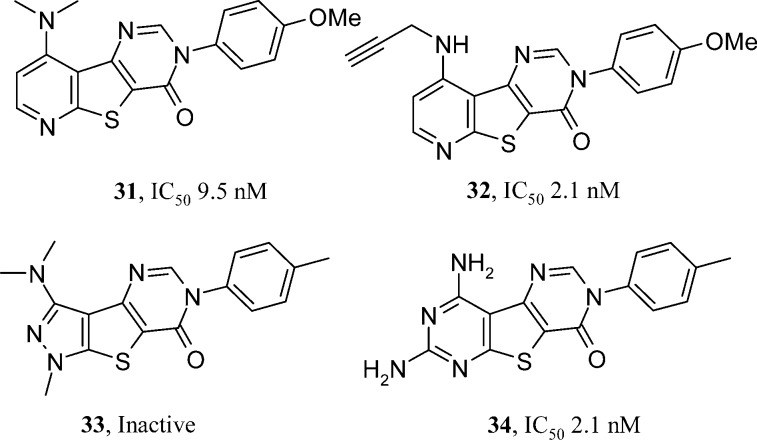
Schering-Plough have published two papers based on the exactly same triazafluorenone HTS hit (14, Figure 3) as Abbott (presumably sourced by both groups from a commercial file enrichment library). Given the same HTS starting position, it is interesting to see the directions pursued by two separate groups. As reviewed earlier, Abbott primarily looked at nitrogen regioisomers in the pyridine ring of the tricyclic core and the pyrimidinone N-substituent.16 Although Scherring-Plough also mined the pyrimidinone group (exculsively with aryl groups (31, Figure 6) unlike the cycloalkyls identified by Abbott), they expanded more on the dimethylamine group substituent in the lead and also inserted a nitrogen atom into the triazafluorenone pyridine ring. The resulting fused pyrimidine ring increased the template polarity and expanded synthetic options somewhat. The 2009 publication shares extensive pharmacology SAR.24 The conclusions were around the impact of allyl and propargylamine groups (32, Figure 6) replacing the dimethylamine of the lead. Use of either group routinely gave compounds of <10 nM. Two compounds were sufficiently robust to be dosed orally in a rat spinal nerve ligation model where they showed efficacy at <10 mg/kg. Despite their oral bioavailability, rat oxidative metabolism was still high. Incubation in rat microsomes and isolation of metabolites showed that, perhaps unsurprsingly, the activated CH2 next to the acetylene in 32 was vulnerable to oxidation and caused a subsequent N-dealkylation. Efforts to block this pathway were incompatible with the pharmacology, losing >100-fold in mGlu1 activity. A second publication from Sasikumar et al.25 makes some more dramatic changes to the A ring of the triazafluorenone core of the HTS hit 14. Five (such as 33, Figure 6) and seven membered rings were weak or inactive, as were compounds where the A ring was branched rather than fused. They returned to the optimized core identified in the previous paper, the tetraazfluorenone (32). This time they explored the position between the two nitogen atoms of the new pyrimidine in the core and, in placing a second NH2 group there, were able to do away with metabolically vulnerable functionality previously used to secure actvity elsewhere in the molecule (allyl/propargyl). With the allyl or propargyl groups no longer necessary, a free NH2 at the 9-position of the tricylic core was sufficient to keep compounds (such as 34, Figure 6) within 10-fold of the original activity. An improved rat pharmacokinetic profile was obtained with compound 34, but overall the best compound’s merits were equivalent to those from the first publication (compound 34 vs 32).
Figure 6.
Schering-Plough mGlu1 antagonists.
Merz
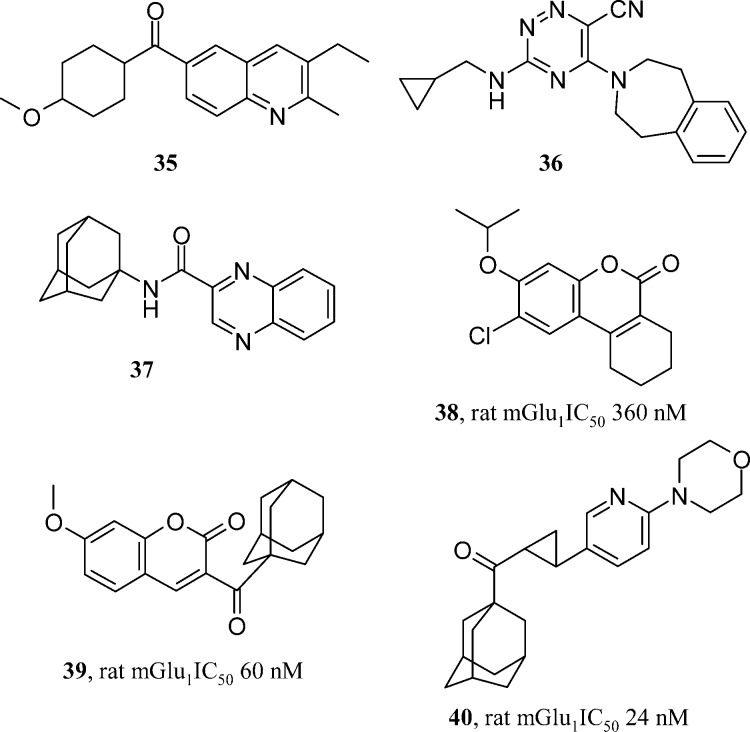
All of the series reviewed so far have depended on the large resources used in HTS efforts to identify lead matter, mainly carried out by major pharmaceutical companies. The Merz collaboration with academic groups in Latvia and Russia sought to use virtual screening methods based on commercial compounds and literature leads to identify novel series of their own. Merz have published three publications in the mGlu1 field in the last five years. One example used six probe compounds (examples of which are compounds 35−37, Figure 7) in a topological pharmacophore search of a virtual screening pool of hundreds of compounds.26 Of the compounds subsequently screened, six were found to have a Ki value of <15 μM. The most active was a 360 nM coumarin (38, Figure 7), which served as a basis for a library design based on this template. The majority of compounds did not beat the activity of the initial virtual screening derived hit. Only on addition of a large adamantyl substituent did activity go below 100 nM (39, Figure 7). The compound looks highly electrophilic and lipophilic. Although this shows that the virtual screening method was able to consturct a predictive pharmacophore, it did not account for desirable druglike properties in the resulting lead matter. Two further publications from Merz repeated this pharmacophore searching hypothesis, each time identifying novel chemical series; however, each time they needed to use exceptionally lipophilic substituents to drive mGlu1 antagonism below 100 nM (such as 40, Figure 7).27,28 It would be interesting to see how effective these pharmacophore methods could be when applied to more druglike input molecules, perhaps like some of those reviewed in this paper. Virtual screening of the more druglike file of company HTS collections may also be of interest with these methods, as the commercially available pool of compounds used by Merz for virtual screening may also be generally less attractive.
Figure 7.
Merz mGluR1 antagonists.
Perspective
There is a common theme to the publications reviewed in the previous sections: HTS hits generally progressed through to orally bioavailable tool compounds for successful use in rat disease models (mainly in pain). In many cases, relatively small changes to the HTS hits were required to optimize the initial lead in terms of mGlu1 potency. Larger changes proved costly to pharmacology, often directing design hypotheses toward preserving mGlu1 antagonism and setting aside some deeper running issues in the chemical series such as solubility, reactivity, toxicity risk, and metabolic liability. Perhaps only the Merck-Banyu and Pfizer groups looked to tackle multiple parameters from the HTS hit outset, resisting some tempting, but lipohilicity driven pharmacology SAR. Only the Banyu series appears to have yielded a disclosed clinical candidate. The dearth of clinical candidates and the number of chemical series being published, sometimes without supporting patents, suggests that there may be a mechanistic safety issue with mGlu1 antagonism. This is supported by independent reports from Johnson and Johnson,29 Pfizer,30 Merz,31 and Abbott32 related to behavioral side effects and low therapeutic indecies for a range of structurally differentiated mGlu1 chemotypes. Johnson and Johnson29 reported that full occupancy of mGlu1 reeceptors in the cerebellum and thalamus is associated with acquisition and retention impairment in a spatial water maze task. Merz and Abbott both profiled A-841720 in pain efficacy models while also assessing motor and cognitive function side effects.31,32 A-841720 worked at doses beginning at 1 mg/kg in the CFA pain model, going on to give full efficacy at 10 mg/kg. However, significant motor side effects were seen at these analgesic doses with the compound causing impaired cognitive function in water maze tests. In an oral presentation in 2006, Gibson of Pfizer reported that studies in their azaquinazoline series had also identified a low therapeutic index for mGlu1 antagonists in rodents (beam walking test).30 This was not a function of the chemical series, as an mGlu1 inactive azaquinazoline had no behavioral side effects in any species.
A peripherally selective mGlu1 antagonist could be a solution to the CNS side effects, but long-term, chronic administration may still have its risks. Han published on the effect of intra-articular mGlu1 and mGlu5 anatgonists in an inflammed rodent knee joint model in 2007 et al.33 mGlu5 antagonists looked the more promising of the two, but the studies were somewhat limited by the chemical tools chosen. Studies with a designed, non-CNS penetrant mGlu1 anatogonist could be of interest. This does not seem to have been strategy pursued by any group thus far.
Summary and Outlook
It would seem that the most significant advances in the mGlu1 antagonism field in the last five years have been around mechanistic safety learnings. The low therapeutic index between in vivo efficacy and cognitve function suggest that mGlu1 antagonism is not a viable mechanism for clinical use. The science of medicinal chemistry has produced many chemical series for exploring this vital safety risk through the design and optimization of tool compounds. When working with mechanisms that carry such a risk, like the potential cognitive implications for mGlu1 antagonists, medicinal chemists should perhaps simply explore with speed to find an adequate tool (that may not have the potential to be a drug). Although some of the chemical series reviewed may have had potential flaws in terms of being a druglike molecule for human candidate nomination, they served their purpouse in identifying the mGlu1 mechanistic nonviability via early in vivo safety studies. The broad chemical space that the mGlu1 pharmacophore represents has been tapped into in many different ways, by several different groups. The lack of mechanistic safety has been identified through more than one chemotype, giving us strong grounds to believe that, despite efficacy in certain animal disease models, we are unlikely to to see drugs emerge from mGlu1 antagonism for increasingly certain mechanistic safety concerns.
References
- Conn P. J.; Pin J.-P. (1997) Pharmacology and functions of metabotropic glutamate receptors. Ann. Rev. Pharmacol. Toxicol. 37, 205–237. [DOI] [PubMed] [Google Scholar]
- De Blasi A.; Conn P. J.; Pin J. P.; Nicoletti F. (2001) Molecular determinants of metabotropic glutamate receptor signaling. Trends Pharmacol. Sci. 22, 114–120. [DOI] [PubMed] [Google Scholar]
- Kunishima N.; Shimada Y.; Tsuji Y.; Sato T.; Yamamoto M.; Kumasaka T.; Nakanishi S.; Jingami H.; Morikawa K. (2000) Structural basis of glutamate recognition by a dimeric metabotropic glutamate receptor. Nature 407, 971–977. [DOI] [PubMed] [Google Scholar]
- Spooren W.; Ballard T.; Gasparini F.; Amalric M.; Mutel V.; Schreiber R. (2003) Insight into the function of Group I and Group II metabotropic glutamate (mGlu) receptors: behavioural characterization and implications for the treatment of CNS disorders. Behav. Pharmacol. 14, 257–277. [DOI] [PubMed] [Google Scholar]
- Conquet F.; Bashir Z. I.; Davies C. H.; Daniel H.; Ferraguti F.; Bordi F.; Franz-Bacon K.; Reggiani A.; Matarese V.; Conde F.; Collingridge G. L.; Crepel F. (1994) Motor deficit and impairment of synaptic plasticity in mice lacking mGlu1. Nature (London) 372, 237–243. [DOI] [PubMed] [Google Scholar]
- Gravius A.; Pietraszek M.; Schaefer D.; Schmidt W. J.; Danysz W. (2005) Effects of mGlu1 and mGlu5 receptor antagonists on negatively reinforced learning. Behav. Pharmacol. 16, 113–121. [DOI] [PubMed] [Google Scholar]
- Schkeryantz J. M.; Kingston A. E.; Johnson M. P. (2007) Prospects for Metabotropic Glutamate 1 Receptor Antagonists in the Treatment of Neuropathic Pain. J. Med. Chem. 50, 2563–2568. [DOI] [PubMed] [Google Scholar]
- Layton M. E. (2005) Subtype-selective noncompetitive modulators of metabotropic glutamate receptor subtype 1 (mGlu1). Curr. Top. Med. Chem. (Sharjah, United Arab Emirates) 5, 859–867. [DOI] [PubMed] [Google Scholar]
- Pellicciari R.; Filosa R.; Fulco M. C.; Marinozzi M.; Macchiarulo A.; Novak C.; Natalini B.; Hermit M. B.; Nielsen S.; Sager T. N.; Stensbol T. B.; Thomsen C. (2006) Synthesis and preliminary biological evaluation of 2′-substituted 2-(3′-carboxybicyclo[1.1.1]-pentyl)glycine derivatives as group I selective metabotropic glutamate receptor ligands. ChemMedChem 1, 358–365. [DOI] [PubMed] [Google Scholar]
- Jorgensen C. G.; Braeuner-Osborne H.; Nielsen B.; Kehler J.; Clausen R. P.; Krogsgaard-Larsen P.; Madsen U. (2007) Novel 5-substituted 1-pyrazolol analogues of ibotenic acid: Synthesis and pharmacology at glutamate receptors. Bioorg. Med. Chem. 15, 3524–3538. [DOI] [PubMed] [Google Scholar]
- Gasparini F.; Spooren W. (2007) Allosteric modulators for mGlu receptors. Curr. Neuropharmacol. 5, 187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen D. R.; Dodd P. G.; Gayton S.; Greener B. S.; Harbottle G. W.; Mantell S. J.; Maw G. N.; Osborne S. A.; Rees H.; Ringer T. J.; Rodriguez-Lens M.; Smith G. F. (2007) Structure-activity relationships of novel non-competitive mGlu1 antagonists: A potential treatment for chronic pain. Bioorg. Med. Chem. Lett. 17, 486–490. [DOI] [PubMed] [Google Scholar]
- van Hoorn W. P.; Bell A. S. (2009) Searching Chemical Space with the Bayesian Idea Generator. J. Chem. Inf. Model. 49, 2211–2220. [DOI] [PubMed] [Google Scholar]
- Mantell S. J.; Gibson K. R.; Osborne S. A.; Maw G. N.; Rees H.; Dodd P. G.; Greener B.; Harbottle G. W.; Million W. A.; Poinsard C.; England S.; Carnell P.; Betts A. M.; Monhemius R.; Prime R. L. (2009) In vitro and in vivo SAR of pyrido[3,4-d]pyramid-4-ylamine based mGlu1 antagonists. Bioorg. Med. Chem. Lett. 19, 2190–2194. [DOI] [PubMed] [Google Scholar]
- Harbottle G. W.; Feeder N.; Gibson K. R.; Glossop M.; Maw G. N.; Million W. A.; Morel F. F.; Osborne S.; Poinsard C. (2007) Microwave-assisted synthesis of mGlu1 ligands: carbon, nitrogen, and oxygen linked derivatives of pyrido[3,4-d]pyrimidin-4-ylamines. Tetrahedron Lett. 48, 4293–4296. [Google Scholar]
- Zheng G. Z.; Bhatia P.; Daanen J.; Kolasa T.; Patel M.; Latshaw S.; El Kouhen O. F.; Chang R.; Uchic M. E.; Miller L.; Nakane M.; Lehto S. G.; Honore M. P.; Moreland R. B.; Brioni J. D.; Stewart A. O. (2005) Structure−Activity Relationship of Triazafluorenone Derivatives as Potent and Selective mGlu1 Antagonists. J. Med. Chem. 48, 7374–7388. [DOI] [PubMed] [Google Scholar]
- Zheng G. Z.; Bhatia P.; Kolasa T.; Patel M.; El Kouhen O. F.; Chang R.; Uchic M. E.; Miller L.; Baker S.; Lehto S. G.; Honore P.; Wetter J. M.; Marsh K. C.; Moreland R. B.; Brioni J. D.; Stewart A. O. (2006) Correlation between brain/plasma ratios and efficacy in neuropathic pain models of selective metabotropic glutamate receptor 1 antagonists. Bioorg. Med. Chem. Lett. 16, 4936–4940. [DOI] [PubMed] [Google Scholar]
- Wang X.; Kolasa T.; El Kouhen O. F.; Chovan L. E.; Black-Shaefer C. L.; Wagenaar F. L.; Garton J. A.; Moreland R. B.; Honore P.; Lau Y. Y.; Dandliker P. J.; Brioni J. D.; Stewart A. O. (2007) Rapid hit to lead evaluation of pyrazolo[3,4-d]pyrimidin-4-one as selective and orally bioavailable mGlu1 antagonists. Bioorg. Med. Chem. Lett. 17, 4303–4307. [DOI] [PubMed] [Google Scholar]
- Micheli F.; Cavanni P.; Di Fabio R.; Marchioro C.; Donati D.; Faedo S.; Maffeis M.; Sabbatini F. M.; Tranquillini M. E. (2006) From pyrroles to pyrrolo[1,2-a]pyrazinones: A new class of mGlu1 antagonists. Bioorg. Med. Chem. Lett. 16, 1342–1345. [DOI] [PubMed] [Google Scholar]
- Di Fabio R.; Micheli F.; Alvaro G.; Cavanni P.; Donati D.; Gagliardi T.; Fontana G.; Giovannini R.; Maffeis M.; Mingardi A.; Tranquillini M. E.; Vitulli G. (2007) From pyrroles to 1-oxo-2,3,4,9-tetrahydro-1H-b-carbolines: A new class of orally bioavailable mGlu1 antagonists. Bioorg. Med. Chem. Lett. 17, 2254–2259. [DOI] [PubMed] [Google Scholar]
- Tsuritani T.; Mizuno H.; Nonoyama N.; Kii S.; Akao A.; Sato K.; Yasuda N.; Mase T. (2009) Efficient Synthesis of 1,4-Diaryl-5-methyl-1,2,3-triazole, A Potential mGlu1 Antagonist, and the Risk Assessment Study of Arylazides. Org. Process Res. Dev. 13, 1407–1412. [Google Scholar]
- Ito S.; Satoh A.; Nagatomi Y.; Hirata Y.; Suzuki G.; Kimura T.; Satow A.; Maehara S.; Hikichi H.; Hata M.; Kawamoto H.; Ohta H. (2008) Discovery and biological profile of 4-(1-aryltriazol-4-yl)-tetrahydropyridines as an orally active new class of metabotropic glutamate receptor 1 antagonist. Bioorg. Med. Chem. 16, 9817–9829. [DOI] [PubMed] [Google Scholar]
- Satoh A.; Nagatomi Y.; Hirata Y.; Ito S.; Suzuki G.; Kimura T.; Maehara S.; Hikichi H.; Satow A.; Hata M.; Ohta H.; Kawamoto H. (2009) Discovery and in vitro and in vivo profiles of 4-fluoro-N-[4-[6-(isopropylamino)pyrimidin-4-yl]-1,3-thiazol-2-yl]-N-methylbenzamide as novel class of an orally active metabotropic glutamate receptor 1 (mGlu1) antagonist. Bioorg. Med. Chem. Lett. 19, 5464–5468. [DOI] [PubMed] [Google Scholar]
- Sasikumar T. K.; Li Q.; Burnett D. A.; Greenlee W. J.; Li C.; Heimark L.; Pramanik B.; Grilli M.; Bertorelli R.; Lozza G.; Reggiani A. (2009) Tricyclic thienopyridine-pyrimidones/thienopyrimidine-pyrimidones as orally efficacious mGlu1 antagonists for neuropathic pain. Bioorg. Med. Chem. Lett. 19, 3199–3203. [DOI] [PubMed] [Google Scholar]
- Sasikumar T. K.; Qiang L.; Burnett D. A.; Greenlee W. J.; Li C.; Grilli M.; Bertorelli R.; Lozza G.; Reggiani A. (2010) A-ring modifications on the triazafluorenone core structure and their mGlu1 antagonist properties. Bioorg. Med. Chem. Lett. 20, 2474–2477. [DOI] [PubMed] [Google Scholar]
- Noeske T.; Jirgensons A.; Starchenkovs I.; Renner S.; Jaunzeme I.; Trifanova D.; Hechenberger M.; Bauer T.; Kauss V.; Parsons C. G.; Schneider G.; Weil T. (2007) Virtual screening for selective allosteric mGlu1 antagonists and structure-activity relationship investigations for coumarine derivatives. ChemMedChem 2, 1763–1773. [DOI] [PubMed] [Google Scholar]
- Noeske T.; Trifanova D.; Kauss V.; Renner S.; Parsons C. G.; Schneider G.; Weil T. (2009) Synergism of virtual screening and medicinal chemistry: Identification and optimization of allosteric antagonists of metabotropic glutamate receptor 1. Bioorg. Med. Chem. 17, 5708–5715. [DOI] [PubMed] [Google Scholar]
- Vanejevs M.; Jatzke C.; Renner S.; Mueller S.; Hechenberger M.; Bauer T.; Klochkova A.; Pyatkin I.; Kazyulkin D.; Aksenova E.; Shulepin S.; Timonina O.; Haasis A.; Gutcaits A.; Parsons C. G.; Kauss V.; Weil T. (2008) Positive and Negative Modulation of Group I Metabotropic Glutamate Receptors. J. Med. Chem. 51, 634–647. [DOI] [PubMed] [Google Scholar]
- Steckler T.; Oliveira A. F. M.; Van Dyck C.; Van Craenendonck H.; Mateus A. M. A.; Langlois X.; Lesage A. S. J.; Prickaerts J. (2005) Metabotropic glutamate receptor 1 blockade impairs acquisition and retention in a spatial Water maze task. Behav. Brain Res. 164, 52–60. [DOI] [PubMed] [Google Scholar]
- Gibson K. R. (2006) mGlu1 Antagonists as Potential Pain Therapeutics. In Nociception: Taking the Pain out of Drug Discovery, Society of the Chemical Industry, London. [Google Scholar]
- More L.; Gravius A.; Pietraszek M.; Belozertseva I.; Malyshkin A.; Shekunova E.; Barberi C.; Schaefer D.; Schmidt W. J.; Danysz W. (2007) Comparison of the mGlu1 antagonist A-841720 in rat models of pain and cognition. Behav. Pharmacol. 18, 273–281. [DOI] [PubMed] [Google Scholar]
- El-Kouhen O.; Lehto S. G.; Pan J. B.; Chang R.; Baker S. J.; Zhong C.; Hollingsworth P. R.; Mikusa J. P.; Cronin E. A.; Chu K. L.; McGaraughty S. P.; Uchic M. E.; Miller L. N.; Rodell N. M.; Patel M.; Bhatia P.; Mezler M.; Kolasa T.; Zheng G. Z.; Fox G. B.; Stewart A. O.; Decker M. W.; Moreland R. B.; Brioni J. D.; Honore P. (2006) Blockade of mGlu1 receptor results in analgesia and disruption of motor and cognitive performances: effects of A-841720, a novel non-competitive mGlu1 receptor antagonist. Br. J. Pharmacol. 149, 761–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S.; Kim J.; Yoon Y. W.; Lee M.-G.; Hong S. K.; Han H. C. (2007) The peripheral role of group I metabotropic glutamate receptors on nociceptive behaviors in rats with knee joint inflammation. Neurosci. Lett. 416, 123–127. [DOI] [PubMed] [Google Scholar]