Abstract
Dopaminergic systems have been described to functionally interact with the neuromodulatory peptide neurotensin. Employing fluorescence detected coimmunoprecipitation and radioligand binding experiments, we herein demonstrate that coexpression of dopamine D2L receptor and the neurotensin receptor subtype NTS1 leads to physical interaction and the formation of heteromers in transfected human embryonic kidney 293 cells. In this in vitro system, a trans-inhibitory effect on the agonist binding affinity of D2 was observed in presence of neurotensin. To correlate between the functional properties of dopaminergic agents and the magnitude of neurotensin-induced modulation of D2L binding affinities in cells coexpressing D2L and NTS1, a structurally diverse set of dopamine receptor agonists, partial agonists, and antagonists was tested. Ligand specific profiles indicating substantial bias between ligand efficacy and transmodulation were discovered, suggesting a heteromerization-based functional selectivity. In the presence of neurotensin, the novel D2 agonist FAUC 326 displayed a 34-fold decrease of binding affinity in cells coexpressing D2L and NTS1.
Keywords: Dopamine D2L receptor, neurotensin receptor 1, NTS1, G-protein coupled receptor, GPCR, coexpression, intramembrane receptor−receptor interaction, negative cooperativity, binding affinity, coimmunoprecipitation, dimer, heteromer
The ability to form spatial complexes allowing molecular interactions between receptor protomers facilitates cooperative effects and tissue specific control of neuronal activity. Thus, heterodimerization is discussed as an integral feature of G-protein coupled receptor (GPCR) mediated signal transduction.1−3 Intramembrane receptor–receptor interactions of associated protomers may modify binding and activating properties of GPCR ligands.4−7 Using Förster resonance energy transfer (FRET) technique, a recent study on the interactions between α2A-adrenergic and μ-opioid receptors demonstrated a trans-inhibitory effect between the protomers. In detail, morphine binding to the μ-opioid receptor triggered the conformational changes in the norepinephrine-occupied α2A-adrenergic receptor leading to the inhibition of its signaling.8 Physical interactions between δ- and μ-opioid receptors were suggested to modulate μ-mediated tolerance and dependence9 when the development of bivalent ligands was constituted as a useful approach to investigate changes in receptor properties as a consequence to dimerization.10,11 Heterodimerization is also a highly relevant phenomenon for dopamine receptor mediated signaling.12,13 Demonstrating the development of a new complex with enhanced functional activity through hetero-oligomerization, the physical interaction between the dopamine D2L and the somatostatin SST5 receptor was investigated via radioligand binding studies, functional assays, and FRET microscopy.14 The hetero-oligomerization of D2L and SST5, known for their colocalization in the central nervous system (CNS), led to a synergistic effect on binding and signaling as molecular cross-talk and the correlation between activated receptor function and oligomerization was stressed.
Dopaminergic systems have been also described to functionally interact with the neuromodulatory peptide neurotensin (NT, pE-L-Y-E-N-K-P-R-R-P-Y-I-L)15 which is suggested to play a role in the pathophysiology of brain diseases including schizophrenia, Parkinson’s disease. and Morbus Alzheimer.16−18 Thus, neurotensin was shown to negatively alter binding affinity of the dopamine receptor agonist [3H]N-n-propyl-nor-apomorphine in specific brain areas.19−25 However, biomolecular interactions and a putative heteromer formation between dopamine receptors and neurotensin receptors have not been investigated yet.
Employing fluorescence detected coimmunoprecipitation and radioligand binding experiments, we herein demonstrate that coexpression of dopamine D2L receptor and the neurotensin receptor subtype NTS1 in human embryonic kidney cells (HEK293 cells) leads to physical interaction at a molecular level. In this in vitro system, a trans-inhibitory effect on the agonist binding affinity of D2 was observed in the presence of neurotensin. A biochemical fingerprint26 of the D2L-NTS1 heteromer was explored by investigating the effect of both neurotensin and NTS1 on the D2 receptor binding properties of different dopaminergics. Mutagenesis studies on NTS1 in extracellular loop 1 (EL1) were used to investigate that the trans-modulatory effect is exerted by a specific receptor–agonist interaction. Structurally diverse ligands were investigated to determine the relationship between the intrinsic activity of dopaminergics and the modulatory effect of NTS1–neurotensin binding on the affinity of dopaminergics.
Results and Discussion
Colocalization and Heteromer Formation
As an important necessity for the functional interaction between receptor heteromers, a sufficient expression of the interaction partners in the cell membrane is prerequisite. To control the membrane expression of D2L and NTS1, fusion proteins C-terminally tagged by eYFP and eCFP,27 respectively, were employed. The cellular distribution was monitored by confocal microscopy with appropriate excitation and emission wavelengths for the two fluorescent proteins. The fluorescently tagged NTS1 and D2L receptors coexpressed in HEK293 cells displayed colocalization of both GPCRs in the plasma membrane, thus allowing the formation of a new functional entity (Figure 1).
Figure 1.
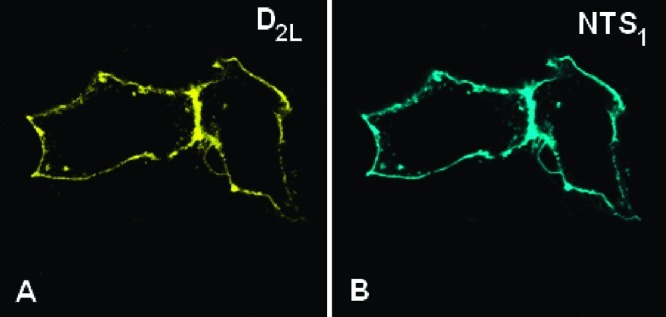
D2L and NTS1 receptors colocalize in the plasma membrane. To determine the cellular distribution of D2L and NTS1 receptors in the HEK293 cells, the cells were transiently transfected with the eYFP-tagged D2L and eCFP-labeled NTS1 receptors. The images were acquired by confocal microscopy. The D2L-eYFP construct (left, A) was shown to be colocalized with NTS1-eCFP (right, B) in the plasma membrane.
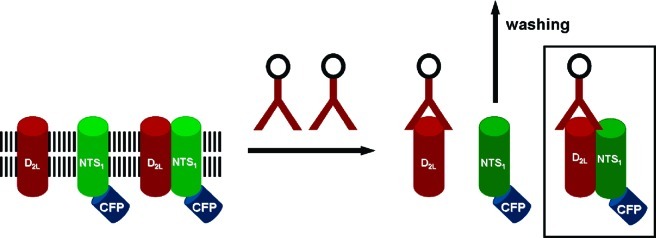
Coimmunoprecipitation followed by Western blotting is a general approach to investigate the formation of physically interacting receptor heteromers.28−30 To circumvent cross-reactions that were associated with only moderate specificity of a commercially available NTS1 antibody, we investigated the formation of D2L-NTS1 heteromers by immunoprecipitation with a D2L antibody and detection of the coprecipitated system by fluorescence spectroscopy. The specificity of the antibody for the D2L receptor was proven by Western blotting of HEK cells transiently expressing D2L receptor revealing a band at 100 kD that corresponds to the dimeric D2L receptor.31 For the fluorescence-assisted coimmunoprecipitation, a C-terminally eCFP-tagged NTS1 was cotransfected with the D2L receptor in HEK293 cells. After lysis, precipitation with the D2L-antibody-bead complex, and washing, heteromer formation was evaluated by detecting the emission spectrum of the eCFP-derived fluorophore (Scheme 1). The quantification of the emission spectra was performed by calculating the area under the curves (AUC).
Scheme 1. General Principle of Fluorescence-Detected Coimmunoprecipitation.
The D2L receptor and the NTS1-eCFP construct were transiently transfected in HEK293 cells. After cell lysis, coimmunoprecipitation of the fluorescently labelled NTS1 with the D2L receptor bound by the anti-D2L-antibody bead complex resulted in an isolation of a fluorescence-emitting complex.
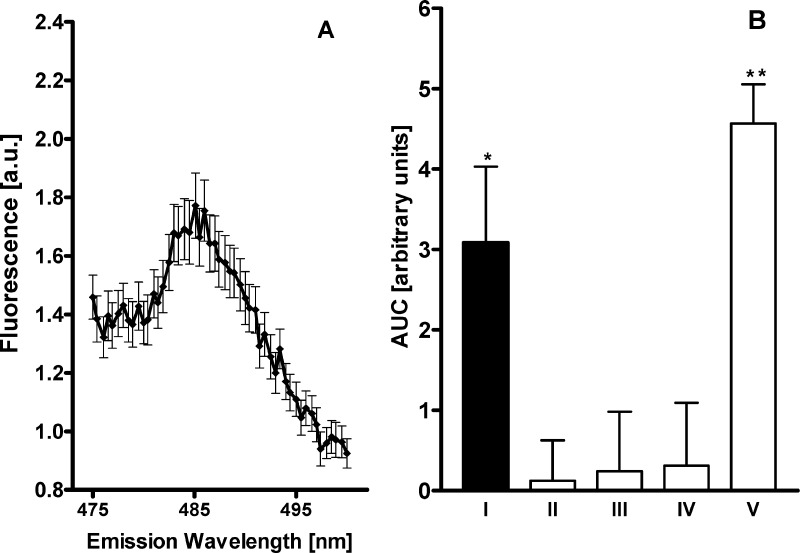
To determine the maximal fluorescence intensity obtained by the fluorescence-detected coimmunoprecipitation, the fluorophore was covalently attached to the D2L receptor (D2L-eCFP). This construct was transiently coexpressed with unlabeled NTS1 in HEK cells. Because we used an antibody specifically recognizing D2L, the detected signal was independent from physical interaction between D2L and NTS1 and thus represents the maximal expected signal (Figure 2A). To ensure that the increase in the detected fluorescence could be assigned to the physical interaction between D2L and NTS1-eCFP, the basal fluorescence of lysis buffer and the protein A/G agarose beads was measured. In contrast to the intensity found for the expression of the D2L-eCFP receptors, these controls displayed fluorescence curves with substantially lower intensity (4–10%) (Figure 2B). The expression of cytosolic eCFP together with D2L and NTS1 did not result in an increased intensity of a fluorescence signal. We observed an increase in the fluorescence intensity solely by the probe containing coexpressed D2L and NTS1-eCFP. Coexpression of D2L and NTS1-eCFP represented 67% of the signal obtained for the immobilized D2L-eCFP receptor. Thus, a stable physical interaction between the two receptor protomers and the formation of a stable D2L-NTS1 heteromer is inferred by the above-mentioned data.
Figure 2.
Fluorescence-detected coimmunoprecipitation assay indicated a stable physical interaction between the receptor NTS1-eCFP and D2L protomers. (A) Emission spectrum from 475 to 500 nm determined after excitation at 430 nm of lysed HEK293 cells coexpressing D2L and the NTS1-eCFP fusion protein. (B) Area under the curve with error bars were calculated from the measured eCFP emission spectra (from 480 to 490 nm) after immunoprecipitation with mouse anti-D2 antibody of six independent experiments of each sample of the (I) coexpression of D2L and NTS1-eCFP; (II) control consisting of lysis buffer with protein A/G agarose beads; (III) single expression of NTS1-eCFP; (IV) coexpression of D2L with NTS1 and cytosolic eCFP; and finally (V) coexpression of D2L-eCFP and NTS1. (* p < 0.01 and ** p < 0.001 compared to control in ANOVA followed by Tukey test).
Modulation of Ligand Binding Affinity across the Receptor Dimer Interface
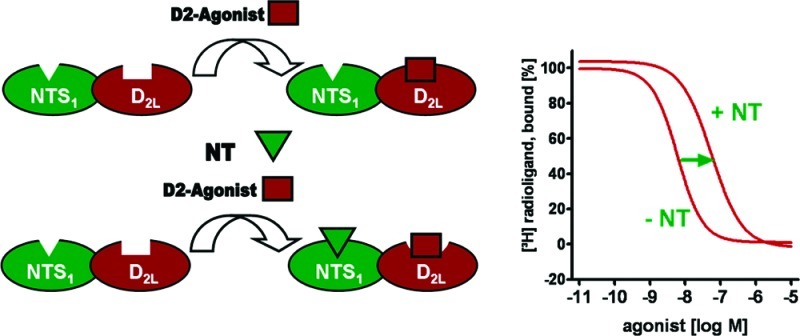
Investigating neuronal tissue, a large body of evidence indicated a negative cooperativity of NT on the receptor binding of dopamine32 and the D2 agonist N-n-propyl-nor-apomorphine19,21,23 apparent in a 20–50% increase of Ki or KD values, respectively. To better understand the molecular origins of these observations, a recombinant in vitro test system was established expressing the long splice variant of the human dopamine D2 receptor (D2L) and the human neurotensin receptor 1 (NTS1). To achieve approximately equimolar expression levels of receptors, transient transfection of HEK293 cells with equal amounts of cDNAs for D2L and NTS1 was performed. The radioligand displacement studies were run on membrane preparations from cells expressing either D2L only, NTS1 only or D2L and NTS1 coexpressed receptors. Binding properties of the transiently expressed NTS1 receptors were determined using [3H]neurotensin. The dopamine receptor agonist 7-OH-DPAT and the antagonist spiperone were not able to alter the NTS1 affinity of neurotensin in the membrane preparation of D2L and NTS1 coexpressing cell line. Thus, the D2L receptor in active or inactive conformation bound by agonist or antagonist, respectively, is not able to disturb the recognition of the NTS1 agonist binding site by its ligand neurotensin.
To determine modulatory effects of neurotensin on D2L receptor agonist affinity, KD values of the D2 agonist [3H]7-OH-DPAT were determined. 7-OH-DPAT is structurally very similar to the parent neurotransmitter dopamine and was therefore expected to simulate the natural receptor ligand interactions most appropriately. Employing the test system expressing D2L and NTS1, homologous competition experiments resulted in a KD value of 7.2 nM (Table 1), which was very similar to the dissociation constants that we obtained from a membrane preparations of D2L expressing cell line and from a mixture of membrane preparations from cells containing singly expressed D2L or NTS1 receptors (8.4 and 7.9 nM, respectively). Homologous competition experiments with membrane preparations of a coexpressed cell line revealed a KD value of 57 nM after addition of neurotensin (10 μM). This indicates an 8-fold decrease of binding affinity. Highly similar Bmax values confirmed that the portion of receptors in the high affinity state was not influenced by the affinity modifying neurotensin-induced effect. Singly expressed D2L receptors and mixed membrane preparations singly expressing D2L or NTS1 did not show any alterations of affinity and receptor density after the addition of neurotensin. To investigate if neurotensin can modulate dopamine receptor antagonist binding,33 saturation experiments were performed using the D2 antagonist [3H]spiperone (Supporting Information). The data displayed that the affinity of D2 antagonists remained unchanged.
Table 1. Influence of Neurotensin on [3H]7-OH-DPAT Binding Shown as KD Values ± SEM [nM]a.
| coexpression of D2L and NTS1 |
D2L |
mixture of singly expressed NTS1 and D2L |
||||
|---|---|---|---|---|---|---|
| KD [nM] | Bmax [fmol/mg] | KD [nM] | Bmax [fmol/mg] | KD [nM] | Bmax [fmol/mg] | |
| 7-OH-DPAT | 7.2 ± 0.62 | 550 ± 43 | 8.4 ± 0.65 | 530 ± 190 | 7.9 ± 0.74 | 790 ± 150 |
| 7-OH-DPAT + NTb | 57 ± 7.5c | 610 ± 110 | 8.9 ± 0.57 | 340 ± 90 | 9.2 ± 0.80 | 850 ± 290 |
KD values derived from 12–25 individual experiments each done in triplicate were determined on membrane preparations of transiently transfected HEK293 cells using the radioloigand [3H]7-OH-DPAT.
Experiments were performed in the presence of 10 μM neurotensin.
Significances were calculated in an unpaired t test compared to control * p < 0.001.
Aiming to better simulate physiological conditions, lower concentrations of neurotensin were investigated for its ability to influence the agonist binding of [3H]7-OH-DPAT at the D2L-NTS1 heteromer. We observed an 8-fold and a 3-fold increase of KD values of [3H]7-OH-DPAT after incubation with 100 and 10 nM NT, respectively (Figure 3). Because the neurotensin concentration of 10 μM did not result in a stronger effect than 100 nM, we concluded that the neurotensin induced effect is saturable.
Figure 3.
Right-shifted curves of [3H]7-OH-DPAT binding of Table 1 at the D2L-NTS1 heteromer and a Hill slope of −0.8 demonstrate the negative cooperativity induced by neurotensin. Homologous competition curves with error bars representing the SEM are shown.
Because the C-terminal hexapeptide NT(8−13) is considered as an endogenously active form of neurotensin,16,34 the influence of NT(8−13) on D2 agonist binding was evaluated. Application of 1, 10, and 100 nM NT(8−13) induced a 3-fold, 6-fold, and 17-fold reduction of 7-OH-DPAT binding affinity, respectively, which is in accordance with recent investigations using rat neostriatum.35 To corroborate that the negative cooperative effect depends on a specific NTS1–agonist interaction, the influence of the nonpeptidic NTS1 ligand SR4869236,37 on [3H]7-OH-DPAT binding was investigated. As expected,36 NTS1 binding of the nonpeptidic antagonist did not alter the agonist binding properties of [3H]7-OH-DPAT. The parallel application of the agonist neurotensin and the antagonist SR48692, both used in concentrations of 100 and 400 nM, respectively, abolished the neurotensin-induced decrease of dopamine agonist binding. Obviously, neurotensin induced transmodulation depends on a receptor–ligand interaction at the primary binding site, and SR48692 was able to inhibit an agonist–dependent conformational change at the D2L–NTS1 interface.
To confirm that the allosteric effect across the receptor–receptor interface is exerted by specific agonist binding, further control experiments were performed. In earlier studies, we described two mutations in the extracellular loop of the NTS1 (W129A and W134A) displaying a complete loss of agonist binding of [3H]NT(8−13) but exhibiting retention of antagonist binding of [3H]SR48692.38 As evident from the images acquired by confocal microscopy, these mutant NTS1 receptors were properly expressed and colocalized with the D2L receptor in the plasma membrane of transiently transfected HEK cells. For coexpressed D2L/NTS1W134A as well as the D2L/NTS1W129A receptors, no binding of [3H]neurotensin was detectable. The D2L affinities of spiperone and 7-OH-DPAT in the absence and presence of 100 nM neurotensin remained unchanged in these systems (Table 2).
Table 2. Binding Data of Coexpressed NTS1 Mutant Receptors with D2L Display No Susceptibility toward the Application of Neurotensina.
| [3H]neurotensin |
[3H]spiperone |
[3H]7-OH-DPAT |
[3H]7-OH-DPAT with 100 nM neurotensin |
|||||
|---|---|---|---|---|---|---|---|---|
| KD | Bmax | KD | Bmax | KD | Bmax | KD | Bmax | |
| D2L-NTS1W129A | no specific binding | 0.10 ± 0.002 | 4400 ± 360 | 4.3 ± 0.15 | 420 ± 43 | 6.2 ± 0.61 | 320 ± 55 | |
| D2L-NTS1W134A | no specific binding | 0.08 ± 0.001 | 4100 ± 380 | 4.0 ± 0.01 | 480 ± 70 | 4.1 ± 0.42 | 300 ± 60 | |
KD values ± SEM [nM] and Bmax values ± SEM [fmol/mg] were determined in 3–4 individual experiments each done in triplicate.
Based on the obtained experimental data, we concluded that the long splice variant of the D2 receptor and the neurotensin receptor NTS1 form a heterodimer with novel functional properties; the binding affinity of the D2 receptor agonist decreases only in the presence of the NTS1 receptor agonists neurotensin and NT(8−13). Thus, the negative cooperative effect of neurotensin depends on a specific NTS1–ligand interaction at the orthosteric neurotensin binding site, obviously leading to cross-receptor interactions within the heterodimer that modulate D2L agonist binding.
Transmodulatory Effects on Diverse Dopamine Receptor Ligands
To correlate between the functional properties of dopaminergic agents in D2L expressing cells and the magnitude of neurotensin-induced modulation of D2L binding affinities in cells coexpressing D2L and NTS1, a structurally diverse set of dopamine receptor ligands consisting of aminoindanes, phenylpiperazines, and phenylpiperidines with full agonist, partial agonist, and antagonist properties, respectively, was tested and compared to the endogenous ligand dopamine and the reference agonist 7-OH-DPAT (Figure 4). Ligand efficacy was determined by measuring the ability of the test compound to modulate the D2L receptor mediated inhibition of cAMP accumulation and the stimulation of extracellular signal-regulated kinase 1/2 (ERK1/2) phosphorylation. As a representative for dopamine D2 receptor antagonists, we chose the classical antipsychotic drug haloperidol. Haloperidol behaved as an antagonist in both functional assays and was not able to significantly alter binding affinity of the D2L receptor in our D2L/NTS1 coexpressing system (Table 3). From the family of phenylpiperazines, the atypical antipsychotic drug aripiprazole and two structurally related congeners FAUC 321 and FAUC 335 were evaluated.39,40 In fact, analysis of the biological data revealed that the test compounds behaved very differently. The 2,3-dichlorophenyl-piperazine aripiprazole, which is known for its partial agonist activity at pre- and postsynaptic D2 receptors41,42 and for functional selective behavior,43 displayed a maximal efficacy of 62% in the functional assays, but it was not able to significantly modulate D2 binding affinity in the D2L/NTS1 coexpressing cell line. The 2-methoxy-phenylpiperazine derivative FAUC 321 exhibited only 35% and 55% in the functional assays, but it was able to reduce dopamine receptor binding 3.7-fold in the coexpressed system. This effect was even stronger for the thiomethyl-substituted analogue FAUC 335 when a ratio of dopamine receptor affinities of 12 indicated a heterodimer-specific allosteric effect that was superior to the full agonist dopamine (Ki+NT/Ki-NT = 9.3). This shows that, even within one family of compounds, ligand efficacy and the heteromer-promoted affinity modulation do not correlate with each other. Whereas aripiprazole and FAUC 335 will have very similar biological activity in D2L expressing tissue of the CNS, the two test compounds will behave differently in brain areas coexpressing D2L and NTS1 leading to a heteromerization-based functional selectivity. Besides this, subtle structural variations can cause substantially different modulatory potencies.
Figure 4.
Chemical structures of the ligands investigated in this study.
Table 3. Affinities on the D2L-NTS1-Heteromer and Functional Properties at the D2L Receptor of the Dopamine Receptor Agonists and Antagonists.
| Ki [nM]a | Ki [nM] + 100 nM NTa | ratio Ki+NT/Ki –NTb | cAMP efficacy in % (pEC50)d | ERK1/2 efficacy in % (pEC50) | |
|---|---|---|---|---|---|
| dopamine | 8.8 ± 1.2 | 82 ± 3.3 | 9.3 | 111 ± 5 (7.7 ± 0.10) | 106 ± 4 (7.8 ± 0.11) |
| 7-OH-DPAT | 7.2 ± 0.62 | 62 ± 11 | 8.6 | 84 ± 4 (8.6 ± 0.11) | 98 ± 2 (9.0 ± 0.08) |
| aripiprazole | 1.7 ± 0.13 | 2.3 ± 0.05 | 1.3 | 62 ± 5 (7.5 ± 0.15) | 62 ± 5 (6.9 ± 0.17) |
| haloperidol | 0.68 ± 0.04 | 1.4 ± 0.21 | 2.0 | –8 ± 1 (8.7 ± 0.27) | n.a. |
| 2-N,N-dipropylaminoindane | 5.0 ± 1.8 | 22 ± 5.2 | 4.4 | 89 ± 10 (7.9 ± 0.8) | n.d. |
| FAUC 326 | 0.32 ± 0.07 | 11 ± 2.4 | 34 | 114 ± 10 (8.7 ± 0.91) | 95 ± 4 (9.0 ± 0.9) |
| FAUC 335 | 0.38 ± 0.09 | 4.7 ± 1.3 | 12 | 46 ± 4 (7.9 ± 0.19) | 55 ± 4 (7.5 ± 0.11) |
| FAUC 321 | 0.86 ± 0.12 | 3.2 ± 0.33 | 3.7 | 35 ± 2 (8.1 ± 0.17) | 55 ± 3 (8.4 ± 0.14) |
The affinities of investigated substances were determined on membrane preparations of transiently transfected HEK293 cells coexpressing D2L and NTS1 using [3H]7-OH-DPAT for competition experiments. Data are derived from 3–8 individual experiments each done in triplicate.
The ratio Ki+NT/Ki-NT indicates the changes in affinity.
KD value in [nM].
Functional data of test compounds presented as pEC50 values and efficacies (%) were determined by measuring the D2L receptor mediated the inhibition of cAMP accumulation and in the stimulation of ERK1/2 phosphorylation. Pooled data of 3–9 experiments performed in triplicate are shown as mean values ± SEM. Legend: n.a., not available; n.d., not determined.
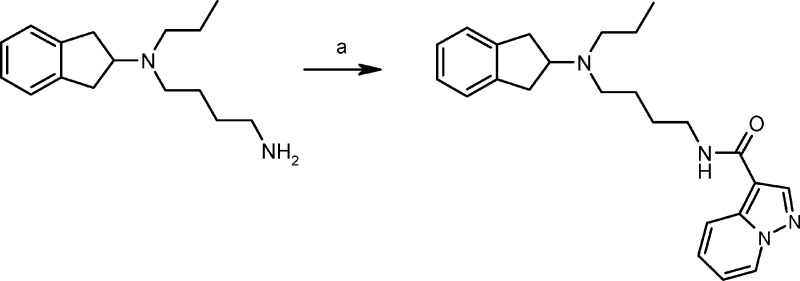
Finally, we evaluated 2-N,N-dipropylaminoindane44 and its long-chain analogue FAUC 326 for their biological profiles. As a derivative of the full agonist 2-N,N-dipropylaminoindane,45 FAUC 326 was newly synthesized for this study because we intended to further improve D2 receptor binding affinity by formally attaching an affinity-generating appendage to one of the propyl substituents,46 which should lead to an energetically favorable interaction with the lipophilic microdomain of the D2 receptor according to our very recent docking studies46 (Scheme 2).
Scheme 2.
Reagents and conditions: (a) pyrazolo[1,5-a]pyridine-3-carboxylic acid, TBTU, DIPEA, CH2Cl2, 4 h, RT.
The full agonists 2-N,N-dipropylaminoindane and FAUC 326 showed significantly distinct modulatory effects on D2 binding in the coexpressing cell line. Whereas neurotensin was able to reduce the binding affinity of 2-N,N-dipropylaminoindane by a factor of 4.4, a 34-fold decrease of binding affinity was observed for the novel D2 agonist FAUC 326.
For the characterization of the influence of NT on the signaling of D2 receptor, we have chosen the firefly (Photinus pyralis) luciferase based PathDetect Elk1 gene reporter assay. The transcription factor Elk-1 is phosphorylated and activated by p42/p44 MAPK.47 Employing a D2L-NTS1 coexpressing cell line, D2L promoted signaling of 7-OH-DPAT and FAUC 326 was observed in a dose dependent manner when coapplication of neurotensin (10 nM) led to a 5- to 6-fold improvement of EC50 values. Further investigations will be necessary to decipher and to better understand the functional system.
Conclusion
Employing fluorescence detected coimmunoprecipitation and radioligand binding experiments, we herein demonstrate that coexpression of dopamine D2L receptor and the neurotensin receptor subtype NTS1 leads to physical interactions at a molecular level in transfected human embryonic kidney 293 cells. In this in vitro system, a trans-inhibitory effect on the agonist binding affinity of D2 was observed in the presence of neurotensin. To correlate between the functional properties of dopaminergic agents and the magnitude of neurotensin-induced modulation of D2L binding affinities in cells coexpressing D2L and NTS1, a structurally diverse set of dopamine receptor agonists, partial agonists, and antagonists was tested, indicating that significant intrinsic activity is necessary. However, ligand specific profiles indicating substantial bias between ligand efficacy and transmodulation were discovered. In the presence of neurotensin, the novel D2 agonist FAUC 326 displayed a 34-fold decrease of binding affinity in cells coexpressing D2L and NTS1. The physiological implication of different NT and NTS1 concentrations depending on the brain region may lead to an individual in vivo activity for drugs. Whereas aripiprazole and FAUC 335 will have very similar biological activity in D2L expressing tissue of the CNS, the two test compounds will behave differently in brain areas coexpressing D2L and NTS1. Such a heteromerization-based functional selectivity might play an important role in neuropathophysiology of Parkinson’s disease. With regard to the increased NT brain levels in the substantia nigra of parkinsonian patients,48 the transmodulatory effects described might have an impact of the therapy with classical dopamine receptor agonists. More efficacious drugs for the treatment of Parkinson’s disease are suggested to have low sensitivity toward NT-induced cross-receptor interactions. The D2L-NTS1 heteromer should be considered as a drug target for the development of novel anti-Parkinson drugs.
Methods
Materials
All cell culture materials were purchased from Invitrogen LifeTechnologies (Karlsruhe, Germany). [3H]Spiperone (102–114 Ci/mmol) and [3H]7-OH-DPAT (157–163 Ci/mmol) were purchased from GE Healthcare (Freiburg, Germany), and [3H]neurotensin (100–112 Ci/mmol) was purchased from PerkinElmer (Rodgau, Germany). cAMP-Glo assay was purchased from Promega (Mannheim, Germany). Dopamine (3,4-dihydroxyphenethylamine), spiperone, haloperidol, 7-OH-DPAT (R-(+)-7-hydroxy-2-(N,N-di-n-propylamino)tetraline hydrobromide), aripiprazole, and other substances were purchased from Sigma (Steinheim, Germany), unless otherwise stated.
Expression Vectors
The wild type hNTS1 and hD2L cDNA was purchased from the UMR cDNA Resource Center and subcloned into a pcDNATM3.1(+) eukaryotic expression vector (Invitrogen, Karlsruhe, Germany) using EcoR1/Xba1 restriction sites.38,46 Oligonucleotidic primers were purchased from Biomers.net (Ulm, Germany). Restriction enzymes were purchased from New England Biolabs (Frankfurt am Main, Germany). NTS1-eCFP-construct was done by the PCR-based mutagenesis method described by Ko and Ma49 with primers for the insertion of the Sap1 restriction site and the removal of the stop codon. NTS1-cDNA was modified using the start primer CGACTCACTATAGGGAGACCCAA and the reverse end primer AAGCTCTTCATGCGTA-CAGCGTCTCGCGGGT. The eCFP-cDNA fragment was obtained by the use of AAGCTCTTCTGCAATGGTGAGCAAGGGCGAG and GCAACTAGAAGGCACAGTCGAGG as start and end primers, respectively. The final cloning in the pcDNATM3.1 vector was performed using Nhe1/Sap1/Apa1 restriction sites. PCR for the D2L-eYFP construct was performed using the D2L cDNA with the forward primer GCTAGCGTTTAAACTTAAGCTTGG and the reverse oligonucleotide CCTCTAGACTCGCCGCGGCAGTGGAG and for the eYFP fragment the primers GCTAGCGCTACCGCGGGGCAGCAATG and CAGCTTGAGTAGCCCCTCTAGAT-TACTAGGCGGCGGTCA. The D2L PCR product was digested with the restriction enzymes HindIII and SacII. SacI and XbaI were utilized for the eYFP PCR fragment. Both digested fragments were ligated in the vector template of the wild type D2L receptor digested with HindIII and XbaI. Fidelity of PCR amplification and introduction of the fluorescence protein tags in the receptor cDNAs were confirmed by sequencing by LGC Genomics (Berlin, Germany)
Cell Culture
Human embryonic kidney cells (HEK293) were grown in DMEM-Ham’s F12 medium (1:1), supplemented with 10% fetal calf serum, 100 U/mL penicillin G, 100 μg/mL streptomycin, and glutamine (2 mM). All cells were grown at 37 °C under a humidified atmosphere with 5% CO2.
Confocal Microscopy
For the preparation of the microscope slides, the coverslips were coated using 0,01% Poly-l-Lysin-Solution (Sigma Adrich, Steinheim, Germany) and HEK293 cells were grown in 6-well plates for 24 h with 10 000 cells/well. Double transfections were done with 2 μL of cDNA per well at a 1:1 ratio of the cDNAs using TransIT-293 transfection reagent (MoBiTec, Göttingen, Germany).
After 48 h cells, were fixed in 4% paraformaldehyde for 30 min and mounted on slides using a PBS/glycerol mixture 1:9 as mounting medium.50 Slides were sealed using colorless nail polish to avoid desiccation. The images were acquired using a Leica TCS SP5 confocal microscope (63× oil objective) of Prof. T. Stamminger at the Institute for Clinical & Molecular Virology (University of Erlangen-Nuremberg, Germany). Images of eCFP were acquired with laser excitation at 405 nm (20% power) and emission collection from 470 to 490 nm. eYFP samples were recorded with excitation by an argon laser at 514 nm (30% power), and the emission was collected from 510 to 530 nm. Fluorescence bleedthrough was minimized by adjusting the pinhole and photomultiplier tubes and confirmed through sequential laser scans.
Fluorescence-Detected Coimmunoprecipitation
HEK293 cells were transiently transfected with 24 μg of DNA per Petri dish (145 × 25 mm) using TransIT-293 transfection reagent (Mirus Bio Corporation). Two days after transfection, the cells were washed once with PBS. The cells (1.5 × 106) were then abraded with a cell scraper into 800 μL of IP-lysis buffer (10× cell lysis buffer, Cell Signaling with 0.5% sodium deoxycholate) and sonicated on ice (10 strokes, 60% amplitude, 30 kHz). The lysate was incubated for 1 h at 4 °C, and insoluble material was removed by centrifugation at 15 000g for 4 min at 4 °C. The supernatant was precleared using 10 μL of protein A/G Plus agarose beads (0.5 mL agarose/2.0 mL, Santa Cruz Biotechnologies, Santa Cruz) for 2 h at 4 °C. The beads were removed by a centrifugation step at 3000g and 4 °C for 30 s, and the supernatant was incubated for 2 h at 4 °C with 1.4 μg of the mouse anti-D2 antibody B-10 (Santa Cruz Biotechnologies, Santa Cruz) against amino acids 1–50 at the N-terminus of the dopamine receptor. After the addition of 25 μL of protein A/G Plus agarose beads, the mixture was incubated overnight at 4 °C under constant rotation and subsequently centrifuged at 3000 g for 30 s at 4 °C. The pellet was washed three times with ice-cold IP-lysis buffer. The beads were resuspendend in 200 μL lysis buffer and transferred in black 96-well plates. The fluorescence emission spectra of the samples were determined using a fluorescence spectrophotometer Cary Eclipse (Agilent, Darmstadt). The eCFP was excited at 430 nm (slit 5 nm) and the emission spectra from 475 to 500 nm were recorded.
Membrane Preparations
For the membrane preparations HEK293 cells were transiently transfected with 24 μg DNA per Petri dish (145 × 25 mm) of the cDNA encoding the proteins of dopamine D2L, of the NTS1 constructs or a mixture of both cDNAs using TransIT-293 transfection reagent according to the protocol given by the manufacturer. Transfected cells were cultivated for 48 h and then harvested. To harvest the cells, the medium was removed and cells were washed once with phosphate buffered saline. Then the cell material was abraded with a cell scraper and resuspended in 10 mL of harvest buffer (10 mM Tris-HCl, 0.5 mM, EDTA, 5.4 mM KCl, and 140 mM NaCl, pH 7.4) into a centrifuge tube. After centrifugation at 220g for 8 min the cellular pellet was resuspended in 5 mL of homogenate buffer (50 mM Tris-HCl, 5 mM EDTA, 1.5 mM CaCl2, 5 mM MgCl2, 5 mM KCl, and 120 mM NaCl, pH 7.4). Cells were used directly or stored at −80 °C. After thawing or directly, the cells were homogenized using a Polytron instrument (20 000 rpm, 5 times for 5 s each in an ice bath) and pelleted at 50 000g for 18 min. The supernatant was discarded, and the membrane pellet was resuspended in binding buffer (50 mM Tris, 1 mM EDTA, 5 mM MgCl2, 100 μg/mL bacitracin, 5 μg/mL soybean trypsin inhibitor, pH 7.4) and homogenized with a Potter-Elvehjem homogenizer. Membrane homogenates were stored in small aliquots at −80 °C. Protein concentration was determined by the method of Lowry et al.,51 using bovine serum albumin as a standard.
Homologous and Nonhomologous Competition Experiments with [3H]7-OH-DPAT
To determine the binding at the high affinity binding site of the D2L receptor, the agonist (R)-7-OH-DPAT (specific activity 157–163 Ci/mmol) was used as tritiated radioligand to determine the KD and Bmax values in homologous competition experiments or Ki values of different test compound in nonhomologous competition experiments. All assays were performed in 24-well plates at a total volume of 500 μL. The [3H]7-OH-DPAT was utilized as 1.5 nM solution for the labeling of 150 μg/mL protein per well. Varying concentrations of unlabeled 7-OH-DPAT or test compounds (0.01 to 100 000 nM) were added to the radioligand. To determine the unspecific binding, 10 μM 7-OH-DPAT was used. Total binding was determined in the absence of test compound. Competition experiments were performed in the absence and presence of NTS1 ligands. After the addition of the membrane homogenates, the mixture was incubated for 1 h at 37 °C. The assay was stopped by rapid filtration through GF/B filters precoated with 0.3% polyethylenimine. Filters were washed five times with ice-cold Tris-EDTA buffer (50 mM Tris, 1 mM EDTA pH 7.4), dried at 50 °C, sealed with MeltiLex solid scintillator (PerkinElmer, Rodgau, Germany), and radioactivity counted in a MicroBeta Trilux (PerkinElmer, Rodgau, Germany).
Saturation Experiments Using [3H]Spiperone and [3H]Neurotensin
Membrane preparations of coexpressed or singly expressed dopamine D2L and NTS1 receptors expressing HEK 293 cells were incubated in 96-well plates with 10 different concentrations (0.005–2 nM) of the tritiated D2 antagonist spiperone (specific activity 102–114 Ci/mmol) or [3H]neurotensin (specific activity 100–112 Ci/mmol). Nonspecific binding was defined in the presence of 10 μM haloperidol or neurotensin, and total binding was measured in absence of any competing drug. To investigate the influence of different NTS1 or D2L receptor ligands, either substance or buffer was added to the reaction mixture. After addition of membrane preparations with protein concentrations of 40 μg/mL, the assay mixture was incubated for 30–60 min at 37 °C and stopped by rapid filtration and further proceeded as described above for [3H]7-OH-DPAT.
Functional Assays
Inhibition of cAMP accumulation assay and phosphoERK1/2 ELISA assay were performed as previously described.45 A detailed description is available in the Supporting Information.
Data Analysis
Fluorescence-detected coimmunoprecipitation assay was analyzed by calculating the area under the curve from 480 to 490 nm using PRISM (GraphPad Software, San Diego, CA). Data are presented as mean ± SEM. Statistical analysis of single group comparisons was performed using an unpaired t test. Significances was set as P < 0.05.
Analysis of the saturation experiments were performed using a nonlinear regression analysis of the data for the determination of KD and Bmax values using PRISM. The resulting competition curves were analyzed by nonlinear regression using the algorithms in PRISM (GraphPad Software, San Diego, CA). The data were initially fitted using a sigmoid model and an IC50 value, representing the concentration corresponding to 50% of maximal inhibition. Data were then calculated for a one-site model using the program PRISM. IC50 values were transformed to Ki values according to the equation of Cheng and Prusoff.52 Data are presented as mean ± SEM. Statistical analysis of single group comparisons was performed using an unpaired t test. Significances was set as P < 0.05.
Chemistry
N-[(N′-Indan-2-yl-N′-propyl)-4-aminobutyl]pyrazolo[1,5-a]pyridine-3-carboxamide (FAUC326)
To a solution of pyrazolo[1,5-a]pyridine-3-carboxylic acid (36.8 mg, 0.23 mmol) in dried CH2Cl2 (4 mL), DIPEA (150 μL, 0.91 mmol) was added. The mixture was cooled to 0 °C before a solution of TBTU (113.8 mg, 0.35 mmol) in dried DMF (1 mL) was added. Then, a solution of N-indane-2-yl-N-propyl)butane-1,4-diamine45 (104.9 mg, 0.43 mmol) in CH2Cl2 (5 mL) was added dropwise. The mixture was stirred for 4 h at room temperature before aqueous NaHCO3 was added. The aqueous layer was extracted several times with CH2Cl2, and the combined organic layers were dried (MgSO4) and evaporated. The residue was purified by flash chromatography (hexane/EtOAc 1:2 + 0.5% NMe2Et) to give FAUC326 as a colorless oil (37.4 mg, 38%): IR 3433 s, 2931 m, 2866 w, 1639 m, 1554 m, 1277 m, 744 m cm–1. 1H NMR (360 MHz, CDCl3) δ 0.87 (t, J = 7.4 Hz, 3 H), 1.44 −1.56 (m, 2 H), 1.56–1.72 (m, 4 H), 2.45–2.54 (m, 2 H), 2.54–2.62 (m, 2 H), 2.88 (dd, J = 15.4 Hz, 8.7 Hz, 2 H), 3.01 (dd, J = 15.4 Hz, 7.7 Hz, 2 H), 3.41–3.57 (m, 2 H), 3.60–3.74 (m, 1 H), 6.04–6.17 (m, 1 H), 6.86–6.96 (m, 1 H), 7.08–7.18 (m, 4 H), 7.33 (ddd, J = 8.9 Hz, 6.8 Hz, 1.1 Hz, 1 H), 8.12 (s, 1 H), 8.47 (ddd, J = 6.9 Hz, 1.0 Hz, 1.0 Hz, 1 H), 8.47 (ddd, J = 6.9 Hz, 1.0 Hz, 1.0 Hz, 1 H). 13C NMR (90 MHz, CDCl3) δ 11.9, 20.2, 25.0, 28.0, 36.4, 39.4, 50.9, 53.4, 63.0, 107.0, 113.5, 119.6, 124.4, 126.2, 128.8, 140.1, 140.6, 141.8, 163.2. EI-MS m/z 390. Anal. (C24H30N4O·1.5H2O) C, H, N. Purity 100% (HPLC).
Acknowledgments
We thank Prof. Thomas Stamminger (Institute for Clinical and Molecular Virology, University of Erlangen-Nuremberg) for the support and for providing the confocal microscope. Dr. Harald Hübner is acknowledged for helpful discussions. Dr. Miriam Ruberg synthesized compound FAUC 326.
Glossary
Abbreviations
- GPCR
G-protein coupled receptor
- FRET
Förster resonance energy transfer
- D2L
dopamine D2L receptor
- NTS1
neurotensin receptor 1
- HEK
human embryonic kidney cells
- eCFP
enhanced cyan fluorescent protein
- eYFP
enhanced yellow fluorescent protein
- AUC
area under the curve
- KD
equilibrium dissociation constant
- Bmax
the maximum of specific binding
- EC50
half maximal effective concentration
- IP
immunoprecipitation
- nd
not determined
- 7-OH-DPAT
7-hydroxy-N,N-dipropyl-2-aminotetraline
- SR48692
2-([1-(7-chloro-4-quinolinyl)-5-(2,6-dimethoxyphenyl)-1H-pyrazole-3-carbonyl]amino)admantane-2-carboxylic acid
- ERK
extracellular-signal-regulated kinases
- cAMP
cyclic adenosine monophosphate
- DA
dopamine
- EDTA
ethylendiaminetetraacetic acid
- Tris
tris-(hydroxymethyl)aminomethane
- PBS
phosphate-buffered saline
- TBTU
O-(benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium tetrafluoroborate
- DIPEA
N,N-diisopropylethylamine
- DMF
N,N-dimethylformamide
Author Contributions
S.K. conducted experiments and contributed to the writing of the manuscript. T.N. performed confocal microscopy experiments and functional assays and contributed to the writing of the manuscript. P.G. designed the research programme, and contributed to the writing of the manuscript.
Supporting Information Available
Western blot, emission spectra of fluorescence detected co-immunoprecipitation, confocal images of the coexpression of D2L and NTS1 as well as receptor binding data for the test compound FAUC 326 at various receptors, binding data and curves of homologous competition experiments using [3H]7-OH-DPAT in the presence of different NTS1 ligands, saturation experiments using [3H]spiperone and [3H]neurotensin, experimental description of the used functional assays, dose response curves of MAPK-driven luciferase reporter gene assay, and analytical data of FAUC326. This material is available free of charge via the Internet at http://pubs.acs.org.
Supplementary Material
References
- Rashid A. J.; O’Dowd B. F.; George S. R. (2004) Minireview: Diversity and complexity of signaling through peptidergic G-protein-coupled receptors. Endocrinology 145, 2645–2652. [DOI] [PubMed] [Google Scholar]
- Rozenfeld R.; Devi L. A. (2010) Receptor heteromerization and drug discovery. Trends Pharmacol. Sci. 3, 124–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pin J.-P.; Neubig R.; Bouvier M.; Devi L.; Filizola M.; Javitch J. A.; Lohse M. J.; Milligan G.; Palczewski K.; Parmentier M.; Spedding M. (2007) International union of basic and clinical pharmacology. LXVII. Recommendations for the recognition and nomenclature of G protein-coupled receptor heteromultimers. Pharmacol. Rev. 59, 5–13. [DOI] [PubMed] [Google Scholar]
- Smith N. J.; Milligan G. (2010) Allostery at G-protein-coupled receptor homo- and heteromers: Uncharted pharmacological landscapes. Pharmacol. Rev. 62, 701–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinster S. C.; Hague C.; Hall R. A. (2005) Receptors: specificity and functional significance. Pharmacol. Rev. 57, 289–298. [DOI] [PubMed] [Google Scholar]
- Fuxe K.; Canals M.; Torvinen M.; Marcellino D.; Terasmaa A.; Genedani S.; Leo G.; Guidolin D.; Diaz-Cabiale Z.; Rivera A.; Lundstrom L.; Langel U.; Narvaez J.; Tanganelli S.; Lluis C.; Ferré S.; Woods A.; Franco R.; Agnati L. F. (2007) Intramembrane receptor–receptor interactions: a novel principle in molecular medicine. J. Neural Transm. 114, 49–75. [DOI] [PubMed] [Google Scholar]
- Birdsall N. J. (2010) Class A GPCR heterodimers: evidence from binding studies. Trends Pharmacol. Sci. 31, 499–508. [DOI] [PubMed] [Google Scholar]
- Vilardaga J.-P.; Nikolaev V. O.; Lorenz K.; Ferrandon S.; Zhuang Z.; Lohse M. J. (2008) Conformational cross-talk between α2A-adrenergic and μ-opioid receptors controls cell signalling. Nat. Chem. Biol. 4, 126–131. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y.; Portoghese P. S.; Takemori A. E. (1993) Involvement of delta 2 opioid receptors in the development of morphine dependence in mice. J. Pharmacol. Exp. Ther. 264, 1141–1145. [PubMed] [Google Scholar]
- Peng X.; Knapp B. I.; Bidlack J. M.; Neumeyer J. L. (2006) Synthesis and preliminary in vitro investigation of bivalent ligands containing homo- and heterodimeric pharmacophores at μ, δ, and κ opioid receptors. J. Med. Chem. 49, 256–262. [DOI] [PubMed] [Google Scholar]
- Daniels D. J.; Lenard N. R.; Etienne C. L.; Law P.-Y.; Roerig S. C.; Portoghese P. S. (2005) Opioid-induced tolerance and dependence in mice is modulated by the distance between pharmacophores in a bivalent ligand series. Proc. Natl. Acad. Sci. U.S.A. 102, 19208–19213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillion J.; Canals M.; Torvinen M.; Casadó V.; Scott R.; Terasmaa A.; Hansson A.; Watson S.; Olah M. E.; Mallol J.; Canela E. I.; Zoli M.; Agnati L. F.; Ibánez C. F.; Lluis C.; Franco R.; Ferré S.; Fuxe K. (2002) Coaggregation, cointernalization, and codesensitization of adenosine A2A receptors and dopamine D2 receptors. J. Biol. Chem. 277, 18091–18097. [DOI] [PubMed] [Google Scholar]
- Lee S. P.; So C. H.; Rashid A. J.; Varghese G.; Cheng R.; Lanca A. J.; O’Dowd B. F.; George S. R. (2004) Dopamine D1 and D2 receptor co-activation generates a novel phospholipase C-mediated calcium signal. J. Biol. Chem. 279, 35671–35678. [DOI] [PubMed] [Google Scholar]
- Rocheville M.; Lange D. C.; Kumar U.; Patel S. C.; Patel R. C.; Patel Y. C. (2000) Receptors for dopamine and somatostatin: formation of hetero-oligomers with enhanced functional activity. Science 288, 154–157. [DOI] [PubMed] [Google Scholar]
- Carraway R.; Leeman S. E. (1973) The isolation of a new hypotensive peptide, neurotensin, from bovine hypothalami. J. Biol. Chem. 248, 6854–6861. [PubMed] [Google Scholar]
- Binder E. B.; Kinkead B.; Owens M. J.; Nemeroff C. B. (2001) Neurotensin and dopamine interactions. Pharmacol. Rev. 53, 453–486. [PubMed] [Google Scholar]
- Boules M; Shaw A; Fredrickson P; Richelson E. (2007) Neurotensin agonists: potential in the treatment of schizophrenia. CNS Drugs 21, 13–23. [DOI] [PubMed] [Google Scholar]
- Fuxe K.; Marcellino D.; Woods A. S.; Giuseppina L.; Antonelli T.; Ferraro L.; Tanganelli S.; Agnai L. F. (2009) Integrated signaling in heterodimers and receptor mosaics of different types of GPCRs of the forebrain: relevance for schizophrenia. J. Neural Trans. 116, 923–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Euler G.; Mailleux P.; Vanderhaeghen J.-J.; Fuxe K. (1990) Neurotensin reduces the affinity of dopamine D2 receptor membranes from post mortem human caudate-putamen. Neurosci. Lett. 109, 325–330. [DOI] [PubMed] [Google Scholar]
- Tanganelli S.; Li X.-M.; Ferraro L.; von Euler G.; O’Connor W. T.; Bianchi C.; Beani L.; Fuxe K. (1993) Neurotensin and cholecystokinin octapeptide control synergistically dopamine release and dopamine D2 receptor affinity in rat neostriatum. Eur. J. Pharmacol. 159–166. [DOI] [PubMed] [Google Scholar]
- Li X.-M.; Ferraro L.; Tanganelli S.; O’Connor W. T.; Hasselrot U.; Ungerstedt U.; Fuxe K. (1995) Neurotensin peptides antagonistically regulate postsynaptic dopamine D2 receptors in rat nucleus accumbens: a receptor binding and microdialysis study. J. Neural Trans. 102, 125–137. [DOI] [PubMed] [Google Scholar]
- Agnati L. F.; Fuxe K.; Benfenati F.; Basttistini N (1983) Neurotensin in vitro markedly reduces the affinity in subcortical limbic 3H-N-propylnorapomorphine binding sites. Acta Physiol. Scand. 119, 459–461. [DOI] [PubMed] [Google Scholar]
- von Euler G.; Fuxe K. (1987) Neurotensin reduces the affinity of D2 dopamine receptors in rat striatal membranes. Acta Physiol. Scand. 131, 625–626. [DOI] [PubMed] [Google Scholar]
- von Euler G.; Fuxe K.; Benfenati F.; Hansson T.; Agnati L. F.; Gustafsson (1989) Neurotensin modulates the binding characteristics of dopamine D2 receptors in rat striatal membranes also following treatment with toluene. Acta Physiol. Scand. 135, 443–448. [DOI] [PubMed] [Google Scholar]
- von Euler G.; Meister B.; Hökfelt T.; Eneroth P.; Fuxe K. (1990) Intraventricular injection of neurotensin reduces dopamine D2 agonist binding in rat forebrain and intermediate lobe of the pituitary gland. Relationship to serum hormone levels and nerve terminal coexistence. Brain Res. 531, 253–262. [DOI] [PubMed] [Google Scholar]
- Ferré S.; Baler R.; Bouvier M.; Caron M. G.; Devi. L. A.; Durroux T.; Fuxe K.; George S. R.; Javitch J. A.; Lohse M. J.; Mackie K.; Milligan G.; Pfleger K. D. G.; Pin J.-P.; Volkow N. D.; Waldhoer M.; Woods A. S.; Franco R. (2009) Building a new conceptual framework for receptor heteromers. Nat. Chem. Biol. 5, 131–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae J. H.; Rubini M.; Jung G.; Wiegand G.; Seifert M. H. J.; Kamran Azim M.; Kim J.-S.; Zumbusch A.; Holak T. A.; Moroder L.; Huber R.; Budisa N. (2003) Expansion of the genetic code Enables Design of a Novel “Gold“ Class of Green Fluorescent Proteins. J. Mol. Biol. 328, 1071–1081. [DOI] [PubMed] [Google Scholar]
- Hall R. A. (2005) Co-Immunoprecipitation as a Strategy to evaluate Receptor-Receptor or Receptor-Protein Interactions. In G Protein-Coupled Receptor-Protein Interactions (George S. R., O’Dowd B. F., Eds.), Chapter 9, pp 165–178, Wiley & Sons, New York; http://www.pharm.emory.edu/rhall/Hall_Co-IP_Chapter.pdf (accessed Febuary 9, 2011). [Google Scholar]
- Kearn C. S.; Blake-Palmer K.; Daniel E.; Mackie K.; Glass M. (2005) Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors enhances heterodimer formation: a mechanism for receptor cross-talk?. Mol. Pharmacol. 67, 1697–1704. [DOI] [PubMed] [Google Scholar]
- Manuela Pfeiffer M.; Koch T.; Schröder H.; Laugsch M.; Höllt V.; Schulz S. (2002) Heterodimerization of somatostatin and opioid receptors cross-modulates phosphorylation, internalization, and desensitization. J. Biol. Chem. 277, 19762–19772. [DOI] [PubMed] [Google Scholar]
- Michel M. C.; Wieland T.; Tsujimoto G. (2009) How reliable are G-protein-coupled receptor antibodies?. Naunyn-Schmiedeberg's Arch. Pharmacol. 379, 385–388. [DOI] [PubMed] [Google Scholar]
- von Euler G. (1991) Biochemical characterization of the intramembrane interaction between neurotensin and dopamine D2 receptors in the rat brain. Brain Res. 561, 93–98. [DOI] [PubMed] [Google Scholar]
- Nemeroff C. B.; Luttinger D.; Hernandez D. E.; Mailman R. B.; Mason G. A.; Davis S. D.; Widerlöv E.; Frye G. D.; Kilts C. A.; Beaumont K.; Breese G. R.; Prange A. J. Jr. (1983) Interactions of neurotensin with brain dopamine systems: biochemical and behavioral studies. J. Pharmacol. Exp. Ther. 225, 337–345. [PubMed] [Google Scholar]
- Tyler-McMahon B. M.; Boules M.; Richelson E. (2000) Neurotensin: peptide for the next millennium. Regul. Pept. 93, 125–136. [DOI] [PubMed] [Google Scholar]
- Li X.-M.; von Euler G.; Hedlund P. B.; Finnmann U.-B.; Fuxe K. (1993) The C-terminal neurotensin-(8–13) fragment potently modulates rat neostriatal dopamine D2 receptors. Eur. J. Pharmacol. 234, 125–128. [DOI] [PubMed] [Google Scholar]
- Díaz-Cabiale Z.; Fuxe K.; Narváez J. A.; Finetti S.; Antonelli T.; Tanganelli S.; Ferraro L. (2002) Neurotensin-induced modulation of dopamine D2 receptors and their function in rat striatum: Counteraction by a NTR1-like receptor antagonist. NeuroReport 13, 763–766. [DOI] [PubMed] [Google Scholar]
- Gully D.; Canton M.; Boigegrain R.; Jeanjean F.; Molimard J. C.; Poncelet M.; Gueudet C.; Heaulme M.; Leyris R.; Brouard A. (1993) Biochemical and pharmacological profile of a potent and selective nonpeptide antagonist of the neurotensin receptor. Proc. Natl. Acad. Sci. U.S.A. 90, 65–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Härterich S.; Koschatzky S.; Einsiedel. J.; Gmeiner P. (2008) Novel insights into GPCR-Peptide interactions: Mutations in extracellular loop 1, ligand backbone methylations and molecular modeling of neurotensin receptor 1. Bioorg. Med. Chem. 16, 9359–9368. [DOI] [PubMed] [Google Scholar]
- Tschammer N.; Bollinger S.; Kenakin T.; Gmeiner P. (2011) Histidine 6.55 is a major determinant of ligand biased signaling in dopamine D2L receptor. Mol. Pharmacol. 79, 575–585. [DOI] [PubMed] [Google Scholar]
- Löber S.; Hübner H.; Tschammer N.; Gmeiner P. (2011) Recent advances in the search for D3- and D4-selective drugs: probes, models and candidates. Trends Pharmacol. Sci. 32, 148–157. [DOI] [PubMed] [Google Scholar]
- Lawler C. P.; Prioleau C.; Lewis M. M.; Mak C.; Jiang D.; Schetz J. A.; Gonzalez A. M.; Sibley D. R.; Mailman R. B. (1999) Interactions of the novel antipsychotic aripiprazole (OPC-14597) with dopamine and serotonin receptor subtypes. Neuropsychopharmacology 20, 612627. [DOI] [PubMed] [Google Scholar]
- Burris K. D.; Molski T. F.; Xu C.; Ryan E.; Tottori K.; Kikuchi T.; Yocca F. D.; Molinoff P. B. (2002) Ariprazole, a novel antipsychotic, is a high-affinity partial agonist at human dopamine D2 receptors. J. Pharmacol. Exp. Ther. 302, 381–389. [DOI] [PubMed] [Google Scholar]
- Mailman R. B. (2007) GPCR functional selectivity has therapeutic impact. Trends Pharmacol. Sci. 28, 309–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon J. G.; Perez J. A.; Pease J. P.; Long j. P.; Flynn J. R.; Rusterholz. D. B.; Dryer S. E. (1980) Comparison of biological effects of N-alkylated congeners of beta.-phenethylamine derived from 2-aminotetralin, 2-aminoindan, and 6-aminobenzocycloheptene. J. Med. Chem. 23, 745–749. [DOI] [PubMed] [Google Scholar]
- Tschammer N.; Dörfler M.; Hübner H.; Gmeiner P. (2010) Engineering a GPCR-ligand pair that simulates the activation of D2L by dopamine. ACS Chem. Neurosci. 1, 25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich K.; Götz A.; Bollinger S.; Tschammer N.; Bettinetti L.; Harterich S.; Hubner H.; Lanig H.; Gmeiner P. (2009) Dopamine D2, D3, and D4 selective phenylpiperazines as molecular probes to explore the origins of subtype specific receptor binding. J. Med. Chem. 52, 4923–4935. [DOI] [PubMed] [Google Scholar]
- Cruzalegui F. H.; Cano E.; Treisman R. (1999) ERK activation induces phosphorylation of Elk-1 at multiple S/T-P motifs to high stoichiometry. Oncogene 18, 7948–7957. [DOI] [PubMed] [Google Scholar]
- Schimpff R.-M.; Avard C.; Fénelon G.; Lhiaubet A.-M.; Tennezé L.; Vidailhet M.; Rostène W. (2001) Increased plasma neurotensin concentrations in patients with Parkinson’s disease. J. Neurol., Neurosurg. Psychiatry 70, 784–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko J.-K.; Ma J. (2005) A rapid and efficient PCR-based mutagenesis method applicable to cell physiology study. Am. J. Physiol. 288, 1273–1278. [DOI] [PubMed] [Google Scholar]
- Schmid J. A., and Malkani N. (2011) Some Secrets of Fluorescent Proteins: Distinct Bleaching in Various Mounting Fluids and Photoactivation of cyan fluorescent proteins at YFP-Excitation. Nat. Precedings, published online April 14, 2011. hdl: 10.1038/npre.2011.4517.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry O. H.; Rosebrough N. R.; Farr A. L.; Randall R. J. (1951) Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265–75. [PubMed] [Google Scholar]
- Cheng Y.-C.; Prusoff W. H. (1973) Relationship between the inhibition constant (KI) and the concentration of inhibitor which causes 50% inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 22, 3099–3108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.