Abstract
Most human malaria deaths are caused by blood-stage Plasmodium falciparum parasites. Cerebral malaria, the most life-threatening complication of the disease, is characterised by an accumulation of Plasmodium falciparum infected red blood cells (iRBC) at pigmented trophozoite stage in the microvasculature of the brain2-4. This microvessel obstruction (sequestration) leads to acidosis, hypoxia and harmful inflammatory cytokines (reviewed in 5). Sequestration is also found in most microvascular tissues of the human body2, 3. The mechanism by which iRBC attach to the blood vessel walls is still poorly understood.
The immortalized Human Brain microvascular Endothelial Cell line (HBEC-5i) has been used as an in vitro model of the blood-brain barrier6. However, Plasmodium falciparum iRBC attach only poorly to HBEC-5i in vitro, unlike the dense sequestration that occurs in cerebral malaria cases. We therefore developed a panning assay to select (enrich) various P. falciparum strains for adhesion to HBEC-5i in order to obtain populations of high-binding parasites, more representative of what occurs in vivo.
A sample of a parasite culture (mixture of iRBC and uninfected RBC) at the pigmented trophozoite stage is washed and incubated on a layer of HBEC-5i grown on a Petri dish. After incubation, the dish is gently washed free from uRBC and unbound iRBC. Fresh uRBC are added to the few iRBC attached to HBEC-5i and incubated overnight. As schizont stage parasites burst, merozoites reinvade RBC and these ring stage parasites are harvested the following day. Parasites are cultured until enough material is obtained (typically 2 to 4 weeks) and a new round of selection can be performed. Depending on the P. falciparum strain, 4 to 7 rounds of selection are needed in order to get a population where most parasites bind to HBEC-5i. The binding phenotype is progressively lost after a few weeks, indicating a switch in variant surface antigen gene expression, thus regular selection on HBEC-5i is required to maintain the phenotype.
In summary, we developed a selection assay rendering P. falciparum parasites a more "cerebral malaria adhesive" phenotype. We were able to select 3 out of 4 P. falciparum strains on HBEC-5i. This assay has also successfully been used to select parasites for binding to human dermal and pulmonary endothelial cells. Importantly, this method can be used to select tissue-specific parasite populations in order to identify candidate parasite ligands for binding to brain endothelium. Moreover, this assay can be used to screen for putative anti-sequestration drugs7.
Keywords: Immunology, Issue 59, Plasmodium falciparum, cerebral malaria, cytoadherence, sequestration, endothelial cell, HBEC-5i
Protocol
General recommendations
Human brain microvascular endothelial cell (HBEC-5i) culture has been previously described in 6, 8. P. falciparum parasites were cultured as in 9. Both HBEC-5i and P. falciparum parasite cultures should be kept in sterile conditions at all times. All reagents should be pre-warmed at 37°C. We recommend to regularly check for mycoplasma contamination10 by PCR (Minevera Biolabs, following manufacturer's instructions). The protocol is summarized in Figure 1.
1. Endothelial cell routine culture
Prepare the necessary reagents.
| Medium to prepare | Reagents | Quantity |
| "DMEM incomplete" | DMEM-F12 Ham | 500ml |
| L-glutamine 200mM | 5ml | |
| Penicillin/streptomycin 100X | 5 ml | |
| NaOH 1M | 1.3 ml (adjust pH to 7.4) | |
| "DMEM complete" | "DMEM incomplete" | 450ml |
| Foetal Bovine Serum heat-inactivated | 50ml | |
| Endothelial cell growth supplement | 5 ml |
Culture HBEC-5i in a vented 25cm2 flask with 10ml DMEM complete medium in a 37°C incubator with 5% CO2.
Passage cells when they become confluent. Remove the old medium by suction and wash twice using DMEM incomplete medium or tissue culture grade PBS (Ca2+ and Mg2+ free) pre-warmed to 37°C.
Add 1ml of pre-warmed Trypsin-EDTA (0.025% Trypsin, 0.5mM EDTA), swirl to cover the entirety of the flask and incubate for ˜2 min at 37°C.
Check under inverted microscope that at least 90% of cells have been detached. If necessary, gently knock the bottom of the flask to dislodge adherent cells. Add 10ml of DMEM complete medium to block the trypsin and transfer cells into a 15ml conical tube. Centrifuge for 4 min at 300 g at room temperature (RT).
Discard the supernatant and resuspend the pellet with 10ml of DMEM complete medium. Pipette the solution up and down to thoroughly resuspend the cells.
Add 1 or 2 ml of the cell suspension into a new culture flask and add 8ml of fresh medium to maintain the culture. Assess culture growth every day under an inverted microscope and change medium every 2 or 3 days before it turns yellow.
2. Preparing endothelial cells for a selection
Two days prior to the day of the selection, add fibronectin diluted in sterile PBS (2 μg/cm2) in one (or more) 60 mm Petri dish. Incubate the dish for 5 to 20 min at 37°C, then remove the fibronectin solution, which can be stored at 4°C for a month and re-used once.
Passage cells as described in sections 1.3. to 1.5. Assuming 100% confluent cells were detached and resuspended in 10ml DMEM complete medium (section 1.6., resuspend with an equivalent volume of medium if the confluency is lower, e.g. resuspend with 8ml if the confluency was 80%), add 1.5ml of the suspended cells to the fibronectin coated Petri dish and another 1.5ml of DMEM complete medium.
Place the seeded Petri dish in the incubator. Note: Ideally, the confluency will be around 90% at the time of selection two days later.
Optional. To activate HBEC-5i, add the TNF (Tumour Necrosis Factor) cytokine at a final concentration of 50μg/ml 24 hours prior to the selection.
3. Plasmodium falciparum routine culture
Prepare the necessary reagents (see table here under). Prepare human red blood cells (RBC) by separating whole blood (group O+) by passage through a leukocyte depletion filter (see "Methods in Malaria Research" publication11 for general malaria parasites culturing). Wash RBC twice by centrifuging at 400 g for 5 min and resuspending them with 10 ml RPMI incomplete. Keep washed RBC at 4°C in incomplete medium at 50% haematocrit.
| Medium to prepare | Reagents | Quantity |
| "RPMI incomplete" | RPMI 1640 (with bicarbonate) | 500ml |
| Hepes 1M | 12.5ml | |
| Glucose 20% | 5ml | |
| L-glutamine 200mM | 5ml | |
| Gentamycin 50mg/ml | 250μl | |
| NaOH 1M | 0.7 ml (adjust pH to 7.2) | |
| "RPMI complete" | "RPMI incomplete" | 450ml |
| Pooled human (non-immune) serum | 50ml |
Culture P. falciparum infected RBC with RPMI complete medium at 2% haematocrit and incubate at 37°C with 3% CO2, 1% O2, and 96% N2. Make Giemsa smear11 daily to assess the stage of development of parasites (Figure 2).
Regularly (approximately once a week) synchronize culture by sorbitol treatment12. The day prior the selection assay, aim for a ring-stage culture at 5% parasitaemia or more (ideally at least 10%).
4. Selection of P. falciparum for cytoadhesion to endothelial cells
On the day of the assay, the parasite culture should be at pigmented trophozoite stage (Figure 2)(ideally 10% parasitaemia) while HBEC-5i culture should be at 50 to 100% confluency (ideally 90%). 30μl packed cell volume of parasite culture is needed per HBEC-5i Petri dish.
Wash parasites twice by centrifuging (500g for 5 min) 1.5ml of parasite culture. Discard supernatant and resuspend with 10ml of freshly made, warmed, DMEM incomplete medium. Repeat the wash a second time. Resuspend the 30μl packed cell volume with 1.5ml of DMEM incomplete with 1% BSA.
Wash the HBEC-5i coated Petri dish twice aspirating medium and adding 3ml of incomplete DMEM.
Add the solution of parasites to the HBEC-5i dish and incubate at 37°C for 75min. Resuspend parasites twice (after 30 and 60 min) during the incubation by gently rocking the dish in the four directions, as well as clockwise and anti-clockwise.
After incubation, wash the dish 5 times by aspirating medium, using a plastic Pasteur pipette to add 3 ml of warm DMEM incomplete medium, and gentle rocking. If many dishes are being used, keep them on a warm surface, such as a large flask filled with water at 37°C.
Check the dish under an inverted microscope. If many uninfected RBC are still visible, do more washes as described above. Handle the Petri dish very carefully to avoid any risk of contamination.
Remove medium from the dish and add 3ml of warm RPMI complete medium with 40μl packed cell volume of fresh uRBC.
Place the dish in an airtight incubating chamber, gas it for 3 min and place the chamber in an incubator at 37°C overnight.
The day after, harvest the parasites by washing with RPMI incomplete medium, in a similar way as described in section 4.4. but more vigorously. Keep all medium used (containing resuspended RBC) in a 15ml conical tube. Check under inverted microscope that all RBC have been removed from the dish.
Pellet the parasites, discard the supernatant, resuspend in 5ml RPMI complete medium and place the mixture in a flask for normal culturing.
5. Representative Results
Unselected parasites show a low level of binding to HBEC-5i (Figure 3A). Thus, after the first round of selection, very few parasites will be harvested and it may take up to a month of culturing to reach a parasitaemia sufficient for the second round of selection. After each round of selection, more and more parasites bind to the endothelial cells and the selections can be repeated at shorter time intervals. After 4-5 rounds of selection, high-binding parasite populations are obtained (Figure 3).
In our hands, the binding of HB3-HBEC to HBEC-5i or TNF activated HBEC-5i was of similar level (data not shown).
The protocol described here was tested with 4 P. falciparum strains: HB3, IT/FCR3, 3D7 and Dd2 (Table 1). Only the latter proved not able to bind to HBEC-5i, even after 5 rounds of selection.
HB3 parasites were also selected for cytoadherence to Human Dermal and Pulmonary Microvascular Endothelial Cells (HDMEC and HPMEC). After 4 rounds of selection, using the method described here, a high-binding population was obtained on both HDMEC and HPMEC (Figure 4).
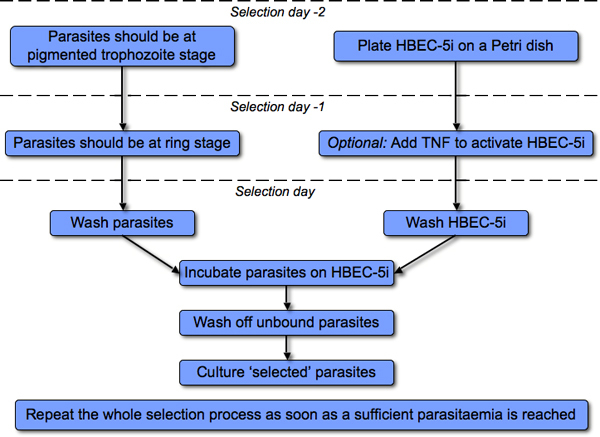 Figure 1. Overview of the selection process.
Figure 1. Overview of the selection process.
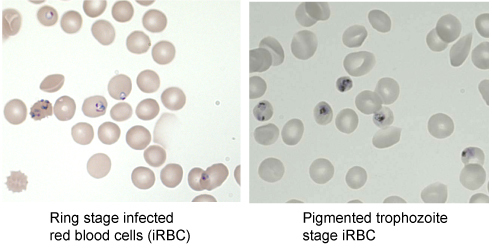 Figure 2.Development stages of P. falciparum infected red blood cells. Giemsa smear visualized under microscope at 1000X magnification.
Figure 2.Development stages of P. falciparum infected red blood cells. Giemsa smear visualized under microscope at 1000X magnification.
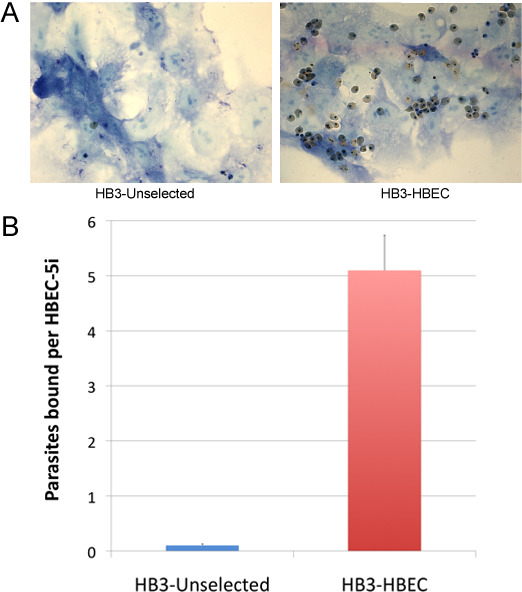 Figure 3.Typical example of parasites binding to HBEC-5i. (A) The blue layer is HBEC-5i fixed with glutaraldehyde and stained with Giemsa, visualized under microscope at 1000X magnification. Pictures were taken after uRBC and unbound iRBC were washed away. The left panel shows a single P. falciparum HB3 (unselected) parasite bound to HBEC-5i. In the right panel the HB3-HBEC parasites had been selected for 5 rounds. (B) Data represents the average of two independent experiments, each performed in duplicate. The number of parasites bound per endothelial cell was counted.
Figure 3.Typical example of parasites binding to HBEC-5i. (A) The blue layer is HBEC-5i fixed with glutaraldehyde and stained with Giemsa, visualized under microscope at 1000X magnification. Pictures were taken after uRBC and unbound iRBC were washed away. The left panel shows a single P. falciparum HB3 (unselected) parasite bound to HBEC-5i. In the right panel the HB3-HBEC parasites had been selected for 5 rounds. (B) Data represents the average of two independent experiments, each performed in duplicate. The number of parasites bound per endothelial cell was counted.
 Table 1. Summary of Plasmodium falciparum strains that were successfully selected for binding to HBEC-5i. Note that HB3 was also selected on TNF-activated HBEC-5i. After 5 rounds of selection, the Dd2 strain showed no increase in binding to HBEC-5i compared to unselected-Dd2.
Table 1. Summary of Plasmodium falciparum strains that were successfully selected for binding to HBEC-5i. Note that HB3 was also selected on TNF-activated HBEC-5i. After 5 rounds of selection, the Dd2 strain showed no increase in binding to HBEC-5i compared to unselected-Dd2.
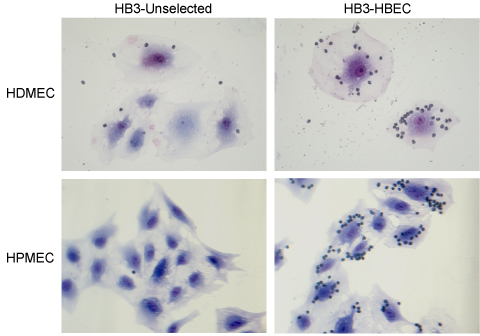 Figure 4.Binding of P. falciparum HB3 parasites to dermal (HDMEC) and pulmonary (HPMEC) endothelial cells, before and after 4 rounds of selection. Although the culture medium for HDMEC and HPMEC slightly differs (see supplier's instructions), the protocol used for the selection was identical as with HBEC-5i. Pictures taken at 400X magnification.
Figure 4.Binding of P. falciparum HB3 parasites to dermal (HDMEC) and pulmonary (HPMEC) endothelial cells, before and after 4 rounds of selection. Although the culture medium for HDMEC and HPMEC slightly differs (see supplier's instructions), the protocol used for the selection was identical as with HBEC-5i. Pictures taken at 400X magnification.
| Name of the reagent | Company | Catalogue number | Comments |
| DMEM-F12 Ham | Sigma | D6421 | For DMEM complete medium |
| L-glutamine 200mM | GIBCO | 25030 | For DMEM and RPMI complete medium |
| Penicillin/streptomycin 100X (10000 units/ml and 10mg/ml) | ScienCell | 0503 | For DMEM complete medium |
| Foetal Bovine Serum heat-inactivated | ScienCell | 0025 | For DMEM complete medium |
| Trypsin-EDTA (0.025% Trypsin, 0.5mM EDTA) | ScienCell | 0103 | |
| Endothelial cell growth supplement | ScienCell | 1052 | For DMEM complete medium |
| Tissue culture treated 60 mm X 15 mm Petri dishes | BD | 353002 | |
| Human Fibronectin | Millipore | FC010 | Use at 2 μg/cm2 |
| TNF | R&D Systems | 210-TA | optional, use at 50 μg/ml |
| RPMI 1640 | Lonza | BE12-167F | For RPMI complete medium |
| Gentamycin 50mg/ml | Lonza | 17-518Z | For RPMI complete medium |
| Hepes 1M | Lonza | BE17-737E | For RPMI complete medium |
| HDMEC | ScienCell | 2000 | Primary cell line |
| HPMEC | ScienCell | 3000 | Primary cell line |
| HBEC-5i | Obtained from Francisco Candal (fcandal@cdc.gov) |
Table 2. Materials
Discussion
The hallmark of cerebral malaria is sequestration of P. falciparum iRBC within the brain microvasculature2, 3. However, in vitro cultures of P. falciparum only poorly cytoadhere to HBEC-5i, a model for human brain microvascular endothelium. Here we developed a straightforward assay to enrich a population for binding to HBEC-5i, a more "in-vivo like" phenotype. Three out of 4 P. falciparum strains were successfully selected using this method. Furthermore, HB3 was also selected on HDMEC and HPMEC, indicating that this protocol can be used for various parasite and endothelial cell types.
Different hypotheses may explain the lack of binding with the Dd2 strain. The most likely being the fact that this parasite line is knobless, which deeply impedes the cytoadherence13, 14.
We recommend culturing unselected parasites alongside the selection process to provide a control for comparison. This will allow, for example, comparing the transcriptome of binding and non-binding parasites, with the hope of discovering parasite ligand candidates.
TNF is a cytokine found at high level in cerebral malaria patients and has been show to induce the expression of many surface proteins of HBEC-5i (Claessens et al, in preparation and 8, 15, 16). In this case, the amount of bound iRBC was similar in normal HBEC-5i compared to activated HBEC-5i.
This "sequestration model" can also be used to study the molecular interaction between the iRBC and the endothelial cell, as well as the effect of putative anti-cytoadherence drugs. In this case, we recommend plating HBEC-5i in smaller wells, such as "8-well chamber slides" (BD 354628) or "CultureWell" (Sigma C7735-20EA).
Disclosures
We have nothing to disclose.
Acknowledgments
We thank Francisco Candal, CDC Technology Transfer Office, Atlanta Georgia for HBEC-5i cells. This work was funded by the Wellcome Trust (4 year PhD studentship to AC and Senior Fellowship in Basic Biomedical Science to JAR, grant number 084226).
References
- Scherf A. Antigenic variation in malaria: in situ switching, relaxed and mutually exclusive transcription of var genes during intra-erythrocytic development in Plasmodium falciparum. Embo. J. 1998;17:5418–5426. doi: 10.1093/emboj/17.18.5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson GG, Warrell MJ, White NJ, Looareesuwan S, Warrell DA. Human cerebral malaria. A quantitative ultrastructural analysis of parasitized erythrocyte sequestration. Am. J. Pathol. 1985;119:385–401. [PMC free article] [PubMed] [Google Scholar]
- Seydel KB, Milner DA, Kamiza SB, Molyneux ME, Taylor TE. The distribution and intensity of parasite sequestration in comatose Malawian children. J. Infect. Dis. 2006;194:208–208. doi: 10.1086/505078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor TE. Differentiating the pathologies of cerebral malaria by postmortem parasite counts. Nat. Med. 2004;10:143–145. doi: 10.1038/nm986. [DOI] [PubMed] [Google Scholar]
- van der Heyde HC, Nolan J, Combes V, Gramaglia I, Grau GE. A unified hypothesis for the genesis of cerebral malaria: sequestration, inflammation and hemostasis leading to microcirculatory dysfunction. Trends. Parasitol. 2006;22:503–508. doi: 10.1016/j.pt.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Dorovini-Zis K, Prameya R, Bowman PD. Culture and characterization of microvascular endothelial cells derived from human brain. Lab. Invest. 1991;64:425–436. [PubMed] [Google Scholar]
- Rowe JA, Claessens A, Corrigan RA, Arman M. Adhesion of Plasmodium falciparum-infected erythrocytes to human cells: molecular mechanisms and therapeutic implications. Expert reviews in molecular medicine. 2009;11:e16–e16. doi: 10.1017/S1462399409001082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L. Plasmodium falciparum: involvement of additional receptors in the cytoadherence of infected erythrocytes to microvascular endothelial cells. Exp. Parasitol. 1996;84:42–55. doi: 10.1006/expr.1996.0088. [DOI] [PubMed] [Google Scholar]
- Corrigan RA, Rowe JA. Strain variation in early innate cytokine induction by Plasmodium falciparum. Parasite. Immunol. 2010;32:512–527. doi: 10.1111/j.1365-3024.2010.01225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JA. Implications of mycoplasma contamination in Plasmodium falciparum cultures and methods for its detection and eradication. Mol. Biochem. Parasitol. 1998;92:177–180. doi: 10.1016/s0166-6851(97)00237-5. [DOI] [PubMed] [Google Scholar]
- Moll K, Ljungström I, Perlmann H, Scherf A, Wahlgren M. Methods in Malaria Research. MR4/ATCC; 2009. [Google Scholar]
- Lambros C, Vanderberg JP. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 1979;65:418–420. [PubMed] [Google Scholar]
- Herricks T, Antia M, Rathod PK. Deformability limits of Plasmodium falciparum-infected red blood cells. Cell. Microbiol. 2009. [DOI] [PMC free article] [PubMed]
- Nakamura K, Hasler T, Morehead K, Howard RJ, Aikawa M. Plasmodium falciparum-infected erythrocyte receptor(s) for CD36 and thrombospondin are restricted to knobs on the erythrocyte surface. J. Histochem. Cytochem. 1992;40:1419–1422. doi: 10.1177/40.9.1380530. [DOI] [PubMed] [Google Scholar]
- Wassmer SC, Cianciolo GJ, Combes V, Grau GE. LMP-420, a new therapeutic approach for cerebral malaria. Med. Sci. (Paris) 2006;22:343–345. doi: 10.1051/medsci/2006224343. [DOI] [PubMed] [Google Scholar]
- Wassmer SC, Combes V, Candal FJ, Juhan-Vague I, Grau GE. Platelets potentiate brain endothelial alterations induced by Plasmodium falciparum. Infect. Immun. 2006;74:645–653. doi: 10.1128/IAI.74.1.645-653.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]


