Abstract
Two-photon Microscopy (TPM) provides image acquisition in deep areas inside tissues and organs. In combination with the development of new stereotactic tools and surgical procedures, TPM becomes a powerful technique to identify "niches" inside organs and to document cellular "behaviors" in live animals. While intravital imaging provides information that best resembles the real cellular behavior inside the organ, it is both more laborious and technically demanding in terms of required equipment/procedures than alternative ex vivo imaging acquisition. Thus, we describe a surgical procedure and novel "stereotactic" organ holder that allows us to follow the movements of Foxp3+ cells within the thymus.
Foxp3 is the master regulator for the generation of regulatory T cells (Tregs). Moreover, these cells can be classified according to their origin: ie. thymus-differentiated Tregs are called "naturally-occurring Tregs" (nTregs), as opposed to peripherally-converted Tregs (pTregs). Although significant amount of research has been reported in the literature concerning the phenotype and physiology of these T cells, very little is known about their in vivo interactions with other cells. This deficiency may be due to the absence of techniques that would permit such observations. The protocol described in this paper provides a remedy for this situation.
Our protocol consists of using nude mice that lack an endogenous thymus since they have a punctual mutation in the DNA sequence that compromises the differentiation of some epithelial cells, including thymic epithelial cells. Nude mice were gamma-irradiated and reconstituted with bone marrows (BM) from Foxp3-KIgfp/gfp mice. After BM recovery (6 weeks), each animal received embryonic thymus transplantation inside the kidney capsule. After thymus acceptance (6 weeks), the animals were anesthetized; the kidney containing the transplanted thymus was exposed, fixed in our organ holder, and kept under physiological conditions for in vivo imaging by TPM. We have been using this approach to study the influence of drugs in the generation of regulatory T cells.
Keywords: Immunology, Issue 59, intravital, in vivo, thymus, 2-photon, regulatory T cells
Protocol
1. Animal preparation
Important notes: BM cell suspensions and thymic transplantations were performed in aseptic conditions. The BM suspensions were prepared inside the hoods of our cell culture room, while the thymic transplantation was performed in the surgical room located within our animal facility. In order to keep and guarantee these aseptic conditions, we were not allowed to record our video in these places. However, all the surgical materials were autoclaved and the surgical bench was previously cleaned with Virkon (Pharmacal Research Laboratories Inc.) and 70% ethanol. All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) and they were in agreement with the Federation of European Laboratory Animal Science Associations (FELASA) directives. The approval ID number is AO10/2010. All the image experiments were terminal procedures and the animals were euthanized immediately following the end of image acquisition.
The detailed procedure is as follows:
Irradiate (with a γ-irradiator) 8 week old Nude mice with 900 rads to destroy the endogenous hematopoietic precursors inside the bone marrows (BM). After irradiation, return the animals to their cages until you prepare the donor BM cells for intravenous injection and further BM reconstitution. This BM transfer was performed 1 h after irradiation.
Separate the BM donor animals. Tibias and femurs of hind limbs of one animal are generally enough to reconstitute up to 3 recipient animals. After euthanasia with CO2, remove the hind limbs and then remove the skin and muscle from the bones.
Cut the closed end of each bone and flush it with PBS (or RPMI) or crush them all in a mortar, using a pestle, with 2 ml of PBS (or RPMI) to remove the BM.
Disrupt the BM structure into a single-cell suspension by repetitions of vigorous aspiration using a P1000 pipette. Alternatively, you can also pass the BM throughout a 21G syringe needle several times. Centrifuge (400g, 5 min, R.T.), re-suspend cells in PBS, and inject intravenously into your nude-irradiated recipients. In our experiments we used BM from Foxp3-KIgfp/gfp mice where all regulatory T cells (Tregs) will express GFP1.
Acceptance of the donor BM occurs in the first days after transfer, since non-BM-reconstituted animals cannot survive more than 14 days. However, one should wait for 4 to 6 weeks to allow fully recovery of all BM compartments. Bactrim (Roche) was added to the water (2 mg/ml) during the first week after irradiation to decrease the risk of bacterial infections.
Embryonic thymus transplantation was already described2. Briefly, start breeding pairs of isogenic mice and check for plugging (evidence of coitus) every day. Separate plugged females and CO2-euthanize them on day 15 of pregnancy (observation of plug is day 1 of pregnancy). Remove the embryos and the thymuses. Place the embryonic thymuses in cold PBS until transfer.
To perform the thymuses transplantation, anesthetize BM-recipient Nude mice with ketamin (120 μg/g of mouse weight) / xylazine (16 μg/g of mouse weight) and keep them on top of a heating pad at 37°C until they get unconscious.
Surgically expose the kidney to perform the transplantation of the thymuses. First scrub the skin with ChloraPrep (Carefusion Inc.) and 70% ethanol. Then, make a 1 cm cut on the skin of the back-lateral body part of the animal, 2 mm bellow the last thoracic rib.
Cut the peritoneal cavity and pull out the kidney of this cavity. Make a small superficial cut in the kidney capsule with a scalpel blade. Using forceps with a very thin tip make a pouch underneath the kidney capsule of the recipient mouse and add up to 4 thymic lobes into this pouch.
Put the kidney back to its place, suture the peritoneal cavity with one or two stitches, and then close the mouse skin using the same suture method. Alternatively, sutures can be done using surgical glue.
Inject analgesic (Butorphanol at 5 mg/kg) subcutaneously right after finishing the surgery to avoid animal pain and keep the animals in a heating pad until they start to recover from anesthesia. Mice are caged individually for the first 3 days after transplantation to prevent cage mates from removing the stitches. Remove stitches after 1 week.
Nude mice do not have T cells. Therefore, you can evaluate the thymus graft acceptance by monitoring the percentage of CD4+ or CD8+ T cells in the blood. Once per week, 2 weeks after thymus transplantation, bleed the animals and stain the blood with anti-CD4 and anti-CD8 monoclonal antibodies (MAbs). When the CD4:CD8 ratio is approximately 2:1, the transplanted thymus is fully functional (Figure 1).
2. Intravital image acquisition
Prepare all the materials you will need in advance and put them aside.
Anesthetize animals with ketamin/xylazine (same doses described at item 1.5) and place them on top of a heating pad at 37°C. Inject 100 μl of Rhodamine B isothiocyanate-Dextran (20 mg/ml in PBS) intravenously to allow future visualization of blood flow.
Expose the kidney containing the transplanted thymus according to the protocol previously described in item 1.6.
To prevent the kidney from returning to its original position, close the lateral sides of the skin incision with stitches.
Place a piece of PBS-soaked cotton on the top of the organ to keep it moist.
Put the heating pad on top of the animal holder stage (Figure 2A).
Put the animal on top of the heating pad in the animal holder, organ up (Figure 2B).
Pinch the organ at both sides with a clamp made of thin aluminum foil (Figure 2C).
Close the animal holder and place the top heater probe as close as possible to your tissue (Figure 2D). This heater probe constantly measures the organ temperature and sends this information to the heater to adjust the electrical current, keeping it at 37°C.
Remove the PBS-soaked cotton and add low-melting agarose (2% in PBS) at 30°C on top of your preparation.
Cover the whole preparation with the top heater (Figure 2E). In the aperture on this heater there is a coverglass isolating the agarose from the objective (Figure 2F). Monitor the mouse anesthesia and inject a half-dose boost every 40 min. After the image acquisition, euthanize the animal with CO2.
Note: pictures of the aluminum foil clip and the top heater are presented in Figure 3.
3. Two-photon imaging acquisition
We used an upright "Prairie Ultima X-Y" two-photon microscope. Our system is equipped with a Ti:Sapphire laser, four top PMTs for simultaneous up to 4 channel acquisitions and a 20x water immersion objective.
Turn on the Ti:Sapphire laser and the whole microscope system.
Add dichroic mirrors and filter sets according to the wavelengths desired. In our case we used an Olympus-BX2 Chroma holder containing a 565 nm dichroic mirror, one 525/50 nm filter (for Foxp3-GFP signal), and one 595/50 nm filter (for Dextran-Rhodamine-B signal inside blood vessels). The laser wavelength range used was between 880 to 900 nm.
Add the animal holder to the microscope, connect and turn on all the heaters, add water on the top heater aperture, carefully lower the objective into the water, and focus on a vessel or some GFP-Tregs.
With the microscope x,y,z external control, quickly survey the tissue to locate a region of interest.
Adjust the gain on the PMTs to optimize color separation and minimize the amount of laser required to achieve sufficient signal over background. Try to use the minimal amount of laser to avoid photo-damage of your tissue.
Once the region of interests is located, begin time-lapse imaging. In our case, a typical 5D (x, y, z, t, and color) acquisition protocol consists of acquiring sequential images of a 50 μm-depth tissue volume, divided into 4.0 μm z-steps, x and y length of 140 μm each. Each volume takes around 30 seconds to be acquired. Therefore, 60 acquisitions will perform around 30 min of image acquisition.
Transfer the data to the institutional server and use ImageJ, Imaris, or Volocity software to perform multi-dimensional rendering and cell tracking.
4. Representative Results
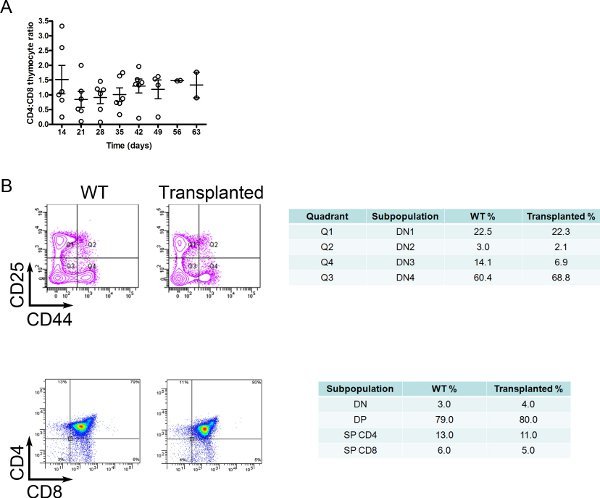 Figure 1. Embryonic thymus acceptance and function. After BM recover, embryonic thymuses were transplanted to BM reconstituted animals; two weeks after thymus transplantation, these mice were bled once per week to monitor the percentages of T cell subtypes by staining with anti-CD4 or anti-CD8 MAbs. We considered the thymus was successfully accepted in animals with a CD4:CD8 ratio around 1.5-2.0:1 (A). To confirm its functionality, we sacrificed some thymus-transplanted animals and compared the percentage of different thymocyte subpopulations with WT mice (B). The percentages of double-negative (DN; CD4-CD8- thymocytes) subpopulations (by staining with anti-CD25 and anti-CD44 MAbs), double-positive (DP; CD4+CD8+ thymocytes) and single-positive (SP) CD4+ or CD8+ thymocytes were similar between these animals.
Figure 1. Embryonic thymus acceptance and function. After BM recover, embryonic thymuses were transplanted to BM reconstituted animals; two weeks after thymus transplantation, these mice were bled once per week to monitor the percentages of T cell subtypes by staining with anti-CD4 or anti-CD8 MAbs. We considered the thymus was successfully accepted in animals with a CD4:CD8 ratio around 1.5-2.0:1 (A). To confirm its functionality, we sacrificed some thymus-transplanted animals and compared the percentage of different thymocyte subpopulations with WT mice (B). The percentages of double-negative (DN; CD4-CD8- thymocytes) subpopulations (by staining with anti-CD25 and anti-CD44 MAbs), double-positive (DP; CD4+CD8+ thymocytes) and single-positive (SP) CD4+ or CD8+ thymocytes were similar between these animals.
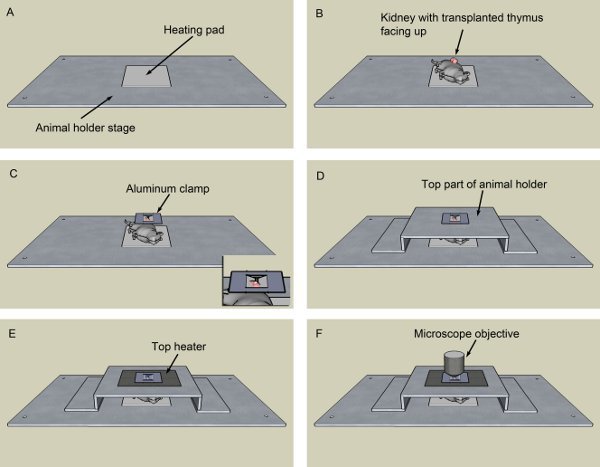 Figure 2. Animal holder assembly. After deeply anesthetized, the animal is put on top of a heating pad, previously mounted on top of the animal holder stage (A). The kidney containing the transplanted thymus is facing up (B). An aluminum foil clamp delicately pinches the whole kidney (C) to keep it in place. Fig. 1C insert shows in detail the clamp. The top part of the animal holder is put in place (D). The PBS-soaked cotton is removed from the top of the organ, replaced by warm low-melting agarose, and the top heater is added (E). Finally, the whole assembly is transferred to the microscope, here represented by the objective (F).
Figure 2. Animal holder assembly. After deeply anesthetized, the animal is put on top of a heating pad, previously mounted on top of the animal holder stage (A). The kidney containing the transplanted thymus is facing up (B). An aluminum foil clamp delicately pinches the whole kidney (C) to keep it in place. Fig. 1C insert shows in detail the clamp. The top part of the animal holder is put in place (D). The PBS-soaked cotton is removed from the top of the organ, replaced by warm low-melting agarose, and the top heater is added (E). Finally, the whole assembly is transferred to the microscope, here represented by the objective (F).
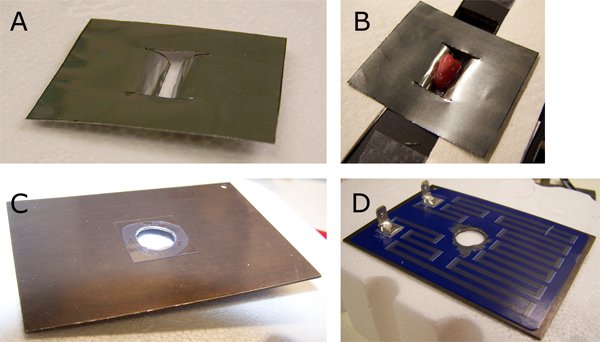 Figure 3. Details of holder parts. These pictures show the aluminum foil clamp (A) holding the kidney (B). Note also the region where the transplanted thymus is located. This approximately indicates the region to cut the thymus capsule and make the pocket to put the embryonic thymus. The top heater coverglass (C) was fixed with silicone glue to its bottom part (D).
Figure 3. Details of holder parts. These pictures show the aluminum foil clamp (A) holding the kidney (B). Note also the region where the transplanted thymus is located. This approximately indicates the region to cut the thymus capsule and make the pocket to put the embryonic thymus. The top heater coverglass (C) was fixed with silicone glue to its bottom part (D).
Movie 1. Intravital imaging of Foxp3-GFP+ thymocytes inside the thymus where the physiological levels of oxygenation and temperature (37°C) were kept during the whole process. Note the blood flow and the movement of Tregs. These speeds can be measured and compared with published data. Click here to view the movie.
Movie 2. Intravital imaging of Tregs inside a thymus where the images were acquired at 30°C. Note the absence of movement and the round shape of the cells despite the maintenance of blood flow. Click here to view the movie.
Discussion
In this paper we demonstrated the procedures for two-photon imaging of thymocytes inside a living animal. We also described some parameters that one should carefully control, such as the continuation of blood flow and the maintenance of organ temperature during the imaging procedures. Nonetheless, despite careful efforts to keep the organ stable, motion artifacts such as "organ drifting" can occur. Posterior image correction can be performed by the development of algorithms specifically designed for this purpose. Further image analysis could also be the source of new protocols development which seeks to minimize errors.
The thymus is the organ where all T cells are produced and, therefore, it is the organ where immunologists interested in understanding the generation of γδ, CD4, or CD8 T cells will focus their attention. Most studies concerning T cells are based upon differences in the numbers and/or stability of these cells after different in vitro/in vivo manipulations. However, only after the in vivo visualization we could observe the interaction between cells of the immune system involved in maintaining homeostasis3-7. Therefore, the in vivo observation of thymocytes is probably one of the most important missing information to better understand T cell biology. Intravital TPM provides a detailed picture of T cell movements and interactions and we demonstrate here how it can be used for detailed thymocyte studies. However, every technique has its limitations. While intravital imaging acquisition is the most accurate system for reflecting cells behavior inside the body, it is also true that explanted image acquisition of organs is less laborious and has been used to collect important information about the immune system8,9. Moreover, one cannot deny intravital imaging methods require surgery to expose tissues and blood vessels in anesthetized animals, which per se could cause an alteration in the whole organ physiology10. Nevertheless, there are non-invasively methods that abolish the artifacts caused by the surgical procedure11 and new methods are being developed that better prepare in advance the animals to be used12. Therefore, new surgical procedures and tolls will minimize or bypass actual limitations of intravital imaging acquisition and become more and more accessible to the scientific community.
We have demonstrated that the method we have described is feasible and it reports all in vivo systemic manipulations, such drug administration, that we have used. Thus, we suggest the use of this method together with ex vivo techniques already available in order to complement and strengthen further studies concerning thymocytes development.
Disclosures
We have nothing to disclose.
Acknowledgments
We would like to thank Dr. David Olivieri for critical review of this manuscript, Dr. Nuno Moreno for the logistic help to build our animal holder and heating pads and Dr. Vijay K. Kuchroo for the kind donation of Foxp3-KIgfp/gfp mice. This work is supported by "Fundação para Ciência e Tecnologia" (FCT, Portugal), grant # PTDC/EBB-BIO/115514/2009.
References
- Bettelli E. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- Ramsdell F, Zúñiga-Pflücker JC, Takahama Y. Coligan JE, editor. In vitro systems for the study of T cell development: fetal thymus organ culture and OP9-DL1 cell coculture. Current protocols in immunology. 2006. [DOI] [PubMed]
- Tadokoro CE. Regulatory T cells inhibit stable contacts between CD4+ T cells and dendritic cells in vivo. J. Exp. Med. 2006;203:505–511. doi: 10.1084/jem.20050783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mempel TR, Henrickson SE, Von Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154–159. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- Miller MJ. Imaging the single cell dynamics of CD4+ T cell activation by dendritic cells in lymph nodes. J. Exp. Med. 2004;200:847–856. doi: 10.1084/jem.20041236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakhar G. Stable T cell-dendritic cell interactions precede the development of both tolerance and immunity in vivo. Nat. Immunol. 2005;6:707–714. doi: 10.1038/ni1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugues S. Distinct T cell dynamics in lymph nodes during the induction of tolerance and immunity. 2004;5:1235–1242. doi: 10.1038/ni1134. [DOI] [PubMed] [Google Scholar]
- Tang Q. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat. Immunol. 2006;7:83–92. doi: 10.1038/ni1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Borgne M. The impact of negative selection on thymocyte migration in the medulla. Nature immunology. 2009;10:823–830. doi: 10.1038/ni.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baez S. An open cremaster muscle preparation for the study of blood vessels by in vivo microscopy. Microvascular research. 1973;5:384–394. doi: 10.1016/0026-2862(73)90054-x. [DOI] [PubMed] [Google Scholar]
- Wang B, Zinselmeyer BH, McDole JR, Gieselman PA, Miller MJ. Non-invasive Imaging of Leukocyte Homing and Migration in vivo. J. Vis. Exp. 2010. pp. e2062–e2062. [DOI] [PMC free article] [PubMed]
- Barretto RPJ. Time-lapse imaging of disease progression in deep brain areas using fluorescence microendoscopy. Nature medicine. 2011;17:223–228. doi: 10.1038/nm.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]


