Abstract
Magnetic nanoparticles biofunctionalized with antibodies against β-amyloid-40 (Aβ-40) and Aβ-42, which are promising biomarkers related to Alzheimer’s disease (AD), were synthesized. We characterized the size distribution, saturated magnetizations, and stability of the magnetic nanoparticles conjugated with anti-Aβ antibody. In combination with immunomagnetic reduction technology, it is demonstrated such biofunctionalized magnetic nanoparticles are able to label Aβs specifically. The ultralow-detection limits of assaying Aβs in vitro using the magnetic nanoparticles via immunomagnetic reduction are determined to a concentration of ∼10 ppt (10 pg/mL). Further, immunomagnetic reduction signals of Aβ-40 and Aβ-42 in human plasma from normal samples and AD patients were analyzed, and the results showed a significant difference between these two groups. These results show the feasibility of using magnetic nanoparticles with Aβs as reagents for assaying low-concentration Aβs through immunomagnetic reduction, and also provide a promising new method for early diagnosis of Alzheimer’s disease from human blood plasma.
Keywords: Magnetic nanoparticles, β-amyloid, immunomagnetic reduction
Magnetic nanoparticles play a role in labeling biomolecules because of their transparent and nonquenched magnetic signals as well as their nanoscaled size. To achieve a specific labeling, specific bioprobes are conjugated onto magnetic nanoparticles. For example, to label a certain kind of protein, the antibodies against the protein are conjugated onto magnetic nanoparticles. With these features, biofunctionalized magnetic nanoparticles are applied as the contrast agent for magnetic resonance imaging,1,2 sorters for target proteins or cells,3 biomolecular markers, and so forth.
In the late 1900s, it was demonstrated biofunctionalized magnetic nanoparticles acted as markers to detect biomolecules.4 By measuring the magnetic signals of magnetic nanoparticles which label target biomolecules, the concentration of the target biomolecules can be detected. Such technologies using biofunctionalized magnetic nanoparticles as labeling markers for assaying biomolecules are referred to as magnetically labeled immunoassay. Until now, several kinds of methods categorized in magnetically labeled immunoassay have been proposed, for example, measurement of saturated magnetization,5 magnetic relaxation,4,6 alternating-current magnetic susceptibility,7 and magnetic remanence.8 By utilizing these proposed methods, the magnetically labeled immunoassay has been demonstrated to be able to quantitatively detect not only proteins but also viruses, carcinogens, chemicals, and nucleic acids. In addition to the versatility of the detected biomolecules, the magnetically labeled immunoassay shows such advantages as low interference by sample color, high accuracy, and low cost; there is a trend to use the magnetically labeled immunoassay in both the academic world and industry.
One of the important issues for the magnetically labeled immunoassay is the preparation of biofunctionalized magnetic nanoparticles. Depending on the to-be-detected biomolecules, magnetic nanoparticles are biofunctionalized with different bioprobes. In this work, concerning the globally growing problem of dementia diseases, the to-be-target biomolecules are focused on the biomarkers related to Alzheimer’s disease. Although the biomarkers for Alzheimer’s disease have not been definitively identified, the promising candidates are β-amyloids, especially β-amyloid-40 (Aβ-40) and β-amyloid-42 (Aβ-42), according to the reported papers.9−13 Pathologically, excessive Aβ-40 and Aβ-42 in the cerebrospinal fluid (CSF) leads to the formation of plaques on the cortex, thus making brain activities dysfunctional. The Aβ-40/Aβ-42 plaques have been evidenced with target magnetic resonance imaging using biofunctionalized Fe2O3 nanoparticles.14,15
Analysis of CSF Aβ-42 shows a significant reduction in AD patients compared to the control while Aβ-40 is unchanged or increased in AD.16 Therefore, it has been suggested the Aβ-42/Aβ-40 ratio can improve AD diagnosis but others have not found these changes. However, the levels and significance of Aβ related proteins in plasma were more controversial.17 Studies have shown plasma Aβ-42 and Aβ-40 levels can be elevated, reduced, or even unchanged in AD versus control patients.18,19 The reasons plasma Aβ-42 levels are unstable are that the peptide is very sticky and binds to plasma proteins such as albumin, lipoproteins, and complement factors.20 In addition, the effect of oligomerization of Aβ-42 on the measurement by enzyme-linked immunosorbent assay (ELISA) is unknown. Both the binding effect and oligomerization could mask Aβ epitopes and decrease the detectable levels. Therefore, we need a brand new method that can counter such nature of Aβ-42, to avoid the contradictory results shown in previous literature. The immunomagnetic reduction of the nanoparticles could be a potential solution.
It is quite inconvenient to diagnose Alzheimer’s disease by traditional molecular detection (e.g., ELISA) of these two biomarkers from the cerebrospinal fluid. An easy and reliable molecule-diagnostic strategy, by testing blood plasma rather than cerebrospinal fluid, is now developing by combination of magnetic nanoparticles and immunomagnetic reduction technology.
To magnetically label Aβ-40 and Aβ-42, magnetic nanoparticles biofunctionalized with antibodies against Aβ-40 and Aβ-42 are synthesized in this work. The physical properties such as the particle size distribution, magnetism, and stability of magnetic nanoparticles dispersed in water are characterized. To investigate the labeling of magnetic nanoparticles onto Aβ-40 and Aβ-42, properties such as a low-detection limit and interference via immunomagnetic reduction assay are examined. Preclinical tests have also been performed by testing plasma samples from normal and AD patients, to verify the feasibility of diagnosis of Alzheimer’s disease according to immunomagnetic reduction signals.
Results and Discussion
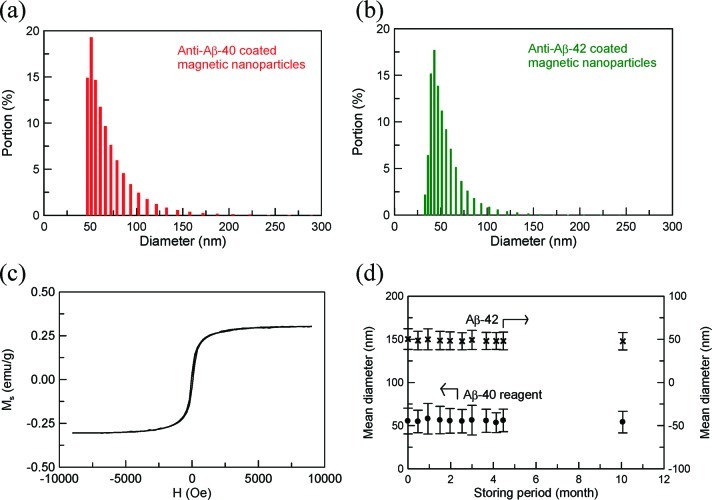
The size distributions of the magnetic nanoparticles coated with anti-Aβ-40 and anti-Aβ-42 are shown in Figure 1a and b. The mean diameters of reagents Aβ-40 and Aβ-42 are 55.3 and 50.3 nm, respectively. Figure 1c plots the magnetic hysteresis curve for either of reagents Aβ-40 and Aβ-42. Clearly, the reagent displays superparamagnetism and the saturated magnetization is 0.3 emu/g (= 8.5 mg Fe/ml).
Figure 1.
Size distributions of reagents (a) Aβ-40 and (b) Aβ-42, (c) magnetic hysteristic curve of reagent, and (d) the mean diameter of reagents as a function of the storage time at 2–8 °C.
For the stability test, the mean diameters of reagents Aβ-40 and Aβ-42 are detected as a function of the storage time. Reagents Aβ-40 and Aβ-42 are stored at 2–8 °C. The results are shown in Figure 1d. Regardless of whether reagent Aβ-40 or Aβ-42 is used, there is no significant variation in the mean diameter when the reagents are stored at 2–8 °C for 10 months. This fact points to the high stability of reagents Aβ-40 and Aβ-42.
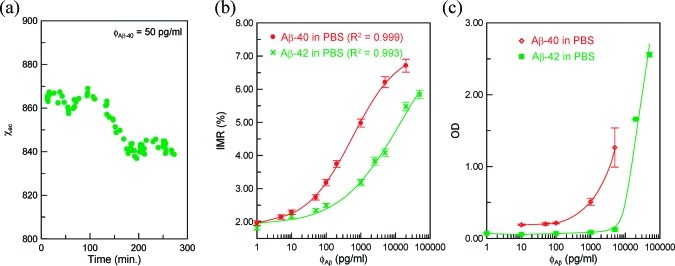
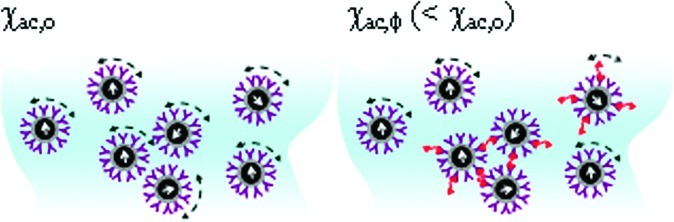
To observe the association between magnetic nanoparticles and to-be-detected Aβs, the real-time χac signal, that is, the χac–t curve, of the mixture of reagent and sample solution is detected using a SQUID-based ac magnetosusceptometer (XacPro-S, MagQu). A typical χac–t curve is shown in Figure 2a for the mixture of reagent Aβ-40 and the 50 pg/mL Aβ-40 solution. The Aβ-40 solution is prepared by spiking Aβ-40 (H-1194, Bachem) into pH 7.4 phosphate buffered saline (PBS) solution. In Figure 2a, at the beginning, the χac signal fluctuates around 865. At the time interval from 100 to 160 min, the χac signal descends. Then, the χac signal remains around 840. The higher-level χac signals at the time interval from 0 to 100 min correspond to the Aβ-40 molecules not associated with magnetic nanoparticles. Once the Aβ-40 molecules bind with the magnetic nanoparticles, the χac signal starts to decrease, as shown by the reduction in the χac signals at the time interval from 100 to 160 min. As the association between Aβ-40 and magnetic nanoparticles finishes, the χac signal comes to an equilibrium level of lower values compared with that at the beginning. By averaging the data point at the time interval from 0 to 100 min, the mean value of the χac signals was found to be 862.6, and the mean value of χac signals beyond 160 min was obtained as 838.3. Thus, the reduction percentage in the χac signal, or so-called IMR signal, of the reagent–sample mixture is calculated to be 2.82%. With the results of the triplicate tests, the IMR signal for 50 pg/mL Aβ-40 solution using reagent Aβ-40 was obtained as (2.73 ± 0.08)%.
Figure 2.
(a) Real-time χac signal of reagent mixed with to-be-detected sample, and Aβ-concentration-dependent (b) IMR (%) via IMR and (c) optical density (OD) via ELISA.
The IMR signals for Aβ-40 solutions of various concentrations were detected, and the results are shown with dot data points in Figure 2b. The detected concentration ϕAβ of Aβ-40 PBS solution is from 1 to 20 000 pg/mL. It was found the IMR signal gently increases with increasing Aβ-40 concentration from 1 to 50 pg/mL, followed by a marked increase in the IMR signal as the Aβ-40 concentration increases to 5000 pg/mL, finally reaching a saturated value at an Aβ-40 concentration higher than 10 000 pg/mL. Such behavior observed for the Aβ-40 concentration dependent IMR signal shown in Figure 3 is very similar to the so-called logistic function
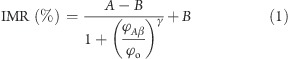 |
1 |
where A, B, ϕo, and γ are the fitting parameters. The dot data points in Figure 2b are fitted with eq 1. The fitting curve is plotted together with the dot data points in Figure 2b. The parameters A, B, ϕo, and γ were found as 1.89, 7.20, 567.3, and 0.65, respectively. The correlation coefficient R2 between the rhombus points and the fitting curve is 0.999.
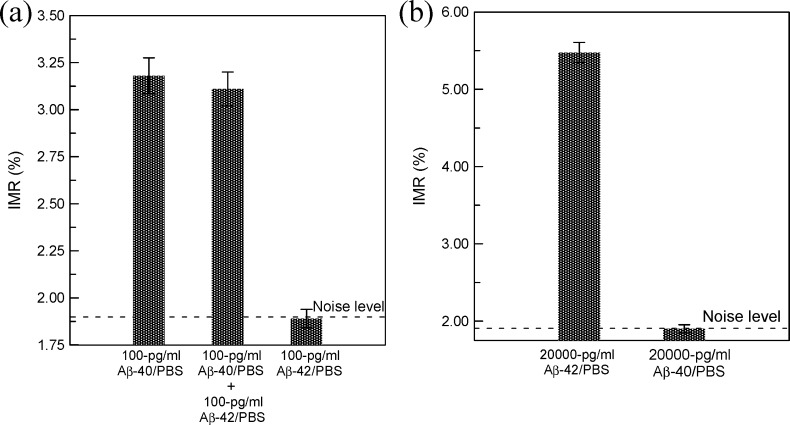
Figure 3.
Interference tests for IMR assays on (a) Aβ-40 and (b) Aβ-42. In (a), reagent Aβ-40 is used, and reagent Aβ-42 is used in (b).
The fitting parameter A in eq 1 denotes the IMR signal as the concentration of Aβ-40 approaches zero. Therefore, the value of A corresponds to the noise level of the IMR signal for assaying Aβ-40. The noise is mainly attributed to the electric noise of SQUID ac magnetosusceptometer. Conventionally, the low-detection limit is defined as the concentration showing an IMR signal higher than the noise level by three times as the standard deviation of IMR signals for a low-concentration test. In this experiment, the standard deviation of low-concentration tests, say 10 pg/mL, is 0.07%. Thus, the low-detection limit is the concentration having an IMR signal of 2.1%. Via eq 1, the low-detection limit for assaying Aβ-40 is found to be 4.28 pg/mL.
As to Aβ-42, the IMR signal as a function of Aβ-42 using reagent Aβ-42 is examined. The experimental data are plotted with cross symbols in Figure 2b. These cross symbols are well fitted to eq 1 with fitting parameters A being 1.90, B being 8.10, ϕo being 14 157.7, and γ being 0.50. The standard deviation for a low-concentration test, say 10 pg/mL, is around 0.07%. Thus, the low-detection limit for assaying Aβ-42 is the concentration having an IMR signal of 2.11%. Using eq 1 with fitting parameters for Aβ-42, the low-detection limit for Aβ-42 is 16.40 pg/mL.
The results shown in Figure 2b are compared with that detected by ELISA. The protocols for detecting Aβ-40 and Aβ-42 are described in the user manuals of the ELISA kits (27718, IBL for Aβ-40; and KHB3441, Invitrogen for Aβ-42). The Aβ-40 concentration dependent optical densities (ODs) are also shown in Figure 2c with hollow tilted squares. It was found there is no significant difference in OD when the concentration of Aβ-40 is lower than 100 pg/mL. However, Figure 2b shows a clear difference in IMR signals between 10 and 1 pg/mL for Aβ-40. These results prove the low-detection limit in Aβ-40 concentration of IMR is lower than that of ELISA by 2 orders of magnitude.
The square points shown in Figure 2c denote the ODs for Aβ-42 solutions by using ELISA. The low-detection limit of ELISA for assaying Aβ-42 is around 105 pg/mL, while IMR measurement shows the low-detection limit to be 16.40 pg/mL. Therefore, SQUID-based IMR assay in Aβ-42 is more sensitive than ELISA by 4 orders of magnitude.
For real samples, such as cerebrospinal fluid or plasma, Aβ-40 and Aβ-42 coexist in samples. Since Aβ-40 is very similar to Aβ-42 in terms of molecular structures, the existing Aβ-42 might interfere with the association between Aβ-40 and anti-Aβ-40 on the magnetic nanoparticles, or Aβ-40 could contribute a false-positive IMR signal when assaying Aβ-42 using reagent Aβ-42. It is necessary to examine the specificity of the associations between Aβ-40/Aβ-42 molecules and reagent Aβ-40/reagent Aβ-42. First, the interference from Aβ-42 to the assay of Aβ-40 using reagent Aβ-40 is examined. To do this, three samples are prepared. The first sample is the 100 pg/mL Aβ-40 PBS solution, the second sample is the mixture solution of 100 pg/mL Aβ-40 and 100-pg/mL Aβ-42 solution. The third sample is the 100 pg/mL Aβ-42 PBS solution. Using reagent Aβ-40, the IMR signals of these three samples are detected. The results are shown in Figure 3a. Clearly, there is a significant nondifference in the IMR signals for the first sample and the second sample. This means the existence of Aβ-42 is not crucial to the assay for Aβ-40 using reagent Aβ-40. The IMR signal of the second sample resulted from Aβ-40, and it had nothing to do with Aβ-42. The independence of Aβ-42 in assaying Aβ-40 using reagent Aβ-40 is shown by the IMR signal of the third sample, which shows the noise level for the IMR signal.
In turn, the effect of Aβ-40 in detecting Aβ-42 using Aβ-42 is checked. Two samples consisting of 20 000 pg/mL Aβ-42 and 20 000 pg/mL Aβ-40 are prepared. The IMR signals for these two samples using reagent Aβ-42 are detected and shown in Figure 3b. The 20 000 pg/mL Aβ-42 sample shows a clear IMR signal around 5.5%, but no significant IMR signal can be found for the 20 000 pg/mL Aβ-40 sample. The results in Figure 3b reveal a high specificity of detecting Aβ-42 using reagent Aβ-42, even with the existence of Aβ-40.
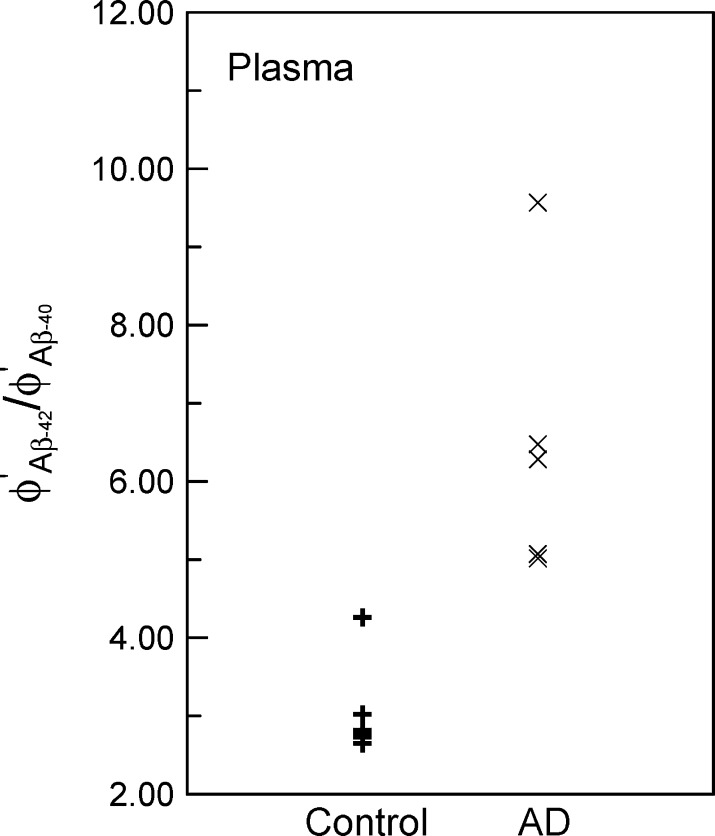
The accuracy of diagnosing AD by assaying the concentrations of Aβ-40 and Aβ-42 in plasma is examined. Twelve human plasma samples are prepared. Six of the samples are from AD patients (AD group), and the other six samples are normal (control group). The human experiments were performed under regulations established by the Internal Review Board of National Taiwan University Hospital. For each sample, the IMR signals for Aβ-40 and Aβ-42 are detected by immunomagnetic reduction assay and using reagents Aβ-40 and Aβ-42. With the relationship in Figure 2b, the concentrations of Aβ-40 and Aβ-42 for each sample are obtained from IMR signals. The detected concentrations of Aβ-40 and Aβ-42 in plasma are denoted by ϕAβ-40′ and ϕAβ-42, respectively. In this work, the parameter ϕAβ-42′/ϕAβ-40 is used for investigating the diagnosing accuracy of AD. The values of the parameter for the control group are plotted with + symbols in Figure 4, and symbols × are for the AD group. There is a clear difference in the parameter ϕAβ-42′/ϕAβ-40 between the control group and AD group. This preliminary result shows the feasibility of diagnosing AD by detecting the concentrations of Aβ-40 and Aβ-42 in plasma through immunomagnetic reduction assay.
Figure 4.
Values of parameter ϕAβ-42′/ϕAβ-40 in human plasma for control group (normal sample) and AD group (AD patients).
In summary, magnetic reagents consisting of magnetic nanoparticles biofunctionalized with antibodies against Aβ-40 and Aβ-42 are synthesized. Through the immunomagnetic reduction assay, the abilities of the reagents associated with Aβ-40 at concentration levels lower than 10 pg/mL and Aβ-42 at concentration levels lower than 20 pg/mL are demonstrated. Further, such reagents show high specificity to detect Aβ-40 and Aβ-42 using the IMR assay. These preliminary results of detecting the concentrations of Aβ-40 and Aβ-42 in human plasma show the promise of high-sensitivity and high-specificity diagnosis for Alzheimer’s disease.
Methods
Preparation of Magnetic Reagent
The processes to synthesize magnetic Fe3O4 nanoparticles are proposed by MagQu Co., Ltd. and are described in detail in refs (21) and (22). The solution consisting of a stoichiometric ratio, 1:2 ferrous sulfate heptahydrate (FeSO4·7H2O) and ferric chloride hexahydrate (FeCl3·6H2O), was mixed with an equal volume of aqueous dextran (molecular weight ∼ 100 000). Dextran formed a hydrophilic layer on the Fe3O4 particles to disperse the particles in water. The mixture was heated to 70–90 °C and titrated with NH4OH solution to form black Fe3O4 particles. Aggregates and excess unbound dextran were removed by centrifugation and gel filtration chromatography to obtain a concentrated magnetic fluid. The reagent with the desired magnetic concentration was obtained by diluting the concentrated magnetic fluid with pH 7.4 PBS solution.
To covalently bind antibodies, that is, anti-Aβ-40 (sc-53822, Santa Cruz Biotech.) or anti-Aβ-42 (437900, Invitrogen), to the dextran of the magnetic nanoparticles, 0.15 M NaIO4 solution was added into the magnetic solution to oxide dextran and create aldehyde groups (−CHO). Dextran reacts with antibodies via −CH=N–. Thus, the antibodies were covalently bound to dextran. Through magnetic separation, the unbound antibodies were separated from the magnetic solution. Hereafter, the magnetic solutions containing magnetic nanoparticles biofunctionalized with anti-Aβ-40 and anti-Aβ-42 are referred as to Aβ-40 reagent and Aβ-42 reagent, respectively.
Size Distribution and Saturated Magnetization Analysis
The size distribution of the magnetic nanoparticles and the magnetic concentration of reagents are characterized. Using dynamic laser scattering (Nanotrac-150, Microtrac), the size distribution of the magnetic nanoparticles biofunctionalized with antibodies was analyzed. The saturated magnetizations of the reagents are obtained by measuring the hysterisis curves at room temperature using a vibrating sample magnetometer (model 4500, EG&G). The maximum applied magnetic field is 9 kGauss.
IMR Measurement
The method to probe the association of to-be-detected Aβs and magnetic nanoparticles in reagents Aβ-40 and Aβ-42 is immunomagnetic reduction (IMR), which detects the reduction percentage in the alternating-current (ac) magnetic susceptibility χac of reagent due to the association of biofunctionalized magnetic nanoparticles and target biomolecules. The detailed mechanism of IMR is described in ref (23). In this work, the reduction percentage in χac of reagent is probed using the ac magnetosusceptometer (XacPro-S, MagQu) equipped with a high-Tc superconducting-quantum-interference-device (SQUID) magnetometer as a magnetic sensor.
For examining the association between Aβs and biofunctionalized magnetic nanoparticles, 80 μL/60-μL Aβ-40/Aβ-42 reagent is mixed with a 40 μL/60 μL sample solution. The time-dependent χac signal of the mixture is recorded with the SQUID-based ac magnetosusceptometer. Once the magnetic nanoparticles bind with the Aβ molecules, the χac signal of the mixture decreases. With the χac values at the initial and the end of the association of magnetic nanoparticles and Aβ molecules, the reduction percentage of the χac signal for the sample is measured. In this paper, the reduction percentage in the χac signal is referred to as the IMR signal.
This work was supported by the National Science Council of Taiwan under Grant Numbers 98-2112-M-003-003, 98-2323-B-003-001-CC2, and NSC98-2752-M-002-016-PAE, by the Department of Health under Grant Numbers DOH98-TD-N-111-008 and DOH99-TD-N-111-008, and by the Ministry of Economic Affairs of Taiwan under Grant Numbers 1Z970688 (SBIR) and S09800226-203 (JAID).
Author Contributions
C.-C.Y. and K.H.C. did the IMR measurements and data analysis for calculating the concentrations of Aβ-40 and Aβ-42. In addition, they performed the ELISA for Aβ-40 and Aβ-42. S.-Y.Y. and B.Y.S. synthesized magnetic particles as well as conjugated antibodies onto magnetic particles. H.-E.H., C.-Y.H., H.-C.Y., and J.-J.C. characterized the properties of magnetic particles with antibodies. For example, the measurements of magnetic hysteresis, particles size distribution, and so forth. T.-F.C. and M.-J.C. collected human plasma for the IMR assays on Aβ-40 and Aβ-42. Also, they provided medical information for assaying Aβ-40 and Aβ-42.
References
- Roberts D.; Zhu W. L.; Frommen C. M.; Rosenzweig Z. (2000) Synthesis of gadolinium oxide magnetoliposomes for magnetic resonance imaging. J. Appl. Phys. 87, 6208. [Google Scholar]
- Wu C. C.; Lin L. Y.; Lin L. C.; Huang H. C.; Liu Y. B.; Tsai M. C.; Gao Y. L.; Wang W. C.; Yang S. Y.; Horng H. E.; Yang H. C.; Tseng W. K.; Lee T. L.; Hsuan C. F.; Tseng I. W. Y. (2008) Biofunctionalized magnetic nanoparticles for in vitro labeling and in vivo locating specific biomolecules. Appl. Phys. Lett. 92, 142504. [Google Scholar]
- Inglis D. W.; Riehn R.; Sturm J. C.; Austin R. H. (2006) Microfluidic high gradient magnetic cell separation. J. Appl. Phys. 99, 08K101. [Google Scholar]
- Kötitz R.; Weitschies W.; Trahms L.; Brewer W.; Semmler W. J. (1999) Determination of the binding reaction between avidin and biotin by relaxation measurements of magnetic nanoparticles. J. Magn. Magn. Mater. 194, 62. [Google Scholar]
- Horng H. E.; Yang S. Y.; Huang Y. W.; Jiang W. Q.; Hong C. Y.; Yang H. C. (2005) Nanomagnetic particles for SQUID-based magnetically labeled immunoassay. IEEE Trans. Appl. Supercond. 15, 668. [Google Scholar]
- Yang H. C.; Yang S. Y.; Liao S. H.; Fang G. L.; Huang W. H.; Liu C. H.; Horng H. E.; Hong C. Y. (2006) Magnetic relaxation measurement in immunoassay using high-transition-temperature superconducting quantum interference device system. J. Appl. Phys. 99, 124701. [Google Scholar]
- Krause H. J.; Wolters N.; Zhang Y.; Offenhäusser A.; Miethe P.; Meyer M. H. F.; Hartmann M.; Keusgen M. (2007) Magnetic particle detection by frequency mixing for immunoassay applications. J. Magn. Magn. Mater. 311, 436. [Google Scholar]
- Enpuku K.; Kuroda D.; Ohba A.; Yang T. Q.; Yoshinaga K.; Nakahara T.; Kuma H.; Hamasaki N. (2003) Biological immunoassay utilizing magnetic marker and high-Tc superconducting quantum interference device magnetometer. Jpn. J. Appl. Phys. 42, L1436. [Google Scholar]
- Tapiola T.; Alafuzoff I.; Herukka S. K.; Parkkinen L.; Hartikainen P.; Soininen H.; Pirttilä T. (2009) Cerebrospinal fluid β-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch. Neurol. 66, 382. [DOI] [PubMed] [Google Scholar]
- Sobow T.; Flirski M.; Liberski P. P.; Kloszewska I. (2007) Plasma Aβ levels as predictors of response to rivastigmine treatment in Alzheimer’s disease. Acta Neurobiol. Exp. 67, 131. [DOI] [PubMed] [Google Scholar]
- Fagan A. M.; Roe C. M.; Xiong C.; Mintun M. A.; Morris J. C.; Holtzman D. M. (2007) Cerebrospinal fluid tau/β-amyloid(42) ratio as a prediction of cognitive decline in nondemented older adults. Arch. Neurol. 64, 343. [DOI] [PubMed] [Google Scholar]
- Frankfort S. V.; Tulner L. R.; van Campen J. P.; Verbeek M. M.; Jansen R. W.; Beijnen J. H. (2008) Amyloid β protein and tau in cerebrospinal fluid and plasma as biomarkers for dementia: a review of recent literature. Curr. Clin. Pharmacol. 3, 123. [DOI] [PubMed] [Google Scholar]
- Blennow K.; Hampel H.; Weiner M.; Zetterberg H. (2010) Is it time for biomarker-based diagnostic criteria for prodromal Alzheimer’s disease?. Nat. Rev. Neurol. 6, 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaat H.; Sorci M.; Belfort G; Margel S. (2008) Effect of maghemite nanoparticles on insulin amyloid fibril formation: Selective labeling, kinetics, and fibril removal by a magnetic field. J. Biomed. Mater. Res., Part A 91, 342. [DOI] [PubMed] [Google Scholar]
- Skaat H.; Margel S. (2009) Synthesis of fluorescent-maghemite nanoparticles as multimodal imaging agents for amyloid-beta fibrils detection and removal by a magnetic field. Biochem. Biophys. Res. Commun. 386, 645. [DOI] [PubMed] [Google Scholar]
- Humpel C. (2010) Identifying and validating biomarkers for Alzheimer’s disease. Trends Biotechnol. 29, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetterberg H.; Blennow K. (2006) Plasma Aβ in Alzheimer’s disease--up or down?. Lancet Neurol. 5, 638. [DOI] [PubMed] [Google Scholar]
- Cedazo-Mnguez A.; Winblad B. (2010) Biomarkers for Alzheimer’s disease and other forms of dementia: clinical needs, limitations and future aspects. Exp. Gerontol. 45, 5. [DOI] [PubMed] [Google Scholar]
- Zetterberg H.; Blennow K.; Hanse E. (2010) Amyloid β and APP as biomarkers for Alzheimer’s disease. Exp. Gerontol. 45, 23. [DOI] [PubMed] [Google Scholar]
- Irizarry M. C. (2004) Biomarkers of Alzheimer disease in plasma. NeuroRx 1, 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W.; Yang H. C.; Yang S. Y.; Horng H. E.; Hung J. C.; Chen Y. C.; Hong C. Y. (2004) Preparation and properties of superparamagnetic nanoparticles with narrow size distribution and biocompatible. J. Magn. Magn. Mater. 283, 210. [Google Scholar]
- Yang S. Y.; Jian Z. F.; Horng H. E.; Hong C. Y.; Yang H. C.; Wu C. C.; Lee Y. H. (2008) Dual immobilization and magnetic manipulation of magnetic nanoparticles. J. Magn. Magn. Mater. 320, 2688. [Google Scholar]
- Chieh J. J.; Yang S. Y.; Jian Z. F.; Wang W. C.; Horng H. E.; Yang H. C.; Hong C. Y. (2008) Hyper-high-sensitivity wash-free magnetoreduction assay on biomolecules using high-Tc superconducting quantum interference devices. J. Appl. Phys. 103, 14703. [Google Scholar]