Abstract
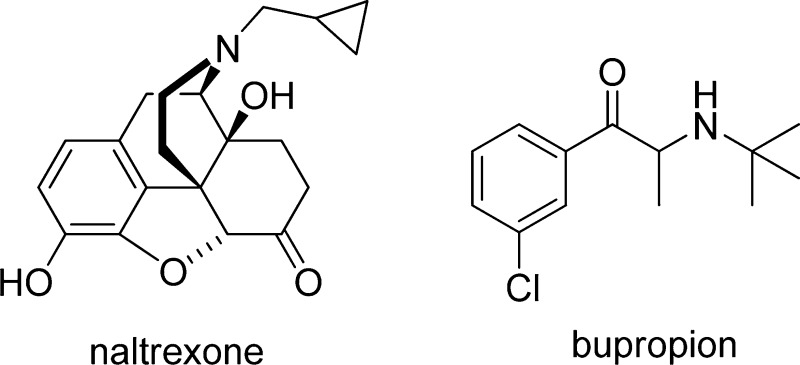
Contrave is an investigational fixed-dose combination drug of naltrexone and bupropion currently in Phase III clinical trials for the treatment of obesity. Orexigen Therapeutics, Inc. has demonstrated efficacy of their product and is currently addressing FDA safety concerns and deciding future actions.
Keywords: Contrave, bupropion, naltrexone, weight-loss, obesity
Contrave is a novel fixed-dose combination product composed of naltrexone and bupropion (NB) in late-stage development by Orexigen Therapeutics, Inc. as a potential treatment for obesity, including weight loss and management, used in conjunction with lifestyle modification. Contrave is recommended for obese patients (BMI ≥ 30 kg/m2) or overweight patients (BMI ≥ 27 kg/m2) with weight-related comorbidities such as type 2 diabetes, dyslipidemia, or hypertension. The NIH reported in 2007 that approximately 68% of adults in the United States are obese or overweight and estimated the United States medical burden of obesity to be approximately $147 billion in 2008.1 Obesity is associated with numerous comorbidities, including diabetes, heart disease, dyslipidemia, hypertension, stroke, cancer and depression. According to the NIH, the initial goal of weight loss therapy is to reduce body weight by approximately 10% from baseline.2 Unfortunately, currently approved pharmacotherapies are associated with <5% weight loss and are often poorly tolerated, leaving bariatric surgery as the only effective, yet invasive, treatment for obesity. These findings have spurred investigation into combination pharmacotherapies, such as Contrave, to potentially fill this treatment gap to safely deliver sufficient and durable weight loss of ≥10%.
Naltrexone and bupropion are both well established drugs with approximately 1 million and 50 million unique patient exposures, respectively.3 Naltrexone monotherapy was approved for the treatment of opioid addiction in 1984 and alcohol dependence in 1995. Bupropion monotherapy was approved in 1985 as an antidepressant and in 1997 as a smoking cessation medication. The Contrave New Drug Application (NDA) was filed with NB available in two tablet strengths: 4 mg naltrexone/90 mg bupropion and 8 mg naltrexone/90 mg bupropion with both naltrexone and bupropion as sustained release. The proposed dosing regimen is two tablets by mouth twice daily (BID), resulting in a total daily maintenance dose of 16 mg naltrexone/360 mg bupropion (NB16) and 32 mg naltrexone/360 mg bupropion (NB32), respectively. Upon treatment initiation, Contrave dosing should be escalated starting with one tablet daily for the first week, followed by the addition of another tablet each subsequent week. Two tablets BID is reached at the start of week 4.
Bupropion is a catecholamine reuptake inhibitor and naltrexone is a mu-opioid receptor antagonist. Both pharmacological mechanisms addressed by NB have been previously explored in obesity. Bupropion has been associated with modest weight loss in clinical trials for both major depression as well as in obese patients. Bupropion has been shown to stimulate hypothalamic pro-opiomelanocortin (POMC) neurons that release alpha-melanocyte stimulating hormone (α-MSH) which, in turn, binds to melanocortin 4 (MC4) receptors. The binding of α-MSH to MC4 receptors initiates a cascade of actions that results in reduced energy intake and increased energy expenditure.4 When α-MSH is released, POMC neurons simultaneously release β-endorphin, an endogenous agonist of the mu-opioid receptor. Binding of β-endorphin to mu-opioid receptors on POMC neurons mediates a negative feedback loop on POMC neurons, leading to a decrease in the release of α-MSH.5,6 Blocking this inhibitory feedback loop with naltrexone is thought to facilitate a more potent and longer-lasting activation of POMC neurons, thereby amplifying effects on energy balance. As a result, coadministration of bupropion and naltrexone produces a substantially greater effect on the POMC firing rate than either compound administered alone, suggesting that the drugs act synergistically.
The efficacy of Contrave was assessed through four randomized, double-blind, placebo-controlled, 56-week phase III clinical studies (NB-301, NB-302, NB-303, and NB-304) in obese and overweight patients. Studies NB-301, NB-302, and NB-303 enrolled a similar patient population, obese patients with either uncomplicated obesity, or obese/overweight patients with controlled hypertension and/or dyslipidemia; whereas NB-304 enrolled obese/overweight patients with type 2 diabetes. NB-301 investigated two doses of NB (NB16 and NB32) and evaluated an abrupt versus tapered discontinuation of NB; the NB32 dose was standard in the other studies. Patients in NB-303 who did not experience or maintain at least 5% weight loss from baseline between weeks 28–44 were rerandomized to either continue taking NB32 or increase to NB48 (48 mg naltrexone/day +360 mg bupropion/day) to determine if the increased naltrexone dose resulted in additional weight loss. NB-303 also evaluated two different dose escalation schemes (3 weeks versus 4 weeks). Patients in NB-302 underwent an intensive behavioral modification program that included regular group counseling sessions, maintenance of food diaries, diet, and exercise.
All four studies demonstrated statistically significant and clinically meaningful weight loss following up to 56 weeks of treatment with NB32 compared with placebo. Percent weight loss from baseline and the percent of patients achieving at least 5% weight loss from baseline were similar between studies NB-301 (−4.8% and 48.0%) and NB-303 (−5.2% and 50.5%), reflecting their similar designs and populations.7 Study NB-302 patients exhibited greater weight loss on average in both the NB- and placebo-treated groups, consistent with the use of intensive behavioral modification; 66.4% of the NB-treated patients achieved ≥5% decrease in body weight relative to 42.5% of placebo group.7 This is an interesting comparison to the other placebo groups which on average 17.5% of subjects achieved at least 5% weight loss from baseline.7 NB32-treated patients with type 2 diabetes in study NB-304 showed the smallest degree of weight loss from baseline (−3.3%), although a significantly greater proportion of NB32-treated patients (44.5%) benefitted with at least 5% weight loss at end point compared to placebo (18.9%).7 The average percent weight loss from baseline observed with NB treatment across the four studies corresponded to between approximately 5 and 9 kg (11–22 pounds).7 Results indicate that treatment with NB32 is efficacious for weight loss and leads to statistically significant and clinically meaningful outcomes in relation to cardiometabolic parameters (waist circumference, HDL-C, triglycerides), blood pressure, and glycemic control. Additionally, NB-treated patients self-reported an improved quality of life most consistently with physical function and self-esteem.
The use of NB was generally well-tolerated with the frequency and distribution of safety findings consistent with the established profile for naltrexone and bupropion. Common adverse events (AEs) included nausea (31.8% total NB group; 6.7% placebo) and vomiting (9.9% total NB group; 2.9% placebo) which tended to occur early in treatment, were mostly mild to moderate in severity, and were generally self-limiting.7 Initiation of treatment with NB was associated with transient increases in blood pressure (∼1 mmHg) and heart rate from baseline, which are consistent with the known hemodynamic effects of bupropion and were attenuated by weight loss in patients who responded to therapy. Additional safety findings include the following: seizure occurrence was consistent with the lowest approved dose of bupropion SR, no increased risk for suicide or depression, no hepatotoxicity with long-term treatment, no prolongation of QTc intervals, and comparable incidence of major adverse cardiac effects (MACE) such as stroke and myocardial infarction.7 However, the number of cardiovascular (CV) events was too low to make a firm conclusion.
In a December 2010 press release, Orexigen announced receipt of a positive recommendation from the FDA Endocrinologic and Metabolic Drugs Advisory Committee on the Contrave NDA. The committee voted 13 to 7 that data demonstrated the potential benefits of Contrave and outweighs the potential risks when used long-term in a population of obese and overweight individuals.8 The committee also voted 11 to 8 that an additional study to examine the drug’s effect on risk for MACE should be conducted as a postapproval requirement versus preapproval.8 Orexigen was later surprised upon receipt of the Complete Response Letter (CRL) from the FDA which stated concerns about the CV safety profile of Contrave when used long-term in a population of overweight and obese subjects.9 The CRL requested Orexigen to conduct a randomized, double-blind, placebo-controlled trial of sufficient size and duration to demonstrate that the risk of MACE in overweight and obese subjects treated with Contrave does not adversely affect the drug’s benefit–risk profile.9
Orexigen recently engaged in discussions with the FDA Division of Metabolic and Endocrinologic Products (DMEP) regarding the CV risk cited in the approval deficiency and also explored approval for a narrowed indication in patients with lower CV risk until data from the proposed CV outcomes trial could be reviewed for label expansion.10 In response, DMEP requested a preapproval CV outcomes trial and will not consider approving Contrave for a narrowed population until review of data from the outcomes trial. Based on DMEP’s feedback, Orexigen plans to (a) appeal DMEP’s response through the formal dispute resolution process, (b) put any further clinical development for its obesity programs in the United States on hold until a clear and feasible path to regulatory approval is identified, and (c) accelerate the exploration of opportunities for product candidates outside of the United States.10
DMEP intends to hold a general advisory committee meeting in early 2012 to discuss CV assessment for obesity therapeutics. Contrave is the third obesity medication disallowed by the FDA in the past 12 months. The last obesity drug approved in the United States was orlistat in 1999.
References
- Flegal K. M.; Carroll M. D.; Ogden C. L.; Curtin L. R. (2010) Prevalence and trends in obesity among US adults, 1999–2008. J. Am. Med. Assoc. 303, 235–241. [DOI] [PubMed] [Google Scholar]
- NIH/NHLBI, NAASO. (2000) The Practical guide: Identification, Evaluation, and Treatment of Overweight and Obesity in Adults, NIH, Bethesda, MD. [Google Scholar]
- Estimates Based on Wolters Kluwer Rx and Patient Data, Jan–Dec 2009; and IMS Health Rx and Persistence Data, Jan 1985–Dec 2009.
- Cowley M. A.; Prortchuk N.; Fan W.; Dinulescu D. M.; Colmers W. F.; Cone R. D. (1999) Integration of NPY, AGRP, and Melanocortin Signals in the Hypothalamic Paraventricular Nucleus: Evidence of a Cellular Basis for the Adipostat. Neuron 24, 155–163. [DOI] [PubMed] [Google Scholar]
- Ibrahim N.; Bosch M. A.; Smart J. L.; Qiu J.; Rubinstein M.; Ronnekleiv O. K.; Low M. J.; Kelly M. J. (2003) Hypothalamic proopiomelanocortin neurons are glucose responsive and express K(ATP) channels. Endocrinology 144, 1331–1340. [DOI] [PubMed] [Google Scholar]
- Kelly M. J.; Loose M. D.; Ronnekleiv O. K. (1990) Opioids hyperpolarize beta-endorphin neurons via mu-receptor activation of a potassium conductance. Neuroendocrinology 52, 268–275. [DOI] [PubMed] [Google Scholar]
- http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM235672.pdf.
- Orexigen Therapeutics, Inc., press release, December 7, 2010. http://ir.orexigen.com/phoenix.zhtml?c=207034&p=irol-newsArticle&ID=1505602&highlight=. [Google Scholar]
- Orexigen Therapeutics, Inc., press release, February 1, 2011. http://ir.orexigen.com/phoenix.zhtml?c=207034&p=irol-newsArticle&ID=1522207&highlight= [Google Scholar]
- Orexigen Therapeutics, Inc., press release, June 3, 2011. http://ir.orexigen.com/phoenix.zhtml?c=207034&p=irol-newsArticle&ID=1570586&highlight= [Google Scholar]


