Abstract
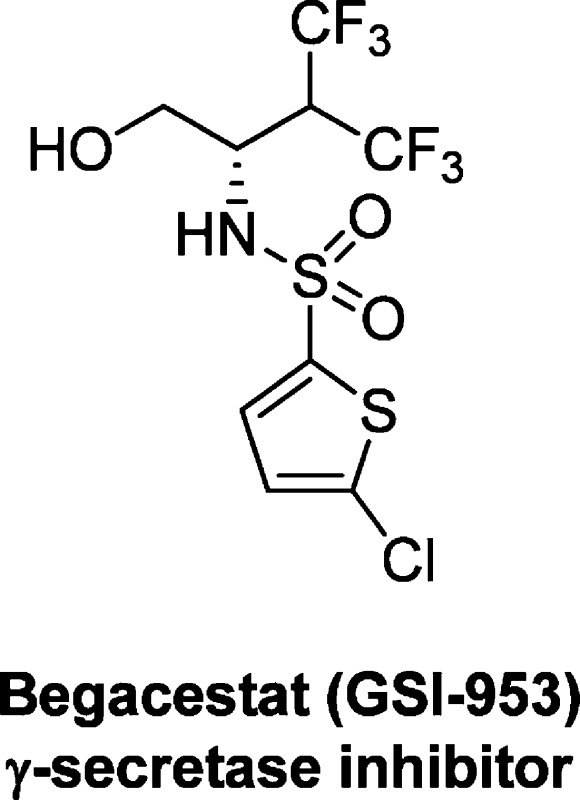
A “second generation” γ-secretase, Begacestat (GSI-953), which is more selective against Notch-signaling, has shown promise in recent Phase I clinical trials. Begacestat, a novel, 2,5-disubsitituted thiophene sulfonamide from Wyeth (now Pfizer) is under evaluation for the treatment of Alzheimer’s disease.
Keywords: Alzheimer's disease, γ-secretase inhibitors, GSI, Aβ-peptides
Alzheimer’s disease (AD) is the most common form of dementia and the most common neurodegenerative disorder, affecting nearly 25 million people worldwide. AD is a progressive disease and current treatments can only temporarily slow the worsening of the dementia symptoms and improve quality of life for a short period of time. There is no known cure for AD; however, there is a massive research effort ongoing in the academic, nonprofit association, and pharmaceutical industry looking for better treatment options and, hopefully, a cure for this devastating disease. Over the past several years, one of the leading hypotheses into the cause and progression of AD is the so-called “amyloid hypothesis”.1 This hypothesis, although not the only hypothesis, nor far from being universally accepted, states that the primary cause of the disease is the accumulation of amyloid β-peptides in the CNS. These Aβ-peptides are thought to initiate the disease progression that ultimately leads to the clinical observations of AD.1c Thus, an appropriate therapeutic intervention would be to inhibit or reverse the accumulation of Aβ-peptides.
One of the more advanced therapeutic avenues for blocking Aβ-peptide formation (namely, Aβ42) studied thus far is selective inhibition of γ-secretase. γ-Secretase (along with β-secretase) produces Aβ42 by cleavage of amyloid precursor protein (APP), thus targeting inhibition of γ-secretase should lead to an overall reduction in Aβ42 levels.2 The pharmaceutical industry has put forth a significant research effort in bringing γ-secretase inhibitor (GSI) compounds into clinical development. Unfortunately, two of the more advanced compounds have failed in the clinic: ELND006 showed significant side effects in the liver, and semagacestat (LY450139) performed worse than placebo in cognition and patients’ ability to complete routine daily tasks.3 Despite these setbacks, a number of compounds remain in the clinic and one of these compounds is being developed by Pfizer, begacestat (GSI-953).
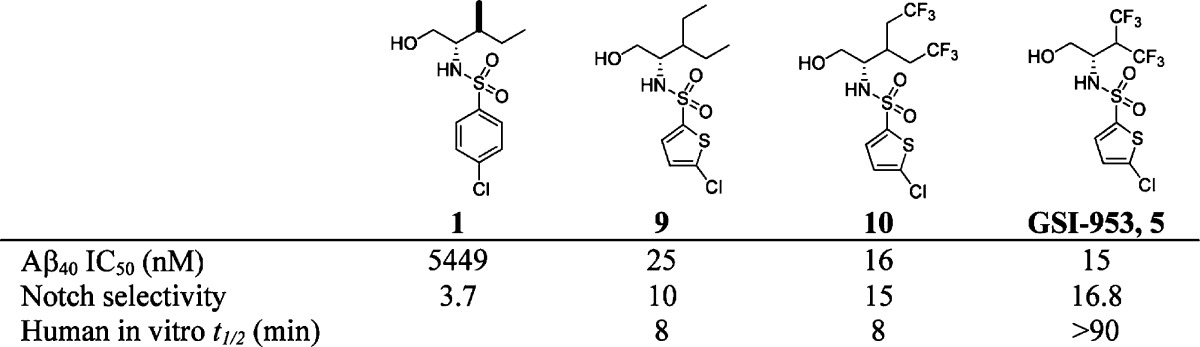
Researchers at Wyeth (now Pfizer) started from a high-throughput screening hit,41, and after extensive optimization discovered compounds 9 and 10, with much improved potency against Aβ production (Table 1).5 Both compounds were >200-fold more potent than the lead compound 1 and showed improved selectivity against Notch, an important protein involved in cell development and differentiation. Unfortunately, these compounds displayed poor stability in human microsomes (t1/2 < 10 min). The metabolic instability was thought to be mainly due to oxidation of the alkyl groups; thus, contraction of the side chain of 10 led to the discovery of GSI-953 (begacestat, 5). GSI-953 displayed significant improvement in human stability (t1/2 > 90 min.) while maintaining potency against Aβ production and selectivity versus Notch.5
Table 1. Lead Optimization of GSI-953, 5.
Begacestat was further characterized in a number of preclinical assays and has been dosed in healthy human volunteers.6 As was reported in the lead optimization paper,5 begascestat was shown to inhibit Aβ production in the low nanomolar range (Aβ40 EC50 = 14.8 nM and Aβ42 EC50 = 12.4 nM) in both cellular and cell-free assays. The group also reported on a close analogue of GSI-953 (WAY-210952, the (R)-isomer), which was shown to have much reduced potency (>10 μM) for Aβ production inhibition. Evaluation in the APP-overexpressing Tg2576 transgenic mice showed that begacestat, when dosed orally, produced significant reduction of Aβ levels in plasma, brain and CSF levels.6 When dosed at 100 mg/kg, begacestat produced an ∼88% reduction in CSF and plasma at 2–6 h time points, and produced an ∼60% reduction in brain levels at 6 h. In addition, a 30 mg/kg dose of begacestat displayed a maximal reduction of Aβ40/42 levels between 4 and 6 h when studied in a 24 h time course study in the brain. Using the same Tg2576 mice, the minimal effective dose (MED) was determined to be 1 mg/kg for Aβ40 reduction, with significant reduction in both Aβ40/42 at 2.5 mg/kg.6 The group also looked at the reversal of contextual memory deficits in Tg2576 mice, a test of hippocampal dependent learning in preplaque animals, and found that begacestat reversed these deficits in a dose-dependent manner from 2.5–10 mg/kg, whereas the (R)-isomer showed no effect (30 mg/kg). Lastly, the group reported the initial study in healthy human volunteers. The study looked at single dose administration (3–600 mg) in young subjects (18–55 years old) and found the compound produced dose-dependent changes in plasma Aβ demonstrating target engagement in humans.6
Based on these exciting preclinical and first-in-human studies, and the lack of Notch-related toxicity in rats and dogs,6 begacestat has been progressed to more advanced clinical trials.7 Although the promise of this molecule is high due to the preliminary results, it still remains to be seen if begacestat can be the first γ-secretase inhibitor to be approved for the treatment of AD.
The authors declare no competing financial interest.
References
- a Hardy J.; Selkoe D. J. (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297, 353–356. [DOI] [PubMed] [Google Scholar]; b Hardy J. A.; Higgins G. A. (1992) Alzheimer’s disease: the amyloid cascade hypothesis. Science 256, 184–185. [DOI] [PubMed] [Google Scholar]; c Selkoe D. J. (1991) The molecular pathology of Alzheimer’s disease. Neuron 6, 487–498. [DOI] [PubMed] [Google Scholar]
- Imbimbo B. P. (2008) Alzheimer’s disease: γ-secretase inhibitors. Drug Discovery Today 5(3), 169–175. [Google Scholar]
- a Hopkins C. R. (2011) ACS Chemical Neuroscience Molecule Spotlight on ELND006: Another γ-Secretase Inhibitor Fails in the Clinic. ACS Chem. Neurosci. 2(6), 279–280. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Hopkins C. R. (2010) ACS Chemical Neuroscience Molecule Spotlight on Semagacestat (LY450139). ACS Chem. Neurosci. 1(8), 533–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Cole D. C.; Stock J. R.; Kreft A. F.; Antane M.; Aschmies S. H.; Atchison K. P.; Casebier, omery T. A.; Diamantidis G.; Ellingboe J. W.; Harrison B. L.; Hu Y.; Jin M.; Kubrak D. M.; Lu P.; Mann C. W.; Martone R. L.; Moore W. J.; Oganesian A.; Riddell D. R.; Sonnenberg-Reines J.; Sun S.-C.; Wagner E.; Wang Z.; Woller K. R.; Xu Z.; Zhou H.; Jacobsen J. S. (2009) (S)-N-(5-Chlorothiophene-2-sulfonyl)-β,β-diethylalaninol a Notch-1-sparing γ-secretase inhibitor. Bioorg. Med. Chem. Lett. 19, 926–929. [DOI] [PubMed] [Google Scholar]; b Kreft A.; Harrison B.; Aschmies S.; Atchison K.; Casebier D.; Cole D. C.; Diamantidis G.; Ellingboe J.; Hauze D.; Hu Y.; Huryn D.; Jin M.; Kubrak D.; Lu P.; Lundquist J.; Mann C.; Martone R.; Moore W.; Oganesian A.; Porte A.; Riddell D. R.; Sonnenberg-Reines J.; Stock J. R.; Sun S.-C.; Wagner E.; Woller K.; Xu Z.; Zhou H.; Jacobsen J. S. (2008) Discovery of a novel series of Notch-sparing γ-secretase inhibitors. Bioorg. Med. Chem. Lett. 18, 4232–4236. [DOI] [PubMed] [Google Scholar]
- Mayer S. C.; K. A. F.; Harrison B.; Abou-Gharbia M.; Antane M.; Aschimes S.; Atchison K.; Chlenov M.; Cole D. C.; Comery T.; Diamantidis G.; Ellingboe J.; Fan K.; Galante R.; Gonzales C.; Ho D. M.; Hoke M. E.; Hu Y.; Huryn D.; Jain U.; Jin M.; Kremer K.; Kubrak D.; Lin M.; Lu P.; Magolda R.; Martone R.; Moore W.; Oganesian A.; Pangalos M. N.; Porte A.; Reinhart P.; Resnick L.; Riddell D. R.; Sonnenberg-Reines J.; Stock J. R.; Sun S.-C.; Wagner E.; Wang T.; Woller K.; Xu Z.; Zaleska M. M.; Zeldis J.; Zhang M.; Zhou H.; Jacobsen J. S. (2008) Discovery of Begacestat, a notch-1-sparing γ-secretase inhibitor for the treatment of Alzheimer’s disease. J. Med. Chem. 51(23), 7348–7351. [DOI] [PubMed] [Google Scholar]
- Martone R. L.; Zhou H.; Atchison K.; Comery T.; Xu J. Z.; Huang X.; Gong X.; Jin M.; Kreft A.; Harrison B.; Mayer S. C.; Aschmies S.; Gonzales C.; Zaleska M. M.; Riddell D. R.; Wagner E.; Lu P.; Sun S.-C.; Sonnenberg-Reines J.; Oganesian A.; Adkins K.; Leach M. W.; Clarke D. W.; Huryn D.; Abou-Gharbia M.; Magolda R.; Bard J.; Frick G.; Raje S.; Forlow S. B.; Balliet C.; Burczynski M. E.; Reinhart P. H.; Wan H. I.; Pangalos M. N.; Jacobsen J. S. (2009) Begacestat (GSI-953): A novel, selective thiophene sulfonamide inhibitor of amyloid precursor protein γ-secretase for the treatment of Alzheimer’s disease. J. Pharmacol. Exp. Ther. 331(2), 598–608. [DOI] [PubMed] [Google Scholar]
- www.clinicaltrials.gov.