Abstract
The ante-mortem diagnosis of Parkinson’s disease (PD) still relies on clinical symptoms. Biomarkers could in principle be used for the early detection of PD-related neuronal damage, but no validated, inexpensive, and simple biomarkers are available yet. Here we report on the breath-print of presymptomatic PD in rats, using a model with 50% lesion of dopaminergic neurons in substantia nigra. Exhaled breath was collected from 19 rats (10 lesioned and 9 sham operated) and analyzed using organically functionalized carbon nanotube sensors. Discriminant factor analysis detected statistically significant differences between the study groups and a classification accuracy of 90% was achieved using leave-one-out cross-validation. The sensors’ breath-print was supported by determining statistically significant differences of several volatile organic compounds in the breath of the lesioned rats and the sham operated rats, using gas chromatography combined with mass spectrometry. The observed breath-print shows potential for cost-effective, fast, and reliable early PD detection.
Keywords: Parkinsonism, early diagnosis, breath analysis, volatile organic compound, sensor, nanomaterial
Parkinson’s disease (PD) is the second most common neurodegenerative disorder, affecting about 1.6% of the elderly population (over age 65) in the United States,1 with a similar prevalence worldwide.2 PD is characterized by resting tremor, bradykinesia, rigidity, and postural instability and is associated with progressive loss of dopaminergic (DAergic) neurons in the compact part of the substantia nigra as well as loss of different neurons in other brain structures.3,4 Neuronal loss also occurs in the peripheral nervous system and might predate neuronal loss in the central nervous system (CNS).
The diagnosis of PD still relies on detection of clinical motor symptoms.5,6 These symptoms are mainly the result of loss of the DAergic neurons in the nigrostriatal pathway, although today increasing reliance is placed on other symptoms such as gastrointestinal disorders, loss of smell (anosmia), and depression.7 Because of compensatory mechanisms in the CNS, motor symptoms are only detected when more than 50% of nigral DAergic neurons have been lost.3,8 Consequently, developing improved methods of early detection is extremely important. Early detection, together with improved neuroprotective strategies, could lead to a reduction in disease progression.9−11 In addition, although idiopathic PD (i.e. disease of unknown cause which encompasses 90% of all PD cases) can be effectively diagnosed in specialist clinics, misdiagnosis occurs frequently outside the specialist setting.12 As a result, a reliable, easy-to-use diagnostic tool applicable in nonspecialist clinics is urgently needed.
Currently, much effort is being made to identify biomarkers in body fluids that indicate disease progression. These biomarkers might enable early diagnosis, differential diagnosis, therapy assessment, and classification of PD subtypes within this heterogeneous disease population.12 Although important progress has been made in biomarker research for PD, no validated, inexpensive biomarker is available to date.3,9,11−15
Cerebrospinal fluid (CSF) is the obvious body fluid of choice in the search for PD biomarkers because of the proximity to the pathology, but CSF testing is invasive, costly, and risky to the patients.15 Recent studies indicate that the relatively subtle metabolic disturbances associated with PD may be traced by metabolomic profiling (i.e., identification of patterns of metabolomic biomarkers that together provide a characteristic fingerprint) of blood or of urine.9,11−13 PD signatures and sets of relevant biomarkers (mostly derivatives of alkylamines, organic acids, and sugar alcohols) were derived through pattern recognition analysis.9,11−13,16 The results of the plasma analysis show promise for the development of early stage diagnostics, and indicate that there might be a connection between disease progression and metabolite variation.11
A novel diagnostic approach relies on the identification of patterns of volatile organic compounds (VOCs) in the exhaled breath.17−27 Disease specific breath-prints could be established as robust and easily accessible biomarkers. The rationale behind this approach is that disease related changes in the blood chemistry may be transmitted in part to the alveolar exhaled breath through exchange in the lung, even at the very onset of the disease.20,22 Breath testing could improve early detection of neurodegenerative diseases, which still lack an objective, technology-based diagnostic procedure, and could be adapted for use in nonspecialist settings.
Nanomaterial-based sensor arrays have already been used to identify and distinguish various different forms of cancer in human subjects from exhaled breath (lung cancer,19,20,26 breast cancer,20,21 colon cancer,20 prostate cancer, and head-and-neck cancer,26 including precursors and subtypes), and also multiple sclerosis24), irrespective of the patients’ age, gender, lifestyle, smoking habits, and other confounding factors.17,18 The concept behind this method relies on the detection of a pattern from a mixture of various exhaled VOCs which would represent the “fingerprint” that indicates the presence of the disease. In the current study, we adapted nanomaterial-based sensors to distinguish a group of rats with a 50% nigrostriatal DAergic neuronal loss from a healthy control group on the basis of the compositional differences in the exhaled breath. This animal model was adopted because it permits the study of the same degree of DAergic neuronal loss which is borderline for detection of PD symptoms in humans. These results could form the basis of a novel biomarker test for early, presymptomatic neuronal damage that could radically reform PD disease management and, in addition, could be used in future clinical trials to support the development of new therapies.
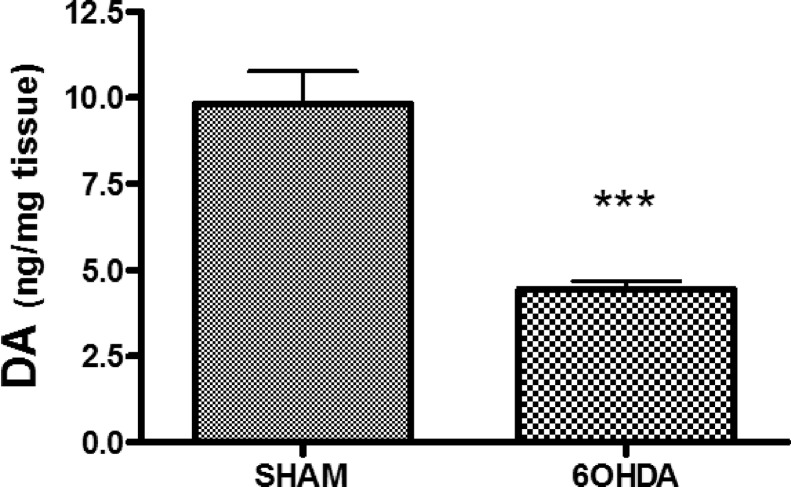
Dopaminergic neurons of the nigrostriatal pathway have their tyrosine hydroxylase-positive cell bodies in the compact part of the substantia nigra, and their axon terminals containing dopamine (DA) project to the striatum. These neurons are destroyed by the neurotoxin 6-hydroxydopamine (6-OHDA) which is taken up selectively by neurons which express a catecholamine transporter (mainly DAergic and noradrenergic neurons in the brain). Rats treated with 6OHDA (250 μg) by intracerebroventricular injection showed a 55 ± 5% lower level of DA in the striatal tissue homogenate compared to the control animals (see Figure 1). This finding is in agreement with our previous experiments,28,29 which found ∼50% extent of DAergic neuronal damage in this model system by immunohistochemistry counting tyrosine hydroxylase-positive neurons in substantia nigra pars compacta. In both rats and humans, PD symptoms are not observed if less than 50% of nigrostriatal neurons have been lost.30 However, the compensatory increased neuronal activity in the remaining neurons30 causes an increase in oxidative stress level in the striatal tissue, which has been detected in our rat model.29 In this context, it is worth mentioning that rats and human are similar in their response to graded striatal dopaminergic depletion, and the rat model enables precise determination of the degree of dopaminergic cell loss, as well as possessing the advantage of a uniform physiological situation without complications of drug treatment, variable dietary conditions, and so forth. However, the applied model does not represent all aspects of PD, since the PD pathology develops over decades, and the majority (∼ 90%) of cases are idiopathic.
Figure 1.
Amount of dopamine in striatal tissue. Rats treated with the toxin 6-hydroxydopamine (6OHDA 250 μg, by intracerebroventricular injection) showed a 55 ± 5% lower level of dopamine in the striatal tissue homogenate compared to control animals (standard error of the mean, SEM, for 9–10 rats per treatment, ***p < 0.001).
Discriminant factor analysis (DFA) was used as a heuristic approach to select two organically functionalized random networks of carbon nanotube (RN-CNT) sensors from a reservoir of 20 available nanomaterial-based sensors that were simultaneously exposed to breath samples (see Methods). The selected organically functionalized RN-CNT sensors achieved optimal separation between the 6-OHDA-lesioned rats and the sham operated animals. Figure 2 shows the first canonical variable (CV1) that was calculated from the sensing responses of the two selected sensors (see Methods). During the statistical signal analysis, the number of DFA input parameters was kept low enough to avoid overfitting. We observed a clear and unambiguous separation between the 6-OHDA-lesioned rats and the sham operated animals in DFA space. Negative values for CV1 around −1.7 were obtained for the 6-OHDA-treated rats, whereas positive CV1 values around 2.0 were obtained for the sham operated rats. The 95% confidence intervals of the CV1 values for the two test groups, represented by the boxes in Figure 2, did not overlap and the two groups were very well separated (p < 0.0001).
Figure 2.
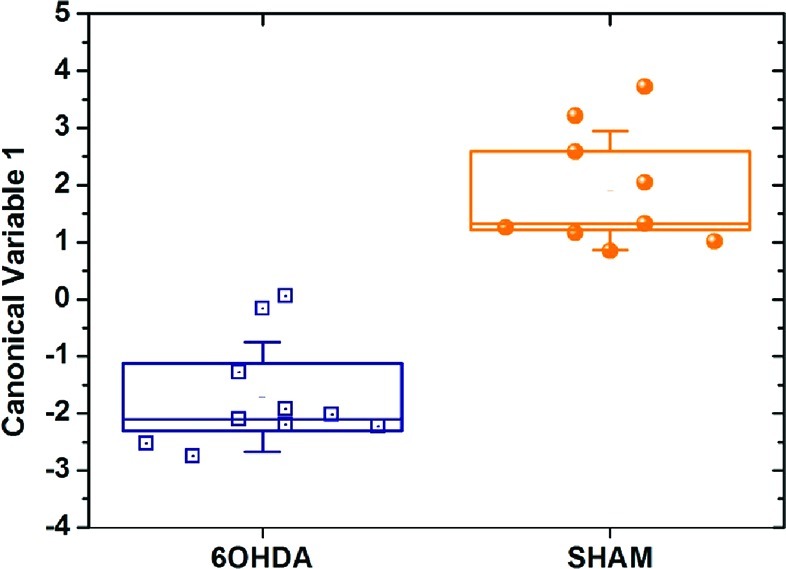
DFA plot of the first canonical variable that was calculated from the responses of two RN-CNT sensors to the 6-OHDA treated rats and the sham operated control animals. Each rat is represented by 1 point in the plot. The standard distribution (SD) of the CV1 values is represented by the error bars. The boxes represent the 95% confidence intervals of the CV1 values, corresponding to 1.96 × SEM.
Leave-one-out cross validation correctly classified eight out of 10 rats with DA-lesions, as well as all nine healthy rats. From this, the sensitivity, specificity, and accuracy of the classification were determined as 80%, 100%, and 90%, respectively. This observed clear separation stems from only ∼50% neuronal damage, while the animal still showed no symptoms of the disease, fed, and gained weight normally. Note that the estimated values for sensitivity and specificity are tentative and a further study with a larger number of animals would be necessary to verify these results. Also, the classification success could be improved in the future through fine-tuning of the sensors.
The chemical composition of the exhaled breath of the 6-OHDA treated rats was analyzed and compared to the breath of the sham controls as a supportive method to validate the patterns that stem from the response of the chemically functionalized RN-CNT sensors to the exhaled breath. Clear differences were observed in the average chemical composition of the breath samples from the two test groups, which could lead to the observed sensor-array breath-print.
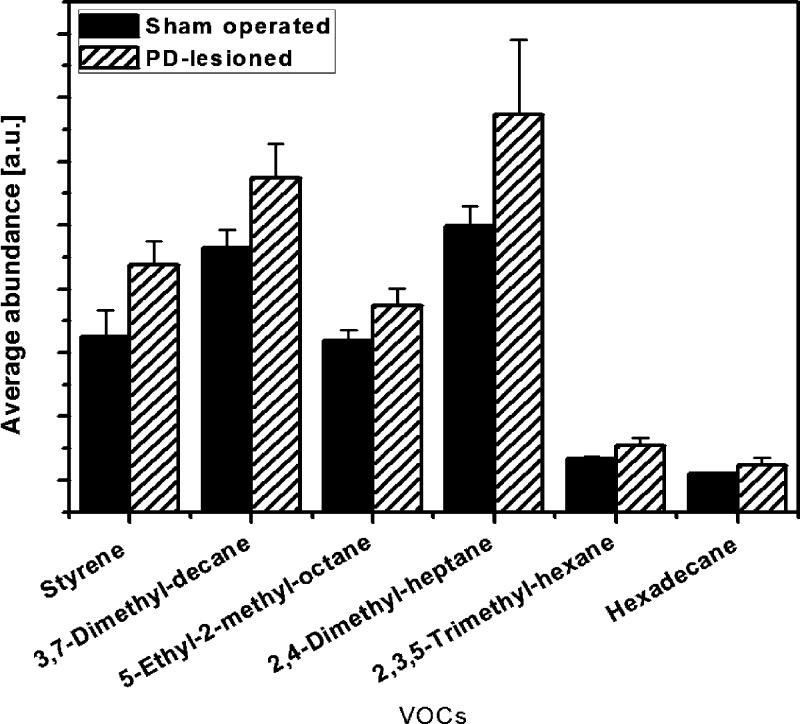
Figure 3 shows six VOCs that were increased in 6-OHDA-lesioned rats, as compared to the sham operated control animals. All these VOCs were present in >80% of the 6-OHDA treated rats and sham rats and showed no (or little) overlap in average abundance (i.e., their average abundances were separated by more than the 90% confidence interval (CI)). We performed a tentative identification of the detected VOCs through spectral library matching and retention times. The confirmation of the VOC names through GC-MS analysis of reference substances is underway and will be published elsewhere. Note that the compositional differences between the two test groups are reliable because the comparison was based on VOC masses and retention times, while the applied preconcentration process increased only the VOCs peaks level captured by the GS-MS but had no influence on the CI separation. Most VOCs can be linked to oxidative stress, which plays a major role in the development and progression of PD. Phospholipids that are associated with cell wall breakdown could lead to the formation of alkanes and methylated alkanes. Figure 3 shows that the hexadecane and four methylated alkanes occur in greater abundance in the breath of the PD-lesioned rats, compared to the sham operated animals. This could result from increased cell death in general, and not specifically dopaminergic neuron death. We have previously shown that oxidative stress level is enhanced in the striatum of the 6-OHDA-lesioned rats, which can lead to lipid peroxidation.31 Although our model of DAergic neuronal loss is stable, in that further neuronal loss does not occur after the 5 weeks postlesion period, the ongoing generation of free radicals in the striatum could lead to enhanced oxidation and turnover of plasma-membrane lipids of postsynaptic neurons with release of alkane derivative metabolites. This hypothesis will be examined in an extended study containing more animals, which will also examine the selectivity of the current breath test analysis for DAergic as opposed to other types of neuronal loss. The origin of the increased styrene levels in the PD-lesioned rats is not well understood (see Figure 3). Styrene is known as an environmental toxin, which can be linked to DNA damage and parkinsonism.32,33 However, the animals were kept in close vicinity of each other and received the same food. A systematic exposure of the PD-lesioned rats to environmental styrene from the environment can be excluded, because all animals were exposed to the same atmosphere at all times. Further studies are necessary to clarify a possible connection of the increased styrene levels in the body with PD-induced oxidative stress.
Figure 3.
Abundance of volatile organic compounds (VOCs) that were found in the breath of >80% of both the 6-OHDA treated and sham operated rats. The columns represent the average abundance and the error bars mark the borders of the 90% CIs. The VOCs were tentatively identified.
Despite the observed differences of abundance in a number of substances, the discriminative abilities of the organically functionalized RN-CNT sensors can be expected to exceed those of the GC-MS/SPME.20 The two methods are fundamentally different: the sensors are broadly cross-reactive and responsive to all (or part of) the PD specific VOCs of interest.17,18,22 The responses of the organically functionalized RN-CNT sensors to a specific VOC at a certain concentration are individually different between the constituent sensors due to the chemical diversity of the organic ligands.17,25,27,34,35 On the other hand, the signals of the same organically functionalized RN-CNT sensors to the mixed VOCs that are present in the breath sample are additive, so that the overall signal of one sensor stems from a total ∼ppm amount of breath VOCs of interest. Hence, the sensors’ responses are less affected by noise than the detected (sub) ppb concentrations of the separate VOCs in the GC-MS/SPME analysis.17,18,20 In contrast, GC-MS detects all confounding VOCs, which introduces noise into the measurement of the abundance of the VOCs of interest and, hence, affects the overall accuracy of the method.
In summary, we have delivered a proof-of-concept for the ability of an organically functionalized RN-CNT sensors array to identify the breath print of presymptomatic PD in rats. The sensor array could distinguish clearly between 6-OHDA treated rats and sham operated animals, and high classification success rates were obtained through leave-one-out cross-validation. In support of these results, we have observed in a complementary approach statistically significant differences in the chemical composition of the exhaled breath of 6-OHDA treated and sham operated rats, using GC-MS/SPME. Eleven VOCs were tentatively identified that are present in more than 80% of healthy and dopamine-lesioned states and might be candidates for volatile biomarkers or their metabolic products in exhaled breath. The presented results could form the basis of a future simple-to-use, noninvasive biomarker breath test with a nanomaterial-based sensor array for the early diagnosis of DAergic neuronal damage that could lead to PD. This method could have potential as a screening test for the aging world population. Considering that currently no adequate diagnostic and screening tests for PD are available, this approach could have a significant impact on disease management and could be used to support the development of new therapies.
Methods
Animals
Studies were conducted on 19 male Sprague–Dawley rats (∼400 g; Harlan, Jerusalem, Israel). The animals were fed with standard rat chow containing 0.5% NaCl and tap water ad libitum. All experiments were authorized by the Technion Animal Care and Use Committee, whose ethical standards are based on those detailed in the National Institutes of Health (Bethesda, MD) Guide for the Care and Use of Laboratory Animals and performed according to their guidelines.
Parkinson’s Disease Model
The model used in this study was described in Aluf et al. 2010. Briefly, rats were anesthetized with ketamine (Fort Dodge Animal Health, Fort Dodge, IA) and xylazine (Kepro, Holland) combination (70/35 mg/kg i.p.) and placed in a stereotaxic frame. Ten rats were administered 6-hydroxydopamine (6-OHDA) hydrochloride 250 μg (in 10 μL 0.85% saline containing 0.1% ascorbic acid) into the left lateral ventricle, in order to induce PD-like neuronal damage; nine control rats (SHAM) were similarly administered vehicle. The noradrenergic neurons were protected by injecting desipramine (10 mg/kg s.c.) 30 min prior to the 6OHDA injection.
Breath Collection
Breath samples were collected under anesthesia 5 weeks after the operation. The rats were anesthetized by intraperitoneal injection of Inactin (thiobutabarbital sodium 100 mg/kg, Sigma) and placed on a thermostatically controlled heating pad to maintain the body temperature at 37 °C. A trachea was exposed and intubated for breath sampling. The plastic tracheal cannula was connected to a one-way valve system, whereby the inlet valve enabled the rat to inhale air from the room, while the outlet valve allowed exhaled air to flow into a sample collection bag.25 Breath samples were collected into 1000 cm3 Tedlar sampling bags (CEL Scientific Corporation). Tedlar gas sampling bags are made of PVF (Tedlar) film which is tough, durable, and considered chemically inert to a wide range of VOCs, and these bags were reused. They were thoroughly cleaned prior to each use with flowing N2 gas. Nevertheless, the bag material may contain small amounts of N,N-dimethylacetamide (DMAC) and phenol, both of which are known to be present in the production process of Tedlar.36
Brain Collection and Dopamine Analysis
Immediately at the end of the breath collection, rats were sacrificed and the striata were removed and homogenized with 10 volumes of 0.1 M perchloric acid in saline (w/v), sonicated for 3 min on ice, and kept frozen at −70 °C until analysis. For DA determination in tissue, 100 μL of striatal homogenate (diluted with 0.1 M perchloric acid in saline 1:10 w/v) were centrifuged for 10 min at 10 000 g. The supernatant was injected into the solvent stream of an HPLC apparatus. The separation of DA was achieved using an Inertsil ODS-2 column 5 μm 4.6 × 150 mm (GL Sciences, Tokyo, Japan) with a mobile phase composed of 100 mM NaH2PO4, 1 mM octanesulfonic acid, 2.5% methanol, 4.5% acetonitrile, and 269 μM sodium EDTA dissolved in HPLC grade deionized water and pH adjusted to 2.75. The flow rate through the system was 1 mL/min. Detection of VOCs was enabled by a model 5011A analytical cell and model 5200 Coulochem II electrochemical detector (ESA, USA) operated in redox mode. Column eluates were initially oxidized at a potential of +300 mV using an ESA guard cell placed before the detector, reduced to +100 mV at detector 1, and measured at −400 mV at detector 2. Dopamine was normalized to tissue weight.
Breath Analysis Using GC-MS
The constituent VOCs of the collected breath were identified using GC-MS (GC- 6890N; MS-5975; Agilent Technologies Ltd.). The GC-MS analysis was preceded by SPME for preconcentrating the VOCs in breath samples. A manual SPME holder with an extraction fiber was inserted into the Mylar bag for 30 min before being delivered to the GC-MS. Fibers with polydimethylsiloxane-divinylbenzene coating were obtained from Sigma-Aldrich. The extracted fiber in the manual SPME holder was inserted into the injector of the GC (splitless mode). The following oven profile was used: 35 °C, 10 min, 7.5 °C/min to 130 °C, 13 °C/min to 290 °C, 1 min at 290 °C. A capillary column SLB-5MS low phenyl methyl siloxane content (30 m length, 0.25 mm i.d., 0.50 μm thickness, from Sigma-Aldrich) was used. The column pressure was set at 8.22 PSI, and the initial flow rate was 1.0 mL/min. The molecular structures of the VOCs were determined. The molecular structures of the VOCs common for >75% of the control and/or lesion samples, as well as their abundance with experimental error (standard error, SE), were identified using the Automated Mass Spectral Deconvolution and Identification System (AMDIS) software. The molecular structures were tentatively identified by spectral library matching and the abundance of the substances was compared via retention time.
Breath Analysis Using the Sensor Array
The breath samples were tested with a nanomaterial-based sensor array developed by Haick and co-workers.17−21,24−27,34,37,38 The array consisted of a reservoir of 20 cross-reactive sensors that were based on different nanomaterials, including organically functionalized gold and platinum nanoparticles and organically functionalized random networks of single-walled carbon nanotubes (RN-CNTs).17,18,27,34 Four features were extracted per sensor: (i) the normalized resistance change after exposure to the breath samples in the middle of the signal; (ii) the normalized resistance change after exposure to the breath samples at the end of the signal; (iii) the area under the response curve at the beginning of the signal; and (iv) the area under the response curve at the end of the signal. For the current study, RN-CNTs functionalized with a layer of α-cyclodextrin (CD) or with a layer of β-CD were selected. The sensor fabrication was described in detail previously.27,34 The organically functionalized RN-CNTs have been fully characterized previously, and typical detection limit for VOCs from the chemical families found to be specific PD biomarkers ranged between ppb to ppm levels.17,25,27,34,35 Each sensor showed a characteristic response to all (or to a certain subset) of the characteristic VOCs found in the exhaled breath samples. The sensing principle was described in detail in refs (17, 18, 20, and 25−27). Different devices with the same coating showed similar results, within ±5% deviations, at the time different types of coatings resulted in differing responses that ranged between −770% to +330%. In other words, the effect of coating type was generally higher than the device-to-device variations, so the observed discrimination effects can be attributed to the influence of the organic coating.17,25,27,34,35
Statistical Analysis
Discriminant factor analysis (DFA) was used to identify PD specific patterns through computerized analysis of the collective response of the sensor array and automatic choice of the most suitable set of sensing features.39,40 DFA is a linear, supervised pattern recognition method, in which the classes to be discriminated are defined before the analysis is performed. The DFA output variables (viz. canonical variables) are obtained in mutually orthogonal dimensions as the linear combinations of the input variables (viz. sensor values) such that the variance within each class is minimized and the variance between classes is maximized. In a two-group classification case, the discrimination is obtained through the first canonical variable (CV1). Thus, DFA effectively reduced the multidimensional experimental data, improved the human perception of the data and allowed the distinction of clusters through visual perception of the first canonical variable. The classification success rate was estimated through leave-one-out cross validation. For this purpose, DFA was computed using a training data set that excluded one test sample. After the DFA computation, the test sample was projected onto the CV1-axis that was calculated using the training set. In this way, the test sample was “blinded” against the DFA model, so that its class affiliation was unknown. All possibilities of leaving out one sample were tested and the left-out sample was classified as true positive (TP), true negative (TN), false positive (FP), or false negative (FN), using standard cluster analysis. Dopamine levels of control and 6-OHDA-treated animals were compared using unpaired t test. The lower the p-value calculated through this test, the more significant the difference between the two groups. A cutoff p-value of 0.05 shows a significant difference between the groups.
Abbreviations
- RN
random network
- CNT
carbon nanotube
- CSF
cerebrospinal fluid
- DFA
discriminant factor analysis
- DA
dopamine
- GC-MS
gas-chromatography/mass-spectrometry
- PD
Parkinson’s disease
- VOC
volatile organic compound
- SPME
solid phase micro extraction
- SEM
standard error of the mean
- CV
canonical variable
- SD
standard deviation
- CI
confidence interval
Acknowledgments
The authors acknowledge Prof. Abraham Marmur for support and fruitful discussions and Mr. A’laa Gharra for assistance.
The authors declare no competing financial interest.
This paper was published on the Web on December 22, 2011, with the fourth author’s name misspelled. The corrected version was reposted on January 3, 2012.
Author Contributions
∥ The authors have contributed equally to the paper.
Author Contributions
U.T. coordinated the breath testing and data analysis, interpreted the data, fabricated the sensors, and drafted the manuscript. Y.A. treated the animals, coordinated the breath collection, determined then dopamine depletion in the striatal tissue, and revised the manuscript. R.I. analyzed the sensing signals and revised the manuscript. M.N. and R.B. analyzed the GC-MS measurements. N.A., D.R., and Y.T. performed the breath tests, the GC-MS measurements, and the exposure of the sensors to the breath samples and extracted the sensing features. J.P.M.F. and H.H. conceived, coordinated, and managed the project and revised the manuscript.
References
- Wright W. A.; Evanoff B. A.; Lian M.; Criswell S. R.; Racette B. A. (2010) Geographic and ethnic variation in Parkinson disease: A population-based study of US medicare beneficiaries. Neuroepidemiology 34, 143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rijk M. C.; Launer L. J.; Berger K.; Breteler M. M.; Dartigues J. F.; Baldereschi M.; Fratiglioni L.; Lobo A.; Martinez-Lage J.; Trenkwalder C.; Hofman A. (2000) Prevalence of Parkinson’s disease in Europe: A collaborative study of population-based cohorts. Neurology 54, S21–S23. [PubMed] [Google Scholar]
- Lozano A. M.; Kalia S. K. (2005) New movement in Parkinson’s. Sci. Am. 293, 68–75. [DOI] [PubMed] [Google Scholar]
- Braak H.; Del Tredici K.; Rub U.; de Vos R. A.; Jansen Steur E. N.; Braak E. (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 24, 197–211. [DOI] [PubMed] [Google Scholar]
- Schrag A.; Ben-Shlomo Y.; Quinn N. (2002) How valid is the clinical diagnosis of Parkinson’s disease in the community?. J. Neurol. Neurosurg. Psychiatry 73, 529–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz C. G.; Tilley B. C.; Shaftman S. R.; Stebbins G. T.; Fahn S.; Martinez-Martin P.; Poewe W.; Sampaio C.; Stern M. B.; Dodel R.; Dubois B.; Holloway R.; Jankovic J.; Kulisevsky J.; Lang A. E.; Lees A.; Leurgans S.; LeWitt P. A.; Nyenhuis D.; Olanow C. W.; Rascol O.; Schrag A.; Teresi J. A.; van Hilten J. J.; LaPelle N. (2008) Movement disorder society-sponsored revision of the unified Parkinson’s disease rating scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov. Disord. 23, 2129–2170. [DOI] [PubMed] [Google Scholar]
- Poewe W. (2008) Non-motor dymptoms in Parkinson’s disease. Eur. J. Neurol. 1, 14–20. [DOI] [PubMed] [Google Scholar]
- Bezard E.; Gross C. E.; Brotchie J. M. (2003) Presymptomatic compensation in Parkinson’s disease is not dopamine-mediated. Trends Neurosci. 26, 215–221. [DOI] [PubMed] [Google Scholar]
- Bogdanov M.; Matson W. R.; Wang L.; Matson T.; Saunders-Pullman R.; Bressman S. S.; Flint Beal M. (2008) Metabolomic profiling to develop blood biomarkers for Parkinson’s disease. Brain 131, 389–396. [DOI] [PubMed] [Google Scholar]
- Coetsee T. N.; Pretorius P. J.; Terre’blanche G.; Bergh J. J. (2008) Investigating the potential neuroprotective effects of statins on DNA damage in mouse striatum. Food Chem. Toxicol. 46, 3186–3192. [DOI] [PubMed] [Google Scholar]
- Ahmed S. S.; Santosh W.; Kumar S.; Christlet H. T. T. (2009) Metabolic profiling of parkinson’s disease: Evidence of biomarker from gene expression analysis and rapid neural network detection. J. Biomed. Sci. 16, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michell A. W.; Mosedale D.; Grainger J. D.; Barker R. A. (2008) Metabolomic analysis of urine and serum in Parkinson’s disease. Metabolomics 4, 191–201. [Google Scholar]
- Quinones M. P.; Kaddurah-Daouk R. (2009) Metabolomics tools for identifying biomarkers for neuropsychiatric diseases. Neurobiol. Dis. 35, 165–176. [DOI] [PubMed] [Google Scholar]
- Barba I.; Fernandez-Montesinos R.; Garcia-Dorado D.; Pozo D. (2008) Alzheimer’s disease beyond the genomic rra: Nuclear magnetic resonance (NMR) spectroscopy-based metabolomics. J. Cell Mol. Med. 12, 1477–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg N.; Grassan A.; Thambisetty M.; Lovestone S.; Legido-Quigley C. (2009) A proposed metabolic strategy for monitoring disease progression in Alzheimer’s disease. Electrophoresis 30, 1235–1239. [DOI] [PubMed] [Google Scholar]
- Baykal A. T.; Jain M. R.; Li H. (2008) Aberrant regulation of choline metabolism by mitochondrial electron transport system inhibition in neuroblastoma cells. Metabolomics 4, 347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisch U.; Haick H. (2010) Nanomaterials for cross-reactive sensor arrays. MRS Bull. 35, 797–803. [Google Scholar]
- Tisch U.; Haick H. (2011) Arrays of chemisensitive monolayer-capped metallic nanoparticles for diagnostic breath testing. Rev. Chem. Eng. 26, 171–179. [Google Scholar]
- Peng G.; Tisch U.; Adams O.; Hakim M.; Shehada N.; Broza Y. Y.; Billan S.; Abdah-Bortnyak R.; Kuten A.; Haick H. (2009) Diagnosing lung cancer in exhaled breath using gold nanoparticles. Nat. Nanotechnol. 4, 669–673. [DOI] [PubMed] [Google Scholar]
- Peng G.; Hakim M.; Broza Y. Y.; Billan S.; Abdah-Bortnyak R.; Kuten A.; Tisch U.; Haick H. (2010) Detection of lung, breast, colorectal, and prostate cancers from exhaled breath using a single array of nanosensors. Br. J. Cancer 103, 542–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuster G.; Gallimidi Z.; Heyman-Reiss A.; Dovgolevsky E.; Billan S.; Abdah-Bortnyak R.; Kuten A.; Engel A.; Shiban A.; Tisch U.; Haick H. (2010) Classification of breast cancer precursors through exhaled breath. Breast Cancer Res. Treat. 126, 791–796. [DOI] [PubMed] [Google Scholar]
- Amann A.; Spanĕl P.; Smith D. (2007) Breath analysis: The approach towards clinical applications. Mini-Rev. Med. Chem. 7, 115–129. [DOI] [PubMed] [Google Scholar]
- Cao W.; Duan Y. (2007) Current status of methods and techniques for breath analysis. Crit. Rev. Anal. Chem. 37, 3–13. [DOI] [PubMed] [Google Scholar]
- Ionescu R.; Broza Y.; Shaltieli H.; Sadeh D.; Zilberman Y.; Feng X.; Glass-Marmor L.; Lejbkowicz I.; Müllen K.; Miller A.; Haick H. (2011) Detection of Multiple Sclerosis from exhaled breath using bilayers of polycyclic aromatic hydrocarbons and single-wall carbon nanotubes. ACS Chem. Neurosci. 2, 687–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haick H.; Hakim M.; Patrascu M.; Levenberg C.; Shehada N.; Nakhoul F.; Abassi Z. (2009) Sniffing chronic renal failure in rat model by an array of random networks of single-walled carbon nanotubes. ACS Nano 3, 1258–1266. [DOI] [PubMed] [Google Scholar]
- Hakim M.; Billan S.; Tisch U.; Peng G.; Dvrokind I.; Marom O.; Abdah-Bortnyak R.; Kuten A.; Haick H. (2011) Diagnosis of head-and-neck cancer from exhaled breath. Br. J. Cancer 104, 1649–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng G.; Trock E.; Haick H. (2008) Detecting simulated patterns of lung cancer biomarkers by random network of single-walled carbon nanotubes coated with nonpolymeric organic materials. Nano Lett. 8, 3631–3635. [DOI] [PubMed] [Google Scholar]
- Aluf Y.; Vaya J.; Khatib S.; Finberg J. P. (2011) Alterations in striatal oxidative stress level produced by pharmacological manipulation of dopamine as shown by a novel synthetic marker molecule. Neuropharmacology 61, 87–94. [DOI] [PubMed] [Google Scholar]
- Aluf Y.; Vaya J.; Khatib S.; Loboda Y.; Kizhner S.; Finberg J. P. (2010) Specific oxidative stress profile associated with partial striatal dopaminergic depletion by 6-hydroxydopamine as assessed by a novel multifunctional marker molecule. Free Rad. Res. 44, 635–644. [DOI] [PubMed] [Google Scholar]
- Zigmond M. J.; Abercrombie E. D.; Berger T. W.; Grace A. A.; Stricker E. M. (1990) Compensations after lesions of central dopaminergic neurons: Some clinical and basic implications. Trends Neurosci. 13, 290–296. [DOI] [PubMed] [Google Scholar]
- Jenner P. (2003) Oxidative stress in Parkinson’s disease. Ann. Neurol. 53, S26–S38. [DOI] [PubMed] [Google Scholar]
- Terre’Blanche G.; Heyer N.; Bergh J. J.; Mienie L. J.; van der Schyf C. J.; Harvey B. H. (2011) The styrene metabolite, phenylglyoxylic acid, induces striatal-motor toxicity in the rat: Influence of dose escalation/reduction over time. Neurotox. Res. 20, 97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wongvijitsuk S.; Navasumrit P.; Vattanasit U.; Parnlob V.; Ruchirawat M. (2011) Low level occupational exposure to styrene: Its effects on DNA damage and DNA repair. Int. J. Hyg. Environ. Health 214, 127–137. [DOI] [PubMed] [Google Scholar]
- Peng G.; Tisch U.; Haick H. (2009) Detection of nonpolar molecules by means of carrier scattering in random networks of carbon nanotubes: Toward diagnosis of diseases via breath samples. Nano Lett. 9, 1362–1368. [DOI] [PubMed] [Google Scholar]
- Zilberman Y.; Tisch U.; Shuster G.; Pisula W.; Feng X.; Müllen K.; Haick H. (2010) Carbon nanotube/hexa-peri-hexabenzocoronene bilayers for discrimination between nonpolar volatile organic compounds of cancer and humid atmospheres. Adv. Mater. 22, 4317–4320. [DOI] [PubMed] [Google Scholar]
- Kushch I.; Schwarz K.; Schwentner L.; Baumann B.; Dzien A.; Schmid A.; Unterkofler K.; Gastl G.; Španel P.; Smith D.; Amann A. (2008) Compounds enhanced in a mass spectrometric profile of smokers’ exhaled breath versus non-smokers as determined in a pilot study using PTR-MS. J. Breath Res. 2, 026002. [DOI] [PubMed] [Google Scholar]
- Dovgolevsky E.; Konvalina G.; Tisch U.; Haick H. (2010) Monolayer-capped cubic platinum nanoparticles for sensing nonpolar analytes in highly humid atmospheres. J. Phys. Chem. C 114, 14042–14049. [Google Scholar]
- Dovgolevsky E.; Tisch U.; Haick H. (2009) Chemically sensitive resistors based on monolayer-capped cubic nanoparticles: towards configurable nanoporous sensors. Small 5, 1158–1161. [DOI] [PubMed] [Google Scholar]
- Ionescu R.; Llobet E.; Vilanova X.; Brezmes J.; Sueiras J. E.; Caldererc J.; Correig X. (2002) Quantitative analysis of NO2 in the presence of CO using a single tungsten oxide semiconductor sensor and dynamic signal processing. Analyst 127, 1237–1246. [DOI] [PubMed] [Google Scholar]
- Brereton R. G. (1990) Chemometrics, Application of Mathematics Statistics to Laboratory Systems, Ellis Horwood, Chichester. [Google Scholar]