Abstract
Defective deep placentation has been associated with a spectrum of complications of pregnancy including preeclampsia, intrauterine growth restriction, preterm labor, preterm premature rupture of membranes, late spontaneous abortion and abruption placentae. The disease of the placental vascular bed that underpins these complications is commonly investigated with targeted biopsies. In this review, we critically evaluate the biopsy technique to summarize the salient types of defective deep placentation and propose criteria for the classification of defective deep placentation into 3 types based on the degree of restriction of remodelling and the presence of obstructive lesions in the myometrial segment of the spiral arteries.
Keywords: Spiral artery, physiological transformation, placental vascular bed, adverse pregnancy outcome
Introduction
It is now well-established that placentation in the humans is associated with unique vascular remodeling. The process of physiological remodeling of the spiral arteries during gestation involves a decidua-associated and a trophoblast-associated stage. Such a process involves the decidual and the junctional zone (JZ) myometrial segments (1,2). Deep placentation involves nearly complete transformation of the decidual and myometrial segments of approximately 100 spiral arteries. Defective deep placentation was first described in preeclampsia and intrauterine growth restriction (IUGR) and was characterized by absent or incomplete remodeling of the JZ segment of the spiral arteries (3,4). In recent years, defective deep placentation has also been associated with other obstetrical syndromes, including late spontaneous abortion (5,6), preterm labor with intact membranes and preterm prelabor rupture of the membranes (PROM) (7,8).
In this review, we critically evaluate the biopsy techniques to assess placental bed vascular pathology, summarize the salient features of defective deep placentation associated with different obstetrical syndromes and propose a new classification that we hope will contribute to a better understanding of the lesions and their pathophysiology.
The study of the placental bed: the beginning
The study of the placental bed began in the late 1950s by two independent groups of investigators using different biopsy techniques. Dixon and Robertson (9), working in Jamaica, obtained biopsies at the time of cesarean delivery using biopsy forceps. At the time of hysterotomy, biopsy samples were obtained under direct visualization from the implantation site after delivery of the placenta. Using curved scissors, the investigators obtained a disk that was approximately 1 cm in diameter. Renaer and Brosens (10), in Leuven, Belgium, obtained biopsy samples after vaginal delivery using a sharpened ovum forceps. The transvaginal technique required the manual localization and removal of the placenta to sample the placental bed. Although the placenta wall peeled away from the wall, the ovum forceps was guided between the palm of the hand and the uterine wall, and a large biopsy including decidua and a few millimeters of the underlying myometrium was obtained. In other studies, different techniques have been used to obtain placental bed biopsy samples. These techniques result in samples in variable size, depth and origin.
Robertson et al. (11) recommended orienting the biopsy so that perpendicular sections of the decidua and myometrium could be obtained. Both groups examined the entire biopsy specimen by using a serial sectioning technique, 1 section being stained for every 5, 10 or 20 sections of the tissue.
Histological confirmation that the biopsy was derived from the placental bed was based on 1) the presence of trophoblast, 2) adherent villi or 3) transformed spiral arteries. However, the absence of these markers does not necessarily mean that the placental bed was not sampled. In IUGR, a small placental bed may affect the success rate of sampling. Unfortunately, the success rates have not been systematically reported in most studies. This is desirable as research in the placental bed moves forward.
A key step in the understanding of the placental bed was made when Brosens (12) systematically studied the placental bed of 14 patients using cesarean hysterectomy specimens. The uteri were obtained from mothers who had preeclampsia, preeclampsia with IUGR, chronic hypertension and nephrotic syndrome. In 3 cases, the placenta was in situ, which aided the precise mapping of the placental bed. However, when the placenta had been detached, the implantation site was identified by the presence of trophoblast. In one specimen obtained from a patient with severe preeclampsia and a fetal death at 31 weeks of gestation, the uterus contained the fetus and placenta in situ. All specimens were processed according to the histological technique of sectioning the uterus with placenta in situ, previously described by Boyd and Hamilton (13). The technique allowed tracing of the radial arteries in the myometrium and then identification of the individual spiral arteries as they traveled through the placental bed. In each specimen, 10–25 spiral arteries in the placental bed and a similar number in the non-placental area were examined. In a large subsequent study of hysterectomy specimens (with the placenta in situ) obtained between 8 and 18 weeks of gestation, Pijnenborg et al. (14–16) examined the transformation of the spiral arteries during the first half of pregnancy.
The uteroplacental blood supply
Spiral artery remodelling
After the physiological changes of the spiral arteries in the placental bed were identified, it was postulated that they resulted from the destructive action of trophoblast on the vascular musculature and the elastic membrane. However, it was soon observed that changes associated with trophoblast invasion were preceded by edema of the wall, disintegration of the elastic elements and changes in smooth muscle cells, such as rounding of the nucleus, the loss of myofibrils and dense bodies and accumulation of glycogen (17).
Subsequent investigation of hysterectomy specimens between 8–18 weeks that were studied by Pijnenborg et al. (14, 16) resulted in 2 major findings. First, vascular changes that included disorganization of the muscular wall could not be exclusively attributed to the presence of trophoblast. It was noted that vascular smooth muscle became disorganized before the arrival of endovascular trophoblast; however, this disorganization was enhanced in the presence of interstitial trophoblast. The second finding was the apparent occurrence of endovascular invasion in the JZ myometrium. This was considered the second “wave” of trophoblast invasion, which occurred after a 4-week period of trophoblast within the decidua. Although the “two-wave concept” is not accepted universally (18, 19), it provided a valuable model to consider the possible mechanisms responsible for defective deep placentation.
A key question has been the relative contribution of the trophoblast and the decidua in vascular remodeling of the spiral arteries. Craven et al. (20) compared the histological characteristics of spiral arteries in the secretory phase of the menstrual cycle using endometrial biopsy specimens and decidual arteries from patients who underwent elective termination of pregnancy. They concluded that the initial stages of physiological change of the spiral arteries occurred without evidence of trophoblast invasion. However, King and Loke (21) noted that “fibrinoid necrosis” of the wall does not occur in the absence of trophoblast invasion. Kam et al. (22) compared the blood vessels from the implantation sites of early human pregnancies with specimens in which trophoblast was absent. The results confirmed that true physiological transformation of the spiral arteries occurred only in the presence of trophoblast. Recently, Smith et al. (23) examined samples of the decidua basalis (8–12 weeks of gestation) using immunohistochemistry, and provided evidence that uterine natural killer cells and macrophages participate in the remodeling through the induction of apoptosis or extracellular matrix degradation. They also reported that in the early stages of spiral artery remodeling, vascular smooth muscle cells showed dramatic disruption and disorganization preceding the presence of endovascular trophoblast.
Deep placentation
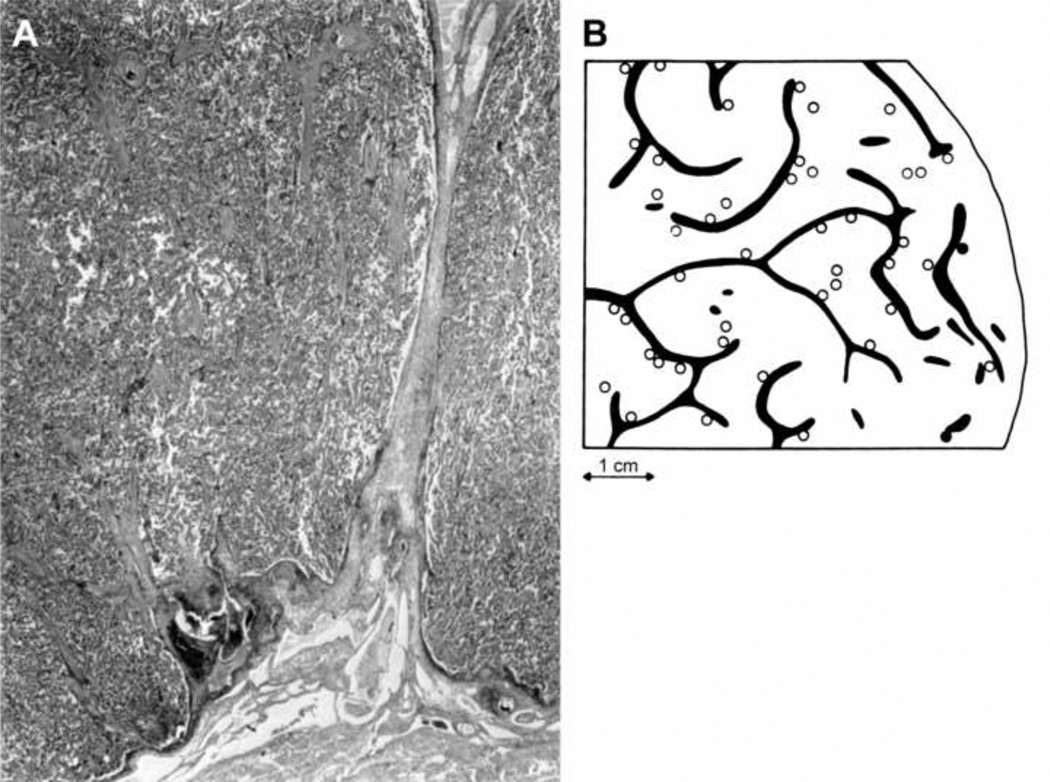
Two major factors determine the maternal blood flow to the placenta. The first is the size of the placental bed, which is determined by the number of spiral arteries that communicate with the intervillous space. In a study that was undertaken to reconstruct the basal plate of the placenta of normal patients, Brosens and Dixon (24) described an irregular distribution of the arterial openings in the intervillous space. They found that arterial openings frequently clustered in groups of 2 or 3 and were located in close proximity to the placental septa (Figure 1). In a careful and detailed study, 48 arterial openings of the spiral arteries were counted (the total was estimated to be 120 openings, based on the examination of a specimen that represented two-fifths of the basal plate). It was also found that each opening corresponded to 1 spiral artery with a density of one artery per 2cm2 of basal plate. Serial sections of hysterectomy specimens demonstrated that the radial arteries were divided approximately 0.5 cm beneath the endometrium (i.e. myometrial JZ) into 2 or 3 arteries with physiological changes or transformation. This may explain the clustering of 2 or 3 openings of spiral arteries in the intervillous space (Figure 1).
Figure 1.
Anatomy of maternal side of placenta. A. Opening of spiral artery at base of septum (left side). Brosens I, Dixon HG. The anatomy of the maternal side of the placenta. J Obstet Gynacol Br Cwth 1966, and Brosend Classification of defective deep placentation. Am J Obstet Gynecol 2010. B. Distribution of spiral artery openings with physiological changes (open circle) in the central area and without physiological changes (black circle) in the peripheral area of the placental bed. Note that the majority of openings are in clusters of 2 or 3 openings, frequently located at the base of a septum. Brosens. The uteroplacental vessels at term: the distribution and extent of physiological changes. Trophoblast Res 1988.
A second feature in determining maternal blood flow to the placenta is that the depth of spiral artery physiologic transformation is greater in the center of the placental bed than in the periphery (15, 16). This is consistent with the observation that the degree of trophoblast invasion is less in the periphery than in the center of the placental bed. Indeed, interstitial trophoblast is absent or scanty in the periphery, and physiologic transformation of the myometrial segment of the spiral arteries is partial or absent, even in normal pregnancy (Figure 1). However, such phenomenon involves approximately 10% of the spiral arteries of the placental bed (12, 24).
Placental bed biopsy studies confirm most of the spiral arteries show full transformation in the JZ myometrial segment (Table 1; Figure 2A). These findings are consistent with the observation reported from ultrasound studies. Color and pulsed-Doppler studies performed during the second trimester of pregnancy have demonstrated a lower impedance to blood flow in the central area of the placental bed than in the periphery (30).
Table 1.
Remodeling of myometrial segment in normal pregnancy versus preeclampsia in placental bed biopsy studies
| Normal | Preeclampsia | ||
|---|---|---|---|
| Gerretsen (25) | 22/23 (96%) | 1/30 (3%) | |
| Khong (5) | 18/18 (100%) | 3/14 (21%) | |
| Frusca (26) | 13/14 (93%) | 6/24 (25%) | |
| Meekins (27) | 16/21 (76%) | 3/24 (12%) | |
| Sagol (28) | 16/20 (80%) | 7/17 (41%) | |
| Kim (7) | 55/59 (93%) | 9/31 (29%) | |
| Kim (8) | 89/103 (86%) | 18/43 (19%) | |
| Guzin (29) | 16/20 (80%) | 11/32 (33%) | |
| Mean | 88% | 27% | |
| Range | 76–100 | 3–41% | |
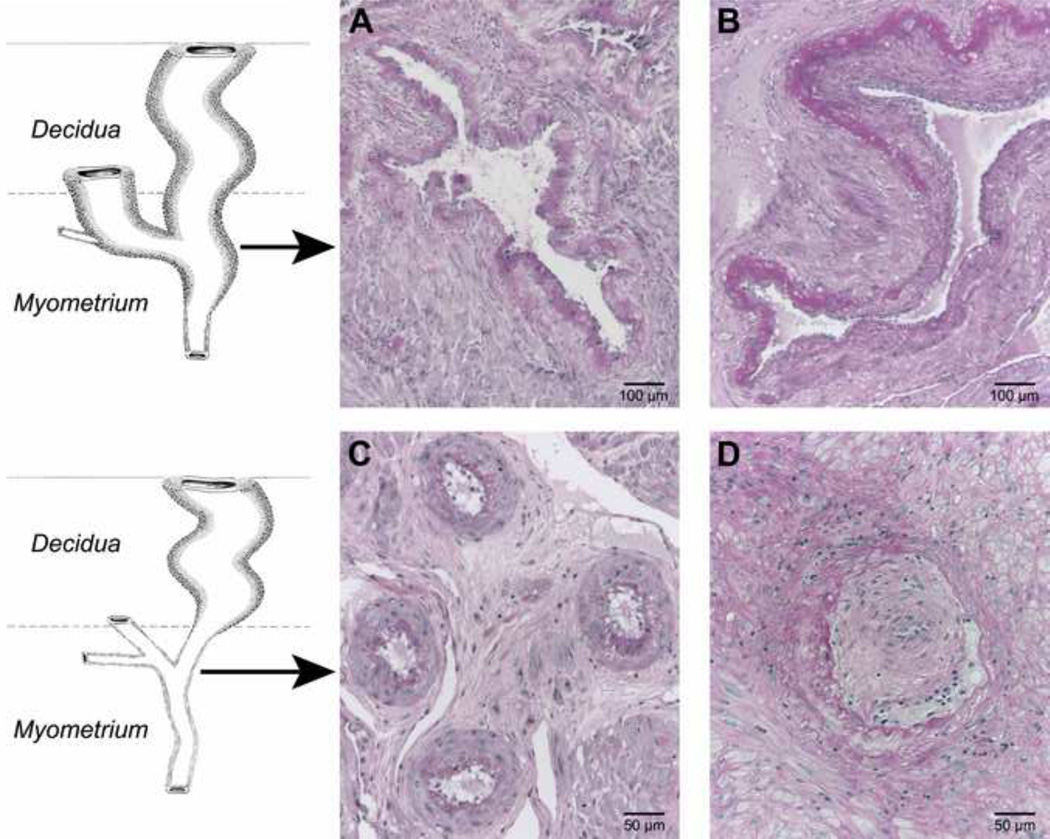
Figure 2.
Spiral artery in the junctional zone myometrium showing: full transformation characterized by the loss of musculo-elastic structure and the presence of fibrinoid with cytotrophoblast (A), partial transformation (top and right) (B), absent transformation (note trophoblastic giant cells surrounding the artery) (C) obstructive lesions by acute atherosis and intimal hyperplasia and absence of transformation and (D) PAS staining, highlighting the fibrinoid in A and B. Brosens. Morphological changes in the utero-placental bed in pregnancy hyptertension. Clin Obstet Gynaecol 1977.
Defective deep placentation
Defective deep placentation is characterized by a significantly increased number of JZ myometrial spiral arteries with absent or partial transformation (Figure 2B and 2C). Physiologic transformation of the spiral arteries is not an “all or none” phenomenon (31). We have noted that there is some confusion in the literature about the definition of partial transformation (5,27,32). An objective assessment of the degree of physiological changes may be achieved by calculating the proportion of the artery that is transformed (31).
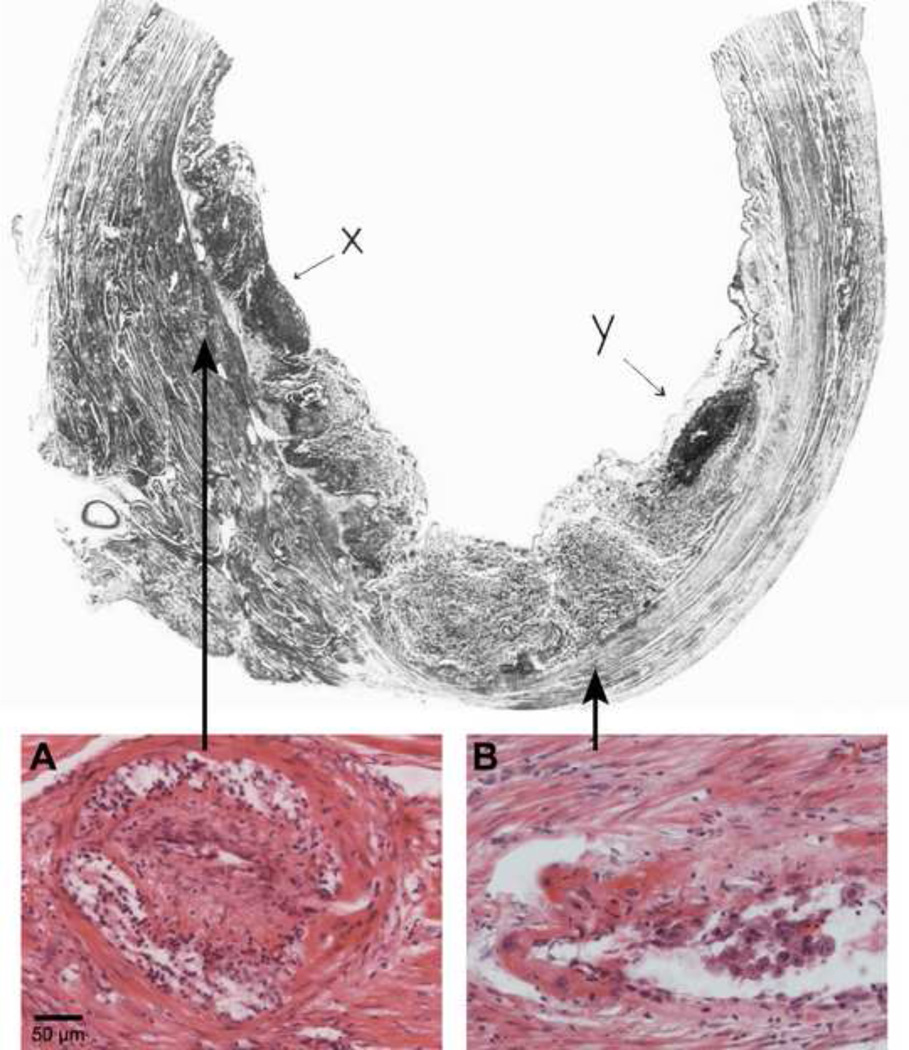
In severe preeclampsia, only a few spiral arteries in the center of the placental bed may show full transformation of the JZ myometrial segment (Figure 3). In addition, obstructive arterial lesions (e.g. thrombosis, acute atherosis) may develop and contribute to the severity of defective deep placentation (Figure 2D).
Figure 3.
Uterus with placenta in situ from patient with severe hypertensive disease and intrauterine growth restriction. A. Spiral arteries in the centre of the placental bed show full transformation of the decidual and myometrial segments. B. Spiral arteries underlying infarcted areas of the placenta (X and Y) show acute atherosis and intimal hyperplasia in the non-transformed myometrial segment. Top: Brosens I, Renaer M. On the pathogenesis of placental infarcts in pre-eclampsia. J OBstet Gyncaec Br Cwth 1972. Bottom: Brosens. Classification of defective deep placentation. Am J Obstet Gynecol 2010.
Although the distribution of interstitial trophoblast in the JZ myometrium is not homogeneous (and varies not only between patients, but also between biopsy specimens from the same patient), placental bed biopsy specimens have limitations because they only provide information about a small segment of the placental bed. It is possible that areas close to the nonbiopsy site may have a completely different degree of vascular transformation (15).
Obstetrical syndromes associated with defective deep placentation
More than 50 years after the original observations, it has become clear that disorders of deep placentation occur in a broader range of clinical complications of pregnancy than initially thought. This underscores the importance of this disorder because it is present in virtually every major obstetrical syndrome.
Preeclampsia
The placental bed of patients with preeclampsia is characterized by a decreased number of spiral arteries with transformation of the myometrial segment (Table 1; Figure 2C). This segment retains a hypertrophic muscular structure, although interstitial trophoblasts are present, sometimes in excessive numbers (3). The defective transformation is more severe in the myometrial, than in the decidual, segments (8, 29).
Preeclampsia with IUGR
The placental bed of patients with preeclampsia associated with IUGR is similar to that described in patients with preeclampsia. It is characterized by a large number of nontransformed myometrial spiral arteries, and such arteries show frequently obstructive lesions, such as acute atherosis and thrombosis (33, 34) (Table 2; Figure 2D). Acute atherosis was first described by Zeek and Assali (35) not only as a distinctive disorder of small decidual arteries, but is also a prominent lesion of the myometrial spiral arteries in cases of preeclampsia with IUGR (33, 34). Defective deep placentation in preeclampsia with IUGR results in a small central region with transformed arteries, as demonstrated by hysterectomy specimens with the placenta in situ (36, 37) (Figure 3). The extent of defective transformation of myometrial spiral arteries and the presence of obstructive myometrial vascular lesions explain the frequent association with placental infarctions.
Table 2.
Obstructive lesions in myometrial segment of placental bed spiral arteries in hysterectomy specimens (12)
| Cesarean hysterectomy n |
Arteries with obstructive lesions n (%) |
|
|---|---|---|
| Normotensive | 8 | 0/103 (0%) |
| Chronic hypertension | 2 | 0/24 (0%) |
| Preeclampsia | 2 | 3/27 (8%) |
| Preeclampsia with IUGR | 2 | 23/33 (70%) |
IUGR: intrauterine growth restriction
Intrauterine growth restriction without hypertension
In placental bed biopsy studies of pregnancies complicated by IUGR, Brosens et al. (34) described in 1977 (in the absence of maternal hypertension) that 55% of the biopsies showed absence of physiological changes in the myometrial segment of the spiral arteries, while no physiological changes were seen in 23 biopsies from women with preeclampsia with or without IUGR. In this condition, partial transformation of the myometrial spiral arteries has been reported by other investigators (5, 25, 38). Khong et al. (5) reported absence of spiral artery remodeling at the level of the decidual segments in women with IUGR without hypertension and indicated that the lack of physiological change may also be confined to part of the circumference of the vessel with the remaining portion of the circumference showing normal remodeling.
Preterm labor and preterm PROM
Preterm labor and preterm PROM are defined as events that occur at <37 weeks of gestation and can be considered as 2 syndromes with various phenotypes. Multiple etiologies include infection/inflammation, ischemia due to vascular disease, cervical disease, uterine overdistension, abnormal allograft reaction, allergy, and endocrine disorders. (39).
In a blinded cross-sectional study, Kim et al. (7) determined the frequency of nontransformed spiral arteries in placental bed biopsy specimens obtained under direct visualization at the time of cesarean delivery in three groups of patients: 1) normal women who delivered at term; 2) patients with preterm PROM who underwent cesarean delivery for obstetric indications; and 3) patients with preeclampsia.
The frequency of failure of physiological transformation of the myometrial segment of the spiral arteries was significantly higher in patients with preterm PROM than in patients who delivered at term. Completely transformed spiral arteries were observed in 59% of patients who delivered at term, 29% of those with preterm PROM, and 4.3% of patients with preeclampsia. Interestingly, the authors observed that preeclampsia had a higher mean number of vessels with defective physiologic changes in the decidual portion than in preterm PROM. They interpreted these observations to suggest that the placentation disorder in preeclampsia (which consistently involves the decidual and myometrial segments) is more severe and probably begins earlier in gestation than the one observed in cases of preterm PROM.
In a similar systematic study, Kim et al. obtained placental bed biopsy specimens at the time of cesarean delivery in patients with preterm labor with intact membranes who had a preterm delivery (8). The study included a control group of women with normal pregnancy and a group of women with preeclampsia. The authors observed that patients with preterm labor with intact membranes who delivered a preterm neonate had a greater degree of failure of transformation of the spiral arteries in the myometrial and decidual segments than women who delivered at term. However, the extent of this defect was much greater in patients with preeclampsia than in those women with preterm labor with intact membranes.
Abruptio placentae
Dommisse and Tiltman (40) reported the results of placental bed biopsy specimens that had been obtained at the time of cesarean section delivery in 18 women with the clinical diagnosis of abruptio placentae. Six biopsies did not include trophoblast in the myometrium and therefore were not considered representative of the placental bed. In 12 cases, at least one spiral artery was seen in the myometrium. Seven of the 12 specimens demonstrated absence of physiological transformation of the spiral arteries. Hemorrhage was observed in 83% of these samples. Brosens (12) reported that 65% (15 of 23 spiral arteries) of non-transformed spiral arteries were affected by acute atherosis in a cesarean hysterectomy specimen from a patient with hypertension and abruptio placentae.
Second trimester abortion
In a preliminary study, Khong et al. (41) described that failure of physiological transformation of the spiral arteries could be observed in women with a spontaneous abortion in the mid trimester. Ball et al. (6) subsequently reported a large series of placental bed biopsy specimens that contained myometrium from late spontaneous abortions from women who had undergone karyotype. The placental implantation site was determined with ultrasound scanning before the termination of pregnancy. A biopsy forceps was introduced through the cervix, and placental bed biopsies were performed under ultrasound visualization (3 or 4 placental bed biopsy specimens of 3–5 mm3 were obtained). When compared with normal pregnancies, myometrial spiral arteries of patients with a second-trimester abortion (late fetal death) showed reduced endovascular and intramural trophoblasts and less extensive fibrinoid deposits in the wall of the spiral artery. Of interest was that the amount of endovascular trophoblast in the decidual segment of the spiral arteries was increased. However, the extent of interstitial trophoblast in the myometrial segment was not significantly lower in patients with a spontaneous abortion. Endovascular trophoblast invasion may become arrested at the decidual level and fail to progress into myometrial segment of the spiral artery. Additionally, musculo-elastic tissue did not persist in myometrial spiral arteries, which suggested that physiological changes may not be entirely dependent on trophoblast invasion. However, it cannot be excluded that a weakening of the elastic layer may be induced by interstitial trophoblast (42).
Comment
Defective deep placentation is associated with a spectrum of obstetrical syndromes that included preeclampsia, IUGR, preterm labor with intact membranes, preterm PROM, abruptio placentae and spontaneous midtrimester abortion. We propose that disorders of deep placentation are characterized by: 1) the degree of restriction of physiologic transformation of the spiral arteries and 2) the presence of arterial lesions in the JZ myometrium of the placental bed.
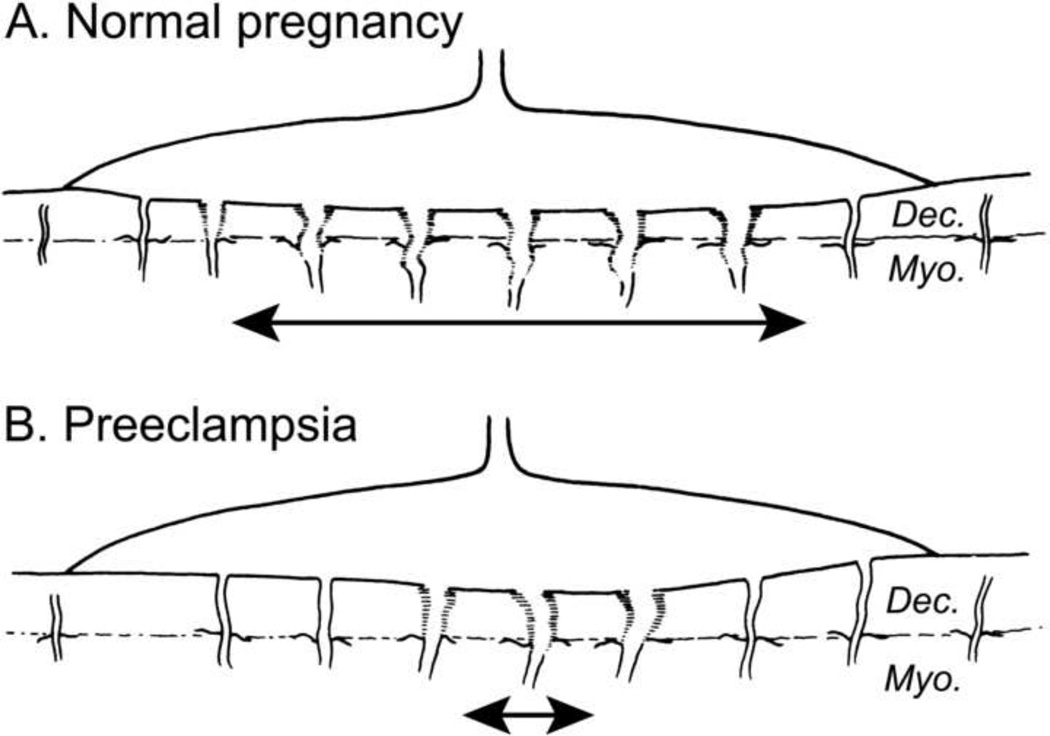
The degree and extent of physiologic transformation of the spiral arteries varies according to the area of the placental bed and is less in the periphery than in the central part of the placental bed. In normal pregnancy, 90% of the JZ myometrial spiral arteries are fully transformed (Figure 4A).
Figure 4.
A. Normal placental bed with full transformation of the myometrial spiral arteries except at the periphery of the placental bed B. Defective deep placentation is characterized by non-transformation of the myometrial spiral arteries reducing the central area with deep placentation.
Three different types of defective spiral artery transformation can be identified in the JZ myometrium: 1) partial transformation; 2) absence of transformation; and 3) absence of transformation with obstructive lesions (Table 3). In preeclampsia, complete physiologic transformation of the spiral arteries in the JZ myometrium is greatly reduced in the central area of the placental bed (Figure 4B). In preeclampsia associated with IUGR, defective deep placentation is frequently observed with the presence of obstructive lesions in the non-transformed myometrial spiral arteries. In preterm labor and IUGR without hypertension, the defective deep placentation may affect only partially affect the spiral arteries in the JZ myometrium.
Table 3.
Types of defective deep placentation in association with adverse pregnancy outcomes
| Type of myometrial spiral artery remodeling | |
|---|---|
| Partial |
|
| Absent |
|
| Absent with obstructive lesions |
|
PROM: premature rupture of membranes; IUGR: intrauterine growth restriction
Spiral artery remodeling has been described as a multistep process that starts at the beginning of pregnancy (43). Based upon histological studies of well-timed early pregnant hysterectomy specimens and numerous third-trimester placental bed biopsies, 4 steps have been distinguished in the spiral artery remodeling: 1) the initial stage of decidua-associated remodeling is followed by 2) intra-arterial trophoblast migration, 3) intramural invasion and trophoblast-associated remodeling, and 4) reendothelialization and other maternal-induced changes. Although the precise mechanisms of defective remodeling are not known, it seems logical to assume that different clinical conditions can lead to various defects of transformation and result in different types of defective deep placentation. Kim et al. (7) interpreted the association of partial transformation with preterm delivery and preterm PROM to suggest that the placentation disorder in preeclampsia is more severe and may begin early in gestation.
Brosens et al (44) recently suggested that the process of cyclic decidualization, followed by menstruation serves as a mechanism to prepare the uterus for deep placentation. Both menstruation and implantation are inflammatory conditions that cause some physiological ischemia-reperfusion tissue stress, albeit much more so in pregnancy. The authors speculated that the emergence of cyclic menstruation may have had a critical role in protecting uterine tissues from the profound inflammatory and oxidative stress associated with deep placentation, a process known as “preconditioning”. In addition, it is interesting to note that normal pregnancy-induced fragmentation of the internal elastic lamina of the myometrial spiral arteries persist following a first pregnancy (45), which could provide an anatomic explanation for higher birthweight in the second and subsequent pregnancies.
The absence of adequate “preconditioning” may explain why pregnancy in the early teenage primigravida women is associated with a significantly increased risk of poor pregnancy outcomes, such as preterm delivery, fetal growth restriction and preeclampsia in comparison with primigravidae women in their early twenties, in whom preconditioning has occurred (46). On the other hand, placentation disorders, even during the subclinical stages, are present in a subset of patients with preeclampsia and fetal growth restriction. Under these circumstances, defective deep placentation is characterized by non-transformed JZ spiral arteries that can be affected severely by obstructive vascular lesions. Arterial lesions such as intimal hyperplasia, acute atherosis and thrombosis can develop in these arteries over a surprisingly short period of time, even with mild hypertension.
The association of obstetrical syndromes with different vascular diseases in the JZ myometrium suggests that the preconditioning of this zone at the time of conception may be critical factor for successful implantation and development of normal placentation. Romero et al. (47) have proposed that more than one mechanism of disease may lead to defective deep placentation and that the common pathophysiologic consequence is ischemia. The extent and timing of ischemia as well as the host response (maternal and fetal) would lead to different clinical phenotypes. Genetic and environmental factors, as well as the time of onset, duration and extent of the ischemic insult, may play a role in the determination of the phenotype. Recently, Roberts and Hubel (48) arrived at a similar conclusion and proposed that factors that increase the risk for preeclampsia are also associated with abnormal implantation.
Progress in understanding the molecular processes that occur during implantation indicates that, among other maternal constitutional factors, the process of endometrial decidualization and angiogenesis is a target for pre-pregnancy diagnosis and therapy. Therefore, the characterization of angiogenesis and the development of biomarkers in early placental development are likely to provide diagnostic and hopefully noninvasive predictive markers to identify mothers at risk for defective deep placentation syndromes such as preeclampsia (49–61), IUGR (62–65), preterm birth (66, 67) and other adverse pregnancy outcomes (68–74).
A proper classification of defective deep placentation also has important implications for fetoplacental research and the diagnosis of placental disease by imaging techniques. Although a placental biopsy specimen may not be representative of the entire vascular placental bed, some authors have attempted to standardize the technique by using ultrasound scanning to target biopsies from the center of the placental bed (28). This technique may reduce the failure rate, but would not address the presence of lesions and their severity in the paracentral region. Similarly, color Doppler and spectral Doppler ultrasound studies of spiral arteries also have limitations, because they do not address the lateral extent of spiral artery remodeling. This means that the conclusions reached with such noninvasive studies should be interpreted with caution (75, 76).
The “Great Obstetrical Syndromes” (77, 78) are associated with defective deep placentation, which may be associated with different degrees of restricted remodeling and obstructive lesions of the spiral arteries in the JZ or inner myometrium. This concept may be used to improve the characterization of the disorders of the placental bed. It is possible that this information will be valuable in refining the existing tools for the assessment of risk before pregnancy outcome.
Acknowledgments
The authors thank Giuseppe Benagiano and Jan J. Brosens for their useful comments.
This work was supported, in part, by the Division of Intramural Research of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH/DHHS.
References
- 1.Brosens I, Robertson WB, Dixon HG. The physiological response of the vessels of the placental bed to normal pregnancy. J Pathol Bacteriol. 1967;93:569–579. doi: 10.1002/path.1700930218. [DOI] [PubMed] [Google Scholar]
- 2.De Wolf F, De Wolf-Peeters C, Brosens I, Robertson WB. The human placental bed: electron microscopy study of trophoblastic invasion of spiral arteries. Am J Obstet Gynecol. 1980;137:58–70. doi: 10.1016/0002-9378(80)90387-7. [DOI] [PubMed] [Google Scholar]
- 3.Brosens IA, Robertson WB, Dixon HG. The role of the spiral arteries in the pathogenesis of preeclampsia. Obstet Gynecol Annu. 1972;1:177–191. [PubMed] [Google Scholar]
- 4.Brosens JJ, Pijnenborg R, Brosens I. The myometrial junctional zone spiral arteries in normal and abnormal pregnancies. Am J Obstet Gynecol. 2002;187:1416–1423. doi: 10.1067/mob.2002.127305. [DOI] [PubMed] [Google Scholar]
- 5.Khong TY, De Wolf F, Robertson WB, Brosens I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol. 1986;93:1049–1059. doi: 10.1111/j.1471-0528.1986.tb07830.x. [DOI] [PubMed] [Google Scholar]
- 6.Ball E, Bulmer JN, Ayis S, Lyall F, Robson SC. Late sporadic miscarriage is associated with abnormalities in spiral artery transformation and trophoblast invasion. J.Pathol. 2006;208:535–542. doi: 10.1002/path.1927. [DOI] [PubMed] [Google Scholar]
- 7.Kim YM, Chaiworapongsa T, Gomez R, Bujold E, Yoon BH, Rotmensch S, et al. Failure of physiologic transformation of the spiral arteries in the placental bed in preterm premature rupture of membranes. Am J Obstet Gynecol. 2002;187:1137–1142. doi: 10.1067/mob.2002.127720. [DOI] [PubMed] [Google Scholar]
- 8.Kim YM, Bujold E, Chaiworapongsa T, Gomez R, Yoon BH, Thaler HT, et al. Failure of physiologic transformation of the spiral arteries in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2003;189:1063–1069. doi: 10.1067/s0002-9378(03)00838-x. [DOI] [PubMed] [Google Scholar]
- 9.Dixon HG, Robertson WB. A study of the vessels of the placental bed in normotensive and hypertensive women. J Obstet Gynaecol Br Emp. 1958;65:803–809. doi: 10.1111/j.1471-0528.1958.tb08876.x. [DOI] [PubMed] [Google Scholar]
- 10.Renaer M, Brosens I. Spiral arterioles in the decidua basalis in hypertensive complications of pregnancy. Ned Tijdschr Verloskd Gynaecol. 1963;63:103–118. [PubMed] [Google Scholar]
- 11.Robertson WB, Khong TY, Brosens I, De Wolf F, Sheppard BL, Bonnar J. The placental bed biopsy: review from three European centers. Am J Obstet Gynecol. 1986;155:401–412. doi: 10.1016/0002-9378(86)90843-4. [DOI] [PubMed] [Google Scholar]
- 12.Brosens I. Thesis. University of London: 1965. The placental bed. [Google Scholar]
- 13.Boyd JD, Hamilton WJ. The human placenta. W. Cambridge: Heffer & Sons; 1970. [Google Scholar]
- 14.Pijnenborg R, Dixon G, Robertson WB, Brosens I. Trophoblastic invasion of human decidua from 8 to 18 weeks of pregnancy. Placenta. 1980;1:3–19. doi: 10.1016/s0143-4004(80)80012-9. [DOI] [PubMed] [Google Scholar]
- 15.Pijnenborg R, Bland JM, Robertson WB. The pattern of interstitial trophoblastic invasion of the myometrium in early human pregnancy. Placenta. 1981;2:303–316. doi: 10.1016/s0143-4004(81)80027-6. [DOI] [PubMed] [Google Scholar]
- 16.Pijnenborg R, Bland JM, Robertson WB, Brosens I. Uteroplacental arterial changes related to interstitial trophoblast migration in early human pregnancy. Placenta. 1983;4:397–413. doi: 10.1016/s0143-4004(83)80043-5. [DOI] [PubMed] [Google Scholar]
- 17.Brosens IA. Morphological changes in the utero-placental bed in pregnancy hypertension. Clin Obstet Gynaecol. 1977;4:573–593. [PubMed] [Google Scholar]
- 18.Robson SC, Ball E, Lyall F, Simpson H, Ayis H, Bulmer JN. Endovascular trophoblast invasion and spiral artery transformation: The "two wave" theory revisited. Placenta. 2001;22:25. [Google Scholar]
- 19.Lyall F. The human placental bed revisited. Placenta. 2002;23:555–562. doi: 10.1053/plac.2002.0850. [DOI] [PubMed] [Google Scholar]
- 20.Craven CM, Morgan T, Ward K. Decidual spiral artery remodelling begins before cellular interaction with cytotrophoblasts. Placenta. 1998;19:241–252. doi: 10.1016/s0143-4004(98)90055-8. [DOI] [PubMed] [Google Scholar]
- 21.King A, Loke YW. Placental vascular remodeling. Lancet. 1997;350:220–221. doi: 10.1016/s0140-6736(05)62389-6. [DOI] [PubMed] [Google Scholar]
- 22.Kam EPY, Gardner L, Loke YW, King A. The role of trophoblast in the physiological change in decidual spiral arteries. Hum Reprod. 1999;14:2131–2138. doi: 10.1093/humrep/14.8.2131. [DOI] [PubMed] [Google Scholar]
- 23.Smith SD, Dunk CE, Aplin JD. Evidence for immune cell involvement in decidual spiral arteriole remodeling in early human pregnancy. Am J Pathol. 2009;174:1959–1971. doi: 10.2353/ajpath.2009.080995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brosens I, Dixon HG. The anatomy of the maternal side of the placenta. J Obstet Gynaec Brit Cwth. 1966;73:357–363. doi: 10.1111/j.1471-0528.1966.tb05175.x. [DOI] [PubMed] [Google Scholar]
- 25.Gerretsen G, Huisjes HJ, Elema JD. Morphological changes of the spiral arteries in the placental bed in relation to pre-eclampsia and fetal growth retardation. Br J Obstet Gynaecol. 1981;88:876–881. doi: 10.1111/j.1471-0528.1981.tb02222.x. [DOI] [PubMed] [Google Scholar]
- 26.Frusca T, Morassi L, Pecorelli S, Grigolato P, Gastaldi A. Histological features of uteroplacental vessels in normal and hypertensive patients in relation to birthweight. Br J Obstet Gynaecol. 1989;96:835–839. doi: 10.1111/j.1471-0528.1989.tb03324.x. [DOI] [PubMed] [Google Scholar]
- 27.Meekins JW, Pijnenborg R, Hanssens M, McFadyen IR, Van Assche A. A study of placental bed spiral arteries and trophoblast invasion in normal and severe pre-eclamptic pregnancies. Br J Obstet Gynaecol. 1994;101:669–674. doi: 10.1111/j.1471-0528.1994.tb13182.x. [DOI] [PubMed] [Google Scholar]
- 28.Sagol S, Ozkinay E, Oztekin K, Ozdemir N. The comparison of uterine artery Doppler velocimetry with the histopathology of the placental bed. Aust N Z J Obstet Gynaecol. 1999;39(3):324–329. doi: 10.1111/j.1479-828x.1999.tb03407.x. [DOI] [PubMed] [Google Scholar]
- 29.Guzin K, Tomruk S, Tuncay YA, Naki M, Sezginsoy S, Zemheri E, Yucel N, Kanadikirik F. The relation of increased uterine artery blood flow resistance and impaired trophoblast invasion in pre-eclamptic pregnancies. Arch Gynecol Obstet. 2005;272:283–288. doi: 10.1007/s00404-005-0005-2. [DOI] [PubMed] [Google Scholar]
- 30.Matijevic R, Meekins JW, Walkinshaw SA, Neilson JP, McFadyen IR. Spiral artery blood flow in the central and peripheral areas of the placental bed in the second trimester. Obstet Gynecol. 1995;86:289–292. doi: 10.1016/0029-7844(95)00129-f. [DOI] [PubMed] [Google Scholar]
- 31.Espinoza J, Romero R, Yeon MK, Kusanovic JP, Hassan S, Erez O, Gotsch F, Chong JK. Normal and abnormal transformation of the spiral arteries during pregnancy. J Perinat Med. 2006;34:447–458. doi: 10.1515/JPM.2006.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aardema MW, Oosterhof H, Timmer A, Van Rooy I, Aarnoudse JG. Uterine artery Doppler flow and uteroplacental vascular pathology in normal pregnancies and pregnancies complicated by pre-eclampsia and small for gestational age fetuses. Placenta. 2001;22:405–411. doi: 10.1053/plac.2001.0676. [DOI] [PubMed] [Google Scholar]
- 33.Robertson WB, Brosens I, Dixon HG. The pathological response of the vessels of the placental bed to hypertensive pregnancy. J Pathol Bacteriol. 1967;93:581–592. doi: 10.1002/path.1700930219. [DOI] [PubMed] [Google Scholar]
- 34.Brosens I, Dixon HG, Robertson WB. Fetal growth retardation and the arteries of the placental bed. Br J Obstet Gynaecol. 1977;84:656–664. doi: 10.1111/j.1471-0528.1977.tb12676.x. [DOI] [PubMed] [Google Scholar]
- 35.Zeek PM, Assali NS. Vascular changes in the decidua associated with toxemia of pregnancy. Am J Clin Pathol. 1950;20:1099–1109. doi: 10.1093/ajcp/20.12.1099. [DOI] [PubMed] [Google Scholar]
- 36.Brosens I, Renaer M. On the pathogenesis of placental infarcts in pre-eclampsia. J Obstet Gynaec Brit Cwth. 1972;79:794–799. doi: 10.1111/j.1471-0528.1972.tb12922.x. [DOI] [PubMed] [Google Scholar]
- 37.Brosens IA. The Uteroplacental Vessels at Term - The distribution and extent of physiological changes. Trophoblast Research. 1988;3:61–67. [Google Scholar]
- 38.Hanssens M, Pijnenborg R, Keirse MJ, Vercruysse L, Verbist L, Van Assche FA. Renin-like immunoreactivity in uterus and placenta from normotensive and hypertensive pregnancies. Eur J Obstet Gynecol Reprod Biol. 1998;81:177–184. doi: 10.1016/s0301-2115(98)00187-0. [DOI] [PubMed] [Google Scholar]
- 39.Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, Chaiworapongsa T, Mazor M. The preterm parturition syndrome. Br J Obstet Gynaecol. 2006;113(SUPPL. 3):17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dommisse J, Tiltman AJ. Placental Bed Biopsies in Placental Abruption. Br J Obstet Gynaecol. 1992;99:651–654. doi: 10.1111/j.1471-0528.1992.tb13848.x. [DOI] [PubMed] [Google Scholar]
- 41.Khong TY, Liddell HS, Robertson WB. Defective haemochorial placentation as a cause of miscarriage: a preliminary study. Br J Obstet Gynaecol. 1987;94:649–655. doi: 10.1111/j.1471-0528.1987.tb03169.x. [DOI] [PubMed] [Google Scholar]
- 42.Pijnenborg R, Vercruysse L, Verbist L, Van Assche FA. Relative contribution of interstitial and endovascular trophoblast to elastica breakdown in placental bed spiral arteries. Am.J.Obstet.Gynecol. 1999;180:S43. [Google Scholar]
- 43.Pijnenborg R, Brosens I. Deep trophoblaqst invasion and spiral artery remodeling. In: Pijnenborg R, Brosens I, Romero R, editors. Placental Bed Disorders. Cambridge University Press; 2010. pp. 97–108. [Google Scholar]
- 44.Brosens JJ, Parker MG, McIndoe A, Pijnenborg R, Brosens IA. A role for menstruation in preconditioning the uterus for successful pregnancy. Am.J.Obstet.Gynecol. 2009;200:615.e1–615e6. doi: 10.1016/j.ajog.2008.11.037. [DOI] [PubMed] [Google Scholar]
- 45.Khong TY, Adema ED, Erwich JJHM. On an anatomical basis for the increase in birth weight in second and subsequent born children. Placenta. 2003;24:348–353. doi: 10.1053/plac.2002.0922. [DOI] [PubMed] [Google Scholar]
- 46.Fraser AM, Brockert JE, Ward RH. Association of young maternal age with adverse reproductive outcomes. N Engl J Med. 1995;332:1113–1117. doi: 10.1056/NEJM199504273321701. [DOI] [PubMed] [Google Scholar]
- 47.Romero R, Kusanovic JP, Kim CJ. Placental disorders in the genesis of the great obstetrical disorders. In: Pijnenborg R, Brosens I, Romero R, editors. Placental Bed Disorders. Cambridge University Press; 2010. pp. 271–289. [Google Scholar]
- 48.Roberts JM, Hubel CA. The two stage model of preeclampsia: variations on the theme. Placenta. 2009;30(SUPPL.):32–37. doi: 10.1016/j.placenta.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chaiworapongsa T, Romero R, Espinoza J, Bujold E, Mee KY, Goncalves LF, et al. Evidence supporting a role for blockade of the vascular endothelial growth factor system in the pathophysiology of preeclampsia. Young Investigator Award. Am J Obstet Gynecol. 2004;190:1541–1547. doi: 10.1016/j.ajog.2004.03.043. [DOI] [PubMed] [Google Scholar]
- 50.Chaiworapongsa T, Romero R, Kim YM, Kim GJ, Kim MR, Espinoza J, Bujold E, Goncalves LF, Gomez R, Edwin S, Mazor M. Plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated prior to the clinical diagnosis of pre-eclampsia. J Matern Fetal Neonatal Med. 2005;17:3–18. doi: 10.1080/14767050400028816. [DOI] [PubMed] [Google Scholar]
- 51.Erez O, Romero R, Espinoza J, Fu W, Todem D, Kusanovic JP, et al. The change of concentrations of angiogenic and anti-angiogenic factors in maternal plasma between the first and second trimesters in risk assessment for the subsequent development of preeclampsia and small-for-gestational age. J Matern Fetal Neonatal Med. 2008;21:279–287. doi: 10.1080/14767050802034545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Espinoza J, Romero R, Nien JK, Gomez R, Kusanovic JP, Goncalves LF, et al. Identification of patients at risk for early onset and/or severe preeclampsia with the use of uterine artery Doppler velocimetry and placental growth factor. Am J Obstet Gynecol. 2007;196:326–313. doi: 10.1016/j.ajog.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kusanovic JP, Romero R, Chaiworapongsa T, Erez O, Mittal P, Vaisbuch E, et al. A prospective cohort study of the value of maternal plasma concentrations of angiogenic and anti-angiogenic factors in early pregnancy and midtrimester in the identification of patients destined to develop preeclampsia. J Matern Fetal Neonatal Med. 2009;22:1021–1038. doi: 10.3109/14767050902994754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Levine RJ, Maynard SE, Qian C, Lim KH, England LF, Yu KF, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 55.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thadhani R, Mutter WP, Wolf M, Levine RJ, Taylor RN, Sukhatme VP, et al. First trimester placental growth factor and soluble fms-like tyrosine kinase 1 and risk for preeclampsia. J Clin Endocrinol Metab. 2004;89:770–775. doi: 10.1210/jc.2003-031244. [DOI] [PubMed] [Google Scholar]
- 57.Venkatesha S, Tororsian M, Lam C, Hanai J, Mammoto T, Kim YM, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12:642–649. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 58.Romero R, Nien JK, Espinoza J, Todem D, Fu W, Chung H, et al. A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. J Matern Fetal Neonatal Med. 2008;21:9–23. doi: 10.1080/14767050701830480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chaiworapongsa T, Romero R, Kusanovic JP, Mittal P, Kim SK, Gotsch F, et al. Plasma soluble endoglin concentration in pre-eclampsia is associated with an increased impedance to flow in the maternal and fetal circulations. Ultrasound Obstet Gynecol. 2010;35:155–162. doi: 10.1002/uog.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chaiworapongsa T, Romero R, Tarca AL, Kusanovic JP, Gotsch F, Mittal P, et al. A decrease in maternal plasma concentrations of sVEGFR-2 precedes the clinical diagnosis of preeclampsia. Am J Obstet Gynecol. 2010;202:550–510. doi: 10.1016/j.ajog.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ogge G, Romero R, Kusanovic JP, Chaiworapongsa T, Dong Z, Mittal P, et al. Serum and plasma determination of angiogenic and anti-angiogenic factors yield different results: the need for standardization in clinical practice. J Matern Fetal Neonatal Med. 2010;23:820–827. doi: 10.3109/14767050903366119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chaiworapongsa T, Romero R, Gotsch F, Espinoza J, Nien JK, Goncalves LF, et al. Low maternal concentrations of soluble vascular endothelial growth factor receptor-2 in preeclampsia and small for gestational age. J Matern Fetal Neonatal Med. 2008;21:41–52. doi: 10.1080/14767050701831397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chaiworapongsa T, Espinoza J, Gotsch F, Kim YM, Kim GJ, Goncalves LF, et al. The maternal plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated in SGA and the magnitude of the increase relates to Doppler abnormalities in the maternal and fetal circulation. J Matern Fetal Neonatal Med. 2008;21:25–40. doi: 10.1080/14767050701832833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stepan H, Kramer T, Faber R. Maternal plasma concentrations of soluble endoglin in pregnancies with intrauterine growth restriction. J Clin Endocrinol Metab. 2007;92:2831–2834. doi: 10.1210/jc.2006-2774. [DOI] [PubMed] [Google Scholar]
- 65.Gotsch F, Romero R, Kusanovic JP, Chaiworapongsa T, Dombrowski M, Erez O, et al. Preeclampsia and small-for-gestational age are associated with decreased concentrations of a factor involved in angiogenesis: soluble Tie-2. J Matern Fetal Neonatal Med. 2008;21:389–402. doi: 10.1080/14767050802046069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chaiworapongsa T, Romero R, Tarca A, Pedro KJ, Mittal P, Kwon KS, et al. A subset of patients destined to develop spontaneous preterm labor has an abnormal angiogenic/anti-angiogenic profile in maternal plasma: evidence in support of pathophysiologic heterogeneity of preterm labor derived from a longitudinal study. J Matern Fetal Neonatal Med. 2009;22:1122–1139. doi: 10.3109/14767050902994838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Savasan ZA, Romero R, Chaiworapongsa T, Kusanovic JP, Kim SK, Mazaki-Tovi S, et al. Evidence in support of a role for anti-agniogenic factors in preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med. 2010;23:828–841. doi: 10.3109/14767050903440471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Romero R, Chaiworapongsa T, Erez O, Tarca AL, Gervasi MT, Kusanovic JP, et al. An imbalance between angiogenic and anti-angiogenic factors precedes fetal death in a subset of patients: results of a longitudinal study. J Matern Fetal Neonatal Med. 2010;23:1384–1399. doi: 10.3109/14767051003681121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chaiworapongsa T, Romero R, Kusanovic JP, Savasan ZA, Kim SK, Mazaki-Tovi S, et al. Unexplained fetal death is associated with increased concentrations of anti-angiogenic factors in amniotic fluid. J Matern Fetal Neonatal Med. 2010;23:794–805. doi: 10.3109/14767050903443467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chaiworapongsa T, Kusanovic JP, Savasan ZA, Mazaki-Tovi S, Kim SK, Vaisbuch E, et al. Fetal death: a condition with a dissociation in the concetnrations of soluble vascular endothelial growth factor receptor-2 between the maternal and fetal compartments. J Matern Fetal Neonatal Med. 2010;23:960–972. doi: 10.3109/14767050903410664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Espinoza J, Romero R, Nien JK, Kusanovic JP, Richani K, Gomez R, et al. A role of the anti-angiogenic factor sVEGFR-1 in the ‘mirror syndrome’ (Ballantyne’s syndrome) J Matern Fetal Neonatal Med. 2006;19:607–613. doi: 10.1080/14767050600922677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Espinoza J, Chaiworapongsa T, Romero R, Kim YM, Kim GJ, Nien JK, et al. Unexplained fetal death: another anti-angiogenic state. J Matern Fetal Neonatal Med. 2007;20:495–507. doi: 10.1080/14767050701413022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, Sibai BM, Karumanchi SA. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355:992–1005. doi: 10.1056/NEJMoa055352. [DOI] [PubMed] [Google Scholar]
- 74.Luft FC. Soluble endoglin (sEng) joins the soluble fms-like tyrosine kinase (sFlt) receptor as a pre-eclampsia molecule. Nephrol Dial Transplant. 2006;21:3052–3054. doi: 10.1093/ndt/gfl439. [DOI] [PubMed] [Google Scholar]
- 75.Aardema MW, Saro MCS, Lander M, De Wolf BTHM, Oosterhof H, Aarnoudse JG. Second trimester Doppler ultrasound screening of the uterine arteries differentiates between subsequent normal and poor outcomes of hypertensive pregnancy: Two different pathphysiological entities? Clin Sci (Lond) 2004;106:377–382. doi: 10.1042/CS20030385. [DOI] [PubMed] [Google Scholar]
- 76.Deurloo KL, Spreeuwenberg MD, Bolte AC, Van Vugt JMG. Color Doppler ultrasound of spiral artery blood flow for prediction of hypertensive disorders and intra uterine growth restriction: A longitudinal study. Prenat Diagn. 2007;27:1011–1016. doi: 10.1002/pd.1822. [DOI] [PubMed] [Google Scholar]
- 77.Romero R. Prenatal medicineL the child is the father of the man. 1996. J Matern Fetal Neonatal Med. 2009;22:636–639. doi: 10.1080/14767050902784171. [DOI] [PubMed] [Google Scholar]
- 78.Di Renzo GC. The great obstetrical syndromes. J Matern Fetal Neonatal Med. 2009;22:633–635. doi: 10.1080/14767050902866804. [DOI] [PubMed] [Google Scholar]