Abstract
Liposome nanoparticles (LNs) with a targeting ligand were used in a semi-continuous flow electroporation (SFE) device to enhance in vitro delivery of exogenous oligonucleotides (ODN). Nanoparticles comprising transferrin-targeted lipoplex encapsulating ODN G3139 were mixed with K562 cells (a chronic myeloid leukemia cell line) and incubated for half an hour to accomplish nanoparticle binding. The mixture was then flowed through a SFE channel where electric pulses were given. Better ODN delivery efficiency was achieved with an increase of ~24% to the case in combination of non-targeted LNs and SFE, and ~60% to the case using targeted LNs alone, respectively. The MTS assay results confirmed cell viability greater than 75%.
Keywords: Electroporation, Lipoplex, Microfluidics, Gene Delivery, Antisense ODN
1. Introduction
Efficient delivery of exogenous cargos (such as nucleic acids, proteins, and small drugs) has long been pursued to increase our understanding on gene regulation mechanism and to yield promising medical benefits, such as in cancer treatment and regenerative medicine (Templeton, 2004, Toneguzzo and Keating, 1986). Such delivery relies on either viral infection or nonviral membrane perturbation (including chemical or physical approaches). Viral vectors offer stable and efficient transduction (Hamer and Leder, 1979; Mulligan et al., 1979) but have safety concerns related to oncogenesis and inflammation (Verma and Somia, 1997; Marshall, 1999). Nonviral vectors, such as lipoplex and polyplex, have been explored as replacements for their low toxicity and immunogenicity (Lunggwitz et al., 1996; Gao and Huang, 1996). Great improvements have been achieved over the years regarding to their delivery efficiency and targeting specificity (Boussif et al., 1996; Abdallah et al., 1996; Felgner et al., 1997; Schmid et al., 1997; Li and Ma, 2001). However, they have yet to reach levels competitive to natural viruses on delivery efficacy and on cell viability. The emerging physical methods, electroporation in particular, offer surgery-like treatment, quick delivery response, and almost no restrictions on cell type and exogenous material properties (Neumann et al., 1982; Chang et al., 1992; Gehl, 2003). These have successfully been applied as research tools to understand biological functions and transport of various molecular probes at the cellular level as well as clinical tools to deliver anticancer drugs and various genes, oligo DNA, and RNAi (Teissie and Role, 1993; Neumann and Kakorin, 1998; Hamm et al., 2002; Lorenz and Morgenstern, 2004; Schakowski et al., 2004).
Conventional electroporation has been reasonably successful while carrying several major drawbacks, including high electric voltage, limited cell population (~105–106 cells/ml), large DNA consumption, low efficiency and cell viability. The recent introduction of microtechnology offers advantages, such as low imposed pulse voltage and in situ monitoring of intracellular transport (Huang and Rubinsky, 1999; Lin et al., 2001; McClain et al., 2003; Lu et al., 2005; Fei et al., 2007; Khine et al., 2005; Kim et al., 2007; Skelley et al., 2009). Flow-through electroporation has recently drawn great attentions to deal with various cell populations with either continuous directional current signals (Wang and Lu, 2006) or semicontinuous electric pulses (Wang et al., 2009). In these approaches, cells are mixed with DNA plasmids and flowed through an electroporation zone. In batch-type electorporation protocols, each cell would on average be surrounded by ~0.5–1.0 × 106 DNA molecules to achieve reasonable transfection efficacy (Note: the estimation is calculated by considering using 2 μg DNA plasmids in an electroporation step with 106 cells). A large percentage of DNA molecules are believed to be wasted. The additional directional flow in flow-through electroporation, even only with slow motions, could greatly reduce the number of DNA molecules that are actually taken by electroporated cells. To reduce the consumption of DNA and increase the DNA delivery efficiency, there is a need to concentrate/capture DNA molecules around cell surface before the cell membrane becomes permeable. In this way, when the cell membrane is polarized and becomes temporarily porous during the imposed electric pulses, the uptake pathway distance for DNA molecules can be significantly shortened. This would allow more molecules to enter the cell cytoplasm before the disappearance of the transient pores and the recovery of the cell membrane.
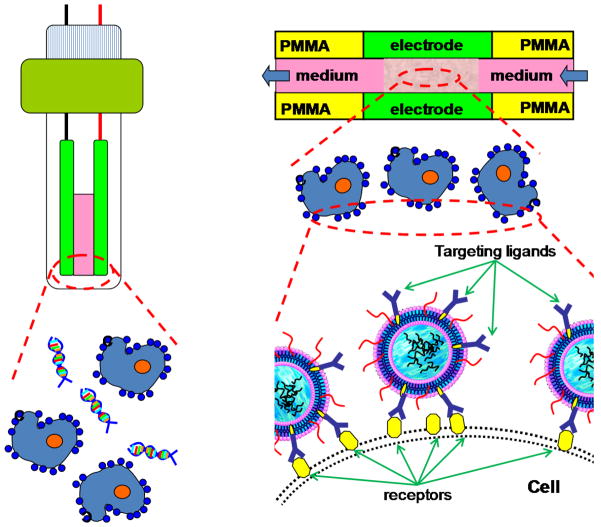
To test our hypothesis, we propose to encapsulate DNA in lipoplex with targeting ligands (e.g., antibodies, transferrin, and folate) and incubate them with cells first. In this way, lipoplex will be attached to or concentrated around the cell membrane surface through targeting ligand-receptor specific binding (Figure 1). As for demonstration, a hard-to-transfect myelogenous leukemia cell line (e.g., K562) and a fluorescence-labeled antisense oligo DNA G3139 were used as our model system. As transferrin receptors (TfR) was often over expressed on K562 cells surface (Sato et al., 2000), transferrin (Tf) was used as our targeting ligand and incorporated in liposome nanoparticles (LNs). ODN G3139, an antisense ODN for anti-cancer therapy, was encapsulated to form transferin targeted lipoplex (Tf-LNs). We expect such Tf-LNs will specifically bind to the TfRs on K562 cell membrane to facilitate the delivery of G3139 during the flow-through electroporation. A semi-continuous flow electroporation (SFE) device was used and electric pulses were applied through the aluminum channel wall of its electroporation zone. This SFE platform has previously been demonstrated to provide high cellular throughput and great improvements on transgenic expression and cell viability in gene transfer studies (Wang et al., 2009).
Figure 1.
Schematic illustration on how Tf-LNs enhance flow-through electroporation through ligand-receptor binding on the cell membrane surface.
2. Materials and Methods
2.1 Materials and reagents
3-[N-(N',N'-Dimethylaminoethane)-carbamoyl] cholesterol (DC-chol), egg phosphatidylcholine (egg PC), and distearoyl phosphatidylethanolamine-N-[maleimidopolyethylene glycol, MW 2000] (Mal-PEG2000-DSPE) were purchased from Avanti Polar Lipids (Alabaster, AL). Methoxy-PEG2000-DSPE (PEG2000-DSPE) was purchased from Genzyme Corporation (Cambridge, MA). Human holo-transferrin (Tf), 2-iminothiolane (Traut’s reagent), protamine sulfate, and other chemicals were purchased from Sigma (St. Louis, MO). All tissue culture media and supplies were purchased from Invitrogen (Carlsbad, CA). The phosphorothioate ODN (G3139, 5'-TCT CCC AGC GTG CGC CAT-3' and its fluorescence labeled derivative, G4243 or FITC-G3139) were generously provided by Genta Inc. (Berkeley Heights, NJ) or were custom synthesized by alpha-DNA (Montreal, QC, Canada).
2.2 Preparation of Tf-conjugated LNs containing G3139 (Tf-LNs-G3139)
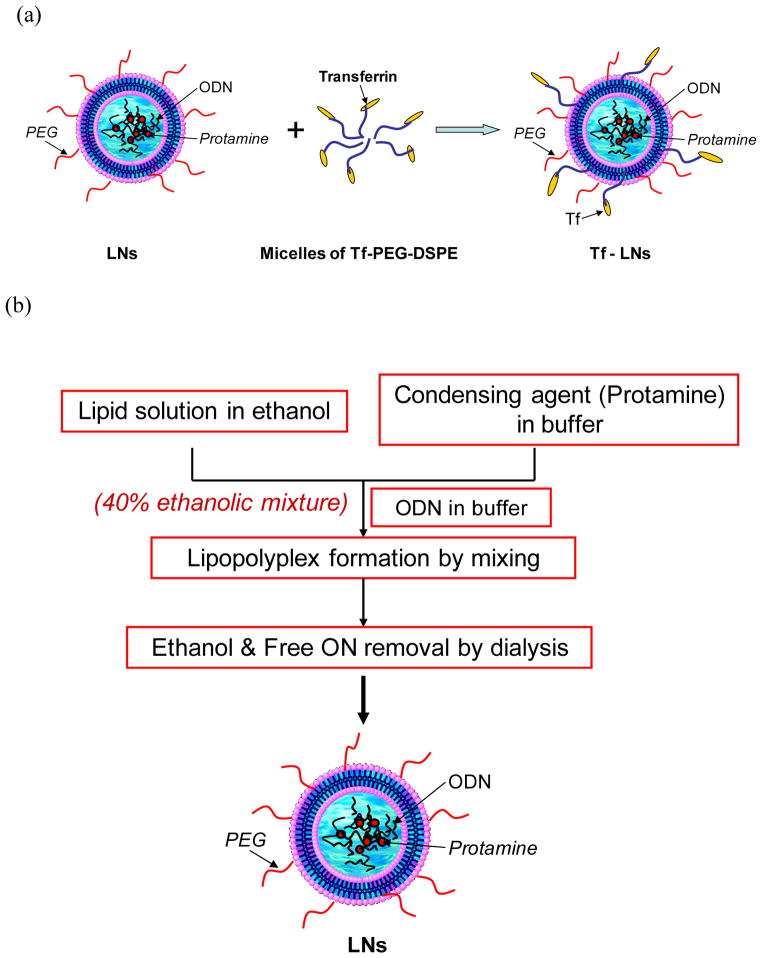
An ethanol dilution method was modified for the synthesis of LNs containing G3139 (Figure 2, Jeffs et al, 2005, Chiu et al., 2006, Yang et al., 2009). Briefly, a lipid mixture egg PC/DC-Chol/PEG2000-DSPE at a molar ratio of 65:30:5 was dissolved in ethanol (EtOH) and then mixed with protamine in a citrate buffer (20 mM, pH 4) at ratios for lipid/protamine of 12.5:0.3 (w/w) and EtOH/water of 2:1 (v/v). G3139 was dissolved in citrate buffer (20 mM, pH=4.0) and then added into the lipid/protamine solution under vortexing to spontaneously form “pre-LNs” at an EtOH concentration of 40% (v/v). The complexes were then dialyzed against citrate buffer (20 mM, pH=4.0) at room temperature for 2 h and then against HEPES buffered saline (HBS, 20 mM HEPES, 145 mM NaCl, pH=7.4) overnight at room temperature, using a MWCO 10,000 Dalton Spectra/Por Float-A-Lyzer device (Spectrum Laboratories, Rancho Dominguez, CA) to remove free G3139 and to adjust the pH value to the physiological range.
Figure 2.
Schematic illustration of the Tf-LNs synthesis with an ethanol dilution approach.
A post insertion method as adopted to incorporate lipid conjugated Tf ligand onto G3139-loaded LNs (Xu et al., 1999; Allen et al., 2002; Chiu et al., 2006). Briefly, holo-(diferric)Tf in HEPES-buffered saline (HBS, pH 8, containing 5 mM EDTA) was reacted with 5× Traut’s reagent to yield holo-Tf-SH. Free Traut’s reagent was removed by dialysis using a MWCO 10,000 Dalton Float-A-Lyzer device against HBS. Holo-Tf-SH was coupled to micelles of Mal-PEG2000-DSPE at a protein-to-lipid molar ratio of 1:10. The resulting Tf-PEG2000-DSPE micelles were then incubated with the G3139-loaded LNs for 1 h at 37°C at a Tf-PEG2000-DSPE-to-total lipid ratio of 1:100. G3139 was spiked with 10% fluorescent oligonucleotide FITC-G3139 to obtain fluorescence-labeled Tf-LNs.
2.3 Design and fabrication of semi-continuous electroporation (SFE) chips
The fabrication of SFE chips was described in detail elsewhere (Wang et al., 2009). Briefly, a polished aluminum bar was pressed into a poly(methyl methacrylate) (PMMA) chip via hot embossing. A serpentine channel was created via cutting the embedded Al piece into two halves using a micromilling machine (Aerotech, Inc). The formed Al edges serve as both electrodes and channel walls in SFE. After ultrasonic cleaning, the chip was sealed by lamination with a thin (~ 60 μm in thickness) plastic film. Here aluminum was used as the electrode material in order to enable easy comparison with commercial systems. For the same reason, the width of the SFE channels was fixed at 2 mm, identical to the distance between the two electrodes widely used in commercial systems.
2.4 Semi-continuous flow electroporation setup and procedure
Conductive copper tapes (McMaster Carr) and wires were used to connect the on-chip Al electrodes to electrodes inside the shockpod chamber of a Bio-Rad Gene Pulser Xcell™ system. Square waves electric pulses were applied through the CE module of the Bio-Rad system. Cells (~0.5×106 cells/mL) mixed with therapeutic materials (i.e., Tf-LNs loaded with fluorescent ODN) flowed through the SFE channel at a pre-specified flow rate controlled by a programmable syringe pump (Pump 22, Harvard Apparatus, Holliston, MA). An electric pulse was imposed when cells were pumped through the serpentine channel at a low flow rate (e.g., 0.2 mL/hr). After electroporation, the channel was filled with Opti-MEM I (a reduced serum media from Invitrogen, widely used for cationic lipid transfections) and the porated cells were flushed out at a higher flow rate (e.g., 5 mL/hr). After twice the amount of Opti-MEM I (the washing solution) was pumped through the SFE channel, fresh cell solution was loaded again for the next electroporation cycle. The cell suspension in each cycle took up about 95% of the channel volume, sandwiched by the washing solutions from two adjacent cycles. Detailed operation procedure is described elsewhere (Wang et al., 2009). In each electroporation cycle, 75 μL cell solutions were pumped into the channel, the same amount which is also used in a 2 mm cuvette for batch mode bulk electroporation (BE). All samples from the channel outlet were collected and transferred to a 6-well cell culture plate or a Nunc T-25cm2 filtered flask.
2.5 Cell culture and LNs targeting
K562 cells (ATCC, CCL-243) were routinely cultured in RPMI 1640 media supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 U/mL penicillin, 100 μg/mL streptomycin, and 100 μg/mL L-glutamine. Cells were seeded in a T-25 cm2 flask at a concentration of 3×105 cells/mL and maintained at 37 °C in a humidified atmosphere containing 5% CO2.
Both non-targeted and Tf targeted G3139 encapsulated lipoplex nanoparticles were used in our studies. The fluorescence-labeled Tf-LNs were mixed with K562 cells in Opti-MEM I at a ratio of 2 μg/106 cells for half an hour before loading into the SFE channel. After electroporation, cells were transferred into 6-well tissue culture plates at 106 cells/well in 1.2mL RPMI1640 medium containing 10% FBS. Phosphate-buffered saline (PBS) (300 μL/well) was added to 6-well plates and the cells were incubated for 4 h at 37 °C. The cells were then transferred to fresh medium, incubated for another 24 h, and analyzed for delivery efficiency and cell viability.
2.6 Cellular uptake of ODN encapsulated lipoplex nanoparticles
Cellular binding and uptake of lipoplex nanoparticles in K562 cells were also examined by laser scanning confocal microscopy. Cells were washed twice with 1x PBS followed by fixation with 2% para-formaldehyde for 30 min. Nuclei were stained with 20 μM of DRAQ5 (Biostatus Limited, Leicestershire, U.K.) for 5 min at room temperature. The cells were mounted on a poly-D-lysine coated cover glass slide (Sigma-Aldrich, St. Louis, MO). Green fluorescence of FITC-ODNs and blue fluorescence of DRAQ5 were analyzed, and then merged images were produced using Zeiss 510 META Laser Scanning Confocal Imaging Systems and LSM Image software (Carl Zeiss MicroImaging, Inc., NY).
2.7 ODN Delivery efficiency and cell viability
K562 cells were seeded in T-25 flasks containing 5 mL of cell culture medium at 5 × 105 viable cells/mL. The cells were incubated at 37°C in a humidified atmosphere containing 5% CO2 for 2 h. Free G3139, non-targeted G3139 encapsulated lipoplex nanoparticles (non-Tf-LNs G3139) and Tf-LNs-G3139 were then added into the medium. The cells were incubated at 37 °C for half an hour and then some samples were taken for electroporation. Cells were further incubated in fresh medium for 24 h and then harvested for analysis.
Tf-LNs delivery was first evaluated qualitatively, by visualizing the number of cells with green fluorescence within a representative area selected from the entire culture surface under an inverted fluorescence microscope (TE 100, Nikon, Japan). It was then evaluated quantitatively on basis of the mean fluorescence intensity (MFI) determined by flow cytometry (FACS Calibur, BD Biosciences, CA) at 24 h post transfection. Briefly, an aliquot of cells at 1.5×106 cells/mL was collected after being cultured for 24 h and the percentage of FITC-positive cells was determined quantitatively via flow cytometry. The unstained samples were run first to adjust the voltage setting and compensation of the flow cytometry. Then the test samples were processed by CellQuest. At least 10,000 events were collected for each sample.
Cell viability was evaluated by an MTS cell proliferation assay (Promega, Madison, WI). Briefly, the cells in 100 μL/well of medium were transferred to a 96-well plate and incubated. 20 μL of CellTiter 96 AQueous One solution (Promega, Madison, WI) was added to each well and the cells were incubated at 37°C for another 4 h. Absorbance was measured at 492 nm on an automated plate reader (GENios Pro, Tecan, Switzerland). Data points were represented as the mean ± standard deviation (SD) of triplicates, unless otherwise indicated.
3. Results and discussion
3.1 TfR targeting and binding enhancement for LNs on the cell membrane
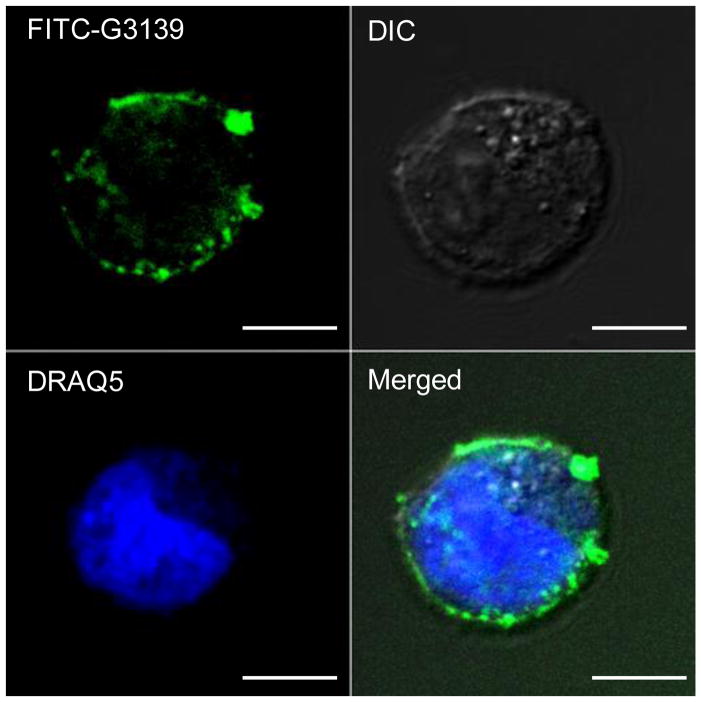
To determine the efficiency of targeted delivery, Tf-LNs encapsulating FITC-ODNs were incubated with K562 cells in Opti-MEM I medium at a ratio of 2 μg/106 cells for about half an hour. The samples were then fixed on a poly-D-lysine coated cover glass slide and examined under confocal microscopy. As shown in Figure 3, a clear green color line is shown around the cell outline, indicating that many fluorescence-labeled Tf-LNs have been successfully bound on the cell membrane surface through TfR. TfR is a dimeric transmembrane glycoprotein (180k Dalton), which is frequently up regulated in proliferating cells including cancer cells like K562. The corresponding ligand for TfR is Tf, which is an 80 kDa iron-transporting glycoprotein. After incubation, ligand-receptor specific binding were created and the encapsulated ODN were brought to cell surface by Tf-LNs. In this way, the intercellular transport distance of ODNs to temporary pores during electroporation is significantly shortened. It must be pointed out that some Tf-LNs had already entered cells before electroporation treatment. This is not surprising as liposomes alone can facilitate the endocytosis of molecule probes. Such Tf-conjugated cationic liposomes have previously shown to successfully deliver both plasmid DNA and oligonucleotides (Chiu et al., 2006; Yang et al., 2009).
Figure 3.
Confocal microscopy images show the cellular binding of Tf-LNs G3139 on K562 cells. Cells were incubated with Tf-LNs encapsulating FITC-G3139 and fixed on poly-D-lysine coated cover glass slides. The scale bars represent 10 μm.
3.2 ODN delivery efficiency in K562 cells
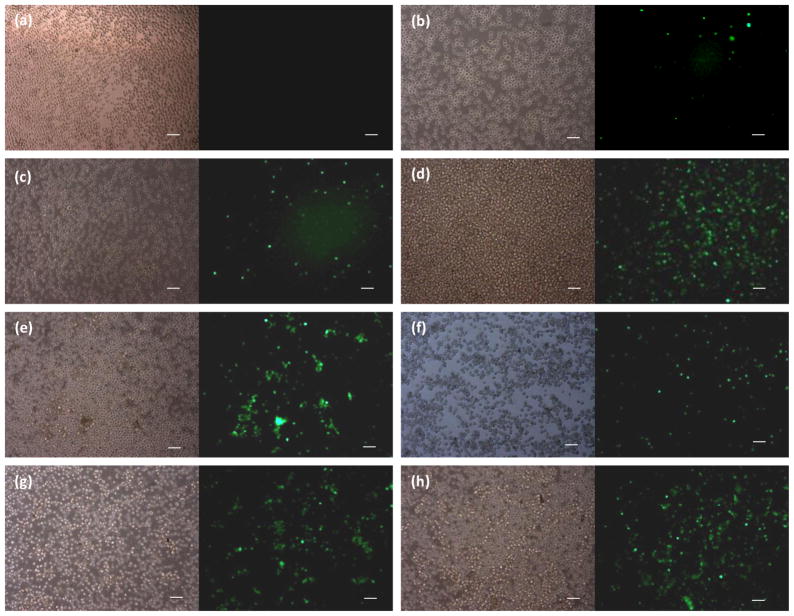
To examine the delivery enhancement of Tf-LNs in flow-through electroporaiton, K562 leukemia cells were incubated with Opti-MEM I medium only (negative control), fluorescence-labeled free ODN G3139 (free ODN), non-Tf-LNs G3139, and Tf-LNs-G3139 for about half an hour at a ratio of 2 μM /106 cells. Electroporation was then carried out in some cases via either a Bio-Rad Gene Pulser Xcell system (namely BE) or a semi-continuous electroporation device (namely SFE). Unipolar square wave electric pulses with a pulse duration of 25 ms were used for all cases requiring electroporation. Successful delivery of ODNs was observed when inspecting 24 hours post electroporation (Figure 4). Compared to large DNA plasmids, ODN G3139 is a quite small probe molecule and the uptake of ODN by cells was less difficult. Therefore, it was not surprising to find some FITC-ODNs in cells even when free ODNs were used alone (Figure 4b). When encapsulating ODNs in liposome nanoparticles, their delivery efficiency was slight improved when non-targeted LNs were used (Figure 4c), while much improved when the incubation was followed by electroporation (Figures 4c and 4d). The Tf-LNs alone were shown to facilitate ODN delivery (Figure 4f) as well, which were consistent with previous reports (Chiu et al., 2006; Yang et al., 2009). When combined with electroporation (with both BE and SFE treatment) after a pre-incubation step, the number of FITC-ODNs uptake by K562 cells was significantly increased (Figures 4g and 4h).
Figure 4.
Phase contrast and fluorescence microscopic images of FITC-ODNs uptake by K562 cells, which were treated with (a) medium only (negative control); (b) free ODNs; nanotargeted FITC-ODNs encapsulated lipoplex nanoparticles, (c) non-Tf-LNs-G3139 alone, (d) non-Tf-LNs-G3139 + BE, and (e) non-Tf-LNs-G3139 + SFE; Tf-targeted FITC-ODNs encapsulated lipoplex nanoparticles, (f) Tf-LNs-G3139 alone, (g) Tf-LNs-G3139 + BE, and (h) Tf-LNs-G3139 + SFE. The FITC-ODNs concentration was set at 2 μM. BE: Bio-Rad Xcell Pulser Bulk Electorporation, 2 mm cuvette; SFE: semi-continuous flow electroporation. Electroporation conditions: unipolar square wave pulses with a pulse duration of 25 ms for both BE and SFE. The scale bars represent 100 μm.
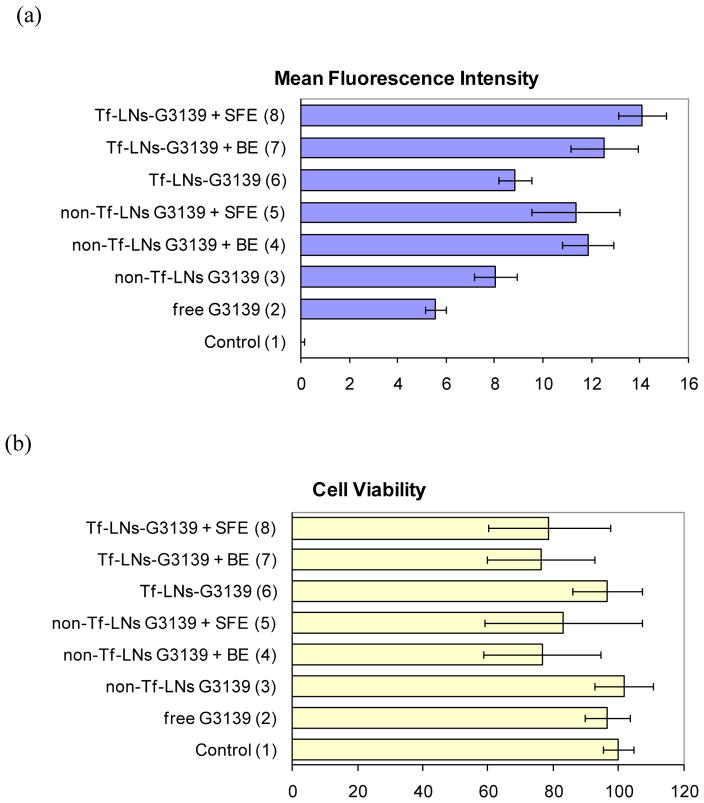
The cellular uptake enhancement was further confirmed quantitatively with flow cytometry results (Figure 5a). The fluorescence signal of cells treated with non-targeted LNs and electroporation (cases #4 and #5) was about double of those treated with free ODNs (case 2) or increased ~40–50% relative to those using non-targeted LNs alone (case #3). Similar enhancement was also found when using targeted LNs (cases #7 and #8) with an increase of ~60% (case #8, Tf-LNs G3139 in SFE) and ~40% (case #7, Tf-LNs G3139 in BE) compared to cells treated with Tf-LNs G3139 alone (case #6). With the similar combination (i.e., LNs followed by electropration), the enhancement for targeted LNs in SFE (case #8) exhibited better efficiency (an increase of ~24% to case #5 with non-targeted LNs in SFE) than in BE (case #7, almost no gain to case #4 with non-targeted LNs in BE).
Figure 5.
Quantitatively analysis of the ODN delivery efficiency in K562 cells via flow cytometry (a) and the cell viability via MTS assay (b). The FITC-ODNs concentration was set at 2 μM. The error bars correspond to triplicate experiments made with independently produced batches of lipoplex nanoparticles. Electroporation conditions: unipolar square wave pulses with a pulse duration of 25 ms in both BE and SFE.
Such improvement is attributed to the pre-concentration/binging of ODNs on cells. A pre-incubation of targeted ODNs and cells was done before they were flowed to the electroporation zone in SFE. This helps create ligand-receptor binding between targeted LNs and the cell membrane surface. Consequently, ODNs encapsulated in LNs were brought close to (or fixed on) the cell surface. The flow motions in SFE would not affect much the local concentration of exogenous probes around cells. Later imposed electric pulses were believed to enhance the delivery dosage in two ways: (1) help make the lipid bilayer structure permeable and generate pores on the cell membrane; (2) help destroy lipoplex structure to release exogenous probes. Since ODNs had been concentrated around/on the cell membrane, this will significantly shorten their transport distance to cross the cell membrane through many temporary pathways created by electric pulses. More probes could gain chances and time to enter the cell cytoplasm before the transient pores on lipid bilayer close. Therefore, it is reasonable to observe the increase of the ODNs uptake dosage when Tf-LNs were used in electroporation.
Our results seem to defer from an early observation (Weecharangsan et al 2007). Based on their observations, such combination offered negative impacts on both the delivery efficiency and the cell viability. The difference likely was resulted from the introduction of the additional pre-incubation step in the current experiments. Without pre-incubation (in Weecharangsan’s experiments), when liposome structures were destroyed by electric pulses and encapsulated probes were released, many of them were still far away from the porous cell membrane surface. The travel distance of exogenous probes in LNs was about the same as free probes alone. When cell membrane became porous, no probe concentration happened to allow more of them to enter cells. On the other hand, the destroyed lipoplex lost its functions on facilitating the endocytosis of molecule probes and released a large number of free lipid molecules. These free lipids, together with additional harsh electric pulses, would further lower the overall cell viability. The situation changed with the extra pre-incubation step. With pre-incubation (in current experiments), lipoplex nanoparticles could gain enough time to interact with cell membrane and create ligand-receptor binding for pre-concentration benefit aforementioned.
With the similar combination, the enhancement in SFE exhibited better efficiency than in BE. This is consistent with our early observations in which plasmid DNA probes were used (Wang et al., 2009). Considering the strong directional flow motions in the SFE operation, the uptake of exogenous probes across cell membrane could be further improved because the probe transport across the cell membrane was also affected by the disturbance of local flow motions. In another word, such Tf-LNs enhancement on the delivery efficiency in flow electroporation could be much better if optimized flow conditions are used.
3.3 Cell Viability
As the cellular uptake of ODNs is not very challenging, high cell survival rates were achieved in most of cases. Here, the cell viability was quantified by the MTS cell proliferation assay and defined as the ratio of the light absorbance of the electroporated cell sample to that of the negative control cell sample after a certain period of post electroporation culture (e.g., 24 h) at 492 nm. As shown in Figure 5b, 75% or even higher relative cell viability was obtained in all cases, with a decrease of ~20% in cases involving further electroporation treatment. As the delivery of ODN G3139 is not combined with other chemotherapy tools in this study, ODN leading apoptosis is involved. The loss is believed mainly from some unsuccessful recovery of cells after electroporaiton. Considering the large variations of cell viability on K562 cells electroporation (40–80%), such Tf-LNs assisted electroporation does not significantly lower the overall cell survival rate.
4. Conclusions
Tf-LNs encapsulating ODN G3139 were used to enhance in vitro delivery in flow-through electroporation. Through ligand-receptor binding, encapsulated ODNs were brought to the cell membrane surface when cell suspensions were flowed through a continuous electroporation zone. As ODNs were concentrated around when the cell membrane becomes permeable during electroporation, better ODN delivery was achieved with an efficiency increase of 24% to the case in combination of non-targeted LNs and SFE, and ~60% to the case using targeted LNs alone, respectively. The MTS assay results confirmed cell viability greater than 75%. This study provides a new approach on improving the deliver efficiency of nucleic acid or anticancer drugs through the combination of targeted nanoparticles and electroporation.
Acknowledgments
This work was supported in part by NIH/National Institute of Biomedical Imaging and Bioengineering (NIBIB) Grant R21EB008247 and Nanoscale Science and Engineering Center for Affordable Nanoengineering of Polymeric Biomedical Devices (Grant No. EEC-0425626).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abdallah B, Hassan A, Benoist C, Goula D, Behr JP, Demeneix BA. Hum Gene Ther. 1996;7:1947–1954. doi: 10.1089/hum.1996.7.16-1947. [DOI] [PubMed] [Google Scholar]
- 2.Allen TM, Sapra P, Moase E. Cell Mol Biol Lett. 2002;7:889–894. [PubMed] [Google Scholar]
- 3.Boussif O, Zanta MA, Behr JP. Gene Ther. 1996;3:1074–1080. [PubMed] [Google Scholar]
- 4.Chang DC, Chassy BM, Saunder JA. Guide to electroporation and electrofusion. Academic; San Diego: 1992. [Google Scholar]
- 5.Chiu SJ, Liu SJ, Perrotti D, Marcucci G, Lee RJ. J Controlled Release. 2006;112:199–207. doi: 10.1016/j.jconrel.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 6.Fei Z, Wang S, Xie Y, Brian H, Koh C, Lee LJ. Anal Chem. 2007;79:5719–5722. doi: 10.1021/ac070482y. [DOI] [PubMed] [Google Scholar]
- 7.Felgner PL, Barenholz Y, Behr JP, Cheng SH, Cullis P, Huang L, Jessee JA, Seymour L, Szoka F, Thierry AR, Wagner E, Wu G. Hum Gene Ther. 1997;8:511–512. doi: 10.1089/hum.1997.8.5-511. [DOI] [PubMed] [Google Scholar]
- 8.Gehl J. Acta Physiol Scand. 2003;177:437–447. doi: 10.1046/j.1365-201X.2003.01093.x. [DOI] [PubMed] [Google Scholar]
- 9.Hamer DH, Leder P. Cell. 1979;18:1299–1302. doi: 10.1016/0092-8674(79)90240-x. [DOI] [PubMed] [Google Scholar]
- 10.Hamm A, Krott N, Breibach I, Blindt R, Bosserhoff AK. Tiss Eng. 2002;8:235–245. doi: 10.1089/107632702753725003. [DOI] [PubMed] [Google Scholar]
- 11.Huang Y, Rubinsky B. Biomed Microdev. 1999;2:145–150. [Google Scholar]
- 12.Huang Y, Rubinsky B. Sens Actuators A. 2001;89:242–249. [Google Scholar]
- 13.Jeffs LB, Palmer LR, Ambegia EG, Giesbrecht C, Ewanick S, MacLachlan I. Pharm Res. 2005;22:362–372. doi: 10.1007/s11095-004-1873-z. [DOI] [PubMed] [Google Scholar]
- 14.Khine M, Lau A, Ionescu-Zanetti C, Seo J, Lee LP. Lab Chip. 2005;5:38–43. doi: 10.1039/b408352k. [DOI] [PubMed] [Google Scholar]
- 15.Kim J, Cho K, Shin Y, Jung N, Chung C, Chang J. Biosens Bioelectron. 2007;22:3273–3277. doi: 10.1016/j.bios.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 16.Li S, Ma Z. Curr Gene Ther. 2001;1:201–226. doi: 10.2174/1566523013348814. [DOI] [PubMed] [Google Scholar]
- 17.Lin YC, Jen CM, Huang MY, Wu CY, Lin XZ. Sens Actuators B. 2001;79:137–143. [Google Scholar]
- 18.Lorenz P, Harnack U, Morgenstern R. Biotechnol Lett. 2004;26:1589–1592. doi: 10.1023/B:BILE.0000045658.33723.d6. [DOI] [PubMed] [Google Scholar]
- 19.Lu H, Schmidt MA, Jensen KF. Lab Chip. 2005;5:23–29. doi: 10.1039/b406205a. [DOI] [PubMed] [Google Scholar]
- 20.Lungwitz U, Breunig M, Blunk T, Gopferich A. Eur J Pharm Biopharm. 1996;3:137–144. doi: 10.1016/j.ejpb.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 21.Marshall E. Science. 1999;286:2244–2245. doi: 10.1126/science.286.5448.2244. [DOI] [PubMed] [Google Scholar]
- 22.McClain MA, Culbertson CT, Jacobson SC, Allbritton NL, Sims CE, Ramsey JM. Anal Chem. 2003;75:5646–5655. doi: 10.1021/ac0346510. [DOI] [PubMed] [Google Scholar]
- 23.Mulligan RC, Howard BH, Berg P. Nature. 1979;277:108–111. doi: 10.1038/277108a0. [DOI] [PubMed] [Google Scholar]
- 24.Neumann E, Schaefer-Ridder M, Wang Y, Hofschneider PH. EMBO J. 1982;1:841–845. doi: 10.1002/j.1460-2075.1982.tb01257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neumann E, Kakorin S. Radiol Oncol. 1998;32:7–17. [Google Scholar]
- 26.Sato Y, Yamauchi N, Takahashi M, Sasaki K, Fukaura J, Neda H, Fujii S, Hirayama M, Itoh Y, Koshita Y, Kogawa K, Kato J, Sakamaki S, Niitsu Y. FASEB J. 2000;14:2108–2118. doi: 10.1096/fj.99-1052com. [DOI] [PubMed] [Google Scholar]
- 27.Schakowski F, Buttgereit P, Mazur M, Marten A, Schottker B, Gorschluter M, Schmidt-Wolf I. Generic vaccine and therapy. 2004;2:1–11. doi: 10.1186/1479-0556-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmid RM, Weidenbach H, Draenert GF, Liptay S, Luhrs H, Adler G. Gut. 1997;41:549–556. doi: 10.1136/gut.41.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skelley AM, Kirak O, Suh H, Jaenisch R, Voldman J. Nat Methods. 2009;6:147–152. doi: 10.1038/nmeth.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teissie J, Rols MP. Biophys J. 1993;65:409–413. doi: 10.1016/S0006-3495(93)81052-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Templeton NS, editor. Gene and cell therapy: therapeutic mechanism and strategies. 2. Marcel Dekker; New York: 2004. [Google Scholar]
- 32.Toneguzzo F, Keating A. Proc Natl Acad Sci USA. 1986;83:3496–3499. doi: 10.1073/pnas.83.10.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verma IM, Somia N. Nature. 1997;389:239–242. doi: 10.1038/38410. [DOI] [PubMed] [Google Scholar]
- 34.Wang H, Lu C. Anal Chem. 2006;78:5158–5164. doi: 10.1021/ac060733n. [DOI] [PubMed] [Google Scholar]
- 35.Wang S, Zhang X, Wang W, Lee LJ. Anal Chem. 2009;81:4414–4421. doi: 10.1021/ac9002672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weecharangsan W, Opanasopit P, Lee RJ. Anticancer Research. 2007;27:309–314. [PubMed] [Google Scholar]
- 37.Gao X, Huang L. Biochemistry. 1996;35:1027–1036. doi: 10.1021/bi952436a. [DOI] [PubMed] [Google Scholar]
- 38.Xu LA, Pirollo KF, Tang WH, Rait A, Chang EH. Hum Gene Ther. 1999;10:2941–2952. doi: 10.1089/10430349950016357. [DOI] [PubMed] [Google Scholar]
- 39.Yang X, Koh CG, Liu S, Pan X, Santhanam R, Yu B, Peng Y, Pang J, Golan S, Talmon Y, Jin J, Muthusamy N, Byrd JC, Chan KK, Lee LJ, Marcucci G, Lee RJ. Mol Pharmaceutics. 2009;6:221–230. doi: 10.1021/mp800149s. [DOI] [PMC free article] [PubMed] [Google Scholar]