Abstract
Background
The purpose of this study was to evaluate intra- and inter-observer variability of right ventricular (RV) functional parameters as evaluated by cardiac MR in patients with acquired heart disease (AHD), and to identify factors associated with an increased variability.
Methods
Sixty consecutive patients were enrolled. Right and left ventricular (LV) volumes, ejection fraction and mass were determined from short axis cine sequences. All analyzes were performed twice by 3 observers with various training-degree in cardiac MR. Intra- and inter-observer variability was evaluated. The impact on variability of each of the following parameters was assessed: observer’s experience, basal and apical slices selection, end-systolic phase selection and delineation.
Results
Mean segmentation time ranged 9.8–19.0 min for RV and 6.4–9.2 min for LV. Variability of RV functional parameters measurement was strongly influenced by previous observer’s experience: it was 2–3 times superior to that of LV, even for the most experienced observer. High variability in the measurement of RV mass was observed. For both ventricles, selection of the basal slice and delineation were major determinants of variability.
Conclusions
As compared to LV, RV function assessment with cardiac MR in AHD patients is much more variable and time-consuming. Observer’s experience, selection of basal slice, and delineation are determinant.
Keywords: Aged; Diagnostic Imaging; methods; Female; Heart; physiology; Heart Ventricles; pathology; Humans; Magnetic Resonance Imaging; methods; Male; Middle Aged; Observer Variation; ROC Curve; Regression Analysis; Reproducibility of Results; Software; Systole; Tricuspid Valve; pathology; Ventricular Function, Right
Background
The assessment of right ventricular (RV) function is essential in cardiac diseases and its prognostic value was reported in both ischemic and non-ischemic cardiomyopathies [1–4]. Contrary to the left ventricle (LV), RV is often considered uneasy to evaluate with current imaging techniques, mainly because of its complex motion and geometry [5]. Cardiac MR is the reference method of RV functional evaluation, for both clinical and research purposes [6, 7]. Previous studies stated that variability of RV function as estimated by cardiac MR was comparable to that of the LV [8, 9]. However, some of these results were obtained with long processing times (up to 45 min) incompatible with routine practice [9]. Moreover, most published studies have evaluated the RV function variability either in healthy volunteers [10–13] or in patients with congenital heart disease (CHD) [9, 14, 15]. On contrary, few studies have evaluated variability in patients with acquired heart disease (AHD). Most of them comprised limited sample size and were limited to a specific disorder [8, 16–20]. Consequently, the extension of those published results to clinical practice is questionable. Furthermore, to our knowledge, previous reports did not evaluate the factors associated with an increased variability, particularly the effect of cardiac MR experience and processing steps [8–20].
The aim of this study was to evaluate intra- and inter-observer variability of RV functional parameters as evaluated by cardiac MR in patients with AHD, and to identify factors associated with an increased variability.
Methods
This study is the second part of a previously published study that evaluated the diagnostic accuracy of 3 semi-quantitative methods for assessing right ventricular systolic function in patients with acquired heart disease [21]. Study design is presented in Fig. 1.
Figure 1. Study design.

AHD = acquired heart disease; ES = end-systole; ED = end-diastole; LV = left ventricle; RV = right ventricle.
Patients
The institutional review board approved the study and all patients gave written informed consent. From June 2008 to August 2008, all patients referred to our centre with a clinical indication of cardiac MR were invited to participate in the study. Exclusion criteria were as follows: age <18 years; contraindication to MR; arrhythmias during MR examination; CHD; and patients referred for an examination that did not include ventricular function analysis (i.e. MR angiography of pulmonary veins or thoracic aorta). The target sample size (60 patients) was defined from the results of a literature study [8, 9]. Sixty consecutive patients were included. Mean patients’ age was 53.5 ± 17.5 years and 42 (70%) were males. Clinical indications were represented by a panel of the currently most frequent cardiac MRI indications in patients with AHD: myocarditis (n=10); ischaemic cardiomyopathy (n=9); suspicion of arrhythmogenic right ventricular dysplasia (n=8); dilated cardiomyopathy (n=6); hypertrophic cardiomyopathy (n=6); aortic stenosis (n=6); other (n=15) [21].
Cardiac MR protocol
Cardiac MR examinations were performed at 1.5T (Symphony Tim®, Siemens Medical Systems, Erlangen, Germany). A dedicated eight-element phased-array cardiac coil was used. Retrospectively synchronised balanced steady-state free precession sequences were performed for cine analysis, with repeated breath-holds of 10–15 s. All conventional planes (2-, 3- and 4-chamber views) were acquired and a total of 8–12 contiguous cine short axis slices were performed from the base to the apex of the ventricles. Sequence parameters were as follows: TR = 50 ms; TE = 1.7 ms; flip angle = 55°; slice thickness = 7 mm; matrix size = 256 × 216; Field of view = 360–420 mm; 20 images per cardiac cycle. Other sequences (i.e. T2-weighted sequences, first-pass perfusion, phase contrast or late gadolinium enhancement) were performed according to clinical indication, but not considered in the present study.
Cardiac MR analysis
Observers
In order to evaluate the effect of experience on the assessment of RV and LV function (including volumes, mass and ejection fraction), 3 observers with various training-degree in cardiac MR were chosen to participate in the image analysis: observer 1 (Obs1) was an expert with 3 years full time practice, observer 2 (Obs2) had 1 year of training, and observer 3 (Obs3) was a radiology resident with no cardiac MR experience. Before the study, Obs3 received a 3-h basic cardiac MR course including anatomy and the principles of cardiac segmentation. He had to process 5 examinations selected in our database under supervision. Analyses were randomly performed with at least 1-month interval and each measurement was performed blinded to the medical history. All analyses were retrospectively performed after completing the inclusion of the 60 patients. Each observer recorded the time necessary to complete respectively LV and RV analyses (from the first click of segmentation process until final result was displayed). In order to evaluate RV and LV variability in a clinical perspective, i.e. in a reasonable amount of time, observers performed analyses as in daily practice.
RV and LV function assessment
All measurements were performed using commercially available software (Argus, Siemens Medical Solutions).
End-diastole and ES definitions
End diastole and ES were considered identical for RV and LV. End diastole was defined as the first temporal image of each stack (first cine phase of the R-wave triggered acquisition) whereas ES was defined on a mid short axis slice as the image with the smallest ventricular cavity area.
Definition of basal slices
The basal slice of the RV at ED and ES was inferred from the position of the tricuspid annulus as defined on the 4-chamber view at ED/ES (Fig. 2).
Figure 2. Basal short axis slice selection for the right ventricle.

A four-chamber view was used to locate the tricuspid annulus plane.
The basal slice of the LV at ED and ES was defined by the visibility of at least two-thirds of the circumference of the myocardium around the LV cavity.
Definition of apical slices
Apical slice was defined, for both RV and LV, as the last slice with a detectable ventricular cavity.
Endocardial and epicardial delineation
Trabeculae and papillary muscles were included in the ventricular cavity of both ventricles. Observers manually delineated endocardial and epicardial borders of the RV on short axis slices at ED and ES. Semi-automatic segmentation of the LV was performed, followed if required by manual editing. The interventricular septum was included in the LV mass.
Image processing
Each observer had to record the numbers of the slices defined as basal and apical at ED and ES for both ventricles. The selected ES phase was also recorded. Thus, we could retrospectively determine intra and inter-observer agreement regarding each of those selections and their relative influence on variability.
Statistical analysis
Continuous variables are expressed as mean ± SD and qualitative variables as number and percentage. Bland Altman method, coefficient of variation (CV) and intraclass correlation coefficient (ICC) were used to evaluate intra- and inter-observer reproducibility. As the second measurements of each observer were performed to evaluate intra-observer reproducibility, we used only the first measurements to evaluate inter-observer agreement. This choice reflects the clinical practice since only one measurement is usually performed. Chi-square test was performed to compare the frequencies of categorical variables. All Statistical analyses were performed using MedCalc for Windows, version 11.3.2.0 (MedCalc Software, Mariakerke, Belgium).
Results
Processing time
Processing times of each observer for RV and LV are reported in Table 1. The mean segmentation time ranged 9.8–19.0 min and 6.4–9.2 min respectively for RV and LV. Processing time was significantly shorter for LV as compared to RV for all observers, and for their two measurements (p<0.001). A significant decrease of processing time was noted between first vs. second measurement, for both ventricles and for all observers.
Table 1.
Processing times
| Observer 1 | Observer 2 | Observer 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| RV | LV | P value RV vs. LV | RV | LV | P value RV vs. LV | RV | LV | P value RV vs. LV | |
| Measure 1 | 13.4 ± 3.9 | 7.8 ± 2.4 | <0.001 | 19.0 ± 4.0 | 9.2 ± 2.2 | <0.001 | 11.0 ± 2.8 | 7.9 ± 2.4 | <0.001 |
| Measure 2 | 10.5 ± 1.9 | 6.4 ± 1.8 | <0.001 | 14.9 ± 3.1 | 7.0 ± 2.1 | <0.001 | 9.8 ± 2.5 | 7.0 ± 2.1 | <0.001 |
| P value Measure 1 vs. 2 | <0.001 | <0.001 | <0.001 | <0.001 | 0.03 | 0.04 | |||
Note. Data are expressed in min ± SD; RV = right ventricle; LV = left ventricle.
Intra- and inter-observer variability
Intra- and inter-observer variability results are reported in Tables 2 and 3. Intra-observer variability was related to the observer’s experience, for both ventricles and mostly for the RV (Fig. 3). For RV analysis, Obs 1 had the lowest CV and highest ICC for most parameters, followed by Obs2 and Obs3. Also, less variability was observed for LV processing as compared to RV for all parameters (Table 2). Right ventricular mass measurement was the least reproducible parameter for all observers (CV ranged 15.8%–21.3%). On contrary, LV mass measurement was highly reproducible, even for Obs3. Inter-observer variability of RV functional parameters was also influenced by observer’s experience, as demonstrated by CV and ICC including or excluding Obs3 (Table 3). The effect of experience on inter-observer variability was less pronounced for LV parameters.
Table 2.
Intra-observer variability of right and left ventricular parameters.
| Observer 1 | Observer 2 | Observer 3 | ||||
|---|---|---|---|---|---|---|
| RV | LV | RV | LV | RV | LV | |
| EF (%) | ||||||
| Mean | 53.2 ± 11.4 | 55.3 ± 15.2 | 55.5 ± 11.5 | 52.9 ± 15.7 | 52.0 ± 10.6 | 54.8 ± 15.9 |
| Mean difference | − 1.3 ± 4.1 | − 0.7 ± 3.0 | − 0.7 ± 6.5 | 0.5 ± 4.9 | 1.4 ± 10.5 | − 0.5 ± 5.9 |
| CV | 7.8 | 5.4 | 11.7 | 9.4 | 20.2 | 10.7 |
| ICC | 0.931 | 0.980 | 0.854 | 0.952 | 0.605 | 0.935 |
| EDV index (mL/m2) | ||||||
| Mean | 74.7 ± 19.2 | 95.0 ± 33.3 | 74.9 ± 19.8 | 95.2 ± 32.3 | 75.3 ± 21.8 | 93.2 ± 32.4 |
| Mean difference | 4.1 ± 7.0 | 1.2 ± 3.4 | − 2.4 ± 9.5 | 1.3 ± 5.9 | 0.7 ± 9.9 | 1.3 ± 6.3 |
| CV | 9.3 | 3.6 | 12.7 | 6.2 | 13.1 | 6.8 |
| ICC | 0.917 | 0.994 | 0.886 | 0.983 | 0.904 | 0.981 |
| ESV index (mL/m2) | ||||||
| Mean | 35.9 ± 16.5 | 46.2 ± 31.8 | 34.2 ± 16.2 | 48.7 ± 32.5 | 36.9 ± 17.0 | 45.5 ± 31.2 |
| Mean difference | 2.7 ± 4.3 | 1.3 ± 3.5 | − 0.6 ± 4.9 | 0.4 ± 4.4 | − 0.7 ± 6.1 | 0.7 ± 4.5 |
| CV | 12.0 | 7.6 | 14.4 | 9.1 | 16.7 | 10.0 |
| ICC | 0.955 | 0.993 | 0.955 | 0.991 | 0.937 | 0.989 |
| Stroke V index (mL/m2) | ||||||
| Mean | 38.8 ± 10.5 | 48.7 ± 12.4 | 40.9 ± 11.6 | 46.6 ± 12.1 | 38.6 ± 10.9 | 47.5 ± 13.1 |
| Mean difference | 1.4 ± 5.0 | − 0.1 ± 4.0 | −1.5 ± 8.1 | 0.8 ± 7.5 | 1.2 ± 11.5 | 0.4 ± 6.9 |
| CV | 12.8 | 8.3 | 19.9 | 16.2 | 29.7 | 14.4 |
| ICC | 0.890 | 0.949 | 0.777 | 0.824 | 0.567 | 0.874 |
| Mass index (g/m2) | ||||||
| Mean | 27.2 ± 5.8 | 75.9 ± 19.3 | 23.4 ± 5.4 | 69.1 ± 17.2 | 30.0 ± 7.2 | 74.2 ± 18.4 |
| Mean difference | 2.5 ± 3.1 | 1.7 ± 3.2 | 1.6 ± 4.5 | − 0.1 ± 6.4 | −0.2 ± 5.1 | − 0.1 ± 5.0 |
| CV | 11.3 | 4.2 | 19.2 | 9.2 | 17.1 | 6.7 |
| ICC | 0.785 | 0.983 | 0.672 | 0.935 | 0.779 | 0.965 |
Note. RV = right ventricle; LV = left ventricle; EF = ejection fraction; EDV = end diastolic volume; ESV = end systolic volume; Stroke V = Stroke Volume; CV = coefficient of variation, expressed as a percentage; ICC = intraclass correlation coefficient.
Table 3.
Inter-observer variability of right and left ventricular parameters.
| Obs1 vs. Obs2 | Obs1 vs. Obs3 | Obs2 vs. Obs3 | ||||
|---|---|---|---|---|---|---|
| RV | LV | RV | LV | RV | LV | |
| EF (%) | ||||||
| Mean | 53.9 ± 11.3 | 54.0 ± 15.3 | 52.6 ± 10.8 | 54.7 ± 15.2 | 54.0 ± 10.8 | 53.8 ± 15.5 |
| Mean difference | 2.7 ± 7.2 | − 1.8 ± 4.5 | −0.2 ± 9.3 | 0.4 ± 5.9 | 2.5 ± 9.9 | −1.4 ± 5.8 |
| CV | 13.3 | 8.2 | 17.8 | 10.8 | 18.4 | 10.7 |
| ICC | 0.800 | 0.953 | 0.689 | 0.929 | 0.639 | 0.931 |
| EDV index (mL/m2) | ||||||
| Mean | 75.2 ± 19.6 | 95.7 ± 33.0 | 76.2 ± 20.5 | 94.7 ± 32.9 | 74.6 ± 21.1 | 94.8 ± 32.4 |
| Mean difference | −3.1 ± 8.9 | 0.2 ± 5.4 | 1.2 ± 9.9 | 1.8 ± 6.5 | −1.9 ± 10.8 | 2.0 ± 6.6 |
| CV | 11.9 | 5.7 | 13.0 | 6.9 | 14.5 | 7.0 |
| ICC | 0.892 | 0.987 | 0.890 | 0.979 | 0.875 | 0.978 |
| ESV index (mL/m2) | ||||||
| Mean | 35.6 ± 16.1 | 47.9 ± 32.5 | 36.9 ± 16.4 | 46.4 ± 31.5 | 35.2 ± 16.4 | 47.4 ± 31.8 |
| Mean difference | −3.4 ± 5.9 | 1.9 ± 3.7 | 0.8 ± 6.7 | 1.0 ± 5.7 | −2.6 ± 7.4 | 3.0 ± 6.1 |
| CV | 12.4 | 7.7 | 18.1 | 12.3 | 20.9 | 12.8 |
| ICC | 0.917 | 0.992 | 0.921 | 0.983 | 0.894 | 0.978 |
| Stroke V Index (mL/m2) | ||||||
| Mean | 39.8 ± 11.4 | 47.8 ± 11.7 | 39.3 ± 10.8 | 48.2 ± 12.3 | 39.7 ± 11.4 | 47.4 ± 12.0 |
| Mean difference | 0.6 ± 7.3 | −1.7 ± 6.1 | 0.3 ± 10.2 | 1.0 ± 7.0 | 1.0 ± 10.4 | − 0.7 ± 7.2 |
| CV | 18.4 | 12.7 | 26.0 | 14.5 | 26.2 | 15.1 |
| ICC | 0.814 | 0.867 | 0.636 | 0.849 | 0.658 | 0.838 |
| Mass index (g/m2) | ||||||
| Mean | 26.3 ± 6.0 | 72.9 ± 18.0 | 29.3 ± 6.7 | 75.4 ± 18.9 | 29.2 ± 6.5 | 71.6 ± 17.4 |
| Mean difference | − 4.3 ± 5.0 | − 7.8 ± 7.2 | −1.2 ± 6.2 | 2.6 ± 6.7 | − 1.5 ± 5.1 | − 5.1 ± 7.3 |
| CV | 18.9 | 9.9 | 21.2 | 8.8 | 17.5 | 10.2 |
| ICC | 0.540 | 0.848 | 0.638 | 0.932 | 0.713 | 0.881 |
Note. Obs1 = observer 1; Obs2 = observer 2; Obs3 = observer 3; RV = right ventricle; LV = left ventricle; EF = ejection fraction; EDV = end diastolic volume; ESV = end systolic volume; Stroke V = Stroke Volume; CV = coefficient of variation, expressed as a percentage; ICC = intraclass correlation coefficient.
Figure 3. Bland Altman plots: intra-observer variability of RV functional parameters in relation to previous observer’s experience.
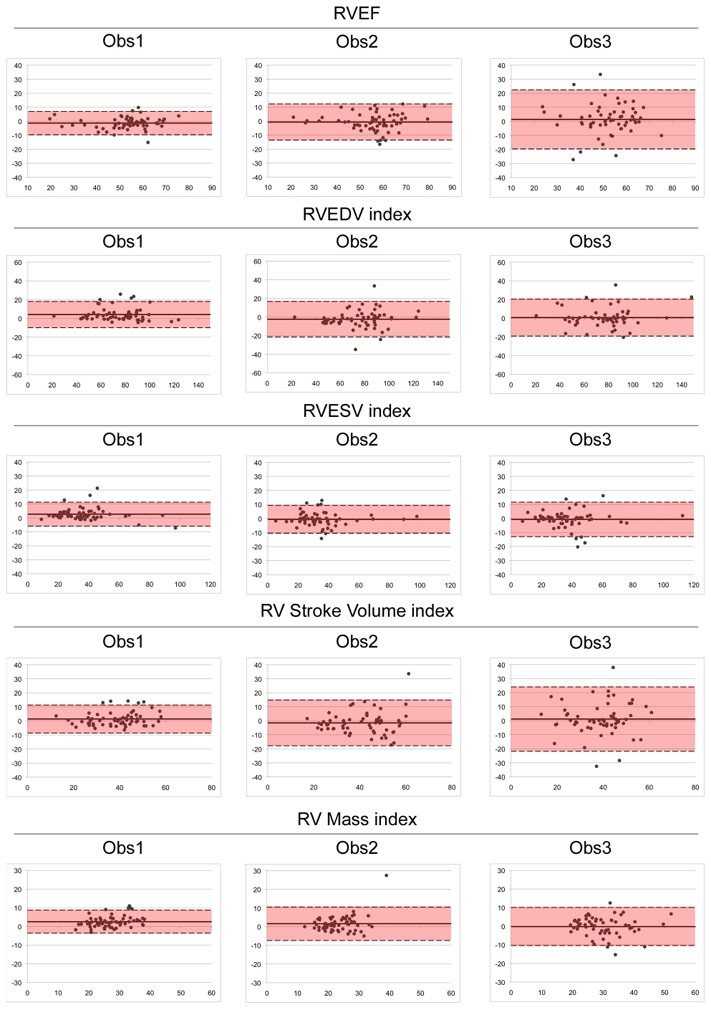
x axis = average of the 2 measurements of the observer; y axis = differences between the 2 measurements of the observer; RV = right ventricle; EF = ejection fraction; EDV = end diastolic volume; ESV = end systolic volume.
Variability of ejection fraction related to each processing steps
Tables 4 and 5 report the effect of each processing steps on intra- and inter-observer variability of RV and LVEF, in function of the different selections made by the observer.
Table 4.
Intra-observer variability of right and left ventricular ejection fraction related to the selection of the same basal/apical slice and end systolic phase during the 2 measurements.
| Observer 1 | Observer 2 | Observer 3 | ||||
|---|---|---|---|---|---|---|
| RV | LV | RV | LV | RV | LV | |
| All measurements | ||||||
| N (%) | 60 (100) | 60 (100) | 60 (100) | 60 (100) | 60 (100) | 60 (100) |
| Mean (%) | 53.2 ± 11.4 | 55.3 ± 15.2 | 55.5 ± 11.5 | 52.9 ± 15.7 | 52.0 ± 10.6 | 54.8 ± 15.9 |
| Mean difference (%) | − 1.3 ± 4.1 | − 0.7 ± 3.0 | − 0.7 ± 6.5 | 0.5 ± 4.9 | 1.4 ± 10.5 | − 0.5 ± 5.9 |
| CV (%) | 7.8 | 5.4 | 11.7 | 9.4 | 20.2 | 10.7 |
| ICC | 0.931 | 0.980 | 0.854 | 0.952 | 0.605 | 0.935 |
| Same basal slice selected | ||||||
| N (%) | 44 (73) | 44 (73) | 34 (57) | 37 (62) | 38 (63) | 39 (65) |
| Mean (%) | 52.5 ± 12.3 | 56.0 ± 15.4 | 55.1 ± 13.7 | 55.9 ± 15.3 | 50.9 | 55.7 ± 16.0 |
| Mean difference (%) | −0.8 ± 3.3 | − 0.2 ± 2.1 | 0.3 ± 3.6 | 0.6 ± 3.3 | 0.7 ± 6.7 | 0.9 ± 4.1 |
| CV (%) | 6.4 | 3.7 | 6.6 | 6.0 | 13.1 | 7.3 |
| ICC | 0.962 | 0.991 | 0.966 | 0.976 | 0.807 | 0.967 |
| Same apical slice selected | ||||||
| N (%) | 44 (73) | 48 (80) | 41 (68) | 44 (73) | 28 (47) | 40 (67) |
| Mean (%) | 54.6 ± 10.7 | 55.3 ± 15.1 | 54.6 ± 11.2 | 50.2 ± 17.0 | 52.1 ± 10.5 | 54.2 ± 17.0 |
| Mean difference (%) | −1.3 ± 4.2 | − 0.5 ± 2.7 | 0.3 ± 5.6 | 0.2 ± 4.8 | 1.2 ± 8.5 | −0.5 ± 5.9 |
| CV (%) | 7.6 | 4.9 | 10.3 | 9.6 | 16.3 | 10.8 |
| ICC | 0.922 | 0.984 | 0.884 | 0.962 | 0.720 | 0.943 |
| Same systolic phase selected | ||||||
| N (%) | 48 (80) | 48 (80) | 36 (60) | 36 (60) | 21 (35) | 21 (35) |
| Mean (%) | 54.5 ± 10.4 | 55.7 ± 15.7 | 58.4 ± 9.6 | 55.2 ± 13.2 | 53.7 ± 9.0 | 55.9 ± 17.4 |
| Mean difference (%) | −1.4 ± 4.1 | − 0.6 ± 3.2 | −0.2 ± 6.1 | 0.3 ± 5.5 | 1.6 ± 7.8 | 1.0 ± 3.9 |
| CV (%) | 7.5 | 5.7 | 10.4 | 9.9 | 14.5 | 7.1 |
| ICC | 0.919 | 0.979 | 0.823 | 0.920 | 0.686 | 0.974 |
| Same basal/apical slices and ES phase selected | ||||||
| N (%) | 25 (42) | 27 (45) | 15 (25) | 16 (27) | 5 (8) | 9 (15) |
| Mean (%) | 54.9 ± 11.6 | 58.3 ± 14.6 | 57.2 ± 11.5 | 55.2 ± 12.3 | 53.8 ± 3.5 | 50.7 ± 18.9 |
| Mean difference (%) | − 0.7 ± 2.9 | 0.1 ± 1.8 | 0.1 ± 3.8 | 0.6 ± 3.4 | −2.9 ± 4.5 | 2.4 ± 3.1 |
| CV (%) | 5.4 | 3.1 | 6.6 | 6.1 | 8.5 | 6.1 |
| ICC | 0.968 | 0.993 | 0.951 | 0.964 | 0.355 | 0.980 |
Note. RV = right ventricle; LV = left ventricle; EF = ejection fraction; CV = coefficient of variation, expressed as a percentage; ICC = intraclass correlation coefficient; ES = end systole.
Table 5.
Inter-observer variability of right and left ventricular ejection fraction related to the selection of the same basal/apical slice and end systolic phase during the 2 measurements.
| Obs1 vs. Obs2 | Obs1 vs. Obs3 | Obs2 vs. Obs3 | ||||
|---|---|---|---|---|---|---|
| RV | LV | RV | LV | RV | LV | |
| All measurements | ||||||
| N (%) | 60 (100) | 60 (100) | 60 (100) | 60 (100) | 60 (100) | 60 (100) |
| Mean (%) | 53.9 ± 11.3 | 54.0 ± 15.3 | 52.6 ± 10.8 | 54.7 ± 15.2 | 54.0 ± 10.8 | 53.8 ± 15.5 |
| Mean difference (%) | 2.7 ± 7.2 | − 1.8 ± 4.5 | −0.2 ± 9.3 | 0.4 ± 5.9 | 2.5 ± 9.9 | −1.4 ± 5.8 |
| CV (%) | 13.3 | 8.2 | 17.8 | 10.8 | 18.4 | 10.7 |
| ICC | 0.800 | 0.953 | 0.689 | 0.929 | 0.639 | 0.931 |
| Same basal slice selected | ||||||
| N (%) | 24 (40) | 38 (63) | 26 (43) | 26 (43) | 14 (23) | 38 (63) |
| Mean (%) | 51.4 ± 14.3 | 53.7 ± 16.9 | 52.5 ± 12.3 | 56.1 ± 14.9 | 51.1 ± 11.6 | 54.9 ± 14.4 |
| Mean difference (%) | 3.8 ± 3.6 | −1.6 ± 3.4 | − 2.5 ± 4.7 | − 0.4 ± 3.3 | 3.8 ± 4.3 | −1.0 ± 4.0 |
| CV (%) | 7.1 | 6.3 | 9.0 | 5.8 | 8.4 | 7.3 |
| ICC | 0.937 | 0.977 | 0.914 | 0.977 | 0.891 | 0.960 |
| Same apical slice selected | ||||||
| N (%) | 21 (35) | 36 (60) | 24 (40) | 41 (68) | 15 (25) | 29 (48) |
| Mean (%) | 55.8 ± 9.9 | 50.1 ± 17.1 | 51.2 ± 11.5 | 53.1 ± 16.3 | 56.2 ± 8.8 | 48.0 ± 16.1 |
| Mean difference (%) | 1.5 ± 8.4 | − 2.2 ± 4.4 | − 0.2 ± 8.9 | − 0.6 ± 5.8 | 2.9 ± 12.5 | − 0.8 ± 5.3 |
| CV (%) | 15.1 | 8.8 | 17.3 | 10.8 | 22.3 | 11.0 |
| ICC | 0.700 | 0.960 | 0.748 | 0.940 | 0.334 | 0.948 |
| Same systolic phase selected | ||||||
| N (%) | 34 (57) | 34 (57) | 25 (42) | 25 (42) | 22 (37) | 22 (37) |
| Mean (%) | 52.7 ± 13.1 | 55.7 ± 15.4 | 51.1 ± 10.8 | 55.3 ± 17.2 | 52.4 ± 12.4 | 54.9 ± 16.1 |
| Mean difference (%) | 3.0 ± 6.4 | −1.4 ± 4.9 | 2.1 ± 8.7 | 1.3 ± 4.8 | 4.9 ± 9.8 | − 3.3 ± 5.3 |
| CV (%) | 12.2 | 8.8 | 17.0 | 8.8 | 18.7 | 9.6 |
| ICC | 0.868 | 0.948 | 0.720 | 0.960 | 0.691 | 0.930 |
| Same basal/apical slices and ES phase selected | ||||||
| N (%) | 4 (7) | 16 (27) | 3 (5) | 7 (12) | 0 (0) | 4 (7) |
| Mean (%) | 54.3 ± 16.0 | 49.4 ± 19.1 | 39.9 ± 13.9 | 61.1 ± 14.8 | - | 53.2 ± 17.8 |
| Mean difference (%) | 2.4 ± 3.0 | − 1.2 ± 3.9 | − 0.1 ± 6.5 | 1.2 ± 3.4 | - | − 3.4 ± 3.7 |
| CV (%) | 5.5 | 7.8 | 16.4 | 5.6 | - | 7.0 |
| ICC | 0.976 | 0.979 | 0.927 | 0.974 | - | 0.966 |
Note. RV = right ventricle; LV = left ventricle; EF = ejection fraction; CV = coefficient of variation, expressed as a percentage; ICC = intraclass correlation coefficient; ES = end systole.
Effect of basal slice selection
The selected basal slice strongly impacted the intra- and inter-observer variability of RVEF measurement. Indeed, when observers have chosen identical ED and ES basal slices, the reproducibility was excellent (Tables 4 and 5, Fig. 4). Besides, intra-observer agreement in the selection of identical basal slices for the 2 measurements improved with experience: 44/60 (73%) of cases for Obs1, 34/60 (57%) for Obs2 and 38/60 (63%) for Obs3 (p=0,15). Identical results were found for the LVEF.
Figure 4. Bland Altman plots: effect of processing steps on intra-observer variability for RV functional parameters.
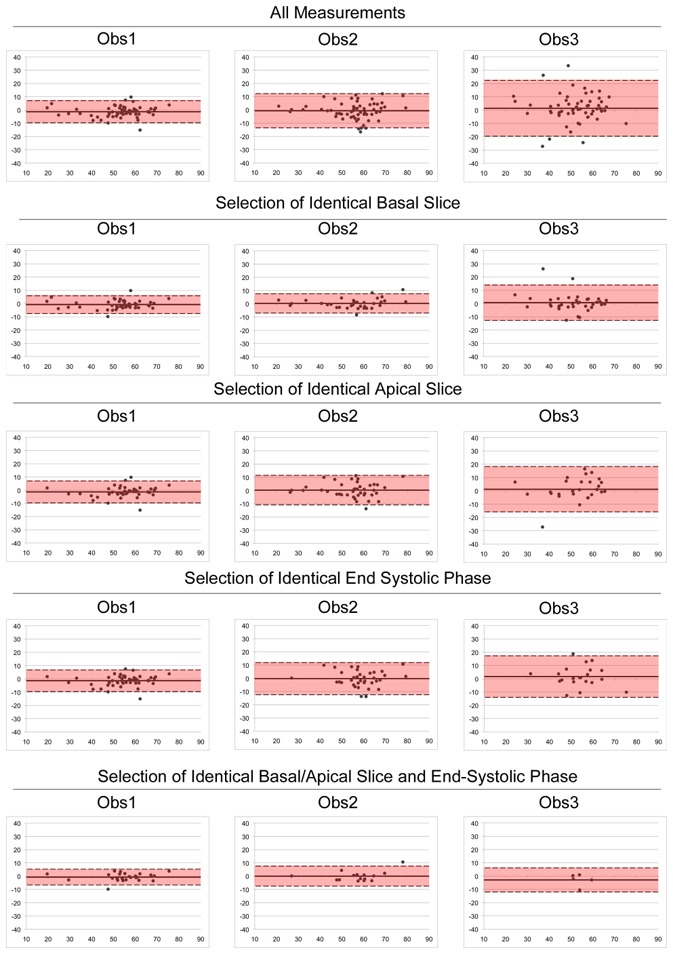
x axis = average of the 2 measurements of the observer; y axis = differences between the 2 measurements of the observer.
Effect of apical slice and ES phase selection
On the other hand, the selection of the apical slice and ES phase hardly influenced the variability (Tables 4 and 5, Fig. 4). Indeed, discordant choices between the 2 measurements did not significantly impair CV or ICC, for both ventricles, except for Obs3. Again, previous experience was determinant in selecting identical apical slice and ES phase for the 2 measurements (Table 4).
Effect of observer’s delineation
Finally, the effect of observer’s delineation was inferred from cases in which a perfect intra-observer agreement was obtained in all processing steps, i.e. identical basal and apical slices in both ED/ES, and identical ES phase. This perfect agreement was related to experience: 25/60 (42%) of cases for Obs 1, 15/60 (25%) for Obs 2 and 5/60 (8%) for Obs 3 (p=0,0001). In these cases, variability was only related to delineation, and remained higher for RV than for LV for all observers (Table 4, Fig. 4). Overall, a perfect inter-observer agreement was poorly achieved in these processing steps, as shown in Table 5.
Discussion
Intra- and inter-observer variability
Effect of observer’s experience
We found that previous experience was a major determinant of intra- and inter-observer variability. Most of previously reported studies evaluated RV and LV function variability without mentioning the observers’ level of experience [14, 15, 19], or they had involved observers of identical experience: inexperienced [9] or experienced [11, 13, 17, 20]. Nevertheless, in a clinical practice perspective, the evaluation of the effect of experience on variability is essential to determine which level of experience provides an accurate diagnosis [12, 16].
Effect of selection of basal slice
The key role of basal slice on variability was mentioned in most cardiac MR studies of ventricular function, though it was never demonstrated. In a limited sample of 10 healthy volunteers, Karamitsos et al. showed the importance of basal slice in the reproducibility of LV functional parameters [12]. To our knowledge, no other study quantified the impact of the basal slice selection on the RV function assessment. In the present study, basal slice selection was more prone to variability for RV than for LV. Indeed, the set of ED short axis slices most often starts at the left atrio-ventricular junction, using the mitral annulus plane as anatomical landmark, as recommended by the Society for Cardiovascular Magnetic Resonance Guidelines [26]. Furthermore, when the basal slice is not perfectly positioned along the mitral annulus plane, the well visible thick LV myocardium allows proper choice of the basal slice in most cases. The problem is more complex for the RV. Firstly the basal positioning of the short axis slices set is not intended to start from the right atrio-ventricular junction at ED, unless the acquisition would be repeated for the RV, lengthening the examination time and increasing the inconvenience for the patient. Secondly, the thinness of the RV myocardium does not help define the atrio-ventricular junction on short axis views. In the present study, we have been selecting the RV basal slice from the 4-chamber view. This method provided a good concordance between the 2 measurements (n = 44/60, 73%, identical to LV) for Obs1, but was less reproducible for less experienced observers. Other methods were proposed to overcome difficulties related to basal slice selection. Strugnell et al. evaluated a modified short axis series, oriented along the outflow tract of the RV [27], whereas Alfakih et al. proposed to evaluate RV volumes from axial sequences [28]. In both models the visualization of tricuspid annulus plane was easier, resulting in a reduced variability. However, partial volume effect is a limitation of both methods, respectively at the level of the RV outflow tract, and at the inferior RV wall. Moreover, these methods require additional acquisitions. Also, the previously published normal values were determined from short axis acquisitions [10, 11, 13, 20]. More recently, it was demonstrated that the combination of the longitudinal cardiac motion (by identifying mitral valve plane and LV apex) with conventional short axis analysis could result in a lesser variability of LV parameters measurements [30], but these results have not been confirmed [31]. Similarly, Maceira et al. determined reference RV systolic and diastolic function in a large sample of healthy subjects using a RV systolic shortening correction [11]. Whether this method could reduce variability of RV measurements needs further investigation.
Effect of selection of apical slice
Apical slice choice was not found to be a major determinant of variability of both RV and LV function. These results are not surprising since small volumes are involved at the apex of ventricles.
Effect of selection of ES phase
Temporal resolution is key factor in cardiac imaging. In the present study, we acquired 20 images per RR interval and found that the effect of the selection of ES phase was negligible in most cases, to the exception of the intra-observer variability of the less experienced observer. This finding is certainly related to the fact that less experienced observers have also the most important variability in delineation, thus enhancing the differences of each discordant choice.
Effect of observer delineation
The effect of observer’s delineation was deduced from intra and inter-observer comparisons for which basal and apical slices in both ED and ES, and same ES phase were selected for the 2 measurements. These concordances were related to experience. Interestingly, the number of cases in which two observers perfectly matched for the RV was very low. These discordances are particularly marked for EF measurements, which represent the end product of all possible choices. Variability related to observers’ delineation was always greater for the RV as compared to the LV. These results highlight the difficulty to perform a correct RV segmentation, due to the RV complex geometry, trabeculations and wall thinness [5]. Improvements of image quality and segmentation software could solve this problem in the future [23, 24, 31].
Processing time
Effect of processing time on variability
In most of previously published studies [8–20], processing times were not reported. Yet, a reasonable time is essential to translate research results into clinical practice. For example, the mean segmentation time of the LV in the present study was in agreement with the results reported by Sardanelli et al. (close to 6 min) [16]. It was much shorter than in the study by Grothues et al. (close to 25 min) [22]. Similarly, the time necessary to segment the RV in our study was much shorter than in the article by Mooij et al. (close to 45 min) [9]. In our study, we performed all measurements as routinely in our clinical practice. This could explain our relatively short processing times and variability results.
Differences between the processing times of LV and RV
Segmentation time of the RV lasted twice as much as that of LV. This reflects the recognized efficacy of semi-automatic LV segmentation. On the other hand, a manual segmentation of the RV remains necessary due to its complex geometry. In this study, it still took about 20–25 min to analyze both ventricles function, an amount of time divided into one-third for LV and two-thirds for RV. These long processing times explain at least partially the under-assessment of RV function in clinical practice. This highlights the need for automatic segmentation software improvement (23, 24) and semi-quantitative methods (tricuspid annulus plane systolic excursion, right ventricular fractional area change) [21, 25].
Clinical and research relevance
Clinical relevance
RVEF and volumes measurements have important diagnostic, prognostic, and therapeutic implications in patients with AHD who need cardiac function follow-up [1–4]. The coefficient of repeatability assesses the minimal significant change between 2 measurements that cannot be attributed to observers’ variability. Coefficient of repeatability can be deduced from Bland-Altman plots or calculated as 2 × SD, SD being the standard deviation of the difference between the 2 measurements. For example, from the results of the present study, a significant RVEF change during patient’s follow-up would be >8.2% for Obs1, >13.0% for Obs2 and >21% for Obs3. In case of perfect consensus on the basal short axis slice definition, this change would be >6.6% for Obs1, >7.2% for Obs 2, and >13.4% for Obs3.
Also, this study demonstrated that RV mass measurement was highly variable, even for an experienced observer. The results of the present study raise question about the accuracy of the measurement and longitudinal follow-up of the RV mass. Thus, in clinical practice, the delineation of the RV could be limited to the endocardial border to assess RV volumes and EF. A precise estimate of volumes and the EF could be obtained in a limited amount of time and would be clinically relevant in most diseases [1–4, 32–35].
Research relevance
Cardiac MRI is being used in evaluating treatments or the prognosis of diseases based on RV function changes [33–35]. The number of subjects required to demonstrate a significant difference is given by the following equation: n = f(α, P) × σ2 x 2/δ2, where n is the sample size; α the significance level; P the study power required; f is a factor deduced from α and P values, e.g. f(α, P) = 10.5 for α = 0.05 and P = 0.90; σ is the inter-measurement standard deviation; and δ the minimal difference to be highlighted [22]. With usual significance level and power (α = 0.05 and P = 0.90), the sample size is strongly dependant on the inter-measurement variance (σ2). For example, with the results of the present study, the sample size of a study taking 3% change of RVEF as an end-point would be extremely modified according to: 1/observer’s experience: n = 40 for Obs1, n = 99 for Obs 2 and n = 258 for Obs3; and 2/perfect inter-measurement agreement for basal short axis slice selection: n = 20 for Obs1, n = 34 for Obs3 and n = 48 for Obs3.
Regarding these findings, we can state that for both clinical and research purposes, 3 conditions seem essential to provide accurate measurement of RV function from cardiac MR: 1/significant previous training, and serial examinations preferably performed by the same observer; 2/consensus about the basal slice selection; 3/delineation limited to the endocardial border of the myocardium.
Limitations
Our study has some limitations. First, this study was monocentric and involved a limited number of patients representative of local recruitment. However, we enrolled 60 patients, which is a common sample size in most studies in this field [8, 9]. Second, all factors influencing variability were not assessed in this study: interstudy variability (related to the repetition of cardiac MRI exam) and inter-instrumentation variability (related to the use of 2 different cardiac MRI equipments) were not evaluated. Interstudy variability of RV/LV function was previously evaluated [8, 22] but only one experienced observer repeated the measurements twice, thus investigating the only intra-observer variability. To date, as far as we know, no study explored inter-instrumentation variability but in most cardiac MRI units, examinations are performed on a dedicated MR scan. Third, we compared measurements of observers with various level of experience. This certainly contributed to increase inter-observer variability. A study including 6 observers spread in 3 pairs with identical level of experience would have been more accurate to evaluate the effect of experience on inter-observer variability.
Conclusions
Assessing the RV function from cardiac MRI in patients with AHD is much more variable and time-consuming than evaluating the left ventricle. Particularly, the measurement of the mass of the RV is highly variable even for a trained observer. Previous experience, basal short axis slice, and delineation are the major determinants of variability. Thus, for an accurate RV functional evaluation, a significant training and a precise definition of the basal short axis slice selection is required. Moreover, delineating the endocardium of the right ventricle seems sufficient in clinical practice and allows time savings.
Acknowledgments
The authors are grateful to Alexandre Klimoff and Agnes Malgouyres (Siemens France) who provided us with the Argus segmentation software used in this study.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
JC carried out data acquisition and interpretation, statistical analysis, manuscript drafting and participated in the study design. JF carried out data acquisition and interpretation, manuscript drafting and participated in the study design. VL carried out data interpretation and manuscript drafting. PHV and CP participated in the study design, statistical analysis and were involved in revising the manuscript critically for important intellectual content. JND was responsible for the initial study concept and the final manuscript. All authors have read and approved the final manuscript.
References
- 1.Ghio S, Gavazzi A, Campana C, Inserra C, Klersy C, Sebastiani R, Arbustini E, Recusani F, Tavazzi L. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol. 2001;37:183–8. doi: 10.1016/s0735-1097(00)01102-5. [DOI] [PubMed] [Google Scholar]
- 2.Mehta SR, Eikelboom JW, Natarajan MK, Diaz R, Yi C, Gibbons RJ, Yusuf S. Impact of right ventricular involvement on mortality and morbidity in patients with inferior myocardial infarction. J Am Coll Cardiol. 2001;37:37–43. doi: 10.1016/s0735-1097(00)01089-5. [DOI] [PubMed] [Google Scholar]
- 3.Mendes LA, Dec GW, Picard MH, Palacios IF, Newell J, Davidoff R. Right ventricular dysfunction: an independent predictor of adverse outcome in patients with myocarditis. Am Heart J. 1994;128:301–7. doi: 10.1016/0002-8703(94)90483-9. [DOI] [PubMed] [Google Scholar]
- 4.Juillière Y, Barbier G, Feldmann L, Grentzinger A, Danchin N, Cherrier F. Additional predictive value of both left and right ventricular ejection fractions on long-term survival in idiopathic dilated cardiomyopathy. Eur Heart J. 1997;18:276–80. doi: 10.1093/oxfordjournals.eurheartj.a015231. [DOI] [PubMed] [Google Scholar]
- 5.Haddad F, Hunt SA, Rosenthal DN, Murphy DJ. Right ventricular function in cardiovascular disease, Part I: Anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation. 2008;117:1436–48. doi: 10.1161/CIRCULATIONAHA.107.653576. [DOI] [PubMed] [Google Scholar]
- 6.Jenkins C, Chan J, Bricknell K, Strudwick M, Marwick TH. Reproducibility of right ventricular volumes and ejection fraction using real-time three-dimensional echocardiography: comparison with cardiac MRI. Chest. 2007;131:1844–51. doi: 10.1378/chest.06-2143. [DOI] [PubMed] [Google Scholar]
- 7.Müller M, Teige F, Schnapauff D, Hamm B, Dewey M. Evaluation of right ventricular function with multidetector computed tomography: comparison with magnetic resonance imaging and analysis of inter- and intraobserver variability. Eur Radiol. 2009;19:278–89. doi: 10.1007/s00330-008-1146-z. [DOI] [PubMed] [Google Scholar]
- 8.Grothues F, Moon JC, Bellenger NG, Smith GS, Klein HU, Pennell DJ. Interstudy reproducibility of right ventricular volumes, function, and mass with cardiovascular magnetic resonance. Am Heart J. 2004;147:218–23. doi: 10.1016/j.ahj.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Mooij CF, de Wit CJ, Graham DA, Powell AJ, Geva T. Reproducibility of MRI measurements of right ventricular size and function in patients with normal and dilated ventricles. J Magn Reson Imaging. 2008;28:67–73. doi: 10.1002/jmri.21407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hudsmith LE, Petersen SE, Francis JM, Robson MD, Neubauer S. Normal human left and right ventricular and left atrial dimensions using steady state free precession magnetic resonance imaging. J Cardiovasc Magn Reson. 2005;7:775–82. doi: 10.1080/10976640500295516. [DOI] [PubMed] [Google Scholar]
- 11.Maceira AM, Prasad SK, Khan M, Pennell DJ. Reference right ventricular systolic and diastolic function normalized to age, gender and body surface area from steady-state free precession cardiovascular magnetic resonance. Eur Heart J. 2006;27:2879–88. doi: 10.1093/eurheartj/ehl336. [DOI] [PubMed] [Google Scholar]
- 12.Karamitsos TD, Hudsmith LE, Selvanayagam JB, Neubauer S, Francis JM. Operator induced variability in left ventricular measurements with cardiovascular magnetic resonance is improved after training. J Cardiovasc Magn Reson. 2007;9:777–83. doi: 10.1080/10976640701545073. [DOI] [PubMed] [Google Scholar]
- 13.Robbers-Visser D, Boersma E, Helbing WA. Normal biventricular function, volumes, and mass in children aged 8 to 17 years. J Magn Reson Imaging. 2009;29:552–9. doi: 10.1002/jmri.21662. [DOI] [PubMed] [Google Scholar]
- 14.Luijnenburg SE, Robbers-Visser D, Moelker A, Vliegen HW, Mulder BJ, Helbing WA. Intra-observer and interobserver variability of biventricular function, volumes and mass in patients with congenital heart disease measured by CMR imaging. Int J Cardiovasc Imaging. 2010;26:57–64. doi: 10.1007/s10554-009-9501-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Winter MM, Bernink FJ, Groenink M, Bouma BJ, van Dijk AP, Helbing WA, Tijssen JG, Mulder BJ. Evaluating the systemic right ventricle by CMR: the importance of consistent and reproducible delineation of the cavity. J Cardiovasc Magn Reson. 2008;10:40. doi: 10.1186/1532-429X-10-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sardanelli F, Quarenghi M, Di Leo G, Boccaccini L, Schiavi A. Segmentation of cardiac cine MR image of left and right ventricles: interactive semiautomated methods and manual contouring by two readers with different education and experience. J Magn Reson Imaging. 2008;27:785–92. doi: 10.1002/jmri.21292. [DOI] [PubMed] [Google Scholar]
- 17.Moon JC, Lorenz CH, Francis JM, Smith GC, Pennell DJ. Breath-hold FLASH and FISP cardiovascular MR imaging: left ventricular volume differences and reproducibility. Radiology. 2002;223:789–97. doi: 10.1148/radiol.2233011181. [DOI] [PubMed] [Google Scholar]
- 18.Plein S, Bloomer TN, Ridgway JP, Jones TR, Brainbridge GJ, Sivananthan MU. Steady-state free precession magnetic resonance imaging of the heart: comparison with segmented k-space gradient echo imaging. J Magn Reson Imaging. 2001;14:230–6. doi: 10.1002/jmri.1178. [DOI] [PubMed] [Google Scholar]
- 19.Clay S, Alfakih K, Messroghli DR, Jones T, Ridgway JP, Sivananthan MU. The reproducibility of left ventricular volume and mass measurements: a comparison between dual inversion-recovery black-blood sequence and SSFP. Eur Radiol. 2006;16:32–7. doi: 10.1007/s00330-005-2853-3. [DOI] [PubMed] [Google Scholar]
- 20.Alfakih K, Plein S, Thiele H, Jones T, Ridgway JP, Sivananthan MU. Normal human left and right ventricular dimensions for MRI as assessed by turbo gradient echo and steady-state free precession imaging sequences. J Magn Reson Imaging. 2003;17:323–9. doi: 10.1002/jmri.10262. [DOI] [PubMed] [Google Scholar]
- 21.Caudron J, Fares J, Vivier PH, Lefebvre V, Petitjean C, Dacher JN. Diagnostic Accuracy and Variability of Three Semi-Quantitative Methods for Assessing Right Ventricular Systolic Function From Cardiac MRI in Patients with Acquired Heart Disease. Eur Radiol. 2011 doi: 10.1007/s00330-011-2152-0. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grothues F, Smith GC, Moon JC, Bellenger NG, Collins P, Klein HU, Pennell DJ. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol. 2002;90:29–34. doi: 10.1016/s0002-9149(02)02381-0. [DOI] [PubMed] [Google Scholar]
- 23.Grosgeorge D, Petitjean C, Caudron J, Fares J, Dacher JN. Automatic cardiac ventricle segmentation in MR images: a validation study. Int J Comput Assist Radiol Surg. 2010 doi: 10.1007/s11548-010-0532-6. in press. [DOI] [PubMed] [Google Scholar]
- 24.Petitjean C, Dacher JN. A review of segmentation methods in cardiac MRI. Med Image Anal. 2010;15:169–84. doi: 10.1016/j.media.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 25.Nijveldt R, Germans T, McCann GP, Beek AM, van Rossum AC. Semi-quantitative assessment of right ventricular function in comparison to a 3D volumetric approach: a cardiovascular magnetic resonance study. Eur Radiol. 2008;18:2399–405. doi: 10.1007/s00330-008-1017-7. [DOI] [PubMed] [Google Scholar]
- 26.Kramer CM, Barkhausen J, Flamm SD, Kim RJ, Nagel E. Standardized cardiovascular magnetic resonance imaging (CMR) protocols, society for cardiovascular magnetic resonance: board of trustees task force on standardized protocols. J Cardiovasc Magn Reson. 2008;10:35. doi: 10.1186/1532-429X-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strugnell WE, Slaughter RE, Riley RA, Trotter AJ, Bartlett H. Modified RV short axis series – a new method for cardiac MRI measurement of right ventricular volumes. J Cardiovasc Magn Reson. 2005;7:769–74. doi: 10.1080/10976640500295433. [DOI] [PubMed] [Google Scholar]
- 28.Alfakih K, Plein S, Bloomer T, Jones T, Ridgway J, Sivananthan M. Comparison of right ventricular volume measurements between axial and short axis orientation using steady-state free precession magnetic resonance imaging. J Magn Reson Imaging. 2003;18:25–32. doi: 10.1002/jmri.10329. [DOI] [PubMed] [Google Scholar]
- 29.Kirschbaum SW, Baks T, Gronenschild EH, Aben JP, Weustink AC, Wielopolski PA, Krestin GP, de Feyter PJ, van Geuns RJ. Addition of the long-axis information to short-axis contours reduces interstudy variability of left-ventricular analysis in cardiac magnetic resonance studies. Invest Radiol. 2008;43:1–6. doi: 10.1097/RLI.0b013e318154b1dc. [DOI] [PubMed] [Google Scholar]
- 30.Berkovic P, Hemmink M, Parizel PM, Vrints CJ, Paelinck BP. MR image analysis: Longitudinal cardiac motion influences left ventricular measurements. Eur J Radiol. 2010;73:260–5. doi: 10.1016/j.ejrad.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 31.Catalano O, Corsi C, Antonaci S, Moro G, Mussida M, Frascaroli M, Baldi M, Caiani E, Lamberti C, Cobelli F. Improved reproducibility of right ventricular volumes and function estimation from cardiac magnetic resonance images using level-set models. Magn Reson Med. 2007;57:600–5. doi: 10.1002/mrm.21157. [DOI] [PubMed] [Google Scholar]
- 32.Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA, Calkins H, Corrado D, Cox MG, Daubert JP, Fontaine G, Gear K, Hauer R, Nava A, Picard MH, Protonotarios N, Saffitz JE, Sanborn DM, Steinberg JS, Tandri H, Thiene G, Towbin JA, Tsatsopoulou A, Wichter T, Zareba W. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the Task Force Criteria. Eur Heart J. 2010;31:806–14. doi: 10.1093/eurheartj/ehq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chin KM, Kingman M, de Lemos JA, Warner JJ, Reimold S, Peshock R, Torres F. Changes in right ventricular structure and function assessed using cardiac magnetic resonance imaging in bosentan-treated patients with pulmonary arterial hypertension. Am J Cardiol. 2008;101:1669–72. doi: 10.1016/j.amjcard.2008.01.055. [DOI] [PubMed] [Google Scholar]
- 34.van Wolferen SA, Marcus JT, Boonstra A, Marques KM, Bronzwaer JG, Spreeuwenberg MD, Postmus PE, Vonk-Noordegraaf A. Prognostic value of right ventricular mass, volume, and function in idiopathic pulmonary hypertension. Eur Heart J. 2007;28:1250–7. doi: 10.1093/eurheartj/ehl477. [DOI] [PubMed] [Google Scholar]
- 35.van Straten A, Vliegen HW, Hazekamp MG, Bax JJ, Schoof PH, Ottenkamp J, van der Wall EE, de Roos A. Right ventricular function after pulmonary valve replacement in patients with tetralogy of Fallot. Radiology. 2004;233:824–9. doi: 10.1148/radiol.2333030804. [DOI] [PubMed] [Google Scholar]


