Abstract
Background
Insecticide resistance is one of the best examples of rapid micro-evolution found in nature. Since the development of the first synthetic insecticide in 1939, humans have invested considerable effort to stay ahead of resistance phenotypes that repeatedly develop in insects. Aphids are a group of insects that have become global pests in agriculture and frequently exhibit insecticide resistance. The green peach aphid, Myzus persicae, has developed resistance to at least seventy different synthetic compounds, and different insecticide resistance mechanisms have been reported worldwide.
Methodology/Principal Findings
To further characterize this resistance, we analyzed genome-wide transcriptional responses in three genotypes of M. persicae, each exhibiting different resistance mechanisms, in response to an anti-cholinesterase insecticide. The sensitive genotype (exhibiting no resistance mechanism) responded to the insecticide by up-regulating 183 genes primarily ones related to energy metabolism, detoxifying enzymes, proteins of extracellular transport, peptidases and cuticular proteins. The second genotype (resistant through a kdr sodium channel mutation), up-regulated 17 genes coding for detoxifying enzymes, peptidase and cuticular proteins. Finally, a multiply resistant genotype (carrying kdr and a modified acetylcholinesterase), up-regulated only 7 genes, appears not to require induced insecticide detoxification, and instead down-regulated many genes.
Conclusions/Significance
This study suggests strongly that insecticide resistance in M. persicae is more complex that has been described, with the participation of a broad array of resistance mechanisms. The sensitive genotype exhibited the highest transcriptional plasticity, accounting for the wide range of potential adaptations to insecticides that this species can evolve. In contrast, the multiply resistant genotype exhibited a low transcriptional plasticity, even for the expression of genes encoding enzymes involved in insecticide detoxification. Our results emphasize the value of microarray studies to search for regulated genes in insects, but also highlights the many ways those different genotypes can assemble resistant phenotypes depending on the environmental pressure.
Introduction
Insecticide resistance is one of the best examples of micro-evolution, or evolution occurring on an ecological time scale [1]–[3]. The study of insecticide resistance is important, both because it leads to a better understanding of evolutionary mechanisms operating in real time, and because of its economic relevance. The development of insecticide resistance in pest insects has been an increasing problem for agriculture, forestry and public health [4], [5]. Agricultural practices usually include the systematic application of a wide array of active compounds at variable dosages and frequencies, which represent a wide range of selective regimes. Therefore, identifying the molecular and genetic adaptations responsible for insecticide resistance will offer new opportunities for developing pest management strategies.
The study of insecticide resistance makes it possible to classify adaptations into three main mechanisms: (i) reduction of insecticide uptake, by reducing the permeability of insect cuticle [6]–[8], (ii) detoxification, through alteration in the levels or enzyme activities that degrade or sequester insecticides [1], [7], [9]–[11] and, (iii) insensitivity due to point mutations in genes encoding for proteins that are the target site of insecticides [12]–[14]. Functional genomics tools have recently been used to disentangle the genetic basis of pesticide resistance in arthropods [15]–[23]. Such studies have shown that insecticide resistance is more complex than previously thought, being mediated by multigenic systems that involve large parts of the insect genomes [10], [18], [24].
Aphids (Hemiptera: Aphididae) are widely distributed herbivorous insects accounting for more than 4,300 described species [25]–[27]. Approximately 100 aphid species have successfully exploited agro-ecosystems to become economically important pests, of whom ∼20 have developed at least one known insecticide resistance mechanism [28], [29]. The peach green aphid, Myzus persicae, of Palearctic origin, is a cosmopolitan aphid species responsible of important economic losses [26], [30], [31]. Is a highly polyphagous, feeding on more than 50 plant families [30], [32], causing losses to agroindustrial crops (including potato, sugar beet and tobacco), horticultural crops (including plants of Brassicaceae, Solanaceae and Cucurbitaceae families) and stone fruits (peach, apricot, and cherry, among others). M. persicae was introduced into Chile with crop plant species [33], and is presently categorized as one of the three most important agricultural pests in this country [34].
M. persicae exhibits a striking capacity for rapid adaptation to insecticides, developing resistance to more active compounds than any other known insect [26], [35]. Six distinct insecticide resistance mechanisms mediating different levels of insensitivity, have been described for the species: (i) Modified acetylcholinesterase (MACE), which confers resistance to organophosphates and carbamate insecticides [36]–[39], (ii) kdr and super kdr mutations in a voltage-gated sodium channel, which is the target of pyrethroids and organochlorines [40]–[42], (iii) the mutation of the GABA receptor, rdl, which is target of organochlorines of the cyclodiene type [43], [44], (iv) the recently described mutation of a key residue in the loop D region of a nAChR b1 subunit [45], (v) the over-production of esterases E4 or FE4 confers resistance to organophosphates, pyrethroids and to a lesser extent carbamates [46]–[51], and (vi) the recently described over-production of a cytochrome P450 confers resistance to neonicotinoids [16], [45], [52].
In Chile, M. persicae has been chemically controlled by the application of almost all classes of insecticides, including neonicotinoids, pyrethroids, organophosphates and carbamates. Pirimicarb, an anti-cholinesterase insecticide, is the most frequently used since the last five years. However, little is known about the insecticide resistance mechanisms of M. persicae in Chile. For instance, esterase-mediated resistance (E4/FE4) has been found in M. persicae on sugar beet crops (Beta vulgaris), with phenotypes ranging from R1 (moderately resistant) to R3 (highly resistant) [53]–[55] In contrast, on tobacco (Nicotiana tabacum) only a single and widely distributed clone has been reported, which exhibits a R1 phenotype for esterases and susceptibility at the site of the kdr mutation [56].
Transcriptomics is an extremely useful approach for the identification of new genes and gene functions related to insecticide resistance [57]. DNA microarrays, one of the most powerful and versatile transcriptomic techniques, make it possible to compare expression profiles for hundreds or thousands of genes simultaneously, thereby linking the study of static genomes to dynamic proteomes [58]. Although the genome of M. persicae has not been sequenced yet, genomic resources are available for this species [24], [59]. Recently, two studies have targeted the identification of insecticide resistance mechanisms in M. persicae using genomic resources in an integrated fashion [16], [45]. In both cases the focus was on discovering the mechanisms responsible for the neonicotinoid resistance, comparing patterns of gene expression between susceptible and resistant aphid clones [16], [45]. Following this methodology, one can identify new genes involved in insecticide resistance in populations, but it is not possible to detect the full potential of a species to evolve in response to insecticides.
In the current study, we took advantage of the recent advances in aphid genomics to examine the transcriptional responses in three genotypes of M. persicae exposed to pirimicarb at the whole-genome level. This approach allowed the comparison of the expression profiles in genotypes carrying different resistance mutations, thereby identifying new genes and mechanisms that are the target of selection.
Results
Insecticide Resistance Characterization
Thirty-two M. persicae genotypes were evaluated constitutive carboxylesterase activity (EST activity), which is indicative of the number of copies for E4/FE4 carboxylesterase genes [60]. EST activity was low for the 32 genotypes evaluated. Indeed, all genotype assayed were “susceptible” according to the classification of Devonshire et al. (1992) [61]. However, broad-sense heritability of EST activity was significant (H2 = 0.61; F31,274 = 15.8, P<0.0001), indicating a larger variation among than within genotypes, which validates the use of this variable in the selection of experimental lineages.
By characterizing the genetic makeup of insecticide resistance mutations (IRM), the 32 genotypes were grouped into three categories. Twenty-one genotypes did not carry any IRM and were labeled as sensitive (i.e. S genotypes). From this group, genotype 13A (hereafter S) exhibited the lowest level of EST activity and was selected for microarray experiments. Nine genotypes were heterozygous for kdr, carrying no MACE or super-kdr mutations, and were labeled as simple resistant (i.e. SR genotypes). From this group, genotype 26A (hereafter SR) was selected due to its intermediate EST activity. Finally, two genotypes were heterozygous for both kdr and MACE mutations and were labeled as multiple resistant (i.e. MR genotypes). From this group, genotype 16A (hereafter MR) was chosen due to its higher levels of EST activity. No other IRM combinations were found.
We found a significant link between the genetic constitution for IRM and the susceptibility of genotypes to insecticide, estimated from insecticide tolerance bioassays. The genotype S showed the lowest lethal dose values for pirimicarb, which results in increased susceptibility (LC50 = 9.27 ppm±0.13 EE), followed by genotype SR (LC50 = 11.44 ppm±0.22 EE); and genotype MR (LC50 = 407.45 ppm±0.13 EE). Descriptive characterizations of the three genotypes selected are presented in Table 1.
Table 1. Characterization of the Myzus persicae genotypes selected for microarray experiments.
| Genotype | MACE | kdr | s.kdr | EST activity* | LC50 pirimicarb (CI 95%) |
| S | SS | SS | SS | 0.150±0.03 | 9.27 (7.2–11.8) |
| SR | SS | SR | SS | 0.207±0.01 | 11.44 (9.4–14.1) |
| MR | SR | SR | SS | 0.291±0.02 | 407 (153–3965) |
(U aphid-equiv. −1) ± SE.
Microarray Experiments
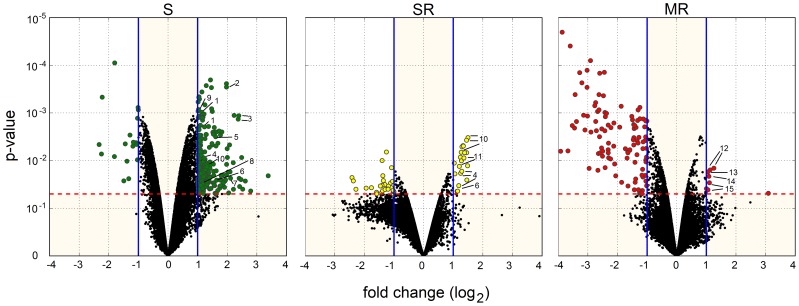
Microarray experiments were performed in order to study the transcriptome responses in three M. persicae genotypes (S, SR and MR) subjected to a dose of pirimicarb. Microarray analysis detected a high variation in transcriptional responses among genotypes. Global gene expression changes are shown in Figure 1 in the form of volcano plots, with threshold of 2-fold-change and a significance threshold of p<0.05. Thus, 183, 17 and 7 genes were significantly up-regulated in S, SR and RM genotypes, respectively. (see Table S1 for the full list of up-regulated genes). Interestingly, the number of down-regulated genes was inverse to the number of up-regulated genes in each genotype. Thus, 17, 28 and 78 genes were significantly down-regulated in S, SR and RM genotypes, respectively. Of the 183 up-regulated genes found in S genotype, 151 had known functions and 51 are potential candidates for being involved in insecticide resistance, including genes encoding for abc transporters, heat shock proteins, cathepsins, cuticle proteins, cytochrome P450s, a carboxylesterase E4/FE4 and glutathione-S-transferases, among others (see some of these genes in Table 2). Of the 17 up-regulated genes in the SR genotype, 12 have known functions and are potentially involved in insecticide resistance, including heat shock proteins, cathepsins, cuticle proteins and cytochrome P450s (Table 3). Finally, of the 7 up-regulated genes in the MR genotype, 3 have unknown functions while the other 4 genes included a histone h3 methyltransferase and a guanine nucleotide-binding protein (Table 4).
Figure 1. Transcriptional responses in three Myzus persicae genotypes (S, SR and MR) subjected to a pirimicarb.
Volcano plots for each genotype show the log2 fold change (x axis) and the statistical significance (y axis) between the controls and treatments. Vertical lines indicate 2-fold expression difference in either direction (−1>log2FC>1). Horizontal line indicates significance threshold (P<0.05). Statistical analysis is based on a Bayesian inference using a lineal model, and reflects both biological and technical replications. Genes showing both 2-fold differential expression and a significant P value are colored. Not all labels appear in the S, SR and MR volcano plot in order to preserve readability (see Table 2 and supporting material for a full listing of significantly over-expressed genes). Gene abbreviations: 1, glutathione s-transferase; 2, cytochrome p450 family CYP6CYP3; 3, carboxylesterase type FE4; 4, cathepsin b; 5, cytochrome p450 family CYP6; 6, cuticle protein; 7, salivary peptide; 8, ABC transporter; 9, glucose transporter; 10, cytochrome p450; 11, heat shock protein 70; 12, heterotrimeric guanine nucleotide-binding protein; 13, histone h3 methyltransferase, 14, eukaryotic initiation factor; 15, unknown protein.
Table 2. Selected genes identified by microarray as significantly up-regulated in S (sensitive) genotype in response to pirimicarb.
| Description | Hit Description | Log2 FC | Contig ID | Probe name |
| heat shock protein 70 | gi|193647903|ref|XP_001945786.1 | 2,.9 | 6029 | M_persicae6029a |
| heat shock protein 70 | gi|193647903|ref|XP_001945786.1| | 2.20 | 8669 | M_persicae8669a |
| heat shock protein 70 | gi|193688192|ref|XP_001951386.1| | 1.03 | 15349 | M_persicae15349a |
| carboxylesterase esterase fe4 | gi|544256|sp|P35502.1| | 2.39 | 9215 | M_persicae9215a |
| carboxylesterase esterase E4 | gi|544255|sp|P35501.1| | 2.30 | 720 | M_persicae720a/b |
| carboxylesterase esterase E4 | gi|544255|sp|P35501.1| | 1.42 | 4586 | M_persicae4586a |
| Esterase | gi|544255|sp|P35501.1| | 2.04 | 3118 | M_persicae3118a/b |
| glutathione s-transferase | gi|193636685|ref|XP_001946604.1| | 1.13 | 1196 | M_persicae1196a |
| glutathione s-transferase | gi|193636685|ref|XP_001946604.1| | 1.09 | 4744 | M_persicae4744a |
| cytochrome p450 cyp6ax1 | gi|193598913|ref|XP_001943150.1| | 1.05 | 3931 | M_persicae3931b |
| cytochrome p450 cyp6ax1 | gi|193671582|ref|XP_001952450.1| | 1.05 | 6957 | M_persicae6957a |
| cytochrome p450 cyp6ax1 | gi|193657143|ref|XP_001948488.1| | 1.61 | 5173 | M_persicae5173a |
| cytochrome p450 cyp6ax1 | gi|193657143|ref|XP_001948488.1| | 1.97 | 497 | M_persicae497a/b |
| cytochrome p450 | gi|193599086|ref|XP_001945361.1| | 1.04 | 1528 | M_persicae1528b |
| cytochrome p450 cyp6ax1 | gi|193657145|ref|XP_001948581.1| | 1.40 | 3798 | M_persicae3798a/b |
| cytochrome p450 cyp6ax1 | gi|193587097|ref|XP_001948421.1| | 1.29 | 9095 | M_persicae9095a |
| cytochrome p450 cyp6ax1 | gi|193657145|ref|XP_001948581.1| | 1.23 | 9584 | M_persicae9584a |
| cytochrome p450 cyp6ax1 | gi|193657145|ref|XP_001948581.1| | 1.73 | 3799 | M_persicae3799a |
| cytochrome p450 cyp6ax1 | gi|193657143|ref|XP_001948488.1| | 1.14 | 749 | M_persicae749a/b |
| aldehyde dehydrogenase | gi|193617714|ref|XP_001949972.1| | 1.37 | 2450 | M_persicae2450b |
| cathepsin b–n | gi|51947600|gb|AAU14266.1| | 1.49 | 256 | M_persicae256a/b |
| cathepsin b–n | gi|51947600|gb|AAU14266.1| | 1.26 | 254 | M_persicae254a/b |
| cathepsin b | gi|161343867|tpg|DAA06114.1| | 1.10 | 3004 | M_persicae3004a |
| cathepsin b | gi|161343867|tpg|DAA06114.1| | 1.09 | 3002 | M_persicae3002b |
| cathepsin b–n | gi|193654855|ref|XP_001943173.1| | 1.04 | 6594 | M_persicae6594a |
| abc transporter | gi|193664711|ref|XP_001950287.1| | 1.15 | 1560 | M_persicae1560a/b |
| abc transporter | gi|193636433|ref|XP_001950956.1| | 1.14 | 7913 | M_persicae7913a |
| abc transporter | gi|193664711|ref|XP_001950287.1| | 1.19 | 2478 | M_persicae2478b |
Table 3. Selected genes identified by microarray as significantly up-regulated in SR (simple resistant) genotype in response to pirimicarb.
| Description | Hit Description | Log2 FC | Contig ID | Probe name |
| heat shock protein 70 | gi|193647903|ref|XP_001945786.1| | 1.36 | 6029 | M_persicae6029a |
| heat shock protein 70 | gi|193647903|ref|XP_001945786.1| | 1.21 | 8669 | M_persicae8669a |
| cytochrome p450 | gi|193713785|ref|XP_001947768.1| | 1.47 | 2519 | M_persicae2519a/b |
| cytochrome p450 | gi|193657315|ref|XP_001944487.1| | 1.20 | 1504 | M_persicae1504a/b |
| cathepsin b | gi|161343867|tpg|DAA06114.1| | 1.06 | 3002 | M_persicae3002b |
| cathepsin b | gi|51947600|gb|AAU14266.1| | 1.20 | 256 | M_persicae256a/b |
| cathepsin b | gi|161343867|tpg|DAA06114.1| | 0.99 | 6891 | M_persicae6891a |
| cuticular protein | gi|240848841|ref|NP_001155592.1| | 1.13 | 10027 | M_persicae10027a |
| cuticular protein | gi|193647875|ref|XP_001945170.1| | 1.00 | 4497 | M_persicae4497a |
| nonstructural protein ns-1 | gi|33235700|ref|NP_874376.1| | 1.26 | 3321 | M_persicae3321a/b |
| protoheme ixfarnesyltransferase | gi|15617066|ref|NP_240279.1| | 1.46 | 9124 | M_persicae9124a |
| zinc mym domain | gi|193704454|ref|XP_001951785.1| | 1.32 | 2558 | M_persicae2558a/b |
Table 4. Selected genes identified by microarray as significantly up-regulated in MR (multiple resistant) genotype in response to pirimicarb.
| Description | Hit Description | Log2 FC | Contig ID | Probe name |
| yellow protein | gi|193683309|ref|XP_001945133.1| | 3.09 | 6351 | M_persicae6351a |
| guanine nucleotide-binding proteinsubunit beta 1 | gi|193596402|ref|XP_001947878.1| | 1.19 | 1180 | M_persicae1180a/b |
| histone h3 methyltransferase | gi|193683706|ref|XP_001947040.1| | 1.06 | 6961 | M_persicae6961a |
| eukaryotic translation initiation factor 4 3 | gi|193657071|ref|XP_001945066.1| | 1.09 | 2227 | M_persicae2227b |
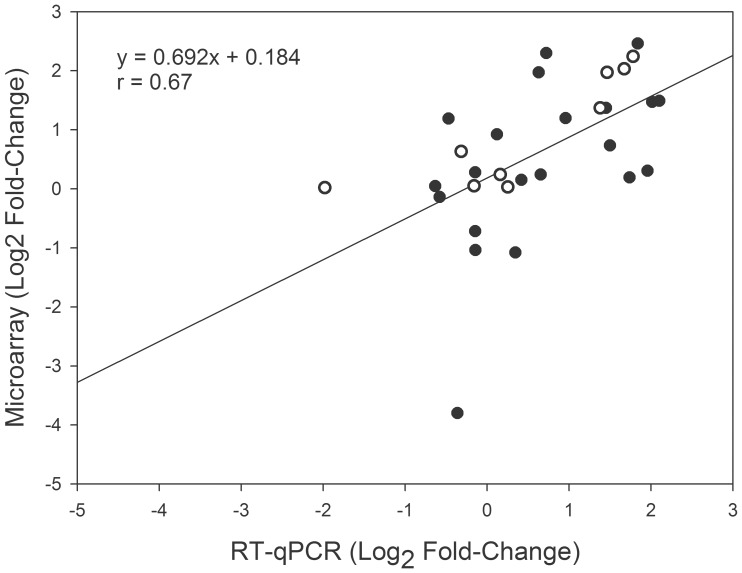
In order to validate the microarray profiles, the transcriptional changes for seven up-regulated genes were studied by RT-qPCR in all the three genotypes, using RNA obtained from new biological replicates. Additionally, transcriptional profiles of three differentially expressed genes were validated using the same RNA samples used for microarray experiments. Comparisons of gene expression between the two techniques are shown in Figure 2 (r = 0.67; P<0.01; Spearman correlation coefficient) and gene expression results for both methodologies are listed in Table S2.
Figure 2. Correlation of gene expression changes measured using DNA microarray analysis and quantitative reverse transcription PCR (RT-qPCR).
The average log2 fold-change values were used, and each point represents the gene expression in a genotype. Open circles correspond to expression using in RT-qPCR the same RNA samples as were used for microarray experiments. Black circles correspond to expression in RT-qPCR experiments using RNA that was obtained from new biological replicates. Spearman correlation coefficient (r) is shown in the graph.
Annotation and Gene Ontology Analysis
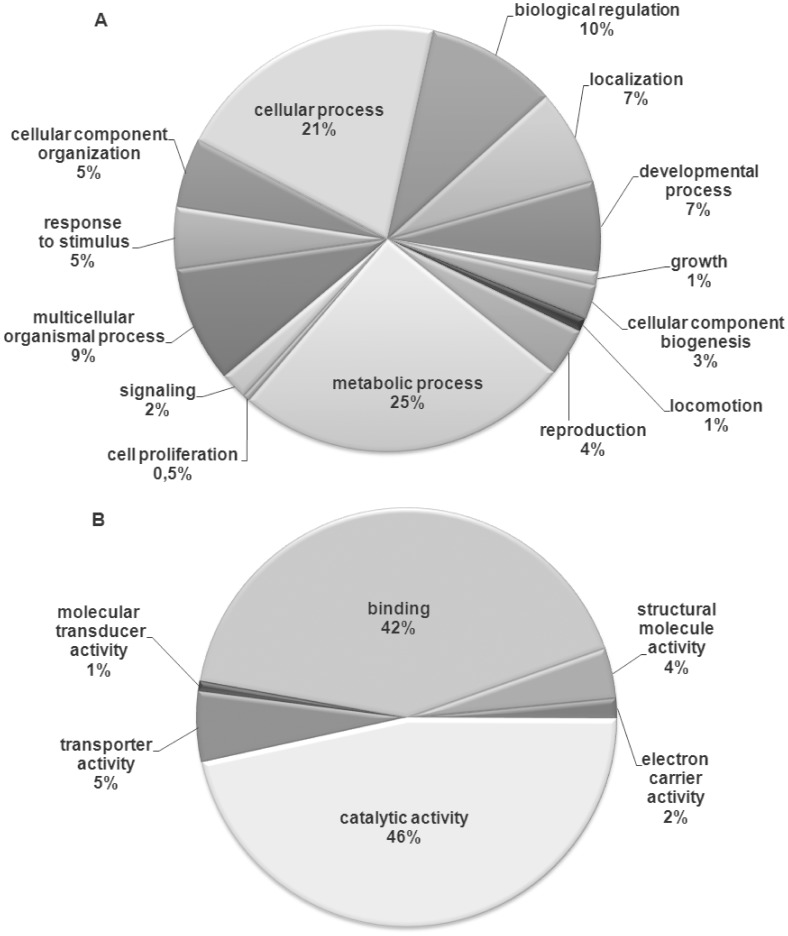
A total of 97 sequences of 183 up-regulated genes in the S genotype were annotated. Gene Ontology (GO) graphs were constructed using percentages of 2nd level GO terms and presented in Figure 3 under biological processes (BP) and molecular functions (MF). GO analysis revealed the participation of 69 putative proteins in 14 BP (Figure 3A). Among them, metabolic processes were the most represented with 49 gene products (25%) involved in primary metabolic processes (protein localization, carbohydrate and lipid biosynthetic and catabolic process, ATP and nucleotide biosynthetic process), cellular metabolic process (including the generation of precursors metabolites and energy) and oxidation reduction processes among others. The second largest represented group corresponded to putative proteins encoded by 40 genes (21%) and involved in cellular processes such as organelle organization, actin filament-based processes, microtubule-based processes, cell division, cytoplasm organization and cell communication. Under the category of molecular functions (MF), 88 gene products were involved in 6 different activities (some in more than one category) (Figure 3B). Most sequences (60 gene products) were related to catalytic activity; among them, the 44% corresponded to hydrolase activity (GO terms associated with esterase and cathepsins), 26% to transferase activity (GO terms associated with glutathione-S-transferase) and 16% to oxidoreductase activity (GO terms associated with cytochrome P450s).
Figure 3. Distribution of GO IDs at the 2nd level.
Based on their participation in biological processes (A) and molecular functions (B) of up-regulated ESTs (putative proteins) in a sensitive genotype (S) of Myzus persicae treated with pirimicarb. Out of 97 annotated EST sequences, 69 presented GO IDs for biological processes and 88 for molecular functions.
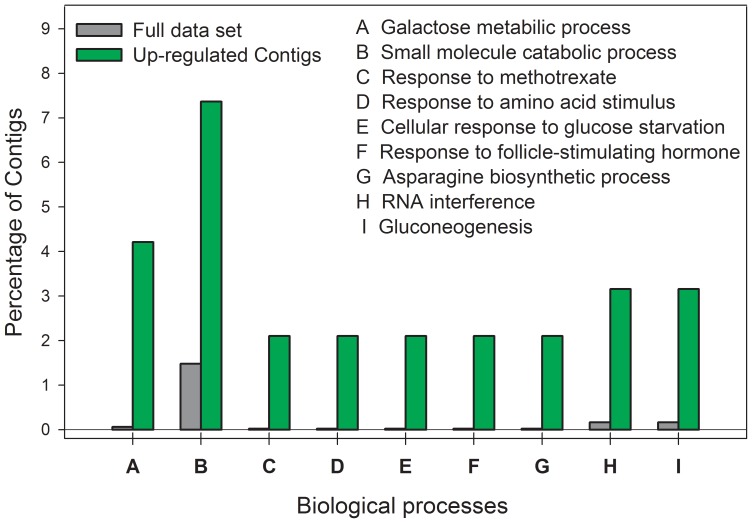
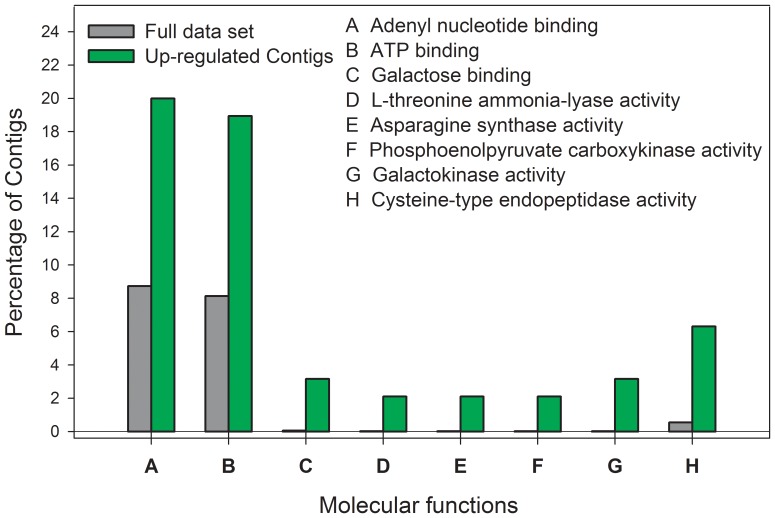
An enrichment analysis (EA) revealed that BP and MF were significantly over-represented among the up-regulated sequences in the genotype S with respect to all sequences in the microarray. The analysis within the BP category revealed that gluconeogenesis, small molecule catabolism, cellular response to glucose starvation, response to amino acid stimulus, among others, were significantly over-represented (Figure 4). The analysis within the MF category showed an over-representation of catalytic activity including peptidase, hydrolase, kinase, and lyase activities (Figure 5).
Figure 4. Biological processes over-represented in the sensitive genotype (S) after an Enrichment Analysis.
The bars show the percentage of contigs associated with each GO term. The dark gray bars show the percentage of contigs associated with each GO term considering the full microarray data set. Green bars show the percentage of contigs associated with each GO terms, but only in the up-regulated date set
Figure 5. Molecular functions over-represented in the sensitive genotype (S) after an Enrichment Analysis.
The bars show the percentage of contigs associated with each GO term. The dark gray show the percentage of contigs associated with each GO term considering the full microarray data set. Green bars show the percentage of contigs associated with each GO term but only in the up-regulated date set.
Transcriptional Levels for Specific Genes
We evaluated the transcriptional expression for seven genes that were found up-regulated in the three studied genotypes, 20 and 30 hours after the application of pirimicarb.
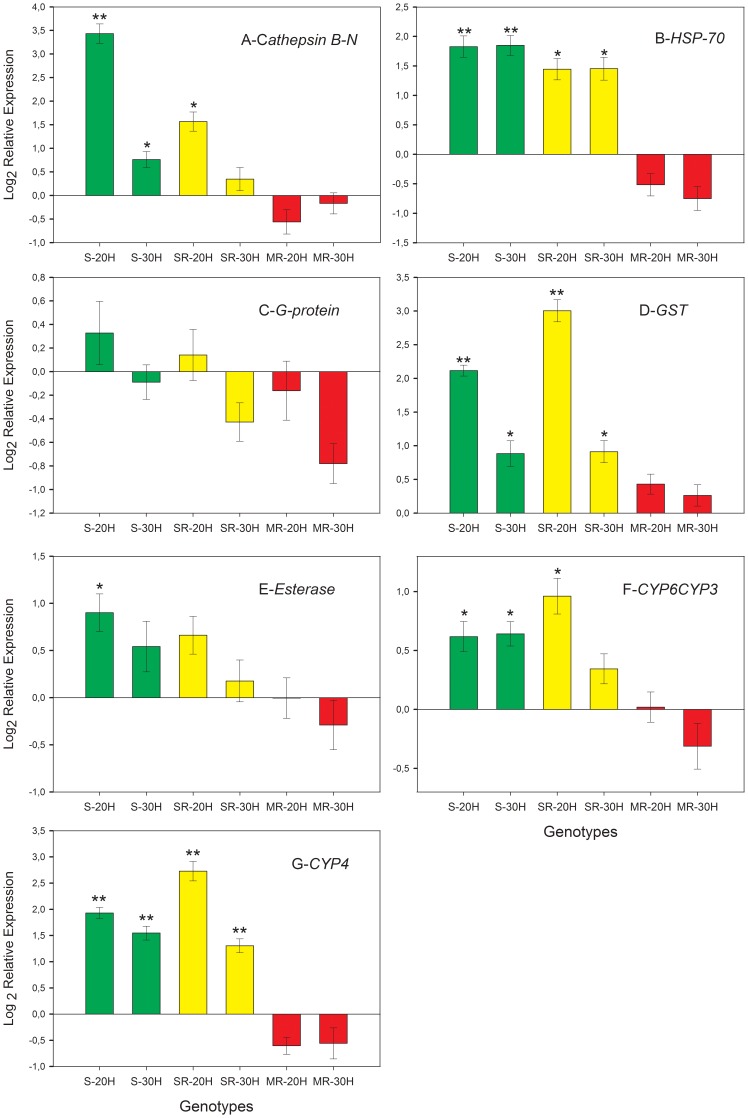
The Cathepsin B gene showed a significant up-regulation in the S and SR genotypes at 20 hours after insecticide application, while at 30 hours up-regulation remained significant only in the S genotype. Genotype MR showed no evidence of regulation for this gene (Figure 6A). The Heat Shock Protein 70 gene showed a significant up-regulation in S and SR genotypes at 20 and 30 hours after the application of the insecticide. In contrast, the MR genotype showed a down-regulation for this gene (Figure 6B). The Heterotrimeric G protein gene did not show a significantly different transcription between treatments in any of the studied genotypes (Figure 6C). In this case, we found transcriptional differences between the results obtained by the microarray analysis compared to the RT-qPCR, which can be explained by intra-clonal variation (see Discussion). The Glutathione-S-transferase gene showed a significantly higher transcription in S and SR genotypes at 20 and 30 hours after the application of insecticide (Figure 6D), while no changes were detected in the MR genotype. The Esterase gene only showed a significant up-regulation at 20 hours after application of insecticide in the S genotype (Figure 6E), while all other genotypes were unaffected. Two genes of the Cytochrome P450 gene family were assessed (CYP6CY3 and CYP4). The genotypes S and SR showed an up-regulation for both genes at 20 and 30 hours after application of insecticide (Figure 6E and 6G), while the genotype MR showed no changes.
Figure 6. Quantification of relative expression in different genotypes of Myzus persicae exposed to pirimicarb.
Graphs represent the relative mRNA expression in aphids sprayed with pirimicarb in comparison to control (water). Data were normalized for interclonal variation using GADPH expression levels. Green bars correspond to the genotype S (sensitive), Yellow corresponds to the genotype SR (simple resistant) and red bars correspond to the genotype MR (multiple resistant). Same color bars represent the time after insecticide spraying, with left bar = 20 hours and right bar = 30 hours. Data are shown as mean ± SE of two independent experiments, with three technical replicates in each case. *p<0.05 and **p<0.01 indicate a significant difference compared to 1, which was used as a reference value for no change in gene expression, using a t-test. Gene abbreviations: (A) cathepsin B–N, cathepsin B clade N; (B) HSP-70, heat shock protein 70; (C) G protein, Heterotrimeric guanine nucleotide-binding protein; (D) GST, glutathione S-transferase; (E) Esterase, carboxylesterase type E4/FE4; (F) CYP6CYP3, cytochrome p450 family CYP6CYP3; (G) CYP4, cytochrome p450 family CYP4.
Discussion
Insecticide resistance is a textbook example of rapid evolution occurring in front of our eyes. The aphid M. persicae holds the world record of insecticide resistance mechanisms, showing resistance to at least seventy different synthetic compounds [62]. This fact alone makes this species an exceptional model for studying the genes and mechanisms that are the target of insecticide selection. In this study, different field-derived genotypes were characterized for three well-described insecticide resistance mechanisms (EST activity, and kdr and MACE mutations). This characterization, together with pirimicarb tolerance bioassays, allowed us to select three genotypes: sensitive (S), resistant only by a kdr mutation (SR) and resistant by kdr and MACE mutations (MR).
The transcriptomic responses of these three genotypes exposed to pirimicarb, provided evidence of a high variation in transcriptional plasticity among genotypes. Although slight discrepancies were observed for the transcriptional profiles given by the microarray and RT-qPCR approaches, these differences can be explained by the use of different biological replicates for each technique, and because aphids are especially known to show intra-clonal variation [63], [64]. In the microarray experiments, the number of up-regulated genes was inversely correlated to insecticide resistance mechanisms. To better understand the observed responses, it is necessary to emphasize that pirimicarb is an anti-cholinesterase insecticide, acting by inhibiting the enzyme acetylcholinesterase [65]. Hence, the MACE mutation (carried only by the MR genotype) confers specific resistance to this class of insecticides.
The different transcriptomic responses found between S and SR genotypes may be associated with kdr mutation, which causes insecticide insensibility in the sodium channel. The kdr mutation should have no effect on resistance to an anti-cholinersterase insecticide. However, epistasis with another insecticide resistance mechanism could be invoked here, as it has been reported in Culex and Aedes mosquitoes [66], [67]. In these cases, epistasis occurs between the kdr mutation and an enhanced detoxification by cytochrome P450 monooxygenases, while in M. persicae a strong linkage disequilibrium between this mutation and insecticide resistance mediated by esterases has been found [68]–[70]. However, we cannot exclude the possibility that the up-regulation shown by the S genotype relative to SR and MR is actually a general stress response due to the inhibition of cholinesterase by the pirimicarb insecticide, rather than a specific resistance response.
General Metabolic Responses to Insecticides
Among the 183 up-regulated genes found in the S genotype after insecticide application, the most interesting observation was the unusual activation of energy metabolism. It was evident the up-regulation of several key enzymes in metabolic pathways affecting glycolysis, gluconeogenesis, the Krebs cycle, galactose, lipids, and amino acid metabolism. This was consistent with the fact that insecticide resistance is usually associated with higher demands of energy in other insect species [7], [71], [72]. In other words, when facing insecticides, aphids of the S genotype experience an increase of general metabolism (both aerobic and anaerobic) that is accompanied by the mobilization of energy stores (glycogen and fats). Our findings suggest that gene expression that promotes mobilization of energy may somehow mitigate the costs of insecticide action (e.g. muscle contractions and insecticide detoxification), even 24 hours after the application of pirimicarb. In contrast, in the SR genotype (17 up-regulated genes, carrying kdr mutation), only detoxifying enzymes were found to be up-regulated, with no evidence for the activation of energetic metabolism and muscle contraction. Interestingly, in the MR genotype (7 genes up-regulated; carrying MACE and kdr mutations), neither metabolic nor detoxifying genes were found to be up-regulated, which strongly suggests that resistance is also related to insecticide entry into the haemolymph.
Detoxification Genes
Transcriptomic responses are discussed separately for genotypes that carry (MR) or do not carry (S and SR) the MACE mutation. Four types of catalytic reactions are known to be involved during insecticide detoxification; hydrolysis, oxidation, reduction and conjugation [65], [73]. Hence, genes coding for enzymes participating in those reactions are putatively involved in resistance. Among the up-regulated unigenes in the S genotype, hydrolase activity was significantly over-represented with 27 unigenes, four of them encoding for carboxylesterase FE4 and its closely variant E4 (contigs ID 3118, 9215, 720, and 4586). Previous studies have reported the constitutive up-regulation (up to 290-fold) of these same contigs in a M. persicae genotype resistant to neonicotinoids [16], [45]. Those contigs showed a good match (E-value ranging between 0 to 3E-172) with E4 gene from clone 794J, which has been characterized as “extremely resistant” (R3) because of the E4 gene amplification involving about 80 copies [47], [74].
The cytochrome P450s (CYP genes) catalyze the oxidation of insecticides, being the only metabolic system involved in resistance to all classes of insecticides [12], [14], [75]–[77]. Eleven P450s unigenes (contigs ID 497, 5173, 1730, 3931, 6957, 1528, 3799, 3798, 9095, 9584, 749) and two (contigs ID 2519 y 1504) were found to be up-regulated in the S and RS genotypes, respectively. It has been estimated that M. persicae has over 150 CYP genes, approximately 40% more than Acyrthosiphon pisum (the only aphid species with a whole genome sequence available) [78]. Why has the expansion of this gene family been favored during the evolution of M. persicae? The number of CYP genes in M. persicae has perhaps granted a range of functional diversity to this aphid, thus promoting insecticide resistance by different metabolic pathways. Indeed, three of the up-regulated contigs found in the S genotype (contigs ID 497, 5173, 749, corresponding to CYPCY6 gene) also have been shown to be constitutively up-regulated (9 to 22 fold) due to gene amplification in a neonicotinoid resistant M. persicae genotype [16], [45].
The consistency in the up-regulation observed for E4 and CYPCY6 genes between the sensitive genotypes studied here, and the constitutive up-regulation by gene duplication in other resistant genotypes, is in agreement with the model for the resistance changes proposed before [1], [79], [80]. In the absence of resistance mutations or when the frequency of resistant alleles is low in populations, most individuals are susceptible, responding to insecticides by the up-regulation of some specific genes. When the selective agent (insecticide) acts within the limits of tolerance of the initial population, a marginal increase in tolerance has been observed, thus promoting selection of different traits with a low but accumulative effect on resistance (i.e., polygenic resistance). Given the massive application of insecticides in agricultural fields, it would be expected that selection for resistance has operated at the extremes of the phenotypic distribution for resistance. Thus, large-effect mutations accumulate, the retention of duplication events for those genes is promoted, and the up-regulation becomes constitutive. This scenario highlights the importance of analyzing the gene expression in susceptible genotypes when one is searching targets of selection.
Cytochrome P450s have traditionally been considered as the only enzymes to oxidize insecticides in insects [11], [65]. However, an aldehyde dehydrogenase (contig ID 2450) was also shown to be up-regulated (2.6-fold) in the S genotype. In mammals, aldehyde dehydrogenases have been described as important enzymes during the detoxification of xenobiotics [81], [82], and have recently been suggested to participate in the detoxification of pyrethroid in insects [22], [83]. Hence, the up-regulation of contig 2450 found in this study provides new evidence for understanding its detoxifying role as part of the insecticide metabolism in insects.
Regarding carbamates metabolism, most literature involves the action of glutathione S transferases (GSTs) in phase II of carbamate detoxification. GSTs are able to conjugate glutathione with phase I metabolites, converting them into non-reactive water-soluble conjugates [11], [65]. Curiously, it has not been possible so far to find a sole empirical study in insects linking GST with carbamate metabolism, whereas other works have characterized the role of GSTs in organophosphate, organochlorines and pyrethroid detoxification (see [12], [14]). GSTs also play an important role in cell protection, participating indirectly in insecticide resistance by reducing the oxidative damage caused by insecticides [9], [84], [85]. In the S genotype, two GST unigenes were found to be up-regulated (contigs ID 1196, 4744), but our experimental design does not allow us to anticipate any mechanism behind this up-regulation.
UDP-glucuronosyltransferases (UGTs) are another group of conjugative enzymes involved in phase II detoxification. In DDT-resistant strains of Drosophila, for example, UGT is constitutively expressed [18], whereas in Anopheles it was shown to be up-regulated after permethrin application [22]. We identified a UGT transcript (contig ID 8298) that is up-regulated in the S genotype, thus extending the range of potential UGT efficacy to carbamate detoxification. Although the SR genotype did not provide evidence of the participation of this enzyme, the transcriptomic response was obtained after 24 hours of pirimicarb treatment (based on a preliminary LC50 experiments in sensitive genotypes), and we cannot exclude the possibility that other genes could be significantly up-regulated at a different time. Indeed, our RT-qPCR profiles of the SR genotype clearly showed an eight-fold up-regulation of contig 1196 encoding GST after 20 hours of insecticide treatment.
Transcripts Coding for Other Potentially Relevant Proteins
Two unigenes (contigs ID 8669 and 6029) encoding heat shock proteins 70 were found up-regulated in the S and SR genotypes. Proteins of the HSP70 family are particularly well studied and correspond to one of the first known mechanisms in stress responses [86]. Insecticide resistance is also commonly associated with the expression of HSP70 [87], [88]. Thus, our results showing a HSP70 induction in S and SR genotypes, would give evidences that insects are trying to restore cellular homeostasis after insecticide application.
Three unigenes encoding ATP-binding cassette (ABC) transporters (contigs ID 1560, 7913, and 2478) were found up-regulated in the S genotype. The ABC transporters belong to a superfamily of proteins involved in extracellular transport of a wide variety of substances, including metabolic products, lipids and xenobiotics [89], [90]. In DDT resistant Drosophila strains, differential transcription of ABC transporters has been found, [18]. In the cotton pest Heliothis virescens, a mutation in the ABC transporter has been associated with resistance to Bt insecticidal toxins [91]. Therefore, gene sequences for ABC transporters in the S genotype appear to play an important role during insecticide elimination.
Another important group of differentially regulated sequences were unigenes coding for peptidases (contigs ID 256, 254, 3002, 3004, 6594, 7762, 3299, 5268 in S and 3299, 5268 in SR genotypes). In addition, a cysteine-type endopeptidase activity (a feature of cathepsin B) was notably over-represented among the up-expressed sequences in the S genotype. This was consistent with the elevated proteolytic activities observed in insecticide resistant strains of the housefly Musca domestica [92], [93] and the maize weevil Sitophilus zeamais [94]. In addition, the constitutive over-transcription of genes encoding proteins with peptidase activity has been reported in insecticide resistant insects using a transcriptomic approach [18], [22]. Two different explanations have been proposed for an increased proteolytic activity during insecticide resistance. First, peptidases may be involved in protein degradation to fulfill higher energy demands, which, as afore mentioned, is usually a response to stress [18], [94]. Second, peptidases may play a role during protein biosynthesis or in modification of the enzyme conformation related, for example, with the metabolic machinery required to detoxify insecticides [92], [93].
Finally, four unigenes (contigs ID 3486, 7126 in S genotype, and contigs ID 10027, 4497 in SR genotype) whose putative products correspond to cuticular proteins (CPs) were also found up-regulated. This suggests that the transcriptional plasticity of cuticule proteins may play a central role in insecticide resistance of M. persicae, most probably by cuticular thickening or sequestering compounds before entering to the haemolymph. Insecticide resistance through decreased cuticle penetration has been demonstrated in several insect species [7], [95], [96]. A higher constitutive expression of CPs has been reported in insecticide resistant strains of M. persicae and Anopheles gambiae, and, in the case of M. persicae, this was associated with a reduced penetration of the insecticide to the haemolymph [16], [23]. In addition, CPs were found up-regulated in insecticide-resistant strains of the Colorado potato beetle, Leptinotarsa decemlineata [8], and in Aedes aegypti [21].
Transcriptomic Responses in the Multiple Resistance Genotype (MR)
The MR genotype, which carries MACE and kdr mutations, exhibits a low transcriptional plasticity and can be considered a canalized genotype [97], [98]. This genotype showed a lack of responses, even for the expression of genes encoding enzymes involved in insecticide detoxification (at 20 and 30 hour after insecticide treatment). However, no consistent results were obtained using the qRT-PCR or microarray hibridizations for the guanine nucleotide-binding protein (G-protein, Contig ID 1180), one of the regulated genes we found in this genotype.
Conclusions
The varied insecticide resistance mechanisms described for M. persicae illustrate the complexity of the involved evolutionary responses. Modifications such as single mutations or duplications that could occur in some of the up-regulated genes may be responsible for resistance to high doses of insecticides, accounting for the wide range of potential adaptations to insecticides in this species. On the other hand, asexual reproduction in aphids enables the evolution of “general-purpose” genotypes, because the lack of recombination does not rearrange the co-adaptation among genes, which could be the case in the MR genotype. Our results emphasize the value of microarray studies to search for regulated genes in insects and highlight the many ways these different genotypes can assemble resistant phenotypes in response to the environmental pressures. Further experiments will certainly contribute to develop a more thorough and complete understanding of what genes are regulated in different insect species after the application of different insecticide classes and under different environmental circumstances.
Materials and Methods
Aphid Genotypes and Plant Material
Ninety four clonal lineages (genotypes) previously sampled and established in the laboratory were used in this study and genotyped using six microsatellite loci (for details see Castañeda et al. 2011) [99]. Among these, 32 different genotypes were characterized in terms of their insecticide resistance mechanisms. Each genotype was categorized into the following categories: sensitive (S), resistant by a single mutation (SR) and resistant by multiple mutations (MR). Three genotypes, one for each category, were selected for experiments and were maintained in laboratory on leaves of Capsicum annuum var. grossum (hereafter pepper) in controlled environment (20±1°C and 16L:8D photoperiod). Aphids were synchronized for 24 to 48 hours on three-month old pepper plants before starting experiments.
Insecticide Resistance Characterization
Constitutive carboxylesterase activity (EST activity) was evaluated in the 32 genotypes reared on pepper using a microplate bioassay [61], with ten independent biological replicates per genotype and three technical replicates per measurement. Broad-sense heritability of enzyme activity was assessed by computing the ratio of inter-clonal variance to phenotypic variance, using the mean squares of a one-way analysis of variance. The presence of insecticide resistance mutations (IRMs) was screened in the 32 genotypes using allelic discrimination based on the quantitative-PCR assays developed by Anstead et al. (2004) for kdr (L1014F) and super-kdr (M918T) mutations [100], and Anstead et al. (2008) for MACE mutations [101]. See Table S3 for primer and probes sequences. The three genotypes selected for experiments exhibiting low, intermediate and high levels of EST activity.
Insecticide Tolerance Bioassay
In order to verify the correspondence between IRMs and levels of actual resistance, the three selected genotypes were sprayed with pirimicarb, a carbamate insecticide. The bioassay allowed characterizing the level of tolerance to pirimicarb in the selected genotypes. Toxicity bioassay was performed using the leaf-dip technique [102], with five different insecticide concentrations (ranging between 90 and 1.25 ppm in prepared with acetone plus water) and water as control, with 24 technical replicates per treatment. In brief, pepper leaf-discs were dipped into each insecticide solution and placed in Petri dishes; then, 30 adult wingless aphids were place on each disc. All bioassays were scored at the endpoint, 48 h after treatment, by counting the survivors. The insecticide concentrations lethal to 50% (LC50) and 99% (LC99) of aphids were calculated using the Probit statistical method [103].
Insecticide Treatments
Four hundred synchronized adult wingless aphids were placed in groups of 20 individuals on a pepper leaf-disc in Petri dishes containing 2% agar. Then, 10 dishes were sprayed with 1 ml of pirimicarb (20 ppm in acetone plus water) using a Potter-Precision laboratory spray tower (Burkhard) that ensures a homogeneous application [104]. After 24 hours, living aphids were quickly frozen in liquid nitrogen and stored at −70°C until RNA extraction. The other 10 Petri dishes were simultaneously sprayed with water (control). This procedure was performed twice in parallel for the three selected genotypes in order to obtain a minimum of two biological replicates.
Microarray Hybridization
A microarray containing probes for over 10.000 M. persicae unigenes, designed with the Agilent eArray platform (Agilent Technologies) was used [59]. Each slide consisted of eight arrays each containing 60-mers probes (8X60K format).
Total RNA was isolated separately for each experimental condition (genotypes, biological replicates and treatments) from ∼ 40 frozen aphids using the RNeasy Plant Mini Kit (Qiagen, Cat no. 74904). Quantity and quality of RNAs was assessed with a NanoDrop ND-1000 spectrophotometer (NanoDrop® Technologies) and Agilent 2100 Bioanalyzer (Agilent Technologies), respectively. RNA spike-ins (Two-Color RNA Spike-In kit; Agilent) were added to each sample to calibrate the hybridization (the kit was used according to manufacturer’s recommendations only in the case of the S genotype). The Amino Allyl MessageAmp™ II with Cy™3/Cy™5 kit (Ambion) was used to prepare RNA samples for array hybridization. In brief, a reverse transcription from 1.2 mg of total RNA was carried out in each sample using the T7 oligo-dT primer provided in the kit, followed by a second strand cDNA synthesis. Then, double-stranded DNA (dsDNA) was purified with a cDNA filter cartridge and used as template for in vitro transcription with the incorporation of aminoallyl modified UTP, which resulted in amplified RNA (aRNA) containing modified UTP. The aRNA was purified with an aRNA filter cartridge and 5 µg were coupled to Cy3 or Cy5 dyes, checking fluorescence with spectrophotometer (NanoDrop® Technologies). Finally, the labeled aRNA was fragmented at 60°C for 30 min and stopped by the addition of 2× GEx Hybridization Buffer HI-RPM as described in the Agilent two-color microarray-based gene expression analysis protocol. All the hybridizations, washed, and scans of microarrays were performed in the Cornell University Life Sciences Core Laboratories Center (http://cores.lifesciences.cornell.edu).
For each genotype, the isolated aRNAs from each condition (insecticide and water) were mixed altogether using opposite dye colors (Cy3 or Cy5 labels). A dye-swap between samples was conducted for each genotype, performing three biological replicates for the S and MR genotypes, and two for the SR genotype. Hence, a total of eight hybridizations were performed (The microarray data sets reported in this paper have been deposited in NCBI’s Gene Expression Omnibus [105] and are accessible through GEO Series accession number GSE37310 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE37310).
Microarray data analysis was performed using the LIMMA library [106] for the statistical package R freely available at: http://www.r-project.org with Bayesian inference using a lineal model [107]. This method allows the joint analysis of all hybridizations performed with each genotype. Normalization within each array was performed using LOESS method, whereas model adjustment was performed using the lmFit function for each genotype [108]. The overall statistical analysis was performed using the eBayes function. We estimated the ratio between fluorescence (insecticide vs. control) in each spot (hereafter Fold-Change or FC); FC values were Log2−transformed. Spots showing values within −1>log2FC>1 and a P value <0.05, were considered as differentially expressed.
Annotation and Gene Ontology Analysis
Three datasets containing significantly regulated genes in each genotype were obtained. Given the large amount of information, subsequent analyses were focused only on the up-regulated genes. The Gene Ontology analysis of transcripts was performed using the Blast2GO program [109]. Only BLASTX analysis with a cut-off E-value <1E-10 were considered. Then, GO terms were assigned to those sequences using the following parameters: E-Value-Hit-Filter = 1E−6; Annotation Cut-Off = 55; GO Weight = 5. This allows the identification of possible roles for each predicted protein, based on three domains of molecular biology: biological processes, molecular functions and cellular components (http://www.geneontology.org) [110], [111].
An enrichment analysis (EA) was also performed in Blast2GO package GOOSIP (Gene Ontology Significance Statistical Interpretation Program, Microdiscovery, Berlin, Germany) [112], [113], in order to compare up-regulated sequences in the S genotype using the entire set of sequences available in the microarray.
Quantitative Reverse Transcription PCR (RT-qPCR) and Microarray Validation
Two other independent experiments were conducted for each of the three selected genotypes in order to obtain two new biological replicates by treatment. Those experiments were performed as described above, and adding a new level: time after insecticide application (pirimicarb, 20 ppm). Living aphids were recovered 20 and 30 hours after spraying (insecticide or water), and quickly frozen in liquid nitrogen and stored at −70°C until RNA isolation. Seven genes were selected according to significant expression differences (observed in any of the three microarray comparisons) and their putative functions, and expression levels were evaluated by RT-qPCR.
Transcriptional profiles of seven selected genes were validated through RT-qPCR in each of the three selected genotypes. Fresh RNA samples obtained from new biological replicates were used for validation, and included samples isolated 20 and 30 hours after insecticide treatments. Additionally, the transcriptional profiles of three differentially expressed genes were also validated using the same RNA samples used for the microarray experiments in the three genotypes. In the case of new RNA samples, the results were expressed as fold change average obtained in the biological replicates, and in the samples isolated 20 and 30 hours after insecticide application. A correlation coefficient between gene expression measured using microarray and RT-qPCR was calculated using the Spearman’s rho correlation in STATISTICA v.7 [114].
For the new biological replicates, total RNA was isolated from three aphids per genotype using the RNeasy Plant Mini Kit (Qiagen, Cat no. 74904), yielding a range of 100 – 400 ng/µl of RNA (Nanodrop ND-1000, Nanodrop Technologies, USA.). Genomic DNA was removed with DNA-free™ kit (Ambion). Reverse transcription was carried out using the AffinityScript QPCR cDNA Synthesis kit (Agilent) using 1.5 µg of total RNA, which yield about 20 µg of cDNA. Then, the cDNA was diluted to 1∶10, taking 2 µl for PCR reactions. Each PCR reaction mix contained 10 pmol of each primer, 6.25 µl SYBR Green PCR Master Mix (Applied Biosystems) and 0.375 µl of Rox (dilution 1∶500) used as passive reference dye. No template controls (NTC) were included for each PCR to detect external contamination. PCR reactions consisted in 10 min at 95°C, followed by 40 cycles of 15 s at 95°C, 15 s at 57°C and 20 s at 72°C using a Mx3000P QPCR Systems (Stratagene). A dissociation curve was included immediately after each PCR using a ramp of 65–95°C to confirm the absence of nonspecific amplifications and primer dimers. Primers were designed from the sequences of M. persicae contigs for seven target genes (GenBank identifiers EC387286, EE261252, EC387215, EE263862, EE262012, EC388935, EE263097) and one endogenous control gene (DW011095), using the package FastPCR (V 5.4.30) and AmplifX (V 1.3.7), and checked in NCBI/Primer-BLAST. Primer sequences, PCR efficiencies and microarray hybridization with up-regulation in the specific gene study are shown in Table S4.
The relative expression ratio of the target gene was computed by relative quantification using the comparative Ct method (Applied Biosystems User Bulletin No. 2 P/N 4303859, 1997) (Livak & Schmittgen, 2001), with the glyceraldehyde-3-phosphate dehydrogenase (GADPH) gene as normalizing endogenous control. Ratios were calculated from a mean normalized expression (MNE), a value that was obtained between biological replicates, as they show a same trend in all cases; MNE value of aphids sprayed with water was used as calibrator. Several studies have validated the use of GAPDH as a reference gene for normalization [115]–[117], and it is one of M. persicae most stable endogenous genes in response to insecticides (FC range 0.94 – 0.99, on the microarray presented in this study). In addition, the algorithm NormFinder [118] was used to identify the most stable reference genes among: GADPH (DW011095), cyclophilin-10-like (EC388830), ribosomal protein LP0 (DW011949) and ribosomal protein L7 (DW361765). NormFinder identified GADPH as the most stable expressed gene when all samples were grouped together (stability value of 0.009), as well as when samples were classed into treatments (stability value of 0.016). For each relative expression ratio, we performed a t-test between the average and 1, which was used as a reference value for no change in relative expression. The log2 of relative expression ratio was calculated to ease the graphical representation.
Supporting Information
The full list of up-regulated genes, with the log2 fold-change values and descriptions based on the closest BLAST hits.
(XLSX)
Gene expression results for microarray and RT-qPCR methodologies.
(XLSX)
Primers and probes used for insecticide resistance characterization.
(XLSX)
Probe name, gene description, primers sequences, PCR efficiency and microarray hybridization with up-regulation for genes study by RT-qPCR.
(XLSX)
Footnotes
Competing Interests: The authors declare that JSR was a former Ph.D. student of GJ at the Boyce Thomson Institute for Plant Research in Cornell during the time the data reported in this manuscript was collected. Currently, JSR is hired at Agave BioSystems (401 E State St # 200 Ithaca, New York 14850, United States of America). This does not alter the authors’ adherence to all the PLoS ONE policies on sharing data and materials.
Funding: This research was funded by Comisión Nacional de Investigación Científica y Tecnológica doctoral grant to AXS, by Fondo Nacional de Desarrollo Científico y Tecnológico grant N°1090378 to CCF, and by the United States Department of Agriculture grant N°2005-35604-15446 to GJ. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.ffrench-Constant RH, Daborn PJ, Le Goff G. The genetics and genomics of insecticide resistance. Trends in Genetics. 2004;20:163–170. doi: 10.1016/j.tig.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Lenormand T, Bourguet D, Guillemaud T, Raymond M. Tracking the evolution of insecticide resistance in the mosquito Culex pipiens. Nature. 1999;400:861–864. doi: 10.1038/23685. [DOI] [PubMed] [Google Scholar]
- 3.McKenzie JA. Evolutionary Ecology: concepts and case studies. New York: Oxford University Press; 2001. Fox CW, Roff, D.A., Fairbairn, D.J., editor. pp. 347–360. [Google Scholar]
- 4.Whalon ME, Mota-Sanchez D, Hollingworth RM. UK: Oxford University Press; 2008. Global Pesticide Resistance in Arthropods.169 [Google Scholar]
- 5.Onstad DW. London, UK: Academic Press; 2008. Insecticide Resistance Management: Biology, Economics and Prediction.320 [Google Scholar]
- 6.Medina P, Smagghe G, Budia F, del Estal P, Tirry L, et al. Significance of penetration, excretion, and transovarial uptake to toxicity of three insect growth regulators in predatory lacewing adults. Archives of Insect Biochemistry and Physiology. 2002;51:91–101. doi: 10.1002/arch.10053. [DOI] [PubMed] [Google Scholar]
- 7.Ahmad M, Denholm I, Bromilow RH. Delayed cuticular penetration and enhanced metabolism of deltamethrin in pyrethroid-resistant strains of Helicoverpa armigera from China and Pakistan. Pest Management Science. 2006;62:805–810. doi: 10.1002/ps.1225. [DOI] [PubMed] [Google Scholar]
- 8.Zhang J, Goyer C, Pelletier Y. Environmental stresses induce the expression of putative glycine-rich insect cuticular protein genes in adult Leptinotarsa decemlineata (Say). Insect Molecular Biology. 2008;17:209–216. doi: 10.1111/j.1365-2583.2008.00796.x. [DOI] [PubMed] [Google Scholar]
- 9.Vontas JG, Small GJ, Hemingway J. Glutathione S-transferases as antioxidant defence agents confer pyrethroid resistance in Nilaparvata lugens. Biochemical Journal. 2001;357:65–72. doi: 10.1042/0264-6021:3570065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oakeshott JG, Horne I, Sutherland TD, Russell RJ. The genomics of insecticide resistance. Genome Biology. 2003;4:202. doi: 10.1186/gb-2003-4-1-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jackson CJ, Oakeshott JG, Sanchez-Hernandez JC, Wheelock CE. Satoh T, Gupta RC, editors. Carboxylesterases in the Metabolism and Toxicity of Pesticides. Anticholinesterase Pesticides: John Wiley & Sons, Inc. 2011. pp. 57–75.
- 12.Hemingway J, Hawkes NJ, McCarroll L, Ranson H. The molecular basis of insecticide resistance in mosquitoes. Insec Biochemisttry and Molecular Biology. 2004;34:653–665. doi: 10.1016/j.ibmb.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 13.Feyereisen R. Molecular biology of insecticide resistance. Toxicology Letters. 1995;82–83:83–90. doi: 10.1016/0378-4274(95)03470-6. [DOI] [PubMed] [Google Scholar]
- 14.Li XC, Schuler MA, Berenbaum MR. Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annual Review of Entomology. 2007;52:231–253. doi: 10.1146/annurev.ento.51.110104.151104. [DOI] [PubMed] [Google Scholar]
- 15.Daborn PJ, Yen JL, Bogwitz MR, Le Goff G, Feil E, et al. A single P450 allele associated with insecticide resistance in Drosophila. Science. 2002;297:2253–2256. doi: 10.1126/science.1074170. [DOI] [PubMed] [Google Scholar]
- 16.Puinean AM, Foster SP, Oliphant L, Denholm I, Field LM, et al. Amplification of a Cytochrome P450 Gene is associated with resistance to neonicotinoid insecticides in the aphid Myzus persicae. Plos Genetics. 2010;6:e1000999. doi: 10.1371/journal.pgen.1000999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pedra JHF, Festucci-Buselli RA, Sun W, Muir WM, Scharf ME, et al. Profiling of abundant proteins associated with dichlorodiphenyltrichloroethane resistance in Drosophila melanogaster. Proteomics. 2005;5:258–269. doi: 10.1002/pmic.200400914. [DOI] [PubMed] [Google Scholar]
- 18.Pedra JHF, McIntyre LM, Scharf ME, Pittendrigh BR. Genom-wide transcription profile of field- and laboratory-selected dichlorodiphenyltrichloroethane (DDT)-resistant Drosophila. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:7034–7039. doi: 10.1073/pnas.0400580101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Müller P, Donnelly MJ, Ranson H. Transcription profiling of a recently colonised pyrethoid resistent Anopheles gambiae strain from Ghana. BMC Genomics. 2007;8 doi: 10.1186/1471-2164-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strode C, Wondji CS, David JP, Hawkes NJ, Lumjuan N, et al. Genomic analysis of detoxification genes in the mosquito Aedes aegypti. Insect Biochemistry and Molecular Biology. 2008;38:113–123. doi: 10.1016/j.ibmb.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 21.Lertkiatmongkol P, Pethuan S, Jirakanjanakit N, Rongnoparut P. Transcription analysis of differentially expressed genes in insecticide-resistant Aedes aegypti mosquitoes after deltamethrin exposure. Journal of Vector Ecology. 2010;35:197–203. doi: 10.1111/j.1948-7134.2010.00047.x. [DOI] [PubMed] [Google Scholar]
- 22.Vontas J, Blass C, Koutsos AC, David JP, Kafatos FC, et al. Gene expression in insecticide resistant and susceptible Anopheles gambiae strains constitutively or after insecticide exposure. Insect Molecular Biology. 2005;14:509–521. doi: 10.1111/j.1365-2583.2005.00582.x. [DOI] [PubMed] [Google Scholar]
- 23.Vontas J, David JP, Nikou D, Hemingway J, Christophides GK, et al. Transcriptional analysis of insecticide resistance in Anopheles stephensi using cross-species microarray hybridization. Insect Molecular Biology. 2007;16:315–324. doi: 10.1111/j.1365-2583.2007.00728.x. [DOI] [PubMed] [Google Scholar]
- 24.Figueroa CC, Prunier-Leterme N, Rispe C, Sepulveda F, Fuentes-Contreras E, et al. Annotated expressed sequence tags and xenobiotic detoxification in the aphid Myzus persicae (Sulzer). Insect Science. 2007;14:29–45. [Google Scholar]
- 25.Blackman RL, Eastop VF. Aphid on the World’s Trees: An Identification and Information Guide; CAB International Wallingford, United Kingdom. 1994.
- 26.Caillaud M, Edwards O, Field L, Giblot-Ducray D, Gray S, et al. The International Aphid Genomics Consortium (IAGC); 2004. Proposal to sequence the genome of the Pea Aphid (Acyrthosiphon pisum). [Google Scholar]
- 27.Francis F, Gerkens P, Harmel N, Mazzucchelli G, De Pauw E, et al. Proteomics in Myzus persicae: Effect of aphid host plant switch. Insect Biochemistry and Molecular Biology. 2006;36:219–227. doi: 10.1016/j.ibmb.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 28.Simon JC, Dedryver CA, Rispe C, Hulle M. Aphids in a new millenium: Proceedings of the 6th International Symposium on Aphids. 2001.
- 29.van Emden H, Harrington R. CABI North American Office, Cambridge, Massachusetts; 2007. Aphids as Crop Pests. [Google Scholar]
- 30.Blackman RL, Eastop VF. An identification guide; 2nd edn. Wiley, Ltd. Chichester, United Kingdom; 2000. Aphids on the world’s crops. [Google Scholar]
- 31.Srigiriraju L, Semtner PJ, Anderson TD, Bloomquist JR. Monitoring for MACE resistance in the tobacco-adapted form of the green peach aphid, Myzus persicae (Sulzer) (Hemiptera: Aphididae) in the eastern United States. Crop Protection. 2010;29:197–202. [Google Scholar]
- 32.Schoonhoven LM, Loon JJAv, Dicke M. New York: Oxford University Press Inc.; 2005. Insect Plant Biology.421 [Google Scholar]
- 33.Artigas J. Concepción, Chile: Universidad de Concepción; 1994. Insectos de Interés Agrícola, Forestal, Médico y Veterinario.943 [Google Scholar]
- 34.Klein C, Waterhouse DF. Distribution and importance of arthropods associated with agriculture and forestry in Chile. Canberra ACIAR Monograph N. 2000;68 [Google Scholar]
- 35.Georghiou GP, Lagunes-Tejada A. An index of cases reported through 1989. Food and Agriculture Organization of the United Nations, Rome; 1991. The occurrence of resistance to pesticides in arthropods. [Google Scholar]
- 36.Moores GD, Devine GJ, Devonshire AL. Insecticide-insensitive acetylcholinesterase can enhance esterase-based resistance in Myzus persicae and Myzus nicotianae. Pesticide Biochemistry and Physiology. 1994;49:114–120. [Google Scholar]
- 37.Moores GD, Devonshire AL, Stumpf N, Nauen R. A fluorometric method to detect insensitive acetycholinesterase in resistant pests. Proceedings of the Brighton Crop Protection Conference – Pests and Diseases. 2000;1:447–452. [Google Scholar]
- 38.Nabeshima T, Kozaki T, Tomita T, Kono Y. An amino acid substitution on the second acetylcholinesterase in the pirimicarb-resistant strains of the peach potato aphid, Myzus persicae. Biochemical and Biophysical Research Communications. 2003;307:15–22. doi: 10.1016/s0006-291x(03)01101-x. [DOI] [PubMed] [Google Scholar]
- 39.Criniti A, Mazzoni E, Cassanelli S, Cravedi P, Tondelli A, et al. Biochemical and molecular diagnosis of insecticide resistance conferred by esterase, MACE, kdr and super-kdr based mechanisms in Italian strains of the peach potato aphid, Myzus persicae (Sulzer). Pesticide Biochemistry and Physiology. 2008;90:168–174. [Google Scholar]
- 40.Martinez-Torres D, Devonshire AL, Williamson MS. Molecular studies of knockdown resistance to pyrethroids: Cloning of domain II sodium channel gene sequences from insects. Pesticide Science. 1997;51:265–270. [Google Scholar]
- 41.Martinez-Torres D, Foster SP, Field LM, Devonshire AL, Williamson MS. A sodium channel point mutation is associated with resistance to DDT and pyrethroid insecticides in the peach-potato aphid, Myzus persicae (Sulzer) (Hemiptera : Aphididae). Insect Molecular Biology. 1999;8:339–346. doi: 10.1046/j.1365-2583.1999.83121.x. [DOI] [PubMed] [Google Scholar]
- 42.Eleftherianos I, Foster SP, Williamson MS, Denholm I. Characterization of the M918T sodium channel gene mutation associated with strong resistance to pyrethroid insecticides in the peach-potato aphid, Myzus persicae (Sulzer). Bulletin of Entomological Research. 2008;98:183–191. doi: 10.1017/S0007485307005524. [DOI] [PubMed] [Google Scholar]
- 43.Anthony N, Unruh T, Ganser D, ffrench-Constant R. Duplication of the Rdl GABA receptor subunit gene in an insecticide-resistant aphid, Myzus persicae. Molecular and General Genetics. 1998;260:165–175. doi: 10.1007/s004380050882. [DOI] [PubMed] [Google Scholar]
- 44.Guillemaud T, Brun A, Anthony N, Sauge MH, Boll R, et al. Incidence of insecticide resistance alleles in sexually-reproducing populations of the peach-potato aphid Myzus persicae (Hemiptera : Aphididae) from southern France. Bulletin of Entomological Research. 2003;93:289–297. doi: 10.1079/ber2003241. [DOI] [PubMed] [Google Scholar]
- 45.Bass C, Puinean A, Andrews M, Cutler P, Daniels M, et al. Mutation of a nicotinic acetylcholine receptor beta subunit is associated with resistance to neonicotinoid insecticides in the aphid Myzus persicae. BMC Neuroscience. 2011;12:51. doi: 10.1186/1471-2202-12-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Devonshire AL, Moores GD. A carboxylesterase with broad substrate specificity causes organophosphorus, carbamate and pyrethroid resistance in peach-potato aphid (Myzus persicae). Pest Biochem Physiol. 1982;18:235–246. [Google Scholar]
- 47.Field LM, Williamson MS, Moores GD, Devonshire AL. Cloning and analysis of the esterase genes conferring insecticide resistance in the peach-potato aphid, Myzus persicae (Sulzer). Biochemical Journal. 1993;294:569–574. doi: 10.1042/bj2940569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Field LM, Devonshire AL. Structure and organization of amplicons containing the E4 esterase genes responsible for insecticide resistance in the aphid Myzus persicae (Sulzer). Biochemical Journal. 1997;322:867–871. doi: 10.1042/bj3220867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Field LM, Blackman RL. Insecticide resistance in the aphid Myzus persicae (Sulzer): chromosome location and epigenetic effects on esterase gene expression in clonal lineages. Biological Journal of the Linnean Society. 2003;79:107–113. [Google Scholar]
- 50.Field LM. Methylation and expression of amplified esterase genes in the aphid Myzus persicae (Sulzer). Biochemical Journal. 2000;349:863–868. doi: 10.1042/bj3490863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Field LM, Devonshire AL. Evidence that the E4 and FE4 esterase genes responsible for insecticide resistance in the aphid Myzus persicae (Sulzer) are part of a gene family. Biochemical Journal. 1998;330:169–173. doi: 10.1042/bj3300169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Philippou D, Field L, Moores G. Metabolic enzyme(s) confer imidacloprid resistance in a clone of Myzus persicae (Sulzer) (Hemiptera: Aphididae) from Greece. Pest Management Science. 2009;66:390–395. doi: 10.1002/ps.1888. [DOI] [PubMed] [Google Scholar]
- 53.Stevens M, Dewar A. Pest and disease problems in Chile: what lessons can be learnt? British Sugar Review. 1996;64:34–38. [Google Scholar]
- 54.Casals P, Silva G. Susceptibilidad a insecticidas del pulgón verde del duraznero (Myzus persicae Sulzer) en remolacha. Agrociencia. 1999;15:55–61. [Google Scholar]
- 55.Casals P, Silva G. Estado actual de susceptibilidad a insecticidas del pulgón verde del duraznero Myzus persicae Sulzer. Revista Fruticola. 2000;21:5–10. [Google Scholar]
- 56.Fuentes-Contreras E, Figueroa CC, Reyes M, Briones LM, Niemeyer HM. Genetic diversity and insecticide. resistance of Myzus persicae (Hemiptera : Aphididae) populations from tobacco in Chile: evidence for the existence of a single predominant clone. Bulletin of Entomological Research. 2004;94:11–18. doi: 10.1079/ber2003275. [DOI] [PubMed] [Google Scholar]
- 57.Zhu YC, Guo ZB, Chen MS, Zhu KY, Liu XFF, et al. Major putative pesticide receptors, detoxification enzymes, and transcriptional profile of the midgut of the tobacco budworm, Heliothis virescens (Lepidoptera: Noctuidae). Journal of Invertebrate Pathology. 2011;106:296–307. doi: 10.1016/j.jip.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 58.Lesk AM. New York: Oxford University Press; 2007. Introduction to Genomics.419 [Google Scholar]
- 59.Ramsey JS, Wilson ACC, de Vos M, Sun Q, Tamborindeguy C, et al. Genomic resources for Myzus persicae: EST sequencing, SNP identification, and microarray design. BMC Genomics. 2007;8:423. doi: 10.1186/1471-2164-8-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Field LM, Blackman RL, Tyler-Smith C, Devonshire AL. Relationship between amount of esterase and gene copy number in insecticide-resistant Myzus persicae (Sulzer). Biochemical Journal. 1999;339:737–742. [PMC free article] [PubMed] [Google Scholar]
- 61.Devonshire AL, Devine GJ, Moores GD. Comparison of microplate esterase assays and immunoassay for identifying insecticide resistant variants of Myzus persicae (Homoptera, Aphididae). Bulletin of Entomological Research. 1992;82:459–463. [Google Scholar]
- 62.Vasquez BL. Resistant to Most Insecticides. In: Walker TJ, editor. Book of Insects Records. University of Florida Gainesville, USA; 1995. [Google Scholar]
- 63.Wilson ACC, Sunnucks P, Hales DF. Heritable genetic variation and potential for adaptive evolution in asexual aphids (Aphidoidea). Biological Journal of the Linnean Society. 2003;79:115–135. [Google Scholar]
- 64.Nespolo RF, Halkett F, Figueroa CC, Plantegenest M, Simon JC. Evolution of Trade-Offs between Sexual and Asexual Phases and the Role of Reproductive Plasticity in the Genetic Architecture of Aphid Life Histories. Evolution. 2009;63:2402–2412. doi: 10.1111/j.1558-5646.2009.00706.x. [DOI] [PubMed] [Google Scholar]
- 65.Yu SJ. The Toxicology and Biochemistry of Insecticides: CRC Press. 2008. 276
- 66.Hardstone MC, Leichter CA, Scott JG. Multiplicative interaction between the two major mechanisms of permethrin resistance, kdr and cytochrome P450-monooxygenase detoxification, in mosquitoes. Journal of Evolutionary Biology. 2009;22:416–423. doi: 10.1111/j.1420-9101.2008.01661.x. [DOI] [PubMed] [Google Scholar]
- 67.Marcombe S, Poupardin R, Darriet F, Reynaud S, Bonnet J, et al. Exploring the molecular basis of insecticide resistance in the dengue vector Aedes aegypti: a case study in Martinique Island (French West Indies). BMC Genomics. 2009;10:494. doi: 10.1186/1471-2164-10-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Foster SP, Harrington R, Devonshire AL, Denholm I, Devine GJ, et al. Comparative survival of insecticide-susceptible and resistant peach-potato aphids, Myzus persicae (Sulzer) (Hemiptera: Aphididae), in low temperature field trials. Bulletin of Entomological Research. 1996;86:17–27. [Google Scholar]
- 69.Foster SP, Denholm I, Poppy GM, Thompson R, Powell W. Fitness trade-off in peach-potato aphids (Myzus persicae) between insecticide resistance and vulnerability to parasitoid attack at several spatial scales. Bulletin of Entomological Research. 2010;101:659–666. doi: 10.1017/S0007485310000623. [DOI] [PubMed] [Google Scholar]
- 70.Foster SP, Harrington R, Devonshire AL, Denholm I, Clark SJ, et al. Evidence for a possible fitness trade-off between insecticide resistance and the low temperature movement that is essential for survival of UK populations of Myzus persicae (Hemiptera: Aphididae). Bulletin of Entomological Research. 1997;87:573–579. [Google Scholar]
- 71.Guedes RNC, Oliveira EE, Guedes NMP, Ribeiro B, Serrao JE. Cost and mitigation of insecticide resistance in the maize weevil, Sitophilus zeamais. Physiological Entomology. 2006;31:30–38. [Google Scholar]
- 72.Lopes KVG, Silva LB, Reis AP, Oliveira MGA, Guedes RNC. Modified alpha-amylase activity among insecticide-resistant and -susceptible strains of the maize weevil, Sitophilus zeamais. Journal of Insect Physiology. 2010;56:1050–1057. doi: 10.1016/j.jinsphys.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 73.Sogorb MA, Vilanova E. Detoxication of Anticholinesterase Pesticides. In: Satoh T, Gupta RC, editors. Anticholinesterase Pesticides. Hoboken, New Jersey; 2011. pp. 121–132. [Google Scholar]
- 74.Field LM, Devonshire AL, Forde BG. Molecular evidence that insecticide resistance in peach potato aphids (Myzus persicae Sulz.) results from amplification of an esterase gene. Biochemical Journal. 1988;251:309–312. doi: 10.1042/bj2510309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Feyereisen R. Insect P450 enzymes. Annual Review of Entomology. 1999;44:507–533. doi: 10.1146/annurev.ento.44.1.507. [DOI] [PubMed] [Google Scholar]
- 76.Berge JB, Feyereisen R, Amichot M. Cytochrome P450 monooxygenases and insecticide resistance in insects. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences. 1998;353:1701–1705. doi: 10.1098/rstb.1998.0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schuler MA. Biochimica Et Biophysica Acta-Proteins and Proteomics 1814: 36–45; 2011. P450s in plant-insect interactions. pp. 36–45. [DOI] [PubMed] [Google Scholar]
- 78.Ramsey JS, Rider DS, Walsh TK, De Vos M, Gordon KHJ, et al. Comparative analysis of detoxification enzymes in Acyrthosiphon pisum and Myzus persicae. Insect Molecular Biology. 2010;19:155–164. doi: 10.1111/j.1365-2583.2009.00973.x. [DOI] [PubMed] [Google Scholar]
- 79.McKenzie JA, Batterham P. The genetic, molecular and phenotypic consequences of selection for insecticide resistance. Trends in Ecology & Evolution. 1994;9:166–169. doi: 10.1016/0169-5347(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 80.McKenzie JA, Batterham P. Predicting insecticide resistance: mutagenesis, selection and response. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences. 1998;353:1729–1734. doi: 10.1098/rstb.1998.0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Choi J, Rose RL, Hodgson E. In vitro human metabolism of permethrin: the role of human alcohol and aldehyde dehydrogenases. Pesticide Biochemistry and Physiology. 2002;74:117–128. [Google Scholar]
- 82.Hodgson E. In vitro human phase I metabolism of xenobiotics I: Pesticides and related compounds used in agriculture and public health, May 2003. Journal of Biochemical and Molecular Toxicology. 2003;17:201–206. doi: 10.1002/jbt.10080. [DOI] [PubMed] [Google Scholar]
- 83.Somwang P, Yanola J, Suwan W, Walton C, Lumjuan N, et al. Enzymes-based resistant mechanism in pyrethroid resistant and susceptible Aedes aegypti strains from northern Thailand. Parasitology Research. 2011;109:531–537. doi: 10.1007/s00436-011-2280-0. [DOI] [PubMed] [Google Scholar]
- 84.Enayati AA, Ranson H, Hemingway J. Insect glutathione transferases and insecticide resistance. Insect Molecular Biology. 2005;14:3–8. doi: 10.1111/j.1365-2583.2004.00529.x. [DOI] [PubMed] [Google Scholar]
- 85.Singh SP, Coronella JA, Benes H, Cochrane BJ, Zimniak P. Catalytic function of Drosophila melanogaster glutathione S-transferase DmGSTS1–1 (GST-2) in conjugation of lipid peroxidation end products. European Journal of Biochemistry. 2001;268:2912–2923. doi: 10.1046/j.1432-1327.2001.02179.x. [DOI] [PubMed] [Google Scholar]
- 86.Gupta SC, Sharma A, Mishra M, Mishra RK, Chowdhuri DK. Heat shock proteins in toxicology: How close and how far? Life Sciences. 2010;86:377–384. doi: 10.1016/j.lfs.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 87.Chowdhuri DK, Saxena DK, Vishwanathan PN. Effect of hexachlorocyclohexane (HCH), its isomers, and metabolites on Hsp70 expression in transgenic Drosophila melanogaster. Pestic Biochem Physiol. 1999;63:15–25. [Google Scholar]
- 88.Nazir A, Mukhopadhyay I, Saxena DK, Chowdhuri DK. Chlorpyrifos induced hsp70 expression and effect on reproductive performance in transgenic Drosophila melanogaster (hsp70- lacZ) Bg9. Arch Environ Contam Toxicol. 2001;41:443–449. doi: 10.1007/s002440010270. [DOI] [PubMed] [Google Scholar]
- 89.Dassa E, Bouige P. The ABC of ABCs: a phylogenetic and functional classification of ABC systems in living organisms. Research in Microbiology. 2001;152:211–229. doi: 10.1016/s0923-2508(01)01194-9. [DOI] [PubMed] [Google Scholar]
- 90.Lee SH, Kang JS, Min JS, Yoon KS, Strycharz JP, et al. Decreased detoxification genes and genome size make the human body louse an efficient model to study xenobiotic metabolism. Insect Molecular Biology. 2010;19:599–615. doi: 10.1111/j.1365-2583.2010.01024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gahan LJ, Pauchet Y, Vogel H, Heckel DG. An ABC Transporter Mutation Is Correlated with Insect Resistance to Bacillus thuringiensis Cry1Ac Toxin. PLoS Genetics. 2010;6:1–11. doi: 10.1371/journal.pgen.1001248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Saleem AM, Shakoori AR, Wilkins RM, Mantle D. In vivo effect of Lambda-cyhalothrin and malathion on the proteolytic enzymes of malathion-resistant and susceptible strains of Musca domestica Pakistan J Zool. 1994;26:327–333. [Google Scholar]
- 93.Ahmed S, Wilkins RM, Mantle D. Comparison of proteolytic enzyme activities in adults of insecticide resistant and susceptible strains of the housefly Musca domestica L. Insect Biochem Mol Biol. 1998;28:629–639. doi: 10.1016/s0965-1748(98)00061-7. [DOI] [PubMed] [Google Scholar]
- 94.Silva LB, Reis AP, Pereira EJG, Oliveira MGA, Guedes RNC. Altered cysteine proteinase activity in insecticide-resistant strains of the maize weevil: Purification and characterization. Comparative Biochemistry and Physiology B-Biochemistry & Molecular Biology. 2010;157:80–87. doi: 10.1016/j.cbpb.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 95.Walter CM, Price NR. The uptake and penetration of pirimiphos-methyl into susceptible and resistant strains of the rust red flour beetle-Tribolium castaneum, herbst (coleoptera: tenebrionidae). Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1989;94:419–423. [Google Scholar]
- 96.Valles SM, Dong K, Brenner R. Mechanisms responsible for cypermethrin resistance in a strain of German cockroach, Blattella germanica. Pestic Biochem Physiol 66. 2000;66:195–205. [Google Scholar]
- 97.Auld JR, Agrawal AA, Relyea RA. Re-evaluating the costs and limits of adaptive phenotypic plasticity. Proceedings of the Royal Society B-Biological Sciences. 2010;277:503–511. doi: 10.1098/rspb.2009.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Van Buskirk J, Steiner UK. The fitness costs of developmental canalization and plasticity. Journal of Evolutionary Biology. 2009;22:852–860. doi: 10.1111/j.1420-9101.2009.01685.x. [DOI] [PubMed] [Google Scholar]
- 99.Castañeda LE, Barrientos K, Cortes P, Figueroa CC, Fuentes-Contreras E, et al. Evaluating reproductive fitness and metabolic costs for insecticide resistance in Myzus persicae from Chile. Physiological Entomology. 2011;36:253–260. [Google Scholar]
- 100.Anstead JA, Williamson MS, Eleftherianos L, Denholm I. High-throughput detection of knockdown resistance in Myzus persicae using allelic discriminating quantitative PCR. Insect Biochemistry and Molecular Biology. 2004;34:871–877. doi: 10.1016/j.ibmb.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 101.Anstead JA, Williamson MS, Denholm I. New methods for the detection of insecticide resistant Myzus persicae in U.K. suction trap network. Agricultural and Forest Entomology. 2008;10:291–295. [Google Scholar]
- 102.Nauen R, Elbert A. European monitoring, of resistance to insecticides in Myzus persicae and Aphis gossypii (Hemiptera : Aphididae) with special reference to imidacloprid. Bulletin of Entomological Research. 2003;93:47–54. doi: 10.1079/BER2002215. [DOI] [PubMed] [Google Scholar]
- 103.Finney DJ. England: Cambridge University Press; 1952. Probit Analysis. [Google Scholar]
- 104.Potter C. An improved laboratory apparatus for applying direct sprays and films, with data on the electrostatic charge on atomized spray fluids. Ann Appl Biol. 1952;39:1–29. [Google Scholar]
- 105.Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Research. 2002;30:207–210. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Smyth GK. Gentleman V. Carey SD, R Irizarry, W Huber, editors. Limma: linear models for microarray data. Bioinformatics and Computational Biology Solutions using R and Bioconductor, R. Springer, New York. 2005. pp. 397–420.
- 107.Smyth GK. Linear models and empirical Bayes methods for assessing dierential expression in microarray experiments. Statistical Applications in Genetics and Molecular Biology 3: 1, Article. 2004;3 doi: 10.2202/1544-6115.1027. [DOI] [PubMed] [Google Scholar]
- 108.Smyth GK, Speed TP. Normalization of cDNA microarray data. Methods. 2003;31:265–273. doi: 10.1016/s1046-2023(03)00155-5. [DOI] [PubMed] [Google Scholar]
- 109.Conesa A, Gotz S, Garcia-Gomez J, Talon J, Robles M. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005;21:3674–3676. doi: 10.1093/bioinformatics/bti610. [DOI] [PubMed] [Google Scholar]
- 110.Harris MA, Clark J, Ireland A, Lomax J, Ashburner M, et al. The Gene Ontology (GO) database and informatics resource. Nucleic Acids Research. 2004;32:D258–D261. doi: 10.1093/nar/gkh036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, et al. Gene Ontology: tool for the unification of biology. Nature Genetics. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Blüthgen H, Brand K, Kielbas SM, Swat M, Herzel H, et al. Biological Profiling of Gene Groups utilizing Gene Ontology – A Statistical Framework. Genome Informatics. 2005;16:106–115. [PubMed] [Google Scholar]
- 113.Bluthgen N, Kielbasa SM, Herzel H. Inferring combinatorial regulation of transcription in silico. Nucleic Acids Research. 2005;33:272–279. doi: 10.1093/nar/gki167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.StatSoft I. 2004. STATISTICA (data analysis software system), version 7. www.statsoft.com.
- 115.Huis R, Hawkins S, Neutelings G. Selection of reference genes for quantitative gene expression normalization in flax (Linum usitatissimum L.). BMC Plant Biology. 2010;10:71. doi: 10.1186/1471-2229-10-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang GP, Xu CS. Reference Gene Selection for Real-Time RT-PCR in Eight Kinds of Rat Regenerating Hepatic Cells. Molecular Biotechnology. 2010;46:49–57. doi: 10.1007/s12033-010-9274-5. [DOI] [PubMed] [Google Scholar]
- 117.Brisson JA, Ishikawa A, Miura T. Wing development genes of the pea aphid and differential gene expression between winged and unwinged morphs. Insect Molecular Biology. 2010;19:63–73. doi: 10.1111/j.1365-2583.2009.00935.x. [DOI] [PubMed] [Google Scholar]
- 118.Andersen CL, Jensen JL, Orntoft TF. Normalization of real-time quantitative reverse transcription-PCR data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Research. 2004;64:5245–5250. doi: 10.1158/0008-5472.CAN-04-0496. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The full list of up-regulated genes, with the log2 fold-change values and descriptions based on the closest BLAST hits.
(XLSX)
Gene expression results for microarray and RT-qPCR methodologies.
(XLSX)
Primers and probes used for insecticide resistance characterization.
(XLSX)
Probe name, gene description, primers sequences, PCR efficiency and microarray hybridization with up-regulation for genes study by RT-qPCR.
(XLSX)