Abstract
Trypanosoma cruzi, the agent of Chagas disease, is a complex of genetically diverse isolates highly phylogenetically related to T. cruzi-like species, Trypanosoma cruzi marinkellei and Trypanosoma dionisii, all sharing morphology of blood and culture forms and development within cells. However, they differ in hosts, vectors and pathogenicity: T. cruzi is a human pathogen infective to virtually all mammals whilst the other two species are non-pathogenic and bat restricted. Previous studies suggest that variations in expression levels and genetic diversity of cruzipain, the major isoform of cathepsin L-like (CATL) enzymes of T. cruzi, correlate with levels of cellular invasion, differentiation, virulence and pathogenicity of distinct strains. In this study, we compared 80 sequences of genes encoding cruzipain from 25 T. cruzi isolates representative of all discrete typing units (DTUs TcI-TcVI) and the new genotype Tcbat and 10 sequences of homologous genes from other species. The catalytic domain repertoires diverged according to DTUs and trypanosome species. Relatively homogeneous sequences are found within and among isolates of the same DTU except TcV and TcVI, which displayed sequences unique or identical to those of TcII and TcIII, supporting their origin from the hybridization between these two DTUs. In network genealogies, sequences from T. cruzi clustered tightly together and closer to T. c. marinkellei than to T. dionisii and largely differed from homologues of T. rangeli and T. b. brucei. Here, analysis of isolates representative of the overall biological and genetic diversity of T. cruzi and closest T. cruzi-like species evidenced DTU- and species-specific polymorphisms corroborating phylogenetic relationships inferred with other genes. Comparison of both phylogenetically close and distant trypanosomes is valuable to understand host-parasite interactions, virulence and pathogenicity. Our findings corroborate cruzipain as valuable target for drugs, vaccine, diagnostic and genotyping approaches.
Introduction
Cathepsin L-like (CATL) are cysteine proteases that play important roles in cell invasion, growth, differentiation, immunity, immune-modulation, virulence, pathogenicity and survival of pathogenic protozoans. Different isoforms of CATL are encoded by a large gene family and perform distinct roles in the interactions of the trypanosomes with vertebrate hosts and vectors, differing in stage, cellular localization and expression level during the life cycle. This functional and structural diversification may have contributed to the adaptation of different trypanosome species to their different life cycles, vertebrate hosts and vectors [1]–[4].
Trypanosoma cruzi is the type species of the subgenus Schizotrypanum and a complex of genetically heterogeneous isolates distributed in 6 intraspecific subdivisions denominated discrete typing units (DTUs), TcI-TcVI [5], [6], and one new genotype (Tcbat) identified in Brazilian bats [7]. Closest relatives of T. cruzi are the bat-restricted T. c. marinkellei followed by T. dionisii, which are referred as T. cruzi-like due to morphologically indistinguishable blood and culture forms [8]–[11]. Development within mammalian cells in vitro is a feature shared by all species of the subgenus Schizotrypanum, while in vivo only T. cruzi has been proven to infect mammals other than chiropterans [7], [9], [10], [12]–[14]. Bats infected by T. cruzi-like species show nests of amastigotes in cardiac, skeletal and stomach muscle cells likewise T. cruzi in a range of hosts including man. Recent studies demonstrated that T. dionisii and T. cruzi invade mammalian cells through a common mechanism involving lysosome mobilization to the site of parasite entry [15], [16]. Previous studies showed that T. cruzi and T. dionisii share similar molecules with important roles in host-parasite interactions such as phospholipids and cysteine proteases [17], [18] as well as epitopes associated to autoimmunity in Chagas disease [19]. Nevertheless, T. dionisii differ from T. cruzi in surface glycoproteins involved in host-cell interactions [16]. Besides morphology and in vitro and in vivo behavior, T. cruzi-like species share cellular, biochemical and immunological features with T. cruzi and, hence, can be valuable as non-infective to humans models for studies of T. cruzi and as targets for trials of drugs, vaccines and diagnosis [10], [20].
Similar to T. cruzi, all T. cruzi-like isolates differentiate from epimastigotes to infective metacyclic trypomastigotes in cultures and in the vector gut; bats are infected by licking vector feces contaminated with trypanosomes on their fur and/or by ingesting the infected vectors themselves [8], [9]. Differently from T. cruzi that can be transmitted by several genera of triatomine bugs, transmission of T. c. marinkellei seems to involve only triatomines of the genus Cavernicola, while cimicids are vectors of T. dionisii [8], [9]. In addition to T. cruzi, T. rangeli was reported infecting humans and non-human primates, chiropterans and mammals of other orders and are both transmitted by triatomines [21], [22]. The mechanisms underpinning vertebrate and vector specificities of these trypanosomes are unknown.
Previous studies demonstrated that two main CATL enzymes are expressed by T. cruzi, the major isoform (>75 copies) addressed in the present study and designated as cruzipain, is the archetype of a large multigene family organized in tandem repeats expressed in all life cycle stages of T. cruzi [4], [23]–[26]. Analysis of polymorphic cruzipain-encoding genes disclosed the isoform cruzipain 2 (∼6 copies), which is expressed preferentially by the mammalian stages and differs markedly from cruzipain with respect to substrate specificity and kinetic properties [27], [28].
Cruzipain plays fundamental functions in T. cruzi life cycle with recognized roles in parasite-host interactions, in establishing, maintaining, exacerbating and controlling infections. There are increasing evidence that the immunopathogenesis of experimental Chagas Disease is, at least in part, due to the activity of cruzipain mediating cell invasion, inflammation, tissue damage and immune evasion [29], [30]. Cruzipain is an immunodominat antigen, expressed on parasite surface and secreted, which elicits potent humoral [31], [32] and cellular immune responses in T. cruzi infected humans and mice [33], [34]. Vaccination with recombinant cruzipain trigger strong humoral and cell-mediated immunity controlilng parasite load and inflammatory tissue damage [34]–[37]. Addition of synthetic irreversible inhibitors to cultures of cells infected with T. cruzi blocks parasite replication, intracellular growth and differentiation [38], [39]. Treatment of T. cruzi infected mice with inhibitors designed to inactivate cruzipain rescued mice from a lethal infection [40]. T. cruzi ability to invade human cells was modulated by the balance between cruzipain and chagasin, a natural endogenous inhibitor of papain-like cysteine proteases [41], [42]. Therefore, inhibitors of cruzipain are among the most promising new drugs for treatment of Chagas disease [1], [4], [43]–[45].
Studies have suggested that the variable levels of cruzipain activity correlate to degrees of metacyclogenesis, cellular invasion and virulence of T. cruzi isolates. Comparison of TcI and TcII strains suggested that cruzipain proteolytic profiles could be useful for separating members of these two DTUs and that high expression levels could be linked to enhanced metacyclogenesis and cell infectivity [46]. In a study using flow cytometry and anti-cruzipain antibody both TcI and TcII isolates showed heterogeneous surface cruzipain patterns, however, expression levels were higher in TcI isolates showing higher metacyclogenesis [47]. In T. cruzi Dm28c (TcI), cruzipain are down regulated during metacyclogenesis [48]. The over expression of this enzyme throughout the parasite life-cycle was associated with enhanced metacyclogenesis but not with increased cell infectivity [49]. Differences in cruzipain expression were correlated with differential virulence for mice of T. cruzi isolates of Z3 (TcIII and IV) [50]. A proteomic analysis suggested that significant differences in the expression of cruzipain by isolates of TcIII and TcIV (Z3) could also contribute to their differential infectivity to cells [51]. Interestingly, proteolytic activity was lower in the virulent T. cruzi CL strain compared to the non-virulent CL-14 clone [52]. Overall, these studies pointed toward noteworthy but still controversial association between levels of cruzipain expression/activity and virulence, metacyclogenesis and cell infectivity of T. cruzi strains. Low levels of activity and transcripts of CATL-like in T. rangeli (rangelipain) were related to lack of pathogenicity and intracellular development [53]. However, a broad study demonstrated that isolates of divergent lineages of T. rangeli express high levels of enzymatic activity and transcripts of rangelipain [54].
The major cruzipain isoform has been the subject of extensive biochemical, structural and immunological studies. However, to date, sequences of cruzipain-encoding genes were not yet comparatively examined in T. cruzi isolates of all DTUs displaying different degrees of cellular invasion, virulence and pathogenicity. Despite limited to a few strains and lacking sequence analysis, studies suggested relevant diversity of cruzipain genes [55], [56]. Genetic diversity and activity of enzymes from T. cruzi of all DTUs and T. cruzi-like species need to be investigated. An understanding of the expression, repertoires and evolutionary relationships of genes encoding cruzipain in T. cruzi of different DTUs and closest related T. cruzi-like species, and comparison with T. rangeli and T. b. brucei, which are phylogenetically distant from T. cruzi with life cycles (which are phylogenetically distant from T. cruzi and unable to develop within mammalian cells in vitro), whose life cycles differ in vertebrate hosts and vectors and differing in vertebrate hosts and vectors, can assist in clarifying the potential role of these enzymes in the host-parasite interactions, virulence and pathogenicity.
Comparative studies between closely related pathogenic (T. cruzi) and non-pathogenic trypanosomes (T. cruzi-like) can contribute for understanding the evolution of the pathogenicity and virulence of T. cruzi and can be helpful for successful design of control, diagnostic and genotyping strategies. With these purpose, our goals in this study were: to characterize the repertoire of genes encoding cruzipain from isolates of all T. cruzi DTUs and compare them with homologues from T. c. marinkellei, T. dionisii, T. rangeli and T. b. brucei; to analyze the relationships among these genes by network genealogies; to investigate their genomic organization by synteny analysis of loci containing cruzipain genes in trypanosome genomes; to assess the expression of cruzipain homologues by the non-pathogenic T. cruzi-like species by northern-blotting and proteolytic assays.
Results
Comparison of whole Genes Encoding CATL Enzymes in Distinct T. cruzi DTUs, T. dionisii, T. rangeli and T. b. brucei
Like mammalian papain-like enzymes, CATL enzymes of trypanosomatids are synthesized as inactive precursors, consisting of pre, pro, and catalytic domains (cd), and a C-terminal extension. Proteolytic cleavage of the N-terminal pro-domain generates the mature enzyme consisting of a cd domain and a C-terminal extension unique of trypanosomatids [24], [26]. To compare the entire cruzipain sequences (∼450 amino acids), sequences from T. cruzi Sylvio X10.6 and G (TcI), Y and Esmeraldo cl3 (TcII), M6241 cl6 (TcIII), CL Brener (TcVI, one sequence from each the Esmeraldo-like and non-Esmeraldo-like haplotypes) and from Tcbat 1994 were aligned with homologous sequences from T. c. marinkellei 344 and T. dionisii 211. Sequences from non-Schizotrypanum species (T. rangeli and T. b. brucei) were included in the alignments [54].
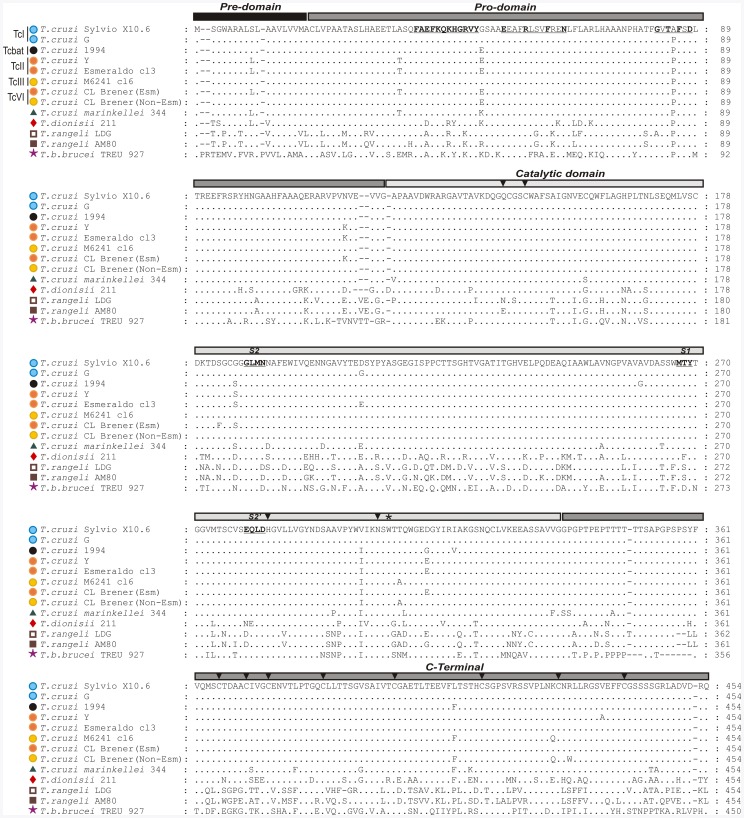
Overall identities were high in the N-terminal region, either in pre- and pro-domains (∼94 and 91%, respectively) and catalytic domains (∼90%), and most variable in the C-terminal regions (∼85%) (Fig. 1). As typically found in peptidases of Clan CA, which are targeted to intracellular compartments and secreted, all cruzipain and homologous genes have a signal peptide at their N-terminal region, as well as the catalytic triad of cysteine, histidine and asparagine residues (Cys25, His159 and Asn179) and the highly conserved Trp181. Important sites for autocatalytic cleavage, the motifs ERFNIN-like and GNFD-like of pro-domains are conserved in all trypanosomes. The clan CA is characterized by having substrate specificity defined by the S2 pocket. In cruzipain genes from all T. cruzi DTUs and homologues from T. c. marinkellei, the S2 subsites are conserved whereas in T. dionisii, T. rangeli and T. b. brucei divergent amino acids were found in these regions, suggesting differences in substrate specificities (Fig. 1).
Figure 1. Alignment of predicted amino acid sequences from entire cruzipain of T. cruzi (TcI, TcII, TcIII, TcVI and Tcbat) and homologues from T. cruzi-like (T. c. marinkellei and T. dionisii), T. rangeli and T. b. brucei.
Pre, pro, catalytic domain and C-terminal extension amino acid sequences of cruzipain genes from T. cruzi Sylvio X10.6 and G (TcI), TCC1994 (Tcbat), Y and Esmeraldo cl3 (TcII), M6241 cl6 (TcIII), CL Brener (TcVI) Non-Esmeraldo-like (TcIII) and Esmeraldo-like (TcII) haplotypes and homologues from T. c. marinkellei (344), T. dionisii (211), T. rangeli (LDG and AM80) and T. b. brucei (TREU 927). The CATL family signatures of pro-domain motifs ERFININ (ERFN) and GNFD (GTFD) are indicated in bold and underlined, the subsites S1, S2 and S2′ are in bold, and the conserved Trp181 are indicated by (*).The glutamine [Q] of the oxyanion hole, cysteine [C], histidine [H] and asparagine [N] of catalytic triad in the catalytic domain, and 8 cysteines in the C-terminal extension are indicated by arrow heads.
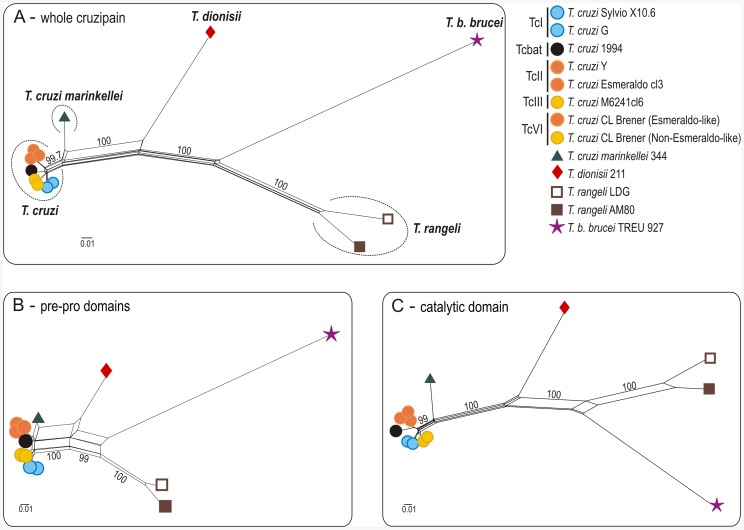
Cruzipain amino acid sequences from T. cruzi isolates were relatively conserved in all domains. The ratio of non-synonymous (dN) to synonymous (dS) substitutions in the catalytic domain was dN/dS <1 by comparing the distinct DTUs of T. cruzi and T. cruzi-like species, suggesting that the enzymatic domain of cruzipain genes has been subjected to stabilizing selection for the conservation of metabolic function within the subgenus Schizotrypanum. Sequences encoding homologous cruzipain genes of T. c. marinkellei were closely related to those of T. cruzi (∼6.5% divergence) but the divergences were larger than those separating the T. cruzi DTUs (maximum of ∼2.5%). Sequences from T. cruzi largely diverged from homologues of T. dionisii (∼20%), T. rangeli (∼33%) and T. b. brucei (∼43%) in all domains (Fig.1). Genealogies based on whole cruzipain genes, or restricted to pre-pro or to catalytic domains, resulted in identical topologies (Fig. 2).
Figure 2. Network genealogies of predicted amino acid sequences from all domains of genes encoding cruzipain in T. cruzi and homologues in other trypanosome species.
Networks produced using the Neighbour-Net algorithm in SplitsTree v4.11.3, excluding all conserved sites and with Uncorrected p-distance. Networks were produced using entire sequences (A), pre- and pro-domains (B) or restricted to catalytic domains (C) of cruzipain encoding genes from the different trypanosomes are indicated by different symbols and colors according to the legend. Numbers in nodes correspond to support values estimated by performing 100 bootstrap replicates using the same parameter optimized for network inferences.
Genealogy of Genes Encoding T. cruzi Cruzipain and Homologues from T. c. marinkellei, T. dionisii and Non-Schizotrypanum Trypanosome Species
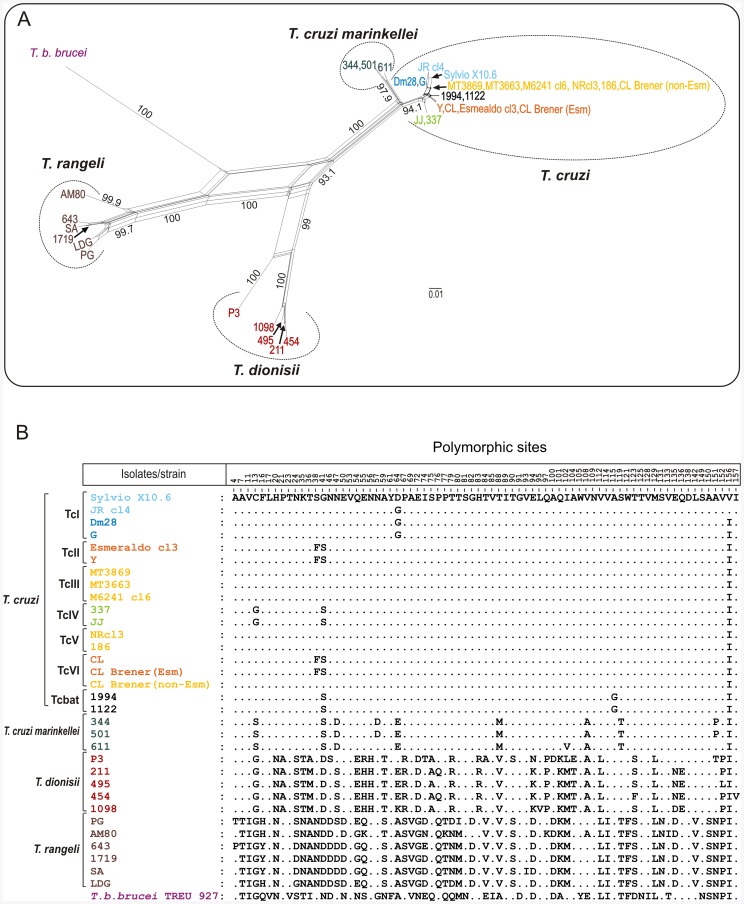
To study the relationships of cruzipain genes from all T. cruzi DTUs, T. c. marinkellei and T. dionisii, and homologues from T. rangeli and T. b. brucei (Table 1), we aligned ∼500 bp cdCATL sequences obtained in this study with the corresponding genes from T. cruzi CL Brener (Esmeraldo-like and non-Esmeraldo-like haplotypes), Esmeraldo cl3, JR cl4, M6241 cl6 and Sylvio X10.6. The analyses of either amino acid (Fig. 3) or nucleotide sequences (data not shown) generated networks of cruzipain genes with similar topologies. Sequences of cd-cruzipain from all T. cruzi DTUs always clustered together in a homogeneous assemblage (∼1.2% amino acid sequence divergence) separated from T. c marinkellei (5.5% divergence), and largely divergent from the cluster formed by T. dionisii from Brazil (20%) and Europe (21%). Amino acid sequences of cruzipain genes of all Schizotrypanum species clustered together and largely separated (28%) from the homologous genes of T. rangeli and T. b. brucei (Fig. 3; Table 1).
Table 1. Trypanosoma cruzi isolates of all DTUs (TcI-TcVI) and other trypanosome species, and their respective sequences of cruzipain and homologous genes determined in this study or retrieved from data banks.
| TCCa code | Trypanosome isolate | Host species | Geographic origin | DTU/genotype | Accession number of cruzipain and homologous sequences | Origin of sequences d | |
| T. cruzi | |||||||
| 1321 | Dm28 | opossum | D. marsupialis | Colombia | TcI | JF421288c/JF421289c | PCR |
| - | Sylvio X10.6 | human | H. sapiens | Brazil | TcI | U41454b | GenBank |
| - | JR cl4 | human | H. sapiens | Venezuela | TcI | TJR4_1_c6805c/TJR4_1_c12312c | draft genome |
| 30 | G | opossum | D. marsupialis | Brazil | TcI | JF421290c/JF421291c/JF421352b/JF825059c/JF825060c | draft genome and PCR |
| 417 | M2542 | bat | T. tricolor | Brazil | TcI | JF421292c/JF421293c | PCR |
| 507 | MO115 | bat | C. perspicillata | Brazil | TcI | JF421294c/JF421295c | PCR |
| 34 | Y | human | H. sapiens | Brazil | TcII | AF314929b/JF421310c/JF421311c | GenBank and PCR |
| 2120 | Esmeraldo cl3 | human | H. sapiens | Brazil | TcII | JF421314c/JF421315c/scf7180000307932b scf7180000305060b/scf7180000304994b | TriTrypDB and PCR |
| 844 | MT3869 | human | H. sapiens | Brazil | TcIII | JF421335c/JF421336c | PCR |
| 845 | MT3663 | triatomine | P. geniculatus | Brazil | TcIII | JF421337c/JF421338c | PCR |
| 1386 | Unidero | dog | C. familiaris | Brazil | TcIII | JF421339c/JF421340c | PCR |
| - | M6241 cl6 | human | H. sapiens | Brazil | TcIII | cCM62_C86 b | draft genome* |
| 85 | JJ (José Julio) | human | H. sapiens | Brazil | TcIV | JF421304c/JF421305c | PCR |
| 337 | Fuscicolis 15 | monkey | S. fuscicolis | Brazil | TcIV | JF421306c/JF421307c | PCR |
| 778 | Rb778 | triatomine | R. brethesi | Brazil | TcIV | JF421308c/JF421309c | PCR |
| 187 | Bertha | human | H. sapiens | Bolivia | TcV | JF421316c - JF421319c | PCR |
| 186 | Tc186 | triatomine | T. infestans | Bolivia | TcV | JF421320c - JF421327c | PCR |
| 967 | NR cl3 | human | H. sapiens | Chile | TcV | JF421328c - JF421334c | PCR |
| 33 | CL | triatomine | T. infestans | Brazil | TcVI | JF421312c/JF421313c/JN701890 c - JN701895 c | PCR |
| CL14 | triatomine | T. infestans | Brazil | TcVI | JF825061c - JF825064c | PCR | |
| - | CL Brener Esmeraldo andNon-Esmeraldo haplotypes unassigned contigs | triatomine | T. infestans | Brazil | TcVI | Tc00.1047053509429.320b/Tc00.1047053507537.20b/Tc00.1047053507603.270b/Tc00.1047053507603.260b/Tc00.1047053507537.10b/AAHK01021104b/AAHK01015705b AAHK01014707b/AAHK01010644b AAHK01012365b/AAHK01018585b AAHK01019951b | TriTrypDB |
| 294 | 998 | bat | M. levis | Brazil | Tcbat | JF421296c/JF421297c | PCR |
| 499 | 1336 | bat | M. nigricans | Brazil | Tcbat | JF421298c/JF421299c | PCR |
| 1994 | MO294 | bat | M. levis | Brazil | Tcbat | JF421300c/JF421301c/JF421353b | draft genome and PCR |
| 1122 | 1122 | bat | M. albescens | Brazil | Tcbat | JF421302c/JF421303c | PCR |
| T. cruzi | marinkellei | ||||||
| 344 | bat | C. perspicillata | Brazil | JF421354b | PCR | ||
| 501 | bat | C. perspicillata | Brazil | JF421343c | PCR | ||
| 611 | bat | A. planirostris | Brazil | JF421344c | PCR | ||
| T. dionisii | |||||||
| - | P3 | bat | P. pipistrellus | England | JF421345c | PCR | |
| 495 | bat | C. perspicillata | Brazil | JF421346c | PCR | ||
| 1098 | bat | Myotis sp | Brazil | JF421347c | PCR | ||
| 454 | bat | D. rotundus | Brazil | JF421348c | PCR | ||
| 211 | bat | E. brasiliensis | Brazil | JF421355b | draft genome | ||
| T. rangeli | Lineagee | ||||||
| 643 | Tra643 | bat | P. lineatus | Brazil | E | FJ997568c | GenBank |
| 1719 | Tra1719 | bat | A. planirostris | Brazil | A | JF421351c | PCR |
| 031 | SA | human | H. sapiens | Colombia | A | FJ997556c | GenBank |
| 086 | AM80 | human | H. sapiens | Brazil | B | JF421356b | draft genome |
| 014 | PG | human | H. sapiens | Panama | C | FJ997564c | GenBank |
| - | LDG cl1 | human | H. sapiens | Colombia | C | L38512b | GenBank |
| T. b. brucei | - | ||||||
| - | T. b. brucei TREU927 | tsetse fly | Glossina sp | Kenya | XM_840125b | GenBank | |
TCC, Code number of the isolates/strains cryopreserved in the Trypanosomatid Culture Collection (TCC); Sequences from cruzipain and homologous genes:
whole genes,
catalytic domains;
cruzipain sequences obtained by sequencing of PCR-amplified genes or from genome databases: GenBank, TriTrypDB and drafts genomes from our Tree of Life project or from the Washington University School of Medicine project*;
Figure 3. Network and polymorphism analyses on catalytic domain of cruzipain genes from different trypanosome species.
Genes from Schizotrypanum species (T. cruzi, T. c. marinkellei and T. dionisii) were compared with homologues from their closest relative species, T. rangeli, and the distant related T. b. brucei. (A) Network of 33 amino acid predicted sequences constructed using the Neighbour-Net algorithm excluding all conserved sites and with Uncorrected p-distance. The numbers in nodes correspond to bootstrap values from 100 replicates. (B) Polymorphism on cruzipain amino acid sequences from the distinct trypanosome species.
Polymorphism of Cruzipain Gene Copies within Isolates and DTUs of T. cruzi
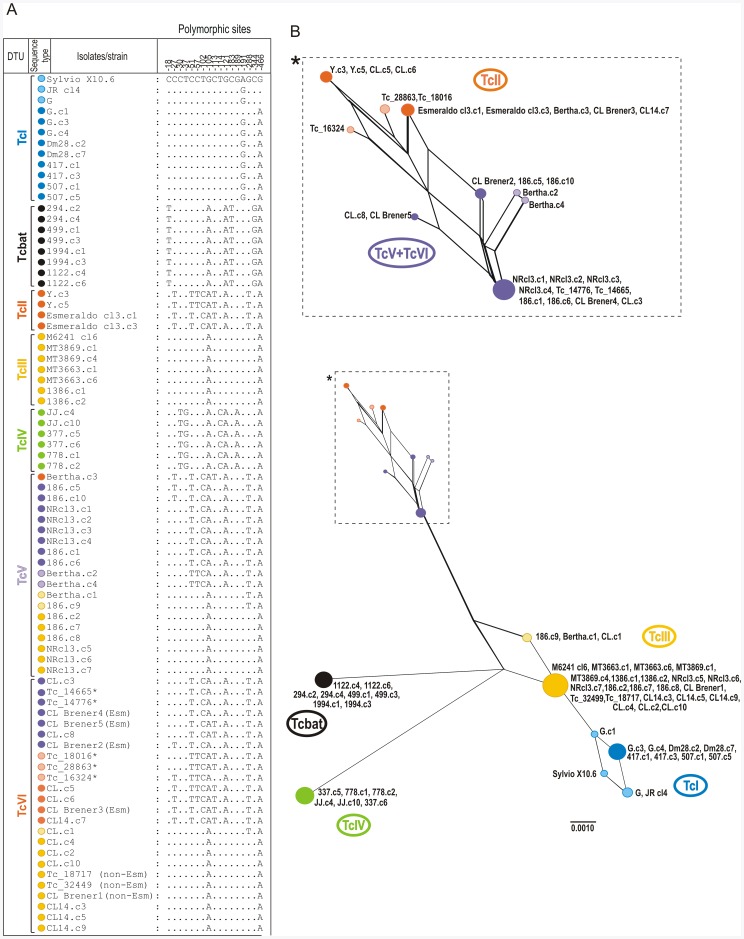
Any attempt to associate cruzipain polymorphisms with biological features of T. cruzi requires a good appraisal of the diversity of gene copies within both one strain/isolate and each DTU. We have assessed the polymorphism on cruzipain gene copies by comparing 3 to 8 sequences from each isolate. Larger number of sequences (7–8) was analyzed from the isolates of hybrid DTUs TcV and TcVI (Table 1). Cruzipain gene copies (paralogous) from isolates of TcI, TcIII, TcIV and Tcbat were identical or highly similar in their amino acid sequences (Fig. 3), whereas diverged in 2 to 6 polymorphic sites in their nucleotide sequences (Fig. 4). We identified a total of 23 variant sequences of cruzipain sequences (Fig. 4). Relatively homogeneous but not identical copies were found by comparing sequences from 6 isolates of TcI and two of TcII. No polymorphic sites were found among sequences from 3 isolates of each TcIII and TcIV. Sequences from Non-Esmeraldo-like haplotype of CL Brener were identical to those of TcIII (Fig. 4).
Figure 4. Polymorphism and network analyses of catalytic domain sequences of cruzipain genes from T. cruzi isolates of TcI-VI and Tcbat.
(A) Polymorphic nucleotide sites on catalytic domains of cruzipain encoding genes; (B) Network based on polymorphic nucleotides constructed with the Neighbour-Net algorithm excluding all conserved sites and with Uncorrected p-distance. The numbers in nodes correspond to bootstrap values from 100 replicates. CLBrener1-5 are sequences from TriTrypDB: Tc00.1047053509429.320, Tc00.1047053507537.20, Tc00.1047053507603.270, Tc00.1047053507603.260 and Tc00.1047053507537.10; *GenBank accession numbers of all sequences included in these analyses are listed on Table1. Major types of sequences from T. cruzi isolates of different DTUs are indicated by different colors according to the legend.
Results disclosed high nucleotide polymorphism on cruzipain gene copies of the heterozygous hybrids assigned to TcV (3 isolates, 4 to 8 sequences of each) and TcVI (3 isolates, 24 sequences). Different sequences were found in TcV (at least 6 sequence types) and TcVI (8 types), including sequences identical to those found in TcII or TcIII, sequences found in both TcV and TcVI, and sequences so far detected exclusively in TcV or TcVI (Fig. 3, 4).
Distinguishing DTUs of T. cruzi according to Polymorphisms on Catalytic Domain of Cruzipain Genes
We evaluated the suitability of the polymorphism on nucleotide sequences of catalytic domain (cd) of cruzipain genes in distinguishing among the T. cruzi DTUs by comparing 80 sequences from 25 isolates of all DTUs (66 sequences from 22 isolates were determined in this study). Sequences differed by 17 polymorphic nucleotide sites and were divided in 5 major types in addition to the hybrid sequences from TcV and TcVI (Fig. 4). Despite polymorphisms within DTUs, unique polymorphic sites distinguished all DTUs. Cruzipain polymorphisms also distinguished Tcbat from all established DTUs (Fig. 4).
To evaluate the suitability of cruzipain polymorphisms as markers for genotyping, we examined at least three isolates from each DTU, excepting TcVI for which CL Brener and CL 14 were analyzed. Despite multiple copies and small polymorphisms among repeats within the same DTUs and even the same strain (Fig. 4), cruzipain encoding genes showed high sequence conservation of catalytic domains from isolates of the same DTU and clustering of sequences was according to DTUs. Thus, the cruzipain analysis agreed with genotyping methods based on either multiple copy or single copy gene markers [6]. In contrast, large polymorphism and unique sequences detected in each TcV and TcVI (Fig. 4) suggested that these markers can be valuable to detect hybrids. Nevertheless, the use of cruzipain genes for T. cruzi genotyping demands the sequencing of several PCR-amplified sequences and comparison of homologous sequences through phylogenetic analyses. A method based on PCR-RFLP analysis of cruzipain genes has been currently developed to facilitate the use of cruzipain as marker for T. cruzi genotyping [Lima et al., in preparation].
Genomic Organization and Synteny of Cathepsin-L Genes in Trypanosome Species
Previous studies showed that cruzipain genes are organized in the genomes of trypanosomes as tandem arrays of duplicated and polymorphic genes located in two or more chromosomes [25]. Analysis of the genomic organization of cruzipain genes in T. cruzi CL Brener Esmeraldo-like and non-Esmeraldo-like haplotypes disclosed polymorphisms in number and organization of genes encoding cruzipain (cruzipain, cruzipain 2 and other putative isoforms). There was substantial variation in number, sequence, chromosome and position of the duplicate genes of the two haplotypes; Esmeraldo-like showed 1–4 cruzipain repeats dispersed in three loci and non-Esmeraldo-like present 3–5 copies in three loci (data from TriTrypDB).
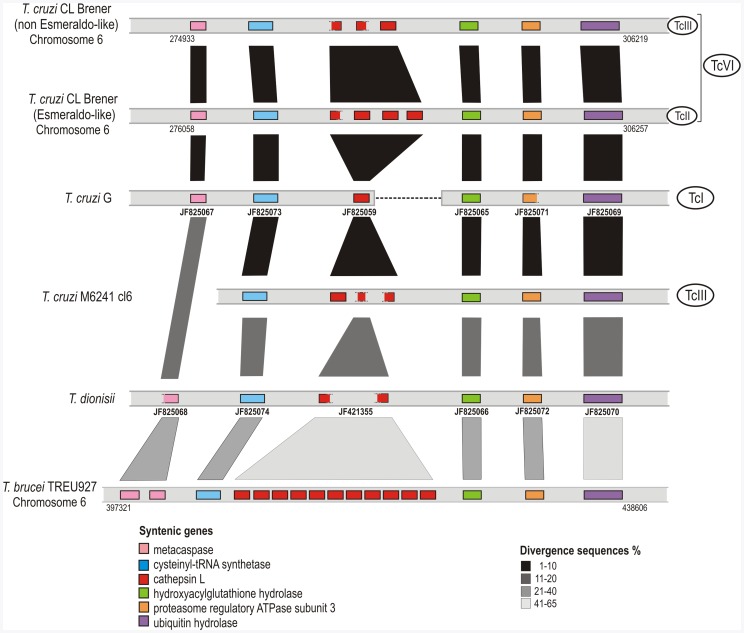
Chromosome segments from the genome of T. cruzi CL Brener (TcVI) containing three and four tandem copies of cruzipain in Esmeraldo and non-Esmeraldo haplotypes, respectively, were compared with data from the genome drafts of T. cruzi G (TcI), M6241 cl6 (TcIII) and T. dionisii. Homologous segments of chromosome 6 from T. cruzi CL Brener containing cruzipain repeats were found in the genomes of other T. cruzi strains: one cruzipain gene copy from T. cruzi G (of three copies detected in the genome) and three from M6241 cl6 (6 copies in the genome). In the genomes of these strains, cruzipain and homologous genes were flanked by five orthologous genes thus constituting a syntenic block (Fig. 5). Homologous cruzipain genes arranged in the same order were detected in the genomes of T. dionisii (two out of three copies found in the genome draft) and T. b. brucei (an array of 11 identical copies of brucipain in the chromosome 1) (Fig. 5). This syntenic block was selected for this study considering the positioning of cruzipain gene copies from distinct T. cruzi strains and the synteny shared with T. dionisii, T. b. brucei (Fig. 5) and other trypanosome species as showed with T. vivax and T. congolense genome drafts (data not shown).
Figure 5. Synteny of a locus containing cruzipain genes in T. cruzi, T. dionisii and T. b. brucei.
Segments from the chromosome 6 of T. cruzi CL Brener non-Esmeraldo-like and Esmeraldo-like haplotypes, corresponding to TcIII and TcII, respectively, showing 3 to 4 cruzipain gene copies (entire or partial) flanked by orthologous genes marked with different colors according to the legend. Data from the draft assembly of T. cruzi G, M6241 cl6 and T. dionisii allowed to place one, three or two cruzipain gene copies, respectively, within the same syntenic region (figure do not reflect their actual position on chromosomes). Syntenic region from the chromosome 6 of T. b. brucei comprising 11 copies in tandem of brucipain genes was included in the alignment. The shades of vertical gray bars indicate the variable degrees of divergence between sequences according to the legend. The accession codes of all contigs/scaffolds and GenBank accession numbers (in bold) are presented below the corresponding sequences.
Assembly of repetitive sequences in tandem arrays is very problematic, and both the copy number and position are very difficult to be accurately determined. Miss-assembly from collapsed repeat sequence frequently arises during automated genome assembly when small sequence reads originating from distinct repeat copies are incorrectly joined to generate a single unit. This artefact could not be ruled out in the genome drafts analyzed. To avoid mis-assembled genes, we selected contigs containing sequences from both cruzipain and adjacent orthologous genes, which warrant the positioning of the genes encoding cruzipain in this syntenic region (Fig. 5). The degrees of sequence identity between each syntenic gene (Fig. 5) agreed with the phylogenetic relationships among all trypanosome species investigated: T. cruzi, T. c. marinkellei, T. dionisii, T. rangeli and T. b. brucei [7], [10], [11], [14], [21], [54].
Comparative Expression Analyses of Cruzipain and Homologues in T. cruzi-like and T. rangeli by Northern Blot Hybridization and Detection of Proteolytic Activity in Gelatin Gels
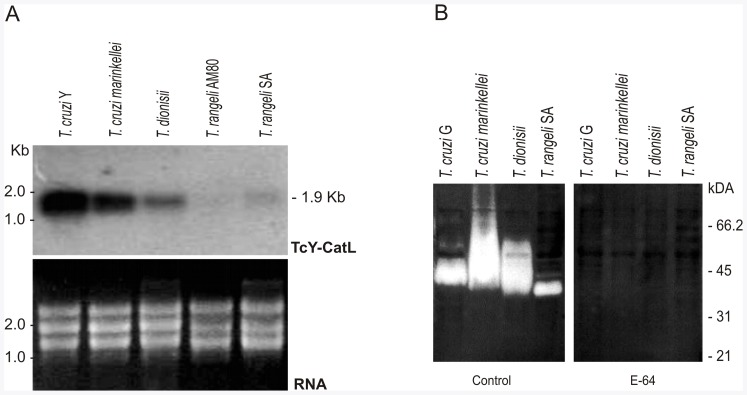
To assess the expression of cruzipain and homologous enzymes from virulent and non-virulent and human-infective or bat-restricted trypanosome species, developing or not within cells, total RNA from epimastigotes of Schizotrypanum species (T. cruzi Y, T. c. marinkellei and T. dionisii) and T. rangeli was compared by northern-blot hybridization using T. cruzi Y derived catalytic domain cruzipain probe that strongly cross-hybridized with RNA from other T. cruzi strains (G, CL and JJ) and from all other trypanosome species using low stringent conditions (data not shown). However, hybridization signals using more stringent conditions correlated very well with sequence identity displaying high cross-hybridization signal with transcripts of T. cruzi, moderate hybridization with T. c. marinkellei, weak hybridization signal with transcripts of T. dionisii and lack of hybrization with T. rangeli isolates. This analysis allowed estimation of transcripts of similar size (∼1.9 Kb) for all the Schizotrypanum species (Fig. 6). In agreement with the differential cross-hybridization among the transcripts of different species, we previously demonstrated that a probe consisting of T. rangeli catalytic domain of CATL (rangelipain) strongly hybridized with T. rangeli while cross-hybridization with Schizotrypanum species was very weak [54].
Figure 6. Comparative expression analysis of cruzipain and homologues in T. cruzi, T. cruzi marinkellei, T. dionisii and T. rangeli.
(A) Northern blotting analysis of cruzipain transcripts from T. cruzi (Y) and cross-hybridization using the probe consisting of PCR-amplified catalytic domains of T. cruzi Y cruzipain labeled with 32P T. cruzi Y (TcY-CatL probe); agarose gel of RNA used for this analysis was stained with ethidium bromide (EtBr). (B) CATL proteolytic activities detected in epimastigote lysates of T. cruzi G, T. cruzi marinkellei, T. dionisii and T. rangeli. Activity banding profiles detected in gelatin gels, pH 5.0 and 5 mM DTT were inhibited in gel incubated with 10 µM E-64.
Cruzipain, a glycoprotein of ∼50–60 kDa, is post-transcriptionally regulated during the T. cruzi life cycle [4], [24], [26]. Here, proteolytic activities related to CATL were detected in epimastigote lysates of all Schizotrypanum species examined (Fig. 6B). In gels incubated at pH 5.0 with 5 mM DTT (a condition found to be optimal for the detection of cysteine proteases in these trypanosomes), remarkable high activities resolving in the region of ∼55 to 35 kDa were detected in lysates of T. c. marinkellei and T. dionisii. Lower cruzipain activity was detected for T. cruzi G of TcI compared to T. cruzi-like trypanosomes (Fig. 6A). We have detected distinct profiles and comparable activity levels for all T. cruzi strains such as the virulent Y and CL (TcII) (data not shown) and the non-virulent JJ (TcIV) [54] using the same conditions for the activity assays (data not shown). Weaker activity was detected in T. rangeli, confirming lower expression of rangelipain compared to cruzipain as previously demonstrated [54]. All activities were fully inhibited by the cysteine protease-specific inhibitor E-64, thus corroborating the activity of CATL enzymes (Fig. 6B).
Discussion
In this study, we compared cruzipain-encoding genes of T. cruzi isolates representatives of all known subspecific phylogenetic diversity, including all DTUs (TcI-TcVI) and the new T. cruzi genotype Tcbat, as well as homologous genes from T. cruzi-like species, T. c. marinkellei and T. dionisii, the closest relatives of T. cruzi. Results demonstrated that cruzipain genes from a large set of T. cruzi isolates representative of the overall biological and genetic diversity compared with homologous CATL genes from other species in a phylogenetic framework disclosed divergences that closely parallel their phylogenetic diversity [5]–[7], [10], [11], [14], [57]. Our findings revealed species-specific and DTU-specific variability that may be valuable for elucidation of the roles of cruzipain in host-parasite interactions, virulence and pathogenicity evolution.
Isolates of T. cruzi show a range of variation in important biological, immunological, pathological (morbidity and mortality) and clinical characteristics [5], [6]. It is likely that much of this variation is probably due to genetic differences among isolates that can be related to specific DTUs. The genomes of the “non virulent” T. cruzi Sylvio X10/1 (TcI) and “virulent” T. cruzi CL Brener (TcVI) are highly similar in their gene-dense ‘‘core’’ coding regions, which show strongly conserved synteny interspersed with variable repetitive sequences [58]. Large differences within and among DTUs have emerged in several repetitive gene families such as cysteine proteases, mucins, trans-sialidases, surface protease gp63 and amastigote surface glycoproteins (amastins) [58]–[61].
In this study, a relevant conservation of amino acid sequences of genes encoding cruzipain was found among T. cruzi of all DTUs, Tcbat and T. c. marinkellei compared to more divergent sequences from T. dionisii. Cruzipain sequences from all these species tightly clustered together and were more related to rangelipain from T. rangeli than to brucipain of T. b. brucei. Notwithstanding hard efforts to find sequences homologous to cruzipain 2, no sequences were found that displayed the signature residues identifying this isoform [28]. Besides sequences homologous to major cruzipain, which comprised the largest part of sequences detected, our searches in the genome data banks and PCR-amplified sequences disclosed a few heterogeneous sequences closely related to cruzipain 2 [Lima et al., in preparation] but lacking the signature residues reported as characteristics for the archetype of this isoform. Variations in residues in the S2 pocket may account for peculiar activity and substrate specificity of cruzipain isoforms, hence, detailed biochemical studies will be necessary to assess expression, activity and functions in order to verify whether these sequences encode new isoforms or variants of cruzipain 2. In any case, our findings confirmed that phylogenetic analyses are valuable to discover new cruzipain isoforms and/or variants as recently shown for congopain variants through combined genetic and functional approaches [62].
In network genealogies, sequences of cruzipain from the same species, as well as from strains/isolates of the same phylogenetic lineages, always clustered together. Even with the high degree of conservation in cruzipain genes from all DTUs, polymorphisms on nucleotide sequences from the catalytic domain of cruzipain generated 5 branches within T. cruzi, each one comprising sequences from one DTU (TcI-TcVI) or Tcbat. In general, sequences from cruzipain gene copies within the same DTU showed small or no variation at all, as we verified for isolates of TcI-TcIV. Data from these DTUs were consistent with their epidemiology and evolutionary histories, with distances among cruzipain genes suggesting that they have had more time segregated from each other than the more recently emerged TcV-TcVI. Isolates of the same DTUs appear to have had a long association with preferential mammalian hosts and vectors and, consequently, naturally circulate separated by biological, ecological and geographical barriers [5]–[7], [63], [64]. TcV and TcVI were confirmed as formed by heterozygous isolates exhibiting polymorphic cruzipain sequences, in addition to sequences identical to those of putative donors TcII or TcIII. In agreement with their hybrid origin, sequences from TcV and TcVI clustered with TcII or TcIII forming a reticulate pattern in the network genealogy of cruzipain genes. Tandem arrays of cruzipain genes in T. cruzi CL Brener genome showed sequences apparently derived from TcII (Esmeraldo-like) and TcIII (Non-Esmeraldo-like), even within a single locus. Unique sequences detected in TcV or TcVI strains could be due to the fact that these strains originated from hybridization between strains of TcII and TcIII not included in this study. In addition, more heterogeneous and unique sequences within hybrid strains could emerge post-hybridization through homologous recombination. Our results corroborate that TcV and TcVI resulted from the hybridization between TcII and TcIII as hypothesized before using other markers. Studies have been performed to better understand the role of hybridization events in shaping the genetic diversity within T. cruzi [65]–[67]. Our findings confirmed Tcbat as a new T. cruzi genotype not yet assigned to any DTU. Sequences from Tcbat clustered closest to TcI but separated from all DTUs as demonstrated with other markers [6], [7], [68].
Genealogy of cruzipain and homologous genes inferred in this study confirmed T. c. marinkellei isolates as closest relative and outgroup for T. cruzi isolates, as previously shown by examination of other genes including SSUrRNA, gGAPDH and Cytb [10], [14], [22]. In a comparative proteomic analysis, T. c. marinkellei was indistinguishable from T. cruzi reinforcing their very close relatedness [69]. To date, Tcbat, T. c. marinkellei and T. dionisii have been found exclusively in bats and all invade and develop in culture cells of a variety of mammals. Tcbat was infective to mice, despite very low virulence. However, these three bat trypanosomes have been reported to be incapable of development in triatomine species commonly infected by T. cruzi [7]–[10], [14], [16].
Evolutionary studies of cruzipain and homologous genes from other trypanosome species corroborated phylogenetic relationships based on SSUrRNA and gGAPDH genes of all species investigated: T. carassii from fish [70], T. rangeli [54], T. vivax [71] and T. theileri [72], [73], the later three from mammals. Evolutionary relationships of cruzipain and homologues from these trypanosome species indicated that this gene family expanded by successive gene duplications followed by divergences giving rise to genes with greater similarity when originated from the same rather than distinct species/genotypes. This suggests that concerted evolution is a widespread homogenizing force in this trypanosome multigene family. Consequently, CATL-like gene duplicates (paralogous) of all trypanosome species examined clustered by species, i.e. like orthologous genes in different species, as shown for several repeated genes in tandem arrays from African trypanosomes [74]. However, gene conversion and positive selection can generate diversity in sequence, quantity and order among tandemly repeated genes as showed in this and in previous studies on trypanosomatids [59]–[61], [74].
Results from this study disclosed molecular markers able to identify the phylogenetically closely related T. cruzi and T. cruzi-like species. Reliable identification and knowledge of the diversity and epidemiology of these species are crucial to understand the shared evolutionary history of T. cruzi and T. cruzi-like bat trypanosomes [7], [10], [11], [14], [57]. We have previously shown that T. cruzi and T. rangeli [54], T. vivax [71] and T. theileri [72] could be diagnosed by specific PCR assays targeting cruzipain sequences. In this study, we demonstrated that cruzipain sequences clustered T. cruzi isolates according to DTUs, similarly to CATL-like gene based genotyping of lineages within T. rangeli [54], T. vivax [71] and T. theileri [72], [73]. Altogether, these previous studies and results herein described support the use of these genes as valuable markers for both inter- and intra-species phylogenetic analyses of trypanosomes.
There are clear evidence that immunization with cruzipain catalytic domain, and not with C-terminal domain, confer important cellular protective immunity against T. cruzi, as evidenced by the reduction in parasitemia, tissue parasitism and mortality in mice, indicating the existence of epitopes important for protective immunity in the catalytic domains and, thus, supporting the use of cruzipain for vaccine development [34]–[37]. The main goal of all vaccination strategies should be a polyvalent vaccine against infection by different strains of T. cruzi of any DTU. Although the polymorphism among cruzipain catalytic domains expressed by distinct strains appears to be reduced, for vaccination purposes it is important to evaluate strain-variant and conserved cross-reactive epitopes in different T. cruzi DTUs, and among strains of the same DTU, and the possible relevance of epitope polymorphism for species- or strain-specific protective immune response [75]–[77]. Therefore, knowledge on the genetic diversity within the plethora of DTUs and strains of any antigen vaccine candidate for Chagas disease can be decisive for the design of efficient and polyvalent vaccines using, if necessary, a pool of stage- and strain-specific antigens. Here, we showed for the first time that all the six DTUs, and even different strains of DTUs, exhibited specific genetic variants of cruzipain. Any possible implications of the cruzipain polymorphisms to warrant efficient cross-protection of a potential vaccine require investigations to demonstrate the immunogenicity of cruzipain variants from different T. cruzi DTUs.
Drugs currently employed for the treatment of Chagas disease have serious limitations due to their failure in chronic patients and side effects. Inhibitors of cruzipain kill the parasite and cure infected mice, thus validating this enzyme as a very promising target for design of new drugs [1], [2], [4], [43], [44]. In this study, we demonstrated that substrate-binding subsites of cruzipain are conserved in all T. cruzi DTUs, but diverge in T. dionisii and other trypanosome species. T. c. marinkellei cruzipain share identical subsites and, hence, can be a valuable non-infective to human model to test drugs against trypomastigotes and intracellular amastigotes. The efficacy of the cruzipain inhibitor K777 was evidenced against 6 T. cruzi strains differing in tissue tropisms and drug susceptibility [44]. More strains must be tested to verify the efficacy of inhibitors for the ample diversity of cruzipain variants expressed by T. cruzi of the different DTUs.
Characterization of genes encoding major cruzipain, genealogies of genetic variants and genomic organization were addressed in this study for the first time to compare T. cruzi of all DTUs and closest related T. cruzi-like species. This is the most comprehensive study using sequences from protein-encoding genes to compare isolates of T. cruzi from all DTUs. Of the strains selected for genome sequencing CL Brener and Esmeraldo are virulent whereas Sylvio X10.6 and G strain are considered of low virulence. Most T. cruzi isolates examined here by PCR-amplification of cruzipain genes were also previously analysed regarding virulence for mice: TcI, Tcbat and TcIV isolates were non-virulent whereas TcIII (TCC1386) and TcV (TCC197) isolates showed to be virulent for mice (data not shown). Interestingly, non-virulent CL14 clone was shown in this study to be a hybrid strain like the virulent CL Brener [52]. All the six DTUs were supported by cruzipain gene markers. Infections caused by different strains of T. cruzi from distinct DTUs extensively diverged in the morbidity and mortality. Attempts to associate T. cruzi DTUs with behavioral phenotypes, including virulence and pathogenicity in mice, metacyclogenesis and cell infectivity, suggested an important degree of association [5], [6], [60]. However, besides some overlap between different DTUs, there is a broad intra-DTU phenotypic diversity (largest differences have been reported within TcI) and even among clones of a given strain such as the virulent CL Brener and non-virulent CL14 clones, both of TcVI. Therefore, we can associate cruzipain polymorphisms to DTUs. However, available data are insufficient to support strong correlations between behavioural phenotypes and DTUs. This goal will require extensive studies of T. cruzi strains from all DTUs through a combination of phylogenetic, biochemical, pathological and immunological approaches.
Our findings indicated conserved major cruzipain in T. cruzi of all DTUs while other trypanosome species express diverse homologous enzymes. Results showed, for the first time, the expression of cruzipain transcripts by T. c. marinkellei and T. dionisii that correlated very well to sequence divergences among all trypanosome species investigated and, in addition, revealed high proteolytic activity in these two non-pathogenic species. An understanding of the cruzipain gene repertoires, expression and functions can help to elucidate the evolutionary history that shaped variability within T. cruzi and its divergence from T. cruzi-like species and the more distantly related T. rangeli. T. cruzi-like trypanosomes, despite sharing genomic and proteomic features [10], [11], [14], [69], exhibit many peculiarities that can potentially explain differences in host preference/restriction, virulence and pathogenesis. Results from this comprehensive study on major cruzipain isoform are the initial steps toward understanding the roles played by genetic repertoires of cruzipain enzymes and homologues in the life cycles and infections caused by T. cruzi of all DTUs, T. cruzi-like species and T. rangeli.
Materials and Methods
PCR Amplification, Sequencing and Phylogenetic Analysis of CATL Sequences
The whole sequences of cruzipain genes (∼1.4 Kb) of T. cruzi Esmeraldo cl3 and CL Brener were retrieved from TriTrypDB. Homologous genes in T. cruzi M6241 cl6 (Project ID:59941) were obtained from the genome drafts produced by the Genome Institute at Washington University School of Medicine (St. Louis, USA). T. cruzi G, Tcbat 1994, T. dionisii 211 and T. rangeli AM80 were obtained from genome drafts that we are currently performing using standard pyrosequencing shotgun methodology according to Roche 454 protocols as described previously [78]. Resulting reads were submitted to Roche’s Newbler software (version 2.3) and contigs containing genes described in this study were assembled in scaffolds (Table 1). Sequences retrieved from genome data banks were aligned with homologues from T. cruzi Sylvio X10.6 and Y, T. rangeli LDG and T. b. brucei TREU 927 from GenBank and T. c. marinkellei 344 determined in this study. The whole sequence from T. c. marinkellei was obtained by PCR-amplification using the primers TDIO5-FOR (5′ ATG ACG AGC TGG GCG CGT G 3′) and CATL3REV2 (5′ TTA GCT TCA GGA GCG GCG ATG 3′) and the conditions described previously [54], [71]. Cathepsin L sequences determined in this study are available in GenBank (Table 1).
PCR products from catalytic domain of cruzipain genes (cd-cruzipain) obtained using primers and reaction conditions described previously [54], [71] were cloned, and 3 to 8 clones from each species/isolates were sequenced. Alignment of sequences encoding cd-cruzipain genes includes 14 sequences from data banks and 66 determined in this study of all DTUs (TcI-TcVI), Tcbat and homologues from T. c. marinkellei and T. dionisii from Brazil and England [7], [14]. Sequences from non-Schizotrypanum trypanosomes, T. rangeli isolates (SA, AM80, PG, 643, 1719 and LDG) representative of its major lineages [21], [22], and T. b. brucei were also added in the alignment. Sequences determined in this study reflecting the spectrum of genetic polymorphism observed were deposited in GenBank (Table 1). All sequences employed in this study are listed in Table 1.
We initially performed a broad search in all available genomes aiming to detect cruzipain isoforms. Most sequences were homologous to the major cruzipain, and no sequences showed the amino acid residues typical of cruzipain 2. A significant number of sequences from distinct strains of T. cruzi, including those obtained by PCR-amplification were found to be different from cruzipain and cruzipain 2 (data not shown). For genealogy analysis, we selected only sequences with the signatures of cruzipain (Fig. 1), thus removing some sequences of other putative isoforms. Sequences were aligned using Clustal X [79] and manually adjusted. The amino acid sequence of cruzipain was used as a template to ensure codon-to-codon correspondence. Phylogenetic relationships were inferred using nucleotide and predicted amino acid sequences from the entire genes or restricted to pre-pro or cdCATL domains. Network genealogy was inferred in SplitsTree v4.11.3 using the neighbor-net method [80]. Internode supports were estimated by performing 100 bootstrap replicates using the same parameters optimized for network inferences.
Synteny and Codon Analyses
Syntenic genes flanking CATL genes were identified in the genomes of T. cruzi CL Brener (Non-Esmeraldo-like and Esmeraldo-like haplotypes) and T. b. brucei 927 genomes (TriTrypDB). Scaffolds comprising cruzipain genes from T. cruzi G and M6241 cl6 and homologues from T. dionisii were aligned with these syntenic segments. Search on the draft genome of Sylvio X10/1 [58], Tcbat 1994 and T. rangeli AM80 disclosed cruzipain sequences, most partial, in small reads that could not be positioned in homologous scaffolds. The ratio of non-synonymous to synonymous (dN/dS) amino acid changes was calculated according to Yang and Nielsen [81] using PAML, v.4.2 software to infer relative selection pressures [82].
Northern Blot and Proteolytic Activities of Cruzipain
For Northern blot analysis, 10 pg of total RNA extracted using Trizol (Gibco BRL) from epimastigotes of T. cruzi Y, T. c. marinkellei 344, T. dionisii 211 and T. rangeli isolates AM80 and SA was electrophoresed in 1.0% agarose gel and blotted onto nylon membrane. The membrane was hybridized for 14–16 hr at 40°C with a probe consisting of PCR-amplified cd-cruzipain of T. cruzi Y labeled with 32P and washed in 1×bufffer (0.3 M NaCl, 0.3 mM Na citrate, pH 7.0 containing 0.1% SDS) at 50°C for 1 h as described previously [54].
To assess the proteolytic activities in gelatin gels, lysates of cultured epimastigotes were subjected to electrophoresis in 10% resolving SDS-acrylamide gels containing 500 µg/ml gelatin as described before [54], [72]. Briefly, gels were incubated in 2.5% (v/v) Triton X-100 in 0.1 M buffer (acetate: pH 4.0 and 5.0; Tris-HCl: pH 7.5) containing 5 mM DTT (dithiothreitol) and then, for ∼18 h in buffer-DTT. Maximum activity was detected in pH 5.0 and was greatly stimulated by DTT. Bands associated to cysteine proteases were identified by incubating gel halves with 10 µM E-64. Gels were fixed with 10% TCA, and stained with Coomassie blue R-250 [54], [72].
Acknowledgments
We would like to thank M. Campaner for culture of parasites and M. M. Yamamoto and Carmem S. A. Takata for sequencing support at USP. We thank V. Lee, S. L. Hendricks and Y. Wang of the Nucleic Acids Research Facilities for the genome drafts at VCU. We are grateful to The Wellcome Trust (TriTrypDB) and to The Genome Institute at Washington University School of Medicine (St. Louis, USA), project ID 59941, for making sequences from complete and draft genomes of trypanosomes freely available.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This research was funded by grants from Brazilian agency CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) to MMGT and from the NSF (USA) Grant: Assembling the Tree of Life: Phylum Euglenozoa (GAB, PI). PAO is doctorate fellow sponsored by CNPq-PROTAX (Programa Nacional de Taxonomia do CNPq). LL, APC and FMS are postdoctoral fellows sponsored by CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
References
- 1.McKerrow JH, Caffrey C, Kelly B, Loke P, Sajid M. Proteases in parasitic diseases. Annu Rev Pathol. 2006;1:497–536. doi: 10.1146/annurev.pathol.1.110304.100151. [DOI] [PubMed] [Google Scholar]
- 2.Atkinson HJ, Babbitt PC, Sajid M. The global cysteine peptidase landscape in parasites. Trends Parasitol. 2009;25:573–581. doi: 10.1016/j.pt.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caffrey CR, Steverding D. Kinetoplastid papain-like cysteine peptidases. Mol Biochem Parasitol. 2009;167:12–19. doi: 10.1016/j.molbiopara.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Alvarez VE, Niemirowicz GT, Cazzulo JJ. The peptidases of Trypanosoma cruzi: digestive enzymes, virulence factors, and mediators of autophagy and programmed cell death. Biochim Biophys Acta. 2012;1824:195–206. doi: 10.1016/j.bbapap.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 5.Miles MA, Llewellyn MS, Lewis MD, Yeo M, Baleela R. The molecular epidemiology and phylogeography of Trypanosoma cruzi and parallel research on Leishmania: looking back and to the future. Parasitology. 2009;136:1509–1528. doi: 10.1017/S0031182009990977. [DOI] [PubMed] [Google Scholar]
- 6.Zingales B, Miles MA, Campbell DA, Tibayrenc M, Macedo AM. The revised Trypanosoma cruzi subspecific nomenclature: rationale, epidemiological relevance and research applications. Infect Genet Evol. 2012;12:240–253. doi: 10.1016/j.meegid.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 7.Marcili A, Lima L, Cavazzana M, Junqueira AC, Veludo HH. A new genotype of Trypanosoma cruzi associated with bats evidenced by phylogenetic analyses using SSU rDNA, cytochrome b and Histone H2B genes and genotyping based on ITS1 rDNA. Parasitology. 2009;136:641–655. doi: 10.1017/S0031182009005861. [DOI] [PubMed] [Google Scholar]
- 8.Hoare CA. The trypanosomes of mammals: a zoological monograph. Oxford: Blackwell Scientific Publications. 749 p. 1972.
- 9.Molyneux DH. Kreier JP, Baker JR, editors. Trypanosomes of bats. 1991. pp. 195–223. Parasitic Protozoa. Academic Press: New York.
- 10.Lima L, Silva FM, Neves L, Attias M, Takata CS. Evolutionary insights from bat trypanosomes: Morphological, developmental and phylogenetic evidence of a new species, Trypanosoma (Schizotrypanum) erneyi sp. nov., in African bats closely related to Trypanosoma (Schizotrypanum) cruzi and allied species. Protist in press. DOI. 2012. http://dx.doi.org/10.1016/j.protis.2011.12.003. [DOI] [PubMed]
- 11.Hamilton PB, Teixeira MMG, Stevens JR. The evolution of Trypanosoma cruzi: the ‘bat seeding’ hypothesis. Trends Parasitol. 2012;28:136–141. doi: 10.1016/j.pt.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Baker JR, Liston AJ. Trypanosoma (Schizotrypanum) dionisii: effect of various agents on attachment and entry to macrophages in vitro and on morphogenesis. J Gen Microbiol. 1978;104:79–89. doi: 10.1099/00221287-104-1-79. [DOI] [PubMed] [Google Scholar]
- 13.Glauert AM, Baker JR, Selden LF. Mechanism of entry and development of Trypanosoma dionisii in non-phagocytic cells. J Cell Sci. 1982;56:371–387. doi: 10.1242/jcs.56.1.371. [DOI] [PubMed] [Google Scholar]
- 14.Cavazzana M, Jr, Marcili A, Lima L, Maia da Silva F, Junqueira AC. Phylogeographical, ecological and biological patterns shown by nuclear (ssrRNA and gGAPDH) and mitochondrial (Cyt b) genes of trypanosomes of the subgenus Schizotrypanum parasitic in Brazilian bats. Int J Parasitol. 2010;40:345–355. doi: 10.1016/j.ijpara.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 15.Oliveira MP, Cortez M, Maeda FY, Fernandes MC, Haapalainen EF. Unique behavior of Trypanosoma dionisii interacting with mammalian cells: invasion, intracellular growth, and nuclear localization. Acta Trop. 2009;110:65–74. doi: 10.1016/j.actatropica.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 16.Maeda FY, Cortez C, Alves RM, Yoshida N. Mammalian cell invasion by closely related Trypanosoma species T. dionisii and T. cruzi. Acta Trop. 2012;121:141–147. doi: 10.1016/j.actatropica.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 17.Branquinha MH, Vermelho AB, Goldenberg S, Bonaldo MC. Characterization of proteinases in trypanosomatids. Braz J Med Biol Res. 1994;27:495–499. [PubMed] [Google Scholar]
- 18.Branquinha MH, Vermelho AB, Almeida IC, Mehlert A, Ferguson MA. Structural studies on the polar glycoinositol phospholipids of Trypanosoma (Schizotrypanum) dionisii from bats. Mol Biochem Parasitol. 1999;30:179–189. doi: 10.1016/s0166-6851(99)00107-3. [DOI] [PubMed] [Google Scholar]
- 19.Petry K, Voisin P, Baltz T, Labouesse J. Epitopes common to trypanosomes (T. cruzi, T. dionisii and T. vespertilionis (Schizotrypanum): astrocytes and neurons. J Neuroimmunol. 1987;16:237–252. doi: 10.1016/0165-5728(87)90078-6. [DOI] [PubMed] [Google Scholar]
- 20.Baker JR. Bat trypanosome models for Trypanosoma cruzi. Parasitol Today. 1985;1:111–113. doi: 10.1016/0169-4758(85)90006-7. [DOI] [PubMed] [Google Scholar]
- 21.Maia da Silva F, Junqueira AC, Campaner M, Rodrigues AC, Crisante G. Comparative phylogeography of Trypanosoma rangeli and Rhodnius (Hemiptera: Reduviidae) supports a long coexistence of parasite lineages and their sympatric vectors. Mol Ecol. 2007;16:3361–3373. doi: 10.1111/j.1365-294X.2007.03371.x. [DOI] [PubMed] [Google Scholar]
- 22.Maia da Silva F, Marcili A, Lima L, Cavazzana M, Jr, Ortiz PA. Trypanosoma rangeli isolates of bats from Central Brazil: genotyping and phylogenetic analysis enable description of a new lineage using spliced-leader gene sequences. Acta Trop. 2009;109:199–207. doi: 10.1016/j.actatropica.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 23.Scharfstein J, Schechter M, Senna M, Peralta JM, Mendonca-Previato L. Trypanosoma cruzi: characterization and isolation of a 57/51,000 m.w. surface glycoprotein (GP57/51) expressed by epimastigotes and bloodstream trypomastigotes. J Immunol. 1986;137:1336–1341. [PubMed] [Google Scholar]
- 24.Eakin AE, Mills AA, Harth G, McKerrow JH, Craik CS. The sequence, organization, and expression of the major cysteine protease (cruzain) from Trypanosoma cruzi. J Biol Chem. 1992;267:7411–7420. [PubMed] [Google Scholar]
- 25.Campetella O, Henriksson J, Aslund L, Frasch AC, Pettersson U. The major cysteine proteinase (cruzipain) from Trypanosoma cruzi is encoded by multiple polymorphic tandemly organized genes located on different chromosomes. Mol Biochem Parasitol. 1992;50:225–234. doi: 10.1016/0166-6851(92)90219-a. [DOI] [PubMed] [Google Scholar]
- 26.Cazzulo JJ, Stoka V, Turk V. Review. Cruzipain, the major cysteine proteinase from the protozoan parasite Trypanosoma cruzi. Biol Chem. 1997;378:1–10. doi: 10.1515/bchm.1997.378.1.1. [DOI] [PubMed] [Google Scholar]
- 27.Lima AP, Tessier DC, Thomas DY, Scharfstein J, Storer AC. Identification of new cysteine protease gene isoforms in Trypanosoma cruzi. Mol Biochem Parasitol. 1994;67:333–338. doi: 10.1016/0166-6851(94)00144-8. [DOI] [PubMed] [Google Scholar]
- 28.Lima AP, dos Reis FC, Serveau C, Lalmanach G, Juliano L. Cysteine protease isoforms from Trypanosoma cruzi, cruzipain 2 and cruzain, present different substrate preference and susceptibility to inhibitors. Mol Biochem Parasitol. 2001;114:41–52. doi: 10.1016/s0166-6851(01)00236-5. [DOI] [PubMed] [Google Scholar]
- 29.Monteiro AC, Schmitz V, Morrot A, de Arruda LB, Nagajyothi F. Bradykinin B2 Receptors of dendritic cells, acting as sensors of kinins proteolytically released by Trypanosoma cruzi, are critical for the development of protective type-1 responses. PLoS Pathog. 2007;11:e185. doi: 10.1371/journal.ppat.0030185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doyle PS, Zhou YM, Hsieh I, Greenbaum DC, McKerrow JH. The Trypanosoma cruzi protease cruzain mediates immune evasion. PLoS Pathog. 2011;7(9):e1002139. doi: 10.1371/journal.ppat.1002139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scharfstein J, Rodrigues MM, Alves CA, de Souza W, Previato JO. Trypanosoma cruzi: description of a highly purified surface antigen defined by human antibodies. J Immunol. 1983;31:972–976. [PubMed] [Google Scholar]
- 32.Martinez J, Campetella O, Frasch AC, Cazzulo JJ. The major cysteine proteinase (cruzipain) from Trypanosoma cruzi is antigenic in human infections. Infect Immun. 1991;59:4275–4277. doi: 10.1128/iai.59.11.4275-4277.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arnholdt AC, Piuvezam MR, Russo DM, Lima AP, Pedrosa RC. Analysis and partial epitope mapping of human T cell responses to Trypanosoma cruzi cysteinyl proteinase.J Immunol. 1993;151:3171–3179. [PubMed] [Google Scholar]
- 34.Schnapp AR, Eickhoff CS, Scharfstein J, Hoft DF. Induction of B- and T-cell responses to cruzipain in the murine model of Trypanosoma cruzi infection. Microbes Infect. 2002;4:805–813. doi: 10.1016/s1286-4579(02)01600-3. [DOI] [PubMed] [Google Scholar]
- 35.Cazorla SI, Frank FM, Becker PD, Corral RS, Guzmán CA. Prime-boost immunization with cruzipain co-administered with MALP-2 triggers a protective immune response able to decrease parasite burden and tissue injury in an experimental Trypanosoma cruzi infection model. Vaccine. 2008;26:1999–2009. doi: 10.1016/j.vaccine.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 36.Cazorla SI, Frank FM, Malchiodi EL. Vaccination approaches against Trypanosoma cruzi infection. Expert Rev Vaccines. 2009;8:921–935. doi: 10.1586/erv.09.45. [DOI] [PubMed] [Google Scholar]
- 37.Cazorla SI, Frank FM, Becker PD, Arnaiz M, Mirkin GA. Redirection of the immune response to the functional catalytic domain of the cystein proteinase cruzipain improves protective immunity against Trypanosoma cruzi infection. J Infect Dis. 2010;202:136–144. doi: 10.1086/652872. [DOI] [PubMed] [Google Scholar]
- 38.Meirelles MN, Juliano L, Carmona E, Silva SG, Costa EM, Murta AC. Inhibitors of the major cysteinyl proteinase (GP57/51) impair host cell invasion and arrest the intracellular development of Trypanosoma cruzi in vitro. Mol Biochem Parasitol. 1992;52:175–184. doi: 10.1016/0166-6851(92)90050-t. [DOI] [PubMed] [Google Scholar]
- 39.Harth G, Andrews N, Mills AA, Engel JC, Smith R. Peptide-fluoromethyl ketones arrest intracellular replication and intercellular transmission of Trypanosoma cruzi. Mol Biochem Parasitol. 1993;58:17–24. doi: 10.1016/0166-6851(93)90086-d. [DOI] [PubMed] [Google Scholar]
- 40.Engel JC, Doyle PS, Hsieh I, McKerrow JH. Cysteine protease inhibitors cure an experimental Trypanosoma cruzi infection. J Exp Med. 1998;188:725–734. doi: 10.1084/jem.188.4.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Santos CC, Sant’anna C, Terres A, Cunha-e-Silva NL, Scharfstein J. Chagasin, the endogenous cysteine-protease inhibitor of Trypanosoma cruzi modulates parasite differentiation and invasion of mammalian cells. J Cell Sci. 2005;118:901–915. doi: 10.1242/jcs.01677. [DOI] [PubMed] [Google Scholar]
- 42.Scharfstein J, Lima AP. Roles of naturally occurring protease inhibitors in the modulation of host cell signaling and cellular invasion by Trypanosoma cruzi. Subcell Biochem. 2008;47:140–154. doi: 10.1007/978-0-387-78267-6_11. [DOI] [PubMed] [Google Scholar]
- 43.Cazzulo JJ, Stoka V, Turk V. The major cysteine proteinase of Trypanosoma cruzi: a valid target for chemotherapy of Chagas disease. Curr Pharm Des. 2001;7:1143–1156. doi: 10.2174/1381612013397528. [DOI] [PubMed] [Google Scholar]
- 44.McKerrow JH, Doyle PS, Engel JC, Podust LM, Robertson SA. Two approaches to discovering and developing new drugs for Chagas disease. Mem Inst Oswaldo Cruz. 2009;104:263–269. doi: 10.1590/s0074-02762009000900034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Doyle PS, Zhou YM, Engel JC, McKerrow JH. A cysteine protease inhibitor cures Chagas’ disease in an immunodeficient-mouse model of infection. Antimicrob Agents Chemother. 2007;51:3932–3939. doi: 10.1128/AAC.00436-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fampa P, Lisboa CV, Zahner V, Jansen AM, Ramirez MI. Wide proteolytic activity survey reinforces heterogeneity among Trypanosoma cruzi TCI and TCII wild populations. Vector Borne Zoonotic Dis. 2010;10:839–845. doi: 10.1089/vbz.2009.0223. [DOI] [PubMed] [Google Scholar]
- 47.Fampa P, Santos AL, Ramirez MI. Trypanosoma cruzi: ubiquity expression of surface cruzipain molecules in TCI and TCII field isolates. Parasitol Res. 2010;107:443–447. doi: 10.1007/s00436-010-1888-9. [DOI] [PubMed] [Google Scholar]
- 48.Bonaldo MC, Scharfstein J, Murta AC, Goldenberg S. Further characterization of Trypanosoma cruzi GP57/51 as the major antigen expressed by differentiating epimastigotes. Parasitol Res. 1991;77:567–571. doi: 10.1007/BF00931014. [DOI] [PubMed] [Google Scholar]
- 49.Tomas AM, Miles MA, Kelly JM. Overexpression of cruzipain, the major cysteine proteinase of Trypanosoma cruzi, is associated with enhanced metacyclogenesis. Eur J Biochem. 1997;244:596–603. doi: 10.1111/j.1432-1033.1997.t01-1-00596.x. [DOI] [PubMed] [Google Scholar]
- 50.Gomes SA, Misael D, Silva BA, Feder D, Silva CS. Major cysteine protease (cruzipain) in Z3 sylvatic isolates of Trypanosoma cruzi from Rio de Janeiro, Brazil. Parasitol Res. 2009;105:743–749. doi: 10.1007/s00436-009-1446-5. [DOI] [PubMed] [Google Scholar]
- 51.Kikuchi SA, Sodré CL, Kalume DE, Elias CG, Santos AL. Proteomic analysis of two Trypanosoma cruzi zymodeme 3 strains. Exp Parasitol. 2010;126:540–551. doi: 10.1016/j.exppara.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 52.Atayde VD, Neira I, Cortez M, Ferreira D, Freymüller E. Molecular basis of non-virulence of Trypanosoma cruzi clone CL-14. Int J Parasitol. 2004;34:851–860. doi: 10.1016/j.ijpara.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 53.Martinez J, Henriksson J, Rydaker M, Cazzulo JJ, Pettersson U. Genes for cysteine proteinases from Trypanosoma rangeli. FEMS Microbiol Lett 29, 135–141. 1995. [DOI] [PubMed]
- 54.Ortiz PA, Maia da Silva F, Cortez AP, Lima L, Campaner M. Genes of cathepsin L-like proteases in Trypanosoma rangeli isolates: markers for diagnosis, genotyping and phylogenetic relationships. Acta Trop. 2009;112:249–259. doi: 10.1016/j.actatropica.2009.07.036. [DOI] [PubMed] [Google Scholar]
- 55.Higo H, Miura S, Agatsuma T, Mimori T, Yanagi T. Identification of Trypanosoma cruzi sublineages by the simple method of single-stranded conformation DNA polymorphism (SSCP). Parasitol Res. 2007;100:1023–1031. doi: 10.1007/s00436-006-0376-8. [DOI] [PubMed] [Google Scholar]
- 56.Rozas M, De Doncker S, Coronado X, Barnabé C, Tibyarenc M. Evolutionary history of Trypanosoma cruzi according to antigen genes. Parasitology. 2008;135:1157–1164. doi: 10.1017/S0031182008004794. [DOI] [PubMed] [Google Scholar]
- 57.Barnabé C, Brisse S, Tibayrenc M. Phylogenetic diversity of bat trypanosomes of subgenus Schizotrypanum based on multilocus enzyme electrophoresis, random amplified polymorphic DNA, and cytochrome b nucleotide sequence analyses. Inf Gen Evol. 2003;2:201–208. doi: 10.1016/s1567-1348(02)00130-2. [DOI] [PubMed] [Google Scholar]
- 58.Franzén O, Ochaya S, Sherwood E, Lewis MD, Llewellyn MS. Shotgun Sequencing Analysis of Trypanosoma cruzi I Sylvio X10/1 and Comparison with T. cruzi VI CL Brener. PLoS Negl Trop Dis. 2011;5:e984. doi: 10.1371/journal.pntd.0000984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cerqueira GC, Bartholomeu DC, DaRocha WD, Hou L, Freitas-Silva DM. Sequence diversity and evolution of multigene families in Trypanosoma cruzi. . Mol Biochem Parasitol. 2008;157:65–72. doi: 10.1016/j.molbiopara.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 60.Minning TA, Weatherly DB, Flibotte S, Tarleton RL. Widespread, focal copy number variations (CNV) and whole chromosome aneuploidies in Trypanosoma cruzi strains revealed by array comparative genomic hybridization. BMC Genomics. 2011;7:139. doi: 10.1186/1471-2164-12-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Freitas LM, dos Santos SL, Rodrigues-Luiz GF, Mendes TA, Rodrigues TS. Genomic analyses, gene expression and antigenic profile of the trans-sialidase superfamily of Trypanosoma cruzi reveal an undetected level of complexity. PLoS One. 2011;6(10):e25914. doi: 10.1371/journal.pone.0025914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pillay D, Boulangé AF, Coetzer TH. Expression, purification and characterisation of two variant cysteine peptidases from Trypanosoma congolense with active site substitutions. Protein Expr Purif. 2010;74:264–271. doi: 10.1016/j.pep.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 63.Marcili A, Valente VC, Valente SA, Junqueira AC, da Silva FM. Trypanosoma cruzi in Brazilian Amazonia: Lineages TCI and TCIIa in wild primates, Rhodnius spp. and in humans with Chagas disease associated with oral transmission. Int J Parasitol. 2009;39:615–623. doi: 10.1016/j.ijpara.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 64.Marcili A, Lima L, Valente VC, Valente SA, Batista JS. Comparative phylogeography of Trypanosoma cruzi TCIIc: new hosts, association with terrestrial ecotopes, and spatial clustering. Infect Genet Evol. 2009;9:1265–1274. doi: 10.1016/j.meegid.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 65.Westenberger SJ, Barnabé C, Campbell DA, Sturm NR. Two hybridization events define the population structure os Trypanosoma cruzi. . Genetics. 2005;171:527–543. doi: 10.1534/genetics.104.038745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Flores-Lopez CA, Machado CA. Analyses of 32 loci clarify phylogenetic relationships among Trypanosoma cruzi lineages and support a single hybridization prior to human contact. PLoS Negl Trop Dis. 2011;5:e1272. doi: 10.1371/journal.pntd.0001272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ferreira RC, Briones MR. Phylogenetic evidence based on Trypanosoma cruzi nuclear gene sequences and information entropy suggest that inter-strain intragenic recombination is a basic mechanism underlying the allele diversity of hybrid strains. Infect Genet Evol. 2012;12:1064–1071. doi: 10.1016/j.meegid.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 68.Hamilton PB, Lewis MD, Cruickshank C, Gaunt MW, Yeo M. Identification and lineage genotyping of South American trypanosomes using fluorescent fragment length barcoding.Infect Genet Evol. 2011;11:44–51. doi: 10.1016/j.meegid.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 69.Telleria J, Biron DG, Brizard JP, Demettre E, Séveno M. Phylogenetic character mapping of proteomic diversity shows high correlation with subspecific phylogenetic diversity in Trypanosoma cruzi. . Proc Natl Acad Sci USA. 2010;10:720411–720416. doi: 10.1073/pnas.1015496107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ruszczyk A, Forlenza M, Savelkoul HF, Wiegertjes GF. Molecular cloning and functional characterization of a cathepsin L-like proteinase from the fish kinetoplastid parasite Trypanosoma carassii. Fish Shellfish Immunol. 2008;24:205–214. doi: 10.1016/j.fsi.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 71.Cortez AP, Rodrigues AC, Garcia HA, Neves L, Batista JS. Cathepsin L-like genes of Trypanosoma vivax from Africa and South America-characterization, relationships and diagnostic implications. Mol Cell Probes. 2009;23:44–51. doi: 10.1016/j.mcp.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 72.Rodrigues AC, Garcia HA, Ortiz PA, Cortez AP, Martinkovic F. Cysteine proteases of Trypanosoma (Megatrypanum) theileri: Cathepsin L-like gene sequences as targets for phylogenetic analysis, genotyping diagnosis. Parasitol Int. 2010;59:318–325. doi: 10.1016/j.parint.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 73.Garcia HA, Rodrigues AC, Martinkovic F, Minervino AH, Campaner M. Multilocus phylogeographical analysis of Trypanosoma (Megatrypanum) genotypes from sympatric cattle and water buffalo populations supports evolutionary host constraint and close phylogenetic relationships with genotypes found in other ruminants. Int J Parasitol. 2011;41:1385–1396. doi: 10.1016/j.ijpara.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 74.Jackson AP. Tandem gene arrays in Trypanosoma brucei brucei: comparative phylogenomic analysis of duplicate sequence variation. BMC Evol Biol. 2007;7:54. doi: 10.1186/1471-2148-7-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Martin DL, Weatherly DB, Laucella SA, Cabinian MA, Crim MT. CD8þT-Cell responses to Trypanosoma cruzi are highly focused on strain-variant trans-sialidase epitopes. PLoS Pathog 2. 2006;(8):e77. doi: 10.1371/journal.ppat.0020077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Claser C, Espíndola NM, Sasso G, Vaz AJ, Boscardin SB. Immunologically relevant strain polymorphism in the amastigote surface protein 2 of Trypanosoma cruzi. Microbes Infect. 2007;8:1011–1019. doi: 10.1016/j.micinf.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 77.Haolla FA, Claser C, de Alencar BC, Tzelepis F, de VasconcelosJR. Strain-specific protective immunity following vaccination against experimental Trypanosoma cruzi infection. Vaccine. 2009;27:5644–5653. doi: 10.1016/j.vaccine.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 78.Alves JM, Voegtly L, Matveyev AV, Lara AM, da Silva FM. Identification and phylogenetic analysis of heme synthesis genes in trypanosomatids and their bacterial endosymbionts. PLoS One. 2011;6(8):e23518. doi: 10.1371/journal.pone.0023518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Huson DH, Bryant D. Application of phylogenetic networks in evolutionary studies. Mol Biol Evol. 2006;23:254–267. doi: 10.1093/molbev/msj030. [DOI] [PubMed] [Google Scholar]
- 81.Yang Z, Nielsen R. Estimating synonymous and nonsynonymous substitution rates under realistic evolutionary models. Mol Biol Evol. 2000;17:32–43. doi: 10.1093/oxfordjournals.molbev.a026236. [DOI] [PubMed] [Google Scholar]
- 82.Yang Z. PAML 4: phylogenetic analysis by maximum likelihood. Mol Biol Evol. 2007;24:1586–1591. doi: 10.1093/molbev/msm088. [DOI] [PubMed] [Google Scholar]