Abstract
Whereas the majority of pathogenic Salmonella serovars are capable of infecting many different animal species, typically producing a self-limited gastroenteritis, serovars with narrow host-specificity exhibit increased virulence and their infections frequently result in fatal systemic diseases. In our study, a genetic and functional analysis of the mannose-specific type 1 fimbrial adhesin FimH from a variety of serovars of Salmonella enterica revealed that specific mutant variants of FimH are common in host-adapted (systemically invasive) serovars. We have found that while the low-binding shear-dependent phenotype of the adhesin is preserved in broad host-range (usually systemically non-invasive) Salmonella, the majority of host-adapted serovars express FimH variants with one of two alternative phenotypes: a significantly increased binding to mannose (as in S. Typhi, S. Paratyphi C, S. Dublin and some isolates of S. Choleraesuis), or complete loss of the mannose-binding activity (as in S. Paratyphi B, S. Choleraesuis and S. Gallinarum). The functional diversification of FimH in host-adapted Salmonella results from recently acquired structural mutations. Many of the mutations are of a convergent nature indicative of strong positive selection. The high-binding phenotype of FimH that leads to increased bacterial adhesiveness to and invasiveness of epithelial cells and macrophages usually precedes acquisition of the non-binding phenotype. Collectively these observations suggest that activation or inactivation of mannose-specific adhesive properties in different systemically invasive serovars of Salmonella reflects their dynamic trajectories of adaptation to a life style in specific hosts. In conclusion, our study demonstrates that point mutations are the target of positive selection and, in addition to horizontal gene transfer and genome degradation events, can contribute to the differential pathoadaptive evolution of Salmonella.
Author Summary
The process of Salmonella host-adaptation is traditionally considered to involve acquisition of novel genetic elements encoding specific virulence factors or loss of genes representing liability for the more pathogenic strains. Here, by analysis of the mannose-sensitive fimbrial adhesin FimH, we demonstrate that in addition to horizontal gene transfer and genome degradation, single amino acid replacement plays an important role in the differential adaptive evolution of Salmonella. We show that acquisition of specific structural mutations in FimH variants of host-adapted (systemically invasive) serovars results in either significantly enhanced or, alternatively, completely inactivated mannose-binding, whereas systemically non-invasive serovars retain a primordial relatively low-binding (shear-dependent) phenotype. A phylogenetic analysis indicates that these mutations are commonly of a convergent nature and occur under strong positive selection illustrating the role of point amino acid changes in convergent evolution of host-adapted Salmonella. Although we show increased bacterial adhesiveness and cell-invasiveness of the high-binding mutants, the physiologic role of the non-binding mutations in FimH remains to be determined.
Introduction
Salmonella enterica is comprised of six subspecies (I, II, IIIa, IIIb, IV and VI) further subdivided into ∼2,500 serovars based on the presence of distinct surface antigens (somatic O, flagellar H and capsular Vi). The vast majority of Salmonella strains pathogenic to humans belong to subspecies I (S. enterica subsp. enterica), which is considered to be adapted to warm-blooded animals unlike the remaining subspecies which are found mostly in reptiles [1], [2]. However, among ∼1,500 serovars of subspecies I, relatively few cause severe systemically invasive infections while most serovars cause milder infections, usually limited to gastroenteritis. Heterogeneity in Salmonella virulence has been traditionally attributed to different distributions of various mobile genetic elements such as chromosomal pathogenicity islands, bacteriophages, transposons, plasmids, etc. [3], [4], [5]. However, the distribution of these factors does not correlate well with differences in clinical features. More recently, gene loss via deletion, insertional inactivation or truncation has been considered important in the evolution of highly pathogenic Salmonella [6], [7], [8]. In this study, however, we demonstrate that amino acid mutations in core genes of Salmonella are also a driving force behind pathoadaptive evolution of Salmonella serovars.
Various Salmonella serovars differ greatly in their host specificity and pathogenicity. Many serovars can infect a broad spectrum of animal hosts, typically producing self-limiting gastroenteritis (e.g., S. Enteritidis and S. Typhimurium). A small number of serovars exhibit narrow host-specificity and show increased virulence in their specific host that usually results in severe typhoid-like disease [9], [10]. For example, human adapted serovars Typhi, Paratyphi A and, Paratyphi C cause typhoid fever exclusively in humans and closely related primates. Avian adapted S. Gallinarum (biovars Gallinarum and Pullorum) cause severe systemic diseases in birds (fowl typhoid and pullorum disease respectively). Some other host-adapted serovars such as Choleraesuis or Dublin, although primarily associated with systemically invasive diseases in specific porcine and bovine hosts, respectively, rarely can infect other animal species including humans [11], [12], [13], [14]. It is noteworthy that in humans, infections with S. Choleraesuis and S. Dublin typically lead to severe invasive disease [14], [15], [16]. Similarly the D-tartrate negative biovar of Paratyphi B is primarily responsible for paratyphoid in humans but can be also sporadically isolated from dairy cattle [17], [18]. In contrast, the broad-host spectrum D-tartrate positive biovar of Paratyphi B (now biovar Java, formerly serovar Java) causes gastroenteritis in humans.
The evolution of pathogenic Salmonella from a non-pathogenic ancestor is primarily attributed to virulence genes acquired by horizontal gene transfer [3]. This includes the acquisition of large chromosomal regions (10–200 kbp) called Salmonella pathogenicity islands (SPI) that contain a number of functionally related genes [19]. Acquisition of small (<5 kbp) genetic loci, bacteriophages, and plasmids also contribute to the evolution of virulence [4], [5]. At least five Salmonella pathogenicity islands (SPI-1 to 5) have been identified in the serovars of S. enterica species, with a further nine islands with characteristics of SPIs identified in genomes of different serovars of subspecies I [20], [21]. Although the molecular effects of virulence traits encoded by these genetic loci have been studied in detail, their distribution does not simply correlate with host specificity or the level of systemic invasiveness of Salmonella serovars. Recently, genome sequencing studies of host-adapted serovars S. Typhi, S. Gallinarum, S. Choleraesuis and newly emerging systemically invasive strains of S. Typhimurium in sub-Saharan Africa revealed that these bacteria undergo extensive gene deletion and truncation [6], [7], [8], [22], [23]. Because the majority of lost genes have functional orthologs in systemically non-invasive Salmonella with key roles in intestinal colonization, it has been suggested that narrow host-adaptation of Salmonella co-evolved with the loss of an intestinal life style and acquisition of the ability to survive in a systemic niche [22].
While high-throughput comparative genomics of different Salmonella strains have identified a large number of single nucleotide polymorphisms (SNPs), very little is known about the functional consequence of these sequence variations and their potential impact on Salmonella ecology and pathogenicity [24], [25], [26]. SNPs that represent random spontaneous mutations in the coding or regulatory regions of genes can result in modification or loss of gene function and/or expression [27]. These so called ‘change of function/loss of function’ mechanisms can confer a strong selective advantage to bacteria during their spread and growth in diverse host environments, improving their survival or increasing their pathogenic potential and thus driving their evolution toward an enhanced pathogenic phenotype. Therefore they are referred to as pathoadaptive mutations [27]. A well characterized example of pathoadaptative mutation is allelic variation in the FimH adhesin of type 1 fimbriae expressed by uropathogenic Escherichia coli [28]. FimH mediates mannose-sensitive bacterial adhesion to target cells [29]. It has been demonstrated that uropathogenic isolates, due to structural point mutations in fimH, express variants of FimH with an increased capability to bind monomannose receptors, conferring a significant advantage for colonization of the bladder compared to most commensal E. coli [30], [31].
Like E. coli, Salmonella have mannose-specific type 1 fimbriae with a tip-associated adhesin also termed FimH [32], [33]. Despite functional and semantic similarity, the fimbriae in these two species are not evolutionarily related, with virtually no significant sequence homology, including fimH [34], [35]. The FimH adhesin is an excellent candidate for the study of evolutionary changes in Salmonella adaptation. This adhesive protein has been reported to play an important role in Salmonella adhesion and invasion [36], [37], [38], [39], [40], [41], [42], and recently was shown to be a crucial mediator of bacterial transcytosis through M-cells, a process which is of great relevance in triggering the mucosal immune response [43], [44]. The expression of FimH on the bacterial surface is tightly controlled by the fim regulatory proteins (FimZ, FimY and FimW) in response to environmental signals [45], [46], [47] and, due to involvement of fim regulatory proteins in the global regulatory network, FimH expression is coupled with the expression of other virulence factors including flagella [48], T3SS [49], [50], [51] and LPS [52]. Thus, it can be expected that diversity of host cell receptors, the host immune response and other indirect mechanisms exert differential selective pressures on FimH adhesins during different types of Salmonella infection.
Recent studies on FimH adhesins of S. Typhimurium demonstrated that there is allelic variability of FimH, where single amino acid substitutions increase bacterial binding to human HEp-2 and murine DC cells, and increase efficiency of biofilm formation in the small intestine of mice [38], [53]. Also, in S. Gallinarum (biovars Pullorum and Gallinarum), FimH variants have lost the ability to mediate mannose-sensitive adhesion due to a single point mutation that eliminates mannose binding [54]. However, the general pattern of FimH variability across different serovars of Salmonella is unknown, and it is not established whether point mutations in FimH are acquired under positive selection and thus are likely to contribute to the evolution of virulence in Salmonella, especially in strains capable of invasive infections.
To determine whether point mutations in FimH are associated with pathoadaptive evolution of specific Salmonella serovars, we performed genetic and functional analyses of FimH adhesin variants from 33 serovars of S. enterica. We found that FimH in host-adapted (systemically invasive) serovars evolve in a convergent way, both structurally and functionally, highlighting the role of point mutations in the differential adaptive evolution of Salmonella.
Results
The phylogenetic relatedness of fimH genes within S. enterica
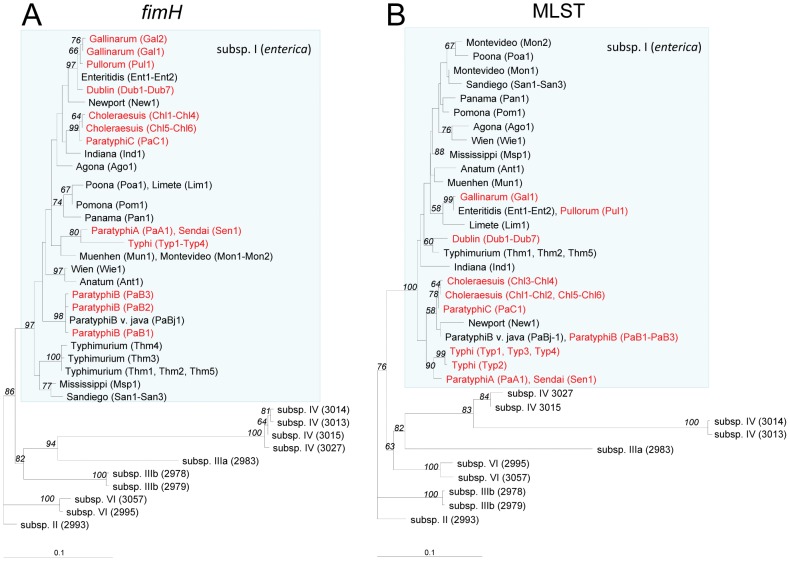
fimH was amplified from 55 of 56 S. enterica isolates, of which 45 represented 22 serovars of subspecies I, and 11 isolates represented other subspecies (Table 1). The isolate of S. enterica subsp. IIIa (2980) carried only part of the fimH gene and was excluded from further analysis. The maximum-likelihood phylogenetic tree constructed based on 55 amplified fimH sequences and five additional fimH alleles obtained from GenBank (fimH of S. Typhimurium AJB3 and LB5010, S. Gallinarum 287/91 and 589/02, and S. Paratyphi C 49 [RKS 4594]) is presented in Figure 1A. The fimH sequences of subspecies I (enterica) were grouped in a distinct phylogenetic clade separate from fimH of subspecies II–VI, which were also separated from one another (with bootstrap values for branch separation higher than 60%).
Table 1. List of bacterial strains used in this study.
| Strain | Strain tag | Strain origin/characteristic relevant for this study (year of strain isolation) | Reference/source |
| Wild-type: | |||
| S. Typhimurium SL1344 | Thm1 | Bovine | [96] |
| S. Typhimurium 1 (RKS4194) | Thm2 | Human, England | [97] |
| S. enterica serogroup B C-24 | Thm5 | Human stool, recurrent gastroenteritis/typhoid fever, (2007) * | F. Fang1 |
| S. Pomona DMSO63 | Pom1 | Unknown | [98] |
| S. Anatum DMSO13 | Ant1 | Unknown | [98] |
| S. Dublin DMSO30 | Dub1 | Unknown | [98] |
| S. Dublin C-29 | Dub2 | Human blood, colitis/sepsis, (2011) | F. Fang |
| S. Dublin 10351 | Dub3 | Bovine (necropsy) (2006) | M. Samadpour2 |
| S. Dublin 11170 | Dub4 | Bovine spleen (2006) | M. Samadpour |
| S. Dublin 11087 | Dub5 | Bovine lung (2006) | M. Samadpour |
| S. Dublin 10928 | Dub6 | Bovine feces (2006) | M. Samadpour |
| S. Dublin 10828 | Dub7 | Bovine necropsy (2006) | M. Samadpour |
| S. Mississippi DMSO49 | Msp1 | Unknown | [98] |
| S. Agona DMSO09 | Ago1 | Unknown | [98] |
| S. Newport DMSO55 | New1 | Unknown | [98] |
| S. Pullorum SL297 | Pul1 | Unknown | [98] |
| S. Paratyphi B S66 | PaB1 | Unknown | [74] |
| S. Paratyphi B S957 | PaB2 | Unknown | [74] |
| S. Paratyphi B 2421 | PaB3 | Unknown | S. Clegg3 |
| S. ParatyphiB var java DMSO45 | PaB-j1 | Unknown | [98] |
| S. Enteritidis 11122-1 | Ent1 | Avian | M. Samadpour |
| S. Enteritidis 12154-1 | Ent2 | Bovine (environmental) | M. Samadpour |
| S. Choleraesuis χ3246 | Chl1 | Porcine | [99] |
| S. Choleraesuis 10411-1 | Chl2 | Bovine lung (necropsy) | M. Samadpour |
| S. Choleraesuis 11175-1 | Chl3 | Porcine lung (necropsy) | M. Samadpour |
| S. Choleraesuis 11359-1 | Chl4 | Porcine (necropsy) | M. Samadpour |
| S. Choleraesuis 1656/04 | Chl5 | Porcine | M. Ugorski4 |
| S. Choleraesuis 1500/05 | Chl6 | Porcine | M. Ugorski |
| S. Typhi 63 (RKS3333) | Typ1 | Dakar | [100] |
| S. Typhi 64 (RKS3320) | Typ2 | Dakar | [100] |
| S. Typhi C-23 | Typ3 | Human blood, typhoid fever (2002) | F. Fang |
| S. Typhi Ty2 (JSG624) | Typ4 | Unknown | J. Gunn5 |
| S. Paratyphi A 42 (RKS4993) | PaA1 | Laboratory strain (ATCC 9150) | [100] |
| S. Sendai 58 (RKS2866) | Sen1 | Human, California | [100] |
| S. Limete 47 (RKS3215) | Lim1 | Human, Africa | [100] |
| S. Indiana 25 (RKS4250) | Ind1 | Scotland | [100] |
| S. Muenchen 32 (RKS3121) | Mun1 | Laboratory strain (ATCC 8388) | [100] |
| S. Montevideo 30 (RKS1762) | Mon1 | Human, Georgia | [100] |
| S. Montevideo 31 (RKS 1740) | Mon2 | Human, Florida | [100] |
| S. Wien 71 (RKS4000) | Wie1 | Human, France | [100] |
| S. Poona MI14a | Poa1 | Marine Iguana, S. Plaza, Galapagos | R.I. Mackie6 |
| S. Panama LI03e | Pan1 | Land Iguana, S. Plaza, Galapagos | R.I. Mackie |
| S. Sandiego MI08d | San1 | Marine Iguana, S. Plaza, Galapagos | R.I. Mackie |
| S. Sandiego LI23b | San2 | Land Iguana, Santa Fe, Galapagos | R.I. Mackie |
| S. Sandiego LI010a | San3 | Land Iguana, S. Plaza, Galapagos | R.I. Mackie |
| S. enterica subsp. II (RKS 2993) | 2993 | Unknown | [97] |
| S. enterica subsp. IIIa (RKS 2980) | 2980 | Corn snake, Oregon | [97] |
| S. enterica subsp. IIIa (RKS 2983) | 2983 | Human, California | [97] |
| S. enterica subsp. IIIb (RKS 2978) | 2978 | Human, Oregon | [97] |
| S. enterica subsp. IIIb (RKS 2979) | 2979 | Human, California | [97] |
| S. enterica subsp. IV (RKS 3015) | 3015 | Animal, Canal zone | [97] |
| S. enterica subsp. IV (RKS 3027) | 3027 | Human, Illinois | [97] |
| S. enterica subsp. IV (RKS 3013) | 3013 | Tonga-T1 | [97] |
| S. enterica subsp. IV (RKS 3014) | 3014 | Human, Florida | [97] |
| S. enterica subsp. VI (RKS 2995) | 2995 | India | [97] |
| S. enterica subsp. VI (RKS 3057) | 3057 | Unknown | [97] |
| Recombinant: | [97] | ||
| S. Typhimurium SL1344H3 | - | fimH::kan mutant of S. Typhimurium SL1344 | [38] |
| S. Typhimurium LBH4 | - | fimH::kan mutant of S. Typhimurium LB5010 | [101] |
| S. Typhi Ty57 | Typ4 | aroA::kan mutant of S. Typhi Ty2 | This study |
Harborview Medical Center, Seattle, WA, USA.
Institute for Environmental Health, Lake Forest Park, WA, USA.
University of Iowa, Iowa City, IA, USA.
Wroclaw University of Environmental and Life Sciences, Wroclaw, Poland.
Ohio State University, Columbus, OH, USA.
University of Illinois, Urbana, IL, USA.
Figure 1. Maximum-likelihood DNA phylograms of S. enterica fimH and concatenated MLST loci (aroC, hisD and thrA).
The fimH tree (A) was built based on an alignment of fimH sequences amplified from 55 isolates including 45 different strains of subspecies I and 10 strains of subspecies II–VI (for details see Table 1). Five additional alleles of fimH obtained from GenBank (S. Typhimurium AJB3 (Thm3), S. Typhimurium LB5010 (Thm4), S. Gallinarum 287/91 (Gal1) and 589/02 (Gal2), and S. Paratyphi C 49 [RKS4594] (PaC1) were included. The MLST loci tree (B) was built on an alignment of concatenated sequences of three genes (aroC, hisD and thrA) obtained for 57 study strains. The trees shown were rooted using S. enterica subsp. II (2993). The italicized values along the branches denote % bootstrap values based on 1000 runs (the bootstrap proportions along the terminal branches separating isolates within single serovars as well as the ones below 50% are not shown). Systemically invasive serovars are shown in red and non-invasive serovars are shown in black. Strain tags are as used in the text.
fimH phylogeny was compared to the phylogenetic relationship of the study strains based on their Multi-Locus Sequence Typing (MLST) profiles by using concatenated sequences of three housekeeping genes aroC, hisD and thrA (Figure 1B). Strains belonging to same serovars had the same, usually distinct, MLST profiles and there was a general congruency between the fimH and MLST trees. In particular, different subspecies of S. enetrica were split into distinct clades on both fimH and MLST trees and, within subspecies I, the fimH phylogeny of serovar clades like Typhi and Paratyphi A, Choleraesuis and Paratyphi C, or Pullorum, Gallinarum, and Enteritidis corresponded well with MLST genotype. Also within the subspecies I, fimH of the same serovars were grouped together, suggesting limited horizontal transfer of fimH among different serovars. However, there was less congruency between the serovar clades defined by fimH and MLST, indicating that, in general, fimH of different host-adapted, systemically invasive serovars have evolved along independent, phylogenetically unlinked pathways.
Structural variability of S. enterica subspecies I FimH
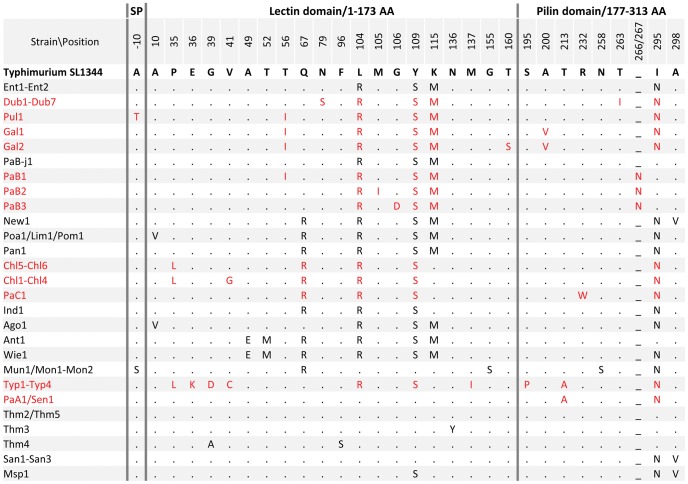
The average nucleotide diversity of fimH from S. enterica subspecies I was 1.7±0.2%, with 28 distinct alleles. The majority of the nucleotide polymorphisms were point mutations with a seven-fold higher rate of synonymous over nonsynonymous mutations. The only other polymorphism type was a three-nucleotide insertion (aat) between nucleotide position 864 and 865 in three fimH alleles of Paratyphi B (PaB1, PaB2 and PaB3). There were a total of 27 protein variants encoded by fimH with an overall protein level identity of 96.6% (Figure 2). There were 45 amino acid replacements in the 335 amino acid polypeptide and an asparagine insertion between positions 266 and 267 in Paratyphi B FimH variants. The majority of serovars contained unique FimH variants, but some serovars, e.g., Montevideo and Muenchen; Paratyphi A and Sendai; or Poona, Limete and Pomona, shared the same protein variants of FimH (Figure 2). For some serovars, e.g. Paratyphi B, Choleraesuis and Gallinarum, within-serovar variability of FimH was observed. FimH variations affected the putative leader peptide (22 aa long) as well as a predicted mannose-binding lectin domain (N-terminal 173 aa in the mature protein) and shaft-anchoring pilin domain (C-terminal 136 aa). There was no significant domain clustering or predominance of either conservative or nonconservative protein changes among FimH variants overall or between FimH from systemically invasive and non-invasive serovars (Figure 2).
Figure 2. Amino acid variation in S. enterica subspecies I FimH.
Residues identical to the amino acid sequence of S. Typhimurium SL1344 (Thm1) FimH are indicated by dots. Systemically invasive serovars are shown in red and non-invasive serovars are shown in black. Position -10 represents the position in the signal peptide of unprocessed FimH upstream of the cleavage site.
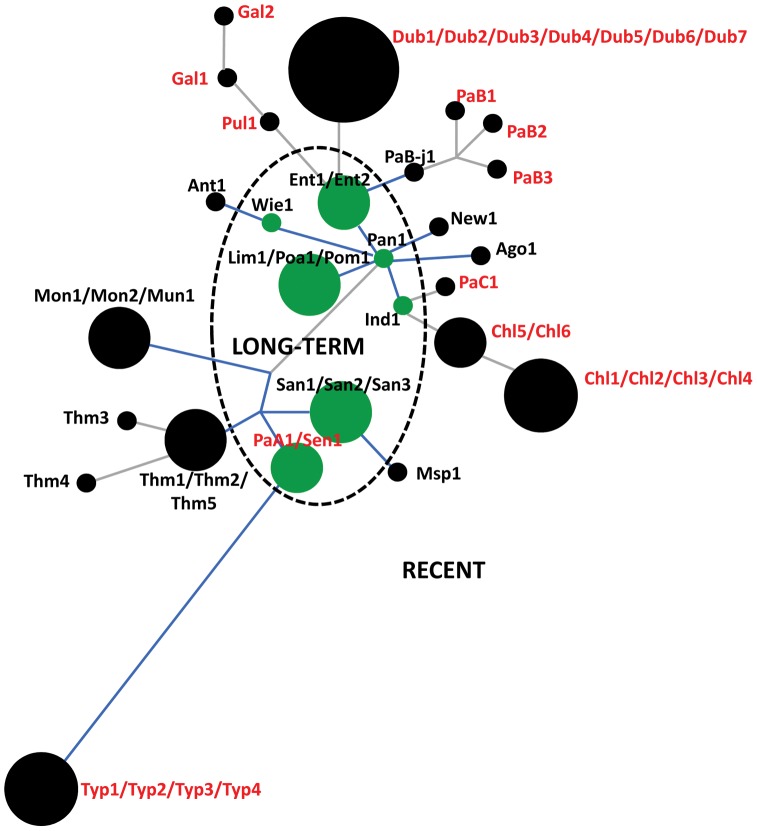
When the FimH protein variants were analyzed for emergence from an evolutionary perspective, most FimH variants (20 out of 27) appeared to have emerged relatively recently, without accumulation of silent changes in the coding alleles (Figure 3). The rest of the FimH variants were of a relatively long-term evolutionary origin, with accumulation of silent changes in the corresponding alleles or along the surrounding branches on the tree. Interestingly, while the systemically non-invasive serovars included nine recent and six long-term FimH variants, only one of twelve FimH variants from systemically invasive serovars was of long-term origin (p = 0.04). Moreover, alleles of the majority (10 of 12) of FimH variants from systemically invasive serovars evolved from the nearest allele exclusively by structural mutations.
Figure 3. DNA-based protein phylogram of S. enterica FimH, derived from ZP analysis.
The tree was built based on the 50 fimH sequences of S. enterica subsp. I. Each circle represents a unique structural variant, and the size of the circle is proportional to the number of representative sequences. The dashed line separates the long-term (green) from the recently emerged variants (black). Branches marked in blue indicate branches containing synonymous mutations. The length of each branch is proportional to the number of non-synonymous mutations that were acquired. The strain tags of systemically invasive serovars are in red and the non-invasive serovars in black.
Thus, while the overall pattern of distribution of FimH variations was not strikingly different between systemically invasive and non-invasive serovars, the evolutionarily recent origin and structural nature of the fimH mutations appeared to be much more typical for the systemically invasive serovars.
High-adhesive and inactive variants of FimH are selected in systemically invasive serovars of S. enterica
We next compared functional properties of different structural variants of FimH by examining the level of bacterial binding to Mannose-BSA (Man-BSA) in an isogenic system. Man-BSA, used as model substrate, contains single (mono-) mannose residues, Man1, covalently coupled to BSA. As controls for assessing the level of binding, we used a fimH-knockout variant (fimHΔ) as well as three structural variants of S. Typhimurium FimH characterized previously: a relatively low-binding variant of S. Typhimurium SL1344 (Thm1) and two high-binding variants from strains AJB3 (Thm3) and 5010 (Thm4) [38], [53], [55] (Figure 4). The majority of FimH variants exhibited relatively low but specific (>95% mannose-inhibitable) binding to Man-BSA (Figure 4 and data not shown). However, with the exception of FimH from S. Paratyphi A/Sendai, all of the low binding FimH variants came from systemically non-invasive serovars of S. enterica. In contrast, all high-binding FimH variants were from systemically invasive serovars such as Typhi (strains Typ1–Typ4), Paratyphi C (PaC1), and Choleraesuis (strains Chl5–Chl6). Also, FimH from another systemically invasive serovar Dublin (Dub1–Dub7) bound Man-BSA significantly stronger than low-adhesive FimH variants, though the binding was weaker in comparison to the other high-binding FimH variants. The binding differences were not due to a difference in the expression level of FimH, as bacteria expressing different variants of FimH bound relatively well to polyclonal anti-FimH antibody (Figure 4). Interestingly, FimH variants expressed by the remainder of the invasive strains did not exhibit detectable mannose-specific binding to Man-BSA, and also failed to bind Man5 oligosaccharides carried by ribonuclease B (RNase B, Figure S1), to which all functionally-active FimH variants bind with much higher affinity than Man1 ligands [55]. However, they still retained their interaction with anti-FimH antibodies (Figure 4). Such an ‘inactive’ FimH phenotype may be similar to that shown previously for FimH variants of S. Gallinarum biovars Pullorum (Figure 4 and Figure S1) and Gallinarum ([54], not tested in this study).
Figure 4. Binding phenotypes of natural S. enterica subspecies I FimH variants.
Static adhesion of S. Typhimurium LBH4 transformed with plasmids encoding different variants of FimH or plasmids carrying fimH deletion (fimHΔ) to Man-BSA (red bars) and anti-FimHSE antibody (grey bars). The binding of 3H- labeled bacteria was determined as described in Material and Methods. Data are the means ± SD of triplicates from one representative experiment of three experiments that were performed. The strain tags of systemically invasive serovars are in red and the non-invasive ones in black. Bacterial binding was >95% inhibitable in the presence of 50 mM methyl-D-mannopyranoside (not shown). * The non-binding FimH variants of S. Gallinarum were not tested in this study.
We also performed the static adhesion assay to examine mannose-dependent binding of wild type strains of Salmonella. As presented in supplementary Figures S2 A and B, the mannose-binding pattern of wild type strains corresponded to the binding pattern of FimH variants expressed in isogenic recombinant system. The wild-type Salmonella carrying fimH alleles encoding low-binding FimH exhibited relatively weak binding to monomannose substrate (Man1) and bacteria with ‘high-binding’ fimH alleles adhered strongly to monomannose (Figure S2 B, red bars). Wild-type isolates with non-binding fimH alleles did not adhere to any of the mannosylated substrates tested (Man1 and Man5). The differences in the level of mannose-specific adhesion between these three groups of wild-type Salmonella were clear, even though variability in the fimbriation level was observed (Figure S2, grey bars). A reduced level of fimbriation was found particularly for isolates of serovar Choleraesuis (Figure S2) while isolates of Partyphi A and Sendai from this study did not produce fimbriae at all (data not shown). Nevertheless, the fimbriated wild-type and recombinant strains displayed the same receptor specificity of binding as assessed by determination of the Man1/Man5 binding ratio (Figure S3).
Selected FimH variants with a range of binding activities (Typ1, PaC1, San1, Chl1 and Pul1) were further analyzed in Man-BSA binding under flow conditions (Figure S4). FimH variants with low-binding phenotypes mediated shear-enhanced (shear-dependent) adhesion to Man-BSA, whereas FimH variants with high-binding phenotypes bound to Man-BSA in a shear-independent manner. Bacteria expressing non-binding variants of FimH did not exhibit binding to Man-BSA under any flow conditions. These results indicate that the low-binding shear-activated phenotype of FimH is predominant among systemically non-invasive serovars of S. enterica, while high-adhesive shear-independent or inactive FimH variants occur only in systemically invasive serovars of Salmonella.
FimH of systemically invasive Salmonella evolved by accumulation of “activating” and/or “inactivating” point mutations
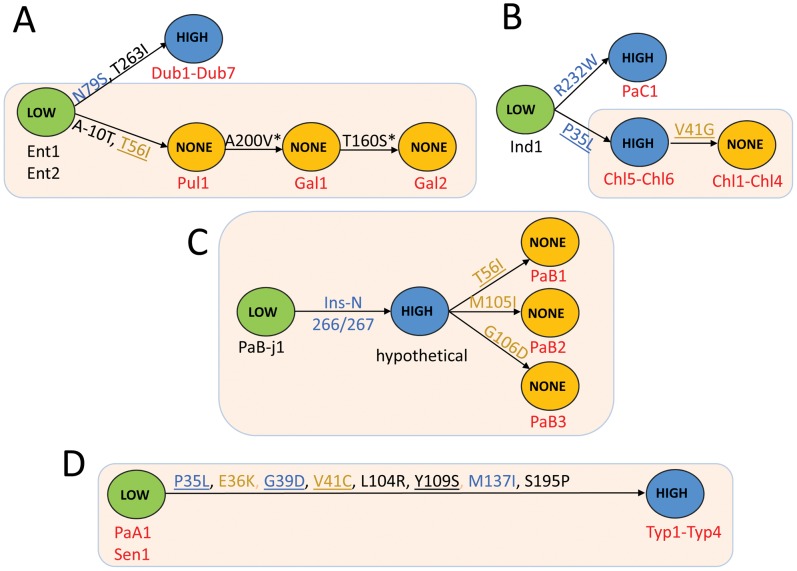
We compared amino acid sequences of high-binding and inactive FimH variants with their closest FimH ancestors exhibiting low-binding phenotypes (Figure 1). According to the fimH phylogeny, the low-binding phenotype is evolutionarily ancestral to the high-binding FimH and, in most cases, non-binding variants evolved from high-binding variants (Figure 5). For example, high-binding variants of FimH of S. Paratyphi C and S. Choleraesuis (Chl5–Chl6) evolved from the low-binding variant of S. Indiana; and the non-binding FimH of S. Choleraesuis (Chl1–Chl4) evolved from the high-binding FimH of S. Choleraesuis (Chl5–Chl6). In the case of S. Paratyphi B, the whole spectrum of phenotypes evolved within the serovar. The non-binding phenotype of S. Gallinarum biovar Pullorum appeared to evolve from low-binding variants of S. Enteriditis, with the former then giving rise to two non-binding FimH variants of S. Gallinarum biovar Gallinarum. Interestingly, with the exception of S. Typhi FimH, all microevolutionary changes in the gene observed in systemically invasive serovars occurred exclusively via amino acid replacements, i.e., without accumulation of any silent mutations, suggesting the action of strong positive selection. In addition, some of the mutations occurred repeatedly (P35L, T56I) or in the same amino acid position (V41G and V41C) indicating convergent evolution of the FimH variants, also a strong indicator of positive selection. Importantly, the evolutionary changes of the fimH variants correspond to serovar phylogeny based on MLST data (Figure 5, light orange boxes).
Figure 5. Schematic representation of evolutionary changes in the FimH binding phenotype of selected S. enterica serovars.
The evolutionary changes in the FimH of S. Enteritidis, S. Pullorum, S. Gallinarum and S. Dublin (A), S. Indiana, S. Paratyphi C and S. Choleraesuis (B), S. Paratyphi B (C) and S. Paratyphi A, S. Sendai and S. Typhi (D). Low (green)-FimH with low-binding phenotype; High (blue)-FimH with high-binding phenotype; None (orange)-inactive variant of FimH. The strain tags of systemically non-invasive serovars are in black and the systemically invasive serovars in red. Structural mutations are given along each arrow. Structural hot-spot mutations are underlined. The activating mutations are in blue and the inactivating mutations are in orange. The FimH variants from strains with phylogenetic relatedness supported by MLST are shown in tan boxes.
The naturally occurring mutations were separately introduced into a plasmid-encoded S. Typhimurium SL1344 (Thm1) fimH (low-binding phenotype) and assayed for functional effects in the isogenic S. Typhimurium LBH4 strain. In the isogenic system, a switch from low- to high-binding phenotype was observed for the single mutations N79S (Ent1/Ent2 to Dub1–Dub72), P35L and R232W (Ind1 to Chl5/Chl6 and to PaC1, respectively) and the N266/267 insertion (PaB-j1 to hypothetical PaB variant) (Figure S5 and Figure 5). Mutation T56I resulted in a change from low- (Ent1/Ent2) to non-binding phenotype (Pul1), while a high- to non-binding switch was confirmed for T56I, M105I and G106D (all in S. Paratyphi B) as well as V41G in S. Choleraesuis. Notably, the non-binding mutation V41G had a deleterious effect on bacterial fimbriation when introduced directly into the low-binding FimH test background (S. Typhimurium SL1344). However, when this mutation was introduced into the high-binding P35L background of its immediate ancestor, fimbriation was normal as indicated by anti-FimH antibody binding while the binding function was lost (Figure S5). When the multiple mutations leading from the low-binding phenotype of S. Paratyphi A/Sendai FimH to the high-binding FimH of S. Typhi were tested individually, three mutations resulted separately in a high-binding phenotype: P35L (as in Chl5–Chl6), G39D and M137I (Figure S5). In contrast, mutations E36K and V41C resulted individually in a significantly decreased or a non-binding phenotype, respectively. Unlike V41G in S. Choleraesuis, the V41C substitution did not eliminate fimbriation in the FimH test background.
Taken together, these results indicate that both high- and non-binding phenotypes of FimH in systemically invasive serovars of S. enterica are acquired under positive selection by convergent evolution, with inactivation of FimH generally preceded by evolution of the high-binding phenotype.
FimH variations affect adhesion to and internalization into epithelial cells and macrophages
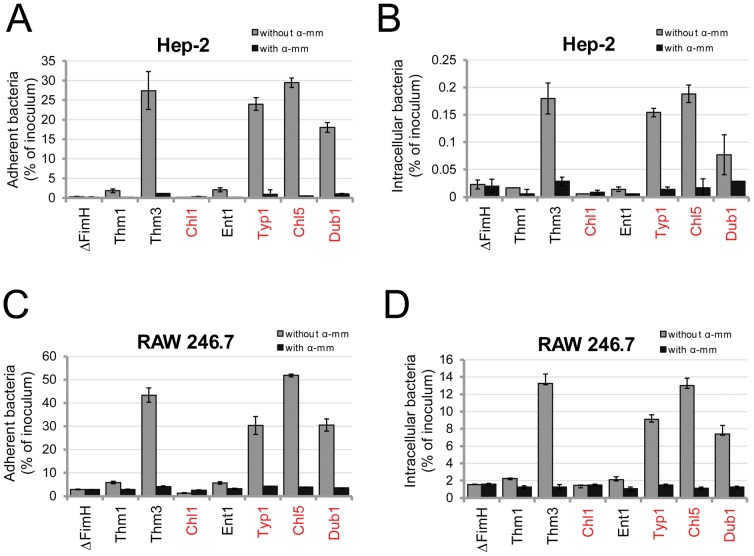
We investigated how variation in mannose-binding by Salmonella FimH affects bacterial cell adhesion and invasion. The human epithelial cell line HEp-2 and the murine macrophage cell line RAW264.7 were used as target cells. We compared isogenic strains that express two previously characterized S. Typhimurium FimH variants, low-binding FimHSL1344 (Thm1) and high-binding FimHAJB3 (Thm3), that differ in a single amino acid N136Y, and FimH variants from five other serovars: Enteritidis (Ent1, low-binding), Dublin (Dub1, medium-binding), Choleraesuis strain Chl5 and Typhi strain Typ1 (both high-binding), and Choleraesuis strain Chl1 (non-binding). Bacterial adhesion and invasion were assessed after allowing a bacterial suspension to interact with the target cell monolayers under static conditions without centrifugation. As shown in Figure 6, the level of bacterial binding to both epithelial cells (6A) and macrophages (6C) corresponded well to the mannose-binding capability of the FimH variants, with the high-binding FimH mediating up to 10-fold higher adhesion than the low-binding variants and up to 100-fold higher adhesion than inactive FimH of Choleraesuis (Chl1) or the FimH knockout strain that does not express type 1 fimbriae (fimHΔ). The binding was strongly inhibited by a soluble mannose derivative (methyl-alpha-D-mannopyranoside). Thus, under our experimental conditions cell adhesion is primarily mediated by FimH, and the variants with activating mutations mediate significantly better cell binding. When bacterial internalization was assessed (Figure 6B and D), the pattern generally was the same. Consequently, the highly-adhering bacteria were internalized to a significantly higher degree than the low-adhering bacteria. However, while the level of FimH-dependent bacterial adhesion to these two types of eukaryotic cells was similar, the invasion level differed significantly with 75 times greater invasion of the macrophage cell line (7.4–13.2% of bacterial inoculum) compared to the epithelial cells (0.07–0.19% of bacterial inoculum). Again, invasion was strongly inhibited by soluble mannose.
Figure 6. FimH-mediated bacterial interaction with epithelial and macrophage cell lines.
Bacterial adhesion to (A and C) and invasion of (B and D) Hep-2 cells (A and B) and RAW264.7 cells (C and D). Different variants of FimH were expressed in S. Typhimurium SL1344H3 and bacterial binding was tested in the absence and presence of 50 mM methyl-D-mannopyranoside (α-mm). Data are the means ± SD of triplicates from one representative experiment of three experiments that were performed.
Although in vitro differences in cell adhesion and invasion were observed for bacteria expressing different FimH variants, no differences in viable bacterial counts from spleen and liver were observed 7 days after oral inoculation of BALB/c mice with Salmonella strains expressing FimH variants (data not shown).
Discussion
In the present study, we have demonstrated that the FimH adhesin in highly pathogenic (systemically invasive) S. enterica serovars undergoes convergent evolution via point mutations that are likely to have adaptive significance for Salmonella ecology and pathogenesis. We have shown that the shear-dependent low-binding phenotype of FimH is preserved in serovars typically associated with gastroenteritis, whereas the majority of systemically invasive serovars carry FimH variants that exhibit one of two alternative but evolutionarily-interconnected phenotypes: increased affinity towards mannose or the outright loss of the mannose-binding activity. The functional diversification of FimH in systemically invasive Salmonella results from repeated, independently acquired structural mutations showing that, in addition to horizontal gene transfer and gene deletion, point mutations are the target of strong positive selective pressure and contribute to the pathoadaptive evolution of Salmonella.
While separation of Salmonella into systemically invasive, host-adapted and systemically non-invasive, broad host-range serovars may seem somewhat arbitrary, this distinction is generally consistent with prevailing views on Salmonella ecology and epidemiology [9], [10]. Subspecies II–VI of Salmonella (salamae, arizonae, diarizonae, houtenae, and indica) that are almost exclusively isolated in nature from reptiles only sporadically cause infections in humans [1], [2], [56]. Subspecies I (enterica) is much more pathogenic for humans than the other five subspecies and is typically isolated from warm-blooded animals. However, the ecology of subspecies I serovars is not as distinct as originally thought, as many (e.g. Poona, Pomona, Abaetetuba, Newport), commonly inhabit wild reptiles, often as dominant Salmonella serovars [57]. Some of the subspecies I strains tested here (serovars Poona, Panama and Sandiego) were isolated from free-living marine or land iguanas from the Galapagos Islands. Thus, many and probably most of the subspecies I serovars truly have a broad range of natural hosts, not limited to warm-blooded animals. However, other serovars such as Typhi, Paratyphi A–C and Choleraesuis are much more distinct in their host association and ability to cause systemic (invasive) disease relative to other serovars of subspecies I. Interestingly, the distinct structural and functional characteristics of FimH in the serovars defined here as systemically invasive support the physiologic validity of their grouping.
To identify adaptive changes in S. enterica FimH, we first used a bioinformatics-based approach called Zonal Phylogeny that is highly sensitive in detecting footprints of positive selection, superior in this regard to more conservative tests such as the dN/dS ratio [28], [58]. Zonal Phylogeny detects an excess of recently evolved protein variants and determines whether or not they evolved via hot-spot mutations (repeated changes in the same amino acid positions). Both parameters are indicative of the source-sink dynamics of adaptive evolution, in which the source is an evolutionarily primary reservoir habitat for a species and the sink is a novel and/or secondary habitat [59]. It has been hypothesized that source-sink dynamics is one of the major types of evolutionary trajectory in highly-pathogenic bacterial lineages within less pathogenic species. FimH of systemically non-invasive Salmonella were found to have accumulated synonymous mutations (with or without non-synonymous changes), whereas the majority of systemically invasive Salmonella FimH variants evolved exclusively by the accumulation of structural mutations, reflecting the recent origin of the latter. While structural mutations accompanied by synonymous mutations indicate neutral accumulation of changes, differentiation of genes exclusively through structural mutation is strong evidence of positive selection. Furthermore, the recent mutations in FimH frequently were found to be repeated hot-spot mutations, i.e. of an evolutionarily convergent nature - another strong indication that they are under positive selection and, thus, functionally adaptive.
Functional analysis of Salmonella FimH revealed that structural variability is associated with diverse binding properties of the adhesin, also of convergent nature, in the systemically invasive serovars. While all tested FimH variants from broad-host range serovars of S. enterica exhibited low binding to mannose under static conditions, FimH of host-adapted serovars had altered functional properties. FimH from systemically invasive serovars Typhi, Paratyphi C, Dublin, and some isolates of serovar Choleraesuis exhibited significantly increased binding to mannose, whereas some other FimH variants of systemically invasive serovars (Paratyphi B and Choleraesuis) did not bind mannose at all. Both the high-binding and non-binding phenotypes evolved, in part, via hot-spot mutations in different systemically invasive serovars. Of note, FimH variants of gastroenteritis–associated serovars, though differing from each other by various structural mutations, preserved low-adhesive properties suggesting the physiological importance of the low-binding FimH phenotype in the intestinal niche.
The low-binding phenotype was found to be significantly increased under shear stress, i.e., shear-enhanced in nature. This phenomenon was originally demonstrated for E. coli FimH and then for several other fimbrial tip-associated adhesins of enterobacteria, including Salmonella FimH (evolutionarily unrelated to E. coli FimH) [55], [60], [61], [62], [63]. The phenotype is based on an allosteric connection between the mannose-binding pocket in the lectin domain of the adhesin and the fimbria-anchoring pilin domain of FimH. When the domains separate from one another under shear-induced tensile force, the binding pocket converts from a wide-open to a tight configuration, increasing the binding affinity for mannose [64]. In contrast, the high-binding phenotype (as observed here for systemically invasive Salmonella) is shear-independent in nature, with already strong binding under low or no shear conditions and no enhancement of binding under shear stress. In E. coli FimH, the high-binding protein variants carry mutations in either lectin or pilin domains that ease the inter-domain interaction [65]. This explains why the high-binding phenotype mutations in Salmonella are found both in the predicted lectin (P35L, G39D and M137I) and pilin (R232W and insertion 266/267N) domains. In contrast, the non-binding phenotype mutations are found only in the lectin domain. Notably, the non-binding phenotype of Salmonella FimH could not be rescued under increased shear, indicating that they might directly affect the function of the mannose-binding pocket.
While the evolutionarily adaptive origin of the FimH mutations in systemically invasive Salmonella serovars is evident from the action of positive selection, and there are hints to their structural basis, the physiological significance of the mutations remains an open question. In E. coli, high-binding mutations that are selected in uropathogenic strains were shown to increase urothelial adhesion and colonization in a mouse model of infection [31]. One might speculate that in Salmonella, increased cell adhesiveness may also be adaptive for systemically invasive serovars. In gastroenteritis, bacterial infection remains localized to the intestine and mesenteric lymph nodes, while in systemic infections, Salmonella transverses the intestinal barrier and disseminates to the liver, spleen and bone marrow. A critical determinant of systemic dissemination of Salmonella is its ability to infect dendritic cells and other CD18-positive phagocytes [66], [67]. Recent studies of Guo at al. (2007) [39] demonstrated that Salmonella uptake by dendritic cells can be mediated by FimH, and the high-binding FimH variant from S. Typhimurium strain AJB3 was shown to be extremely efficient in mediating bacterial internalization into murine cells. Consistently, we found that FimH variants from serovars Typhi, Choleraesuis and Dublin with increased affinity towards mannose confer significantly higher adhesion to and internalization of macrophage cell line RAW 264.7 than low-binding FimH variants. Moreover, the level of RAW264.7 cell internalization mediated by these FimH variants was on average seventy five times higher than internalization of epithelial cells (Hep-2), although the level of bacterial adhesion to both of the cell types was comparable. This indicates that FimH is important factor determining bacterial entry into phagocytic cells but not into epithelial cells for which FimH-dependent internalization was only marginal. These results are consistent with previous reports demonstrating that SPI-1 encoded T3SS is the major invasive factor for epithelial cells whereas the invasion of phagocytic cells is rather SPI-1-T3SS independent [39], [68]. Although the effect of FimH-mediated entry of Salmonella on its intracellular survival into phagocytic cells has not been analyzed, similar studies performed in E. coli indicate that this route of internalization can promote bacterial survival. It was shown that FimH-dependent uptake of E. coli by mouse macrophages results in the formation of morphologically distinct bacteria-containing vacuoles, compared to antibody-opsonized E. coli, correlating with a significant increase in intracellular survival [69], [70], [71]. Thus, highly adhesive variants of FimH with increased capability to mediate internalization in phagocytes could be advantageous for the systemic dissemination of host-adapted Salmonella serovars.
Another important step in Salmonella pathogenesis is the traversing of M cells, whose apical membranes are rich in transcytotic receptor-glycoprotein 2 (GP2), a mannosylated glycoprotein that acts as a receptor for FimH [43], [44]. Although FimH variation in bacterial uptake and transcytosis by M-cells has not been studied, it is possible that allelic variations could affect bacterial delivery to deeper tissues (including Peyer's patches) and alter Salmonella tissue dissemination and triggering of the immune response. Of note, the mechanisms of Salmonella entry into M-cells appear to be important for the distribution of bacteria in tissues and activation of immune cells [72]. It has also been observed that different serovars of Salmonella use distinct mechanisms for M-cells transit. For example, S. Typhi traverses the murine epithelium via M-cells without causing M-cell destruction, followed by the rapid clearance of bacteria from Peyer patches, whereas SPI-1 T3SS-dependent invasion of M-cells by S. Typhimurium is accompanied by M-cell destruction, bacterial replication in Peyer's patches and robust activation of the mucosal immune response [72], [73]. Thus, in addition to the interaction with dendritic cells, allelic variations in FimH may have a significant effect on bacterial uptake by M-cells.
Somewhat unexpectedly, we discovered that some isolates of host-adapted serovars Choleraesuis and Paratyphi B carry variants of FimH that do not bind to mannose. Mannose non-binding type 1 fimbriae have been previously described for Salmonella in serovar Gallinarum (biovars Gallinarum and Pullorum), some isolates of Paratyphi B and Dublin, and were originally referred to as type 2 fimbriae based on the same morphology and antigenic properties as type 1 fimbriae but with an inability to cause mannose-sensitive hemagglutination [32], [74]. More recently, studies in S. Gallinarum revealed that non-hemagglutinating fimbriae represent type 1 fimbriae that have lost the ability to bind to mannose due to a single point mutation (T56I) in FimH [54]. However, it was also shown that although FimH of S. Gallinarum does not bind to murine dendritic cells or other mammalian eukaryotic cells, it does mediate efficient adhesion to chicken leukocytes in vitro and, and most recently, promote systemic dissemination of bacteria in chick model [53], [75]. These results indicate that FimH of these avian-adapted serovars of Salmonella can be functionally active and potentially determine Salmonella host-specificity. Consistent with these findings is the fact that S. Gallinarum FimH (Gal1 and Gal2 strains, Figure 5) has accumulated extra mutations in addition to the original mutation in S. Pullorum (T56I) that cause complete inactivation of FimH. The additional mutations could result in a fine-tuning of some non-mannose type of receptor of as yet unknown identity. Thus, although we did not detect adhesion of S. Choleraesuis or S. Paratyphi B non-binding FimH variants to human epithelial (Hep-2) cells and murine macrophages (RAW264.7), we cannot exclude the possibility that they are active towards a different type of eukaryotic cell or cells of different host origin.
On the other hand, it is possible that the non-binding phenotype is adaptive per se (i.e., conferring a loss-of-function) for the invasive strains. Recent whole-genome comparative analyses revealed that host-adapted) serovars of Salmonella have undergone extensive genome degradation [6], [7], [23], [24], with a high proportion of deleted genes or pseudogenes. Many of these pseudogenes are derived from genes of systemically non-invasive Salmonella that encode proteins important for intestinal colonization and intestinal persistence, including many different types of fimbriae. It has been suggested that gene silencing by pseudogene formation along with other ‘loss of function’ mutations allows host-adapted Salmonella to shed genes that are no longer needed in the systemic niche [7]. However, the footprint of strong positive selection for loss of function in FimH indicates that it is not removed only on a “use-it-or-lose-it” basis, but that the inactivation of FimH in invasive serovars might be adaptive for these bacteria because a functional adhesin presents a liability in the course of systemic infection. This hypothesis is in agreement with the observation that, upon oral infection of mice, a non-fimbriated fim mutant of S. Typhimurium results in significantly higher mortality than wild-type bacteria expressing type 1 fimbriae [76]. Similarly, non-type 1 fimbriated E. coli were selected during the course of experimentally induced bacteremia [77], [78] suggesting that attenuation of type 1 fimbrial function could be beneficial for systemic infection.
In any event, the non-binding phenotype appears to be evolutionarily linked to the highly adhesive phenotype. Indeed, in addition to both being common in systemically invasive strains, in two of three cases the high-binding variant was evolutionarily intermediate to the non-binding phenotype. On the other hand, among eight mutations leading to the high-binding phenotype of FimH in S. Typhi, two mutations resulted in a non-binding phenotype of FimH when tested separately, suggesting that some of the evolutionary intermediates of the human-adapted FimH could be non-binding. However, it remains to be understood how this interplay between seemingly functionally opposite phenotypes could lead to the emergence of systemically-invasive Salmonella from systemically non-invasive serovars. Currently, the emergence of systemically invasive non-typhoidal Salmonella (iNTS) strains uniquely associated with invasive diseases in humans has become a serious health problem in Africa [23]. Whole-genome sequencing of iNTS S. Typhimurium strain D23580 associated with these infections revealed that similarly to the host-adapted Salmonella this strain has undergone evolutionary changes characterized by pseudogene formation and chromosomal deletions. Analysis of fimH from D23580 strain and also the Salmonella serogroup B C-24 (Thm5) isolate from our study (with a documented clinical history of recurrent gastroenteritis/typhoid fever) showed that these strains carry the low-adhesive variant of the FimH. This indicates that these invasive disease-associated NTS strains are likely to be in earlier stages of adaptive evolution as invasive pathogens.
After orally infecting mice with recombinant Salmonella, we did not observe an effect of FimH mutations on bacterial burdens in the liver and spleen. However, these experiments involved only a single inoculum size and a single bacterial strain background (S. Typhimurium) constitutively expressing a plasmid copy of fimH used to infect a single host (BALB/c). One possible explanation of these results could be that the experiment requires most specific settings, e.g. the specific animal-host. In light of recently published findings [75], host-specificity appears to be important factor in the relevance of FimH association with Salmonella pathogenicity in experimental models. In the chick model, clear differences were observed in the virulence of avian adapted Salmonella Gallinarum with an endogenous variant of FimH and S. Gallinarum expressing mannose-sensitive FimH from S. Enteritidis. However, similar studies performed in mice showed no difference in disease for these bacteria [79]. Thus, to investigate the physiological significance of adaptive FimH variants and, in particular, their pathoadaptive role, more detailed future studies will be required.
S. enterica possess a wide repertoire of fimbrial and nonfimbrial adhesins that contribute to the adhesion and the pathogenicity [80], [81], [82], [83], [84], [85], with some of them differently distributed between serovars. Our studies on the mannose-specific type 1 fimbrial adhesin present in all Salmonella serovars demonstrate that point mutations in the FimH are acquired under positive selection and, thus, have functional consequences with an adaptive significance. However, although the FimH adhesin is the primary fimbrial subunit responsible for mannose binding, other type 1 fimbrial proteins and/or bacterial components can affect fimbriae expression and the adhesion pattern [48], [49], [86]. In addition, recently available full-genome sequences for different Salmonella serovars revealed the presence of potential SNP mutations and pseudogenes in fim structural genes and regulatory sequences raising questions about their possible influence on FimH-dependent binding [6], [24], [87]. Here, by the examination of mannose-dependent binding of wild type Salmonella we show that the vast majority of strains tested in our study produced type 1 fimbriae and the pattern of mannose-binding by these wild-type Salmonella corresponded well to the binding mediated by their FimH variants expressed in an isogenic recombinant system. These results are consistent with a previous report [32] describing type 1 fimbriae in 1444 isolates (149 serovars) of Salmonella enterica where most strains of most serovars were found to be fimbriated and possess mannose-dependent hemagglutinating and adhesive properties. In our study, however, some differences in fimbriation level were observed between wild-type strains, and some of the strains (S. Paratyphi A and S. Sendai) appeared to produce no fimbriae, even upon serial passage in conditions inducing type 1 fimbriae expression. Interestingly, such non-fimbriated isolates were also observed previously in Paratyphi A and Sendai serovars as well as in a portion of other serovars. This might suggest that potential loss-of-function mutations in other type 1 fimbrial genes or/and regulatory sequences could be responsible for the abrogation of type 1 fimbriae expression. However, the presence and role of these mutations/pseudogenes remain to be elucidated.
While FimH represents only one of many virulence traits, our studies clearly highlight the importance of investigating the physiological role of naturally-occurring mutations at the level of single nucleotide polymorphisms in genes shared by all Salmonella serovars because those mutations, in addition to horizontal gene transfer and gene loss, are likely to make a significant contribution to the adaptive evolution of Salmonella host adaptation and virulence.
Materials and Methods
Ethics statement
This study was carried out in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was reviewed and approved by the University of Washington Institutional Animal Care and Use Committee (Office of Laboratory Animal Welfare assurance number: A3464-01).
Bacterial strains and knockouts
Salmonella strains used in this study are listed in Table 1. The collection included 45 isolates of S. enterica subspecies I (22 serovars) and 11 isolates of S. enterica subspecies II–VI. The strains were routinely grown overnight in LB (Luria-Bertani) broth without shaking. The non-fimbriated fimH mutants of S. Typhimurium strains SL1344H3 and LBH4 [38], [87] were used as hosts for recombinant plasmids encoding different FimH variants or fimHΔ.pISF255b plasmid with the fimH deletion (supplementary material Table S1). Transformed bacteria were cultured in SB (Super Broth) supplemented with 30 µg/ml chloramphenicol and 50 µg/ml kanamycin. All bacteria used in the adhesion assay were serially subcultured without shaking at 37°C for optimal expression of type 1 fimbriae. Also, for the biosafety reason, the aroA mutant of wild-type S. Typhi JSG624 (Typ4) was used in the adhesion assay. An aroA deletion mutant of S. Typhi JSG624 was constructed using λ red recombinase and primers TYP9 TYAROAP1 CTGACGTTACAACCCATCGCGCGGGTCGATGGCGCCATTAgtgtaggctggagctgcttc and TYP10- CGTACTCATCCGCGCCAGTTGTTCGAAATAATCAGGGAACcatatgaatatcctccttag as described by Datsenko and Wanner (2000). Escherichia coli DH5α, used for recombinant DNA manipulations, were cultured in SB broth supplemented with appropriate antibiotics as indicated.
fimH sequencing and cloning
The fimH and three housekeeping genes (aroC, hisD and thrA) were PCR-amplified from various strains of S. enterica using genomic DNA as a template. The primers for fimH amplification were: fimH5′-CAGGCGATTACGATAGCC-3′ and fimH3′-ATCCACCACGTTACCGCGC-3′; and primers for housekeeping genes were as described at http://mlst.ucc.ie/mlst/dbs/Senterica. PCR products were purified after separation in 1% agarose gel on QIAquick column (Qiagen) and sequenced using BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems).
For cloning, the fimH alleles of interest were PCR-amplified from genomic DNA using fimH-XbaI-F 5′-CTCTCTAGATGTATCCGTCCGGCGTC and fimH-SpeI-R 5′-GAGACTAGTTTAATCATAATCGACTCG-3′ primers, XbaI/SpeI digested and ligated into pISF255b [55]. The resulting plasmids carrying different S. enterica fimH alleles are listed in Table S1 (supplemental material).
Site-directed mutagenesis
The different mutations were introduced into fimH by PCR using the Quick-Change mutagenesis kit (Stratagene). pISF255b carrying fimH from S. Typhimurium SL1344 was used as a template. The mutagenic pairs of primers are listed in Table S2 (supplemental material). Sequencing of fimH was performed to confirm introduced mutations.
Phylogenetic analysis
PhyML 3.0 [88] was used to generate the maximum-likelihood based DNA phylograms of fimH and concatenated MLST loci, and to derive the bootstrap proportion values from 1000 replicates under GTR substitution model. The nucleotide sequences were aligned using ClustalW with default settings [89]. Zonal Phylogeny (ZP) analysis and associated statistics were performed using Zonal Phylogeny Software (ZPS) [58]. The maximum likelihood (ML) phylograms as implemented in ZPS were generated by PAUP* 4.0b using the general time reversible (GTP) substitution model with codon-position specific estimated base frequencies [90]. Sequence diversity was measured by the average pairwise diversity index (π) and the rates of nonsynonymous (dN) and synonymous (dS) mutations [91] using MEGA version 4 [92]. Analysis of statistical significance was performed using the z-test for π and dN/dS values [93]. The presence of structural hotspot mutations was determined using ZPS.
Static adhesion assay
FimH-dependent bacterial adhesion under static condition was analyzed as described previously [94]. Briefly, immulon 4HBX 12 well strips (Thermo Electron Corp.) were coated with 20 µg/ml Man-BSA, yeast mannan, RNaseB or rabbit anti-FimHSE antibody (diluted 1∶500), and quenched with 0.1% BSA in PBS. S. Typhimurium LBH4 expressing different FimH variants were grown overnight with 0.33 µM [methyl-3H]-thymidine (PerkinElmer Life Sciences, Inc.), washed with PBS and then added to each well at 100 µl volume and OD540 = 2. Plates were washed with PBS and radioactivity for each well was counted with a scintillation counter (MP Biomedicals). The number of bound bacteria was determined from calibration curves. For inhibition, bacterial binding was tested in the presence of 50 mM methyl-D-mannopyranoside (α-mm).
Some adhesion experiments were performed without 3H-bacteria labeling. Instead, bacteria bound to the ligands in microtiter plates were dried and then stained with 0.1% crystal violet for 10 min at room temperature. After several washes with water, 100 µl of 50% ethanol were added to each well and after dye solubilization the absorbance at a wavelength of 600 nm were measured.
Parallel plate flow chamber experiment
Binding under flow conditions was performed using parallel plate flow chambers as described previously [55]. Briefly, 35-mm polystyrene cell culture dishes (Corning, Inc.) were coated with Man-BSA (200 µg/ml), and a parallel plate flow chamber (2.5 (long)×0.25 cm (wide)×250 µm (high), GlycoTech) was assembled on the culture dish. The entire assembly was then mounted on a Nikon TE2000-E microscope with a 10× phase-contrast objective and connected to a high resolution CCD Cascade camera (Roper Scientific, Inc.). Bacteria in 0.2% BSA/PBS were flowed into the chamber at different flow rates using a Warner Instruments syringe pump. Bacterial binding to the surface was recorded for 4 min and analyzed using MetaView video acquisition software (Universal Imaging Corp., PA).
Cell-adhesion and invasion assays
S. Typhimurium SL1344H3 expressing different FimH variants were incubated with monolayers of Hep-2 or RAW 264.7 cells at a multiplicity of infection of 25∶1. Bacteria were allowed to interact with the cells for 1 h at 37°C in 5% CO2. The cells were then washed five times with DMEM and lysed with 1% Triton (Sigma) in PBS, or for invasion studies, incubated with DMEM containing 100 µg/ml gentamicin for 1 h at 37°C in 5% CO2. After antibiotic treatment the cells were washed three times with DMEM and lysed with 1% Triton. The number of CFU in each well was quantified by plating serial dilutions of cell lysates on LB plates.
Mouse virulence assays
S. Typhimurium strains expressing different FimH variants from S. Typhimurium SL1344 (Thm1, low-binding), S. Typhi (Typ1, high-binding), and S. Choleraesuis (Chl1, inactive) were administered orally to 6–8 week-old BALB/c mice (Taconic Farms). The fimH genes were expressed from stable plasmid vector pRB3-273C [95]. Bacteria were grown overnight without shaking in SB broth supplemented with 30 µg/ml chloramphenicol, washed and suspended in sterile PBS (pH 7.0) to a final concentration of 107 colony-forming units (CFU) per 25 µl. Food and water were withheld from the mice for 4 hours before bacteria were administered atraumatically from a pipet tip. Mice were sacrificed after 7 days for liver and spleen removal and homogenization in sterile PBS. Serial dilutions of tissue homegenates were plated onto LB agar with or without ampicillin to quantitate CFU per organ.
List of accession/id numbers for genes mentioned in the text
EU445777- fimH of S. Typhimurium AJB3
L19338.1 - fimH of S. Typhimurium LB5010
FN424405- fimH of S. Typhimurium D23580
AY486389 - fimH of S. Gallinarum 589/02
AM933173 - fimH of S. Gallinarum 287/91
NC_012125 - fimH of S. Paratyphi C 49 [RKS 4594]
aroA gene id: 1068996
Supporting Information
Static adhesion of S. Typhimurium LBH4 expressing different variants of FimH to mannose-containing substrates. Binding of 3H-labled bacteria to Man1 (Man-BSA) and Man5 (RNaseB) was determined as described in ‘Materials and Methods’. Data are the means ± SD of triplicates from one representative experiment of three experiments that were performed.
(TIF)
Static adhesion of representative wild-type and recombinant S. enterica to mannose-containing substrates. Binding of wild-type (A) and recombinant (B) S. enterica strains to Man1 (yeast mannan, red), Man5 (RNaseB, blue) and anti-FimHSE antibody (grey). Attached bacteria were stained with crystal violet and the adhesion was quantified by measuring absorbance at 600 nm. Data are the means ± SD of triplicates from one representative experiment of three performed. The strain tags of systemically non-invasive serovars are in black and the invasive serovars in red.
(TIF)
Man1/Man5 binding ratio calculated for representative wild-type and recombinant strains of S. enterica. Data are the means ± SD of triplicates from one representative experiment of three performed. The strain tags of systemically non-invasive serovars are in black and the invasive serovars in red. ND, not determined.
(TIF)
Bacterial accumulation on Man1-coated surfaces in the parallel plate flow chamber. Bacterial binding to Man-BSA under different shear conditions was recorded for 4 min. Data are the means of two independent experiments.
(TIF)
Effects of point mutations on binding phenotype of S. enterica FimH. Static adhesion of 3H-labeled bacteria to Man1 (Man-BSA, red), Man5 (RNaseB, blue) and anti-FimHSE antibody (grey). Data are the means ± SD of triplicates from one representative experiment of three performed.
(TIF)
List of plasmids.
(RTF)
List of primers.
(RTF)
Acknowledgments
We gratefully acknowledge Steve Moseley (University of Washington) for critical reading of the manuscript and helpful advice and Kay Greeson (Institute for Environmental Health, Lake Forest Park, WA, USA) for help with obtaining the strains of S. enterica.
Footnotes
The authors have declared that no competing interests exist.
This work was supported by National Institutes of Health Grant RO1 GM084318 (EVS) and R21 AI91966-02 (FCF). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Scheelings TF, Lightfoot D, Holz P. Prevalence of Salmonella in Australian reptiles. J Wildl Dis. 2011;47:1–11. doi: 10.7589/0090-3558-47.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Briones V, Tellez S, Goyache J, Ballesteros C, del Pilar Lanzarot M, et al. Salmonella diversity associated with wild reptiles and amphibians in Spain. Environ Microbiol. 2004;6:868–871. doi: 10.1111/j.1462-2920.2004.00631.x. [DOI] [PubMed] [Google Scholar]
- 3.Groisman EA, Ochman H. How Salmonella became a pathogen. Trends Microbiol. 1997;5:343–349. doi: 10.1016/S0966-842X(97)01099-8. [DOI] [PubMed] [Google Scholar]
- 4.Helmuth R, Stephan R, Bunge C, Hoog B, Steinbeck A, et al. Epidemiology of virulence-associated plasmids and outer membrane protein patterns within seven common Salmonella serotypes. Infect Immun. 1985;48:175–182. doi: 10.1128/iai.48.1.175-182.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lemire S, Figueroa-Bossi N, Bossi L. Prophage contribution to Salmonella virulence and diversity. In: Hensel M, Schmidt H, editors. Horizontal gene transfer in the evolution of bacterial pathogenesis. Cambridge: Cambridge University Press; 2008. pp. 159–192. [Google Scholar]
- 6.Chiu CH, Tang P, Chu C, Hu S, Bao Q, et al. The genome sequence of Salmonella enterica serovar Choleraesuis, a highly invasive and resistant zoonotic pathogen. Nucleic Acids Res. 2005;33:1690–1698. doi: 10.1093/nar/gki297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McClelland M, Sanderson KE, Clifton SW, Latreille P, Porwollik S, et al. Comparison of genome degradation in Paratyphi A and Typhi, human-restricted serovars of Salmonella enterica that cause typhoid. Nat Genet. 2004;36:1268–1274. doi: 10.1038/ng1470. [DOI] [PubMed] [Google Scholar]
- 8.Holt KE, Thomson NR, Wain J, Langridge GC, Hasan R, et al. Pseudogene accumulation in the evolutionary histories of Salmonella enterica serovars Paratyphi A and Typhi. BMC Genomics. 2009;10:36. doi: 10.1186/1471-2164-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uzzau S, Brown DJ, Wallis T, Rubino S, Leori G, et al. Host adapted serotypes of Salmonella enterica. Epidemiol Infect. 2000;125:229–255. doi: 10.1017/s0950268899004379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clarke RC, Gyles CL. Salmonella. In: Gyles CL, Thoen CO, editors. Pathogenesis of bacterial infections in animals. Ames: Iowa State University Press; 1993. pp. 133–153. [Google Scholar]
- 11.Wray C, Davies RH. Salmonella infections in cattle. In: Wray C, Wray A, editors. Salmonella in domestic animals. London: CABI Publishing; 2000. pp. 169–190. [Google Scholar]
- 12.Fedorka-Cray PJ, Gray JT, Wray C. Salmonella Infections in Pigs. In: Wray C, Wray A, editors. Salmonella in domestic animals. London: CABI Publishing; 2000. pp. 191–207. [Google Scholar]
- 13.Milstein M. Salmonella dublin septicemia in a Scottish terrier recently imported from England. Can Vet J. 1975;16:179–180. [PMC free article] [PubMed] [Google Scholar]
- 14.Jones TF, Ingram LA, Cieslak PR, Vugia DJ, Tobin-D'Angelo M, et al. Salmonellosis outcomes differ substantially by serotype. J Infect Dis. 2008;198:109–114. doi: 10.1086/588823. [DOI] [PubMed] [Google Scholar]
- 15.Vugia DJ, Samuel M, Farley MM, Marcus R, Shiferaw B, et al. Invasive Salmonella infections in the United States, FoodNet, 1996–1999: incidence, serotype distribution, and outcome. Clin Infect Dis. 2004;38(Suppl 3):S149–156. doi: 10.1086/381581. [DOI] [PubMed] [Google Scholar]
- 16.Wollin R. A study of invasiveness of different Salmonella serovars based on analysis of the Enter-net database. Euro Surveill. 2007;12:E070927 070923. doi: 10.2807/esw.12.39.03275-en. [DOI] [PubMed] [Google Scholar]
- 17.Chart H. The pathogenicity of strains of Salmonella paratyphi B and Salmonella java. J Appl Microbiol. 2003;94:340–348. doi: 10.1046/j.1365-2672.2003.01863.x. [DOI] [PubMed] [Google Scholar]
- 18.George JT, Wallace JG, Morrison HR, Harbourne JF. Paratyphoid in man and cattle. Br Med J. 1972;3:208–211. doi: 10.1136/bmj.3.5820.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ochman H, Groisman EA. Distribution of pathogenicity islands in Salmonella spp. Infect Immun. 1996;64:5410–5412. doi: 10.1128/iai.64.12.5410-5412.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marcus SL, Brumell JH, Pfeifer CG, Finlay BB. Salmonella pathogenicity islands: big virulence in small packages. Microbes Infect. 2000;2:145–156. doi: 10.1016/s1286-4579(00)00273-2. [DOI] [PubMed] [Google Scholar]
- 21.Morgan E. Salmonella Pathogenecity Islands. In: Rhen M, Maskell D, Mastroeni P, Threlfall J, editors. Salmonella: molecular biology and pathogenesis. Norfolk: Horizon Bioscience; 2007. pp. 67–88. [Google Scholar]
- 22.Thomson NR, Clayton DJ, Windhorst D, Vernikos G, Davidson S, et al. Comparative genome analysis of Salmonella Enteritidis PT4 and Salmonella Gallinarum 287/91 provides insights into evolutionary and host adaptation pathways. Genome Res. 2008;18:1624–1637. doi: 10.1101/gr.077404.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kingsley RA, Msefula CL, Thomson NR, Kariuki S, Holt KE, et al. Epidemic multiple drug resistant Salmonella Typhimurium causing invasive disease in sub-Saharan Africa have a distinct genotype. Genome Res. 2009;19:2279–2287. doi: 10.1101/gr.091017.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu WQ, Feng Y, Wang Y, Zou QH, Chen F, et al. Salmonella paratyphi C: genetic divergence from Salmonella choleraesuis and pathogenic convergence with Salmonella typhi. PLoS One. 2009;4:e4510. doi: 10.1371/journal.pone.0004510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guard J, Morales CA, Fedorka-Cray P, Gast RK. Single nucleotide polymorphisms that differentiate two subpopulations of Salmonella enteritidis within phage type. BMC Res Notes. 2011;4:369. doi: 10.1186/1756-0500-4-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holt KE, Baker S, Dongol S, Basnyat B, Adhikari N, et al. High-throughput bacterial SNP typing identifies distinct clusters of Salmonella Typhi causing typhoid in Nepalese children. BMC Infect Dis. 2010;10:144. doi: 10.1186/1471-2334-10-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sokurenko EV, Hasty DL, Dykhuizen DE. Pathoadaptive mutations: gene loss and variation in bacterial pathogens. Trends Microbiol. 1999;7:191–195. doi: 10.1016/s0966-842x(99)01493-6. [DOI] [PubMed] [Google Scholar]
- 28.Sokurenko EV, Feldgarden M, Trintchina E, Weissman SJ, Avagyan S, et al. Selection footprint in the FimH adhesin shows pathoadaptive niche differentiation in Escherichia coli. Mol Biol Evol. 2004;21:1373–1383. doi: 10.1093/molbev/msh136. [DOI] [PubMed] [Google Scholar]
- 29.Klemm P, Christiansen G. Three fim genes required for the regulation of length and mediation of adhesion of Escherichia coli type 1 fimbriae. Mol Gen Genet. 1987;208:439–445. doi: 10.1007/BF00328136. [DOI] [PubMed] [Google Scholar]
- 30.Sokurenko EV, Chesnokova V, Doyle RJ, Hasty DL. Diversity of the Escherichia coli type 1 fimbrial lectin. Differential binding to mannosides and uroepithelial cells. J Biol Chem. 1997;272:17880–17886. doi: 10.1074/jbc.272.28.17880. [DOI] [PubMed] [Google Scholar]
- 31.Sokurenko EV, Chesnokova V, Dykhuizen DE, Ofek I, Wu XR, et al. Pathogenic adaptation of Escherichia coli by natural variation of the FimH adhesin. Proc Natl Acad Sci U S A. 1998;95:8922–8926. doi: 10.1073/pnas.95.15.8922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duguid JP, Anderson ES, Campbell I. Fimbriae and adhesive properties in Salmonellae. J Pathol Bacteriol. 1966;92:107–138. doi: 10.1002/path.1700920113. [DOI] [PubMed] [Google Scholar]
- 33.Thankavel K, Shah AH, Cohen MS, Ikeda T, Lorenz RG, et al. Molecular basis for the enterocyte tropism exhibited by Salmonella typhimurium type 1 fimbriae. J Biol Chem. 1999;274:5797–5809. doi: 10.1074/jbc.274.9.5797. [DOI] [PubMed] [Google Scholar]
- 34.Boyd EF, Hartl DL. Analysis of the type 1 pilin gene cluster fim in Salmonella: its distinct evolutionary histories in the 5′ and 3′ regions. J Bacteriol. 1999;181:1301–1308. doi: 10.1128/jb.181.4.1301-1308.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nuccio SP, Baumler AJ. Evolution of the chaperone/usher assembly pathway: fimbrial classification goes Greek. Microbiol Mol Biol Rev. 2007;71:551–575. doi: 10.1128/MMBR.00014-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baumler AJ, Tsolis RM, Heffron F. Fimbrial adhesins of Salmonella typhimurium. Role in bacterial interactions with epithelial cells. Adv Exp Med Biol. 1997;412:149–158. [PubMed] [Google Scholar]
- 37.Horiuchi S, Inagaki Y, Okamura N, Nakaya R, Yamamoto N. Type 1 pili enhance the invasion of Salmonella braenderup and Salmonella typhimurium to HeLa cells. Microbiol Immunol. 1992;36:593–602. doi: 10.1111/j.1348-0421.1992.tb02059.x. [DOI] [PubMed] [Google Scholar]
- 38.Boddicker JD, Ledeboer NA, Jagnow J, Jones BD, Clegg S. Differential binding to and biofilm formation on, HEp-2 cells by Salmonella enterica serovar Typhimurium is dependent upon allelic variation in the fimH gene of the fim gene cluster. Mol Microbiol. 2002;45:1255–1265. doi: 10.1046/j.1365-2958.2002.03121.x. [DOI] [PubMed] [Google Scholar]
- 39.Guo A, Lasaro MA, Sirard JC, Kraehenbuhl JP, Schifferli DM. Adhesin-dependent binding and uptake of Salmonella enterica serovar Typhimurium by dendritic cells. Microbiology. 2007;153:1059–1069. doi: 10.1099/mic.0.2006/000331-0. [DOI] [PubMed] [Google Scholar]
- 40.Ewen SW, Naughton PJ, Grant G, Sojka M, Allen-Vercoe E, et al. Salmonella enterica var Typhimurium and Salmonella enterica var Enteritidis express type 1 fimbriae in the rat in vivo. FEMS Immunol Med Microbiol. 1997;18:185–192. doi: 10.1111/j.1574-695X.1997.tb01044.x. [DOI] [PubMed] [Google Scholar]
- 41.Althouse C, Patterson S, Fedorka-Cray P, Isaacson RE. Type 1 fimbriae of Salmonella enterica serovar Typhimurium bind to enterocytes and contribute to colonization of swine in vivo. Infect Immun. 2003;71:6446–6452. doi: 10.1128/IAI.71.11.6446-6452.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naughton PJ, Grant G, Bardocz S, Allen-Vercoe E, Woodward MJ, et al. Expression of type 1 fimbriae (SEF 21) of Salmonella enterica serotype enteritidis in the early colonisation of the rat intestine. J Med Microbiol. 2001;50:191–197. doi: 10.1099/0022-1317-50-2-191. [DOI] [PubMed] [Google Scholar]
- 43.Hase K, Kawano K, Nochi T, Pontes GS, Fukuda S, et al. Uptake through glycoprotein 2 of FimH(+) bacteria by M cells initiates mucosal immune response. Nature. 2009;462:226–230. doi: 10.1038/nature08529. [DOI] [PubMed] [Google Scholar]
- 44.Ohno H, Hase K. Glycoprotein 2 (GP2): grabbing the FimH bacteria into M cells for mucosal immunity. Gut Microbes. 2010;1:407–410. doi: 10.4161/gmic.1.6.14078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tinker JK, Clegg S. Control of FimY translation and type 1 fimbrial production by the arginine tRNA encoded by fimU in Salmonella enterica serovar Typhimurium. Mol Microbiol. 2001;40:757–768. doi: 10.1046/j.1365-2958.2001.02430.x. [DOI] [PubMed] [Google Scholar]
- 46.Tinker JK, Hancox LS, Clegg S. FimW is a negative regulator affecting type 1 fimbrial expression in Salmonella enterica serovar typhimurium. J Bacteriol. 2001;183:435–442. doi: 10.1128/JB.183.2.435-442.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chuang YC, Wang KC, Chen YT, Yang CH, Men SC, et al. Identification of the genetic determinants of Salmonella enterica serotype Typhimurium that may regulate the expression of the type 1 fimbriae in response to solid agar and static broth culture conditions. BMC Microbiol. 2008;8:126. doi: 10.1186/1471-2180-8-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clegg S, Hughes KT. FimZ is a molecular link between sticking and swimming in Salmonella enterica serovar Typhimurium. J Bacteriol. 2002;184:1209–1213. doi: 10.1128/jb.184.4.1209-1213.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baxter MA, Jones BD. The fimYZ genes regulate Salmonella enterica Serovar Typhimurium invasion in addition to type 1 fimbrial expression and bacterial motility. Infect Immun. 2005;73:1377–1385. doi: 10.1128/IAI.73.3.1377-1385.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saini S, Pearl JA, Rao CV. Role of FimW, FimY, and FimZ in regulating the expression of type i fimbriae in Salmonella enterica serovar Typhimurium. J Bacteriol. 2009;191:3003–3010. doi: 10.1128/JB.01694-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Field TR, Layton AN, Bispham J, Stevens MP, Galyov EE. Identification of novel genes and pathways affecting Salmonella type III secretion system 1 using a contact-dependent hemolysis assay. J Bacteriol. 2008;190:3393–3398. doi: 10.1128/JB.01189-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kwan LY, Isaacson RE. Identification and characterization of a phase-variable nonfimbrial Salmonella typhimurium gene that alters O-antigen production. Infect Immun. 1998;66:5725–5730. doi: 10.1128/iai.66.12.5725-5730.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guo A, Cao S, Tu L, Chen P, Zhang C, et al. FimH alleles direct preferential binding of Salmonella to distinct mammalian cells or to avian cells. Microbiology. 2009;155:1623–1633. doi: 10.1099/mic.0.026286-0. [DOI] [PubMed] [Google Scholar]
- 54.Kisiela D, Sapeta A, Kuczkowski M, Stefaniak T, Wieliczko A, et al. Characterization of FimH adhesins expressed by Salmonella enterica serovar Gallinarum biovars Gallinarum and Pullorum: reconstitution of mannose-binding properties by single amino acid substitution. Infect Immun. 2005;73:6187–6190. doi: 10.1128/IAI.73.9.6187-6190.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kisiela DI, Kramer JJ, Tchesnokova V, Aprikian P, Yarov-Yarovoy V, et al. Allosteric catch bond properties of the FimH adhesin from Salmonella enterica serovar Typhimurium. J Biol Chem. 2011;286:38136–38147. doi: 10.1074/jbc.M111.237511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hall ML, Rowe B. Salmonella arizonae in the United Kingdom from 1966 to 1990. Epidemiol Infect. 1992;108:59–65. doi: 10.1017/s0950268800049505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Franco A, Hendriksen RS, Lorenzetti S, Onorati R, Gentile G, et al. Characterization of Salmonella occurring at high prevalence in a population of the land iguana Conolophus subcristatus in Galapagos Islands, Ecuador. PLoS One. 2011;6:e23147. doi: 10.1371/journal.pone.0023147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chattopadhyay S, Dykhuizen DE, Sokurenko EV. ZPS: visualization of recent adaptive evolution of proteins. BMC Bioinformatics. 2007;8:187. doi: 10.1186/1471-2105-8-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sokurenko EV, Gomulkiewicz R, Dykhuizen DE. Source-sink dynamics of virulence evolution. Nat Rev Microbiol. 2006;4:548–555. doi: 10.1038/nrmicro1446. [DOI] [PubMed] [Google Scholar]
- 60.Thomas WE, Trintchina E, Forero M, Vogel V, Sokurenko EV. Bacterial adhesion to target cells enhanced by shear force. Cell. 2002;109:913–923. doi: 10.1016/s0092-8674(02)00796-1. [DOI] [PubMed] [Google Scholar]
- 61.Nilsson LM, Thomas WE, Trintchina E, Vogel V, Sokurenko EV. Catch bond-mediated adhesion without a shear threshold: trimannose versus monomannose interactions with the FimH adhesin of Escherichia coli. J Biol Chem. 2006;281:16656–16663. doi: 10.1074/jbc.M511496200. [DOI] [PubMed] [Google Scholar]
- 62.Stahlhut SG, Tchesnokova V, Struve C, Weissman SJ, Chattopadhyay S, et al. Comparative structure-function analysis of mannose-specific FimH adhesins from Klebsiella pneumoniae and Escherichia coli. J Bacteriol. 2009;191:6592–6601. doi: 10.1128/JB.00786-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tchesnokova V, McVeigh AL, Kidd B, Yakovenko O, Thomas WE, et al. Shear-enhanced binding of intestinal colonization factor antigen I of enterotoxigenic Escherichia coli. Mol Microbiol. 2010;76:489–502. doi: 10.1111/j.1365-2958.2010.07116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Le Trong I, Aprikian P, Kidd BA, Forero-Shelton M, Tchesnokova V, et al. Structural basis for mechanical force regulation of the adhesin FimH via finger trap-like beta sheet twisting. Cell. 2010;141:645–655. doi: 10.1016/j.cell.2010.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aprikian P, Tchesnokova V, Kidd B, Yakovenko O, Yarov-Yarovoy V, et al. Interdomain interaction in the FimH adhesin of Escherichia coli regulates the affinity to mannose. J Biol Chem. 2007;282:23437–23446. doi: 10.1074/jbc.M702037200. [DOI] [PubMed] [Google Scholar]
- 66.Vazquez-Torres A, Jones-Carson J, Baumler AJ, Falkow S, Valdivia R, et al. Extraintestinal dissemination of Salmonella by CD18-expressing phagocytes. Nature. 1999;401:804–808. doi: 10.1038/44593. [DOI] [PubMed] [Google Scholar]
- 67.Worley MJ, Nieman GS, Geddes K, Heffron F. Salmonella typhimurium disseminates within its host by manipulating the motility of infected cells. Proc Natl Acad Sci U S A. 2006;103:17915–17920. doi: 10.1073/pnas.0604054103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Petrovska L, Aspinall RJ, Barber L, Clare S, Simmons CP, et al. Salmonella enterica serovar Typhimurium interaction with dendritic cells: impact of the sifA gene. Cell Microbiol. 2004;6:1071–1084. doi: 10.1111/j.1462-5822.2004.00419.x. [DOI] [PubMed] [Google Scholar]
- 69.Baorto DM, Gao Z, Malaviya R, Dustin ML, van der Merwe A, et al. Survival of FimH-expressing enterobacteria in macrophages relies on glycolipid traffic. Nature. 1997;389:636–639. doi: 10.1038/39376. [DOI] [PubMed] [Google Scholar]
- 70.Shin JS, Gao Z, Abraham SN. Involvement of cellular caveolae in bacterial entry into mast cells. Science. 2000;289:785–788. doi: 10.1126/science.289.5480.785. [DOI] [PubMed] [Google Scholar]
- 71.Drecktrah D, Knodler LA, Ireland R, Steele-Mortimer O. The mechanism of Salmonella entry determines the vacuolar environment and intracellular gene expression. Traffic. 2006;7:39–51. doi: 10.1111/j.1600-0854.2005.00360.x. [DOI] [PubMed] [Google Scholar]
- 72.Martinoli C, Chiavelli A, Rescigno M. Entry route of Salmonella typhimurium directs the type of induced immune response. Immunity. 2007;27:975–984. doi: 10.1016/j.immuni.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 73.Pascopella L, Raupach B, Ghori N, Monack D, Falkow S, et al. Host restriction phenotypes of Salmonella typhi and Salmonella gallinarum. Infect Immun. 1995;63:4329–4335. doi: 10.1128/iai.63.11.4329-4335.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Old DC, Payne SB. Antigens of the type-2 fimbriae of salmonellae: “cross-reacting material” (CRM) of type-1 fimbriae. J Med Microbiol. 1971;4:215–225. doi: 10.1099/00222615-4-2-215. [DOI] [PubMed] [Google Scholar]
- 75.Kuzminska-Bajor M, Kuczkowski M, Grzymajlo K, Wojciech L, Sabat M, et al. Decreased colonization of chicks by Salmonella enterica serovar Gallinarum expressing mannose-sensitive FimH adhesin from Salmonella enterica serovar Enteritidis. Vet Microbiol. 2012 doi: 10.1016/j.vetmic.2012.01.029. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 76.Lockman HA, Curtiss R., 3rd Virulence of non-type 1-fimbriated and nonfimbriated nonflagellated Salmonella typhimurium mutants in murine typhoid fever. Infect Immun. 1992;60:491–496. doi: 10.1128/iai.60.2.491-496.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alkan ML, Wong L, Silverblatt FJ. Change in degree of type 1 piliation of Escherichia coli during experimental peritonitis in the mouse. Infect Immun. 1986;54:549–554. doi: 10.1128/iai.54.2.549-554.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Saukkonen KM, Nowicki B, Leinonen M. Role of type 1 and S fimbriae in the pathogenesis of Escherichia coli O18:K1 bacteremia and meningitis in the infant rat. Infect Immun. 1988;56:892–897. doi: 10.1128/iai.56.4.892-897.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wilson RL, Elthon J, Clegg S, Jones BD. Salmonella enterica serovars gallinarum and pullorum expressing Salmonella enterica serovar typhimurium type 1 fimbriae exhibit increased invasiveness for mammalian cells. Infect Immun. 2000;68:4782–4785. doi: 10.1128/iai.68.8.4782-4785.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Baumler AJ, Tsolis RM, Bowe FA, Kusters JG, Hoffmann S, et al. The pef fimbrial operon of Salmonella typhimurium mediates adhesion to murine small intestine and is necessary for fluid accumulation in the infant mouse. Infect Immun. 1996;64:61–68. doi: 10.1128/iai.64.1.61-68.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Baumler AJ, Tsolis RM, Heffron F. The lpf fimbrial operon mediates adhesion of Salmonella typhimurium to murine Peyer's patches. Proc Natl Acad Sci U S A. 1996;93:279–283. doi: 10.1073/pnas.93.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kingsley RA, Abi Ghanem D, Puebla-Osorio N, Keestra AM, Berghman L, et al. Fibronectin binding to the Salmonella enterica serotype Typhimurium ShdA autotransporter protein is inhibited by a monoclonal antibody recognizing the A3 repeat. J Bacteriol. 2004;186:4931–4939. doi: 10.1128/JB.186.15.4931-4939.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lara-Tejero M, Galan JE. Salmonella enterica serovar typhimurium pathogenicity island 1-encoded type III secretion system translocases mediate intimate attachment to nonphagocytic cells. Infect Immun. 2009;77:2635–2642. doi: 10.1128/IAI.00077-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Misselwitz B, Kreibich SK, Rout S, Stecher B, Periaswamy B, et al. Salmonella enterica serovar Typhimurium binds to HeLa cells via Fim-mediated reversible adhesion and irreversible type three secretion system 1-mediated docking. Infect Immun. 2011;79:330–341. doi: 10.1128/IAI.00581-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.van der Velden AW, Baumler AJ, Tsolis RM, Heffron F. Multiple fimbrial adhesins are required for full virulence of Salmonella typhimurium in mice. Infect Immun. 1998;66:2803–2808. doi: 10.1128/iai.66.6.2803-2808.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Duncan MJ, Mann EL, Cohen MS, Ofek I, Sharon N, et al. The distinct binding specificities exhibited by enterobacterial type 1 fimbriae are determined by their fimbrial shafts. J Biol Chem. 2005;280:37707–37716. doi: 10.1074/jbc.M501249200. [DOI] [PubMed] [Google Scholar]
- 87.Richardson EJ, Limaye B, Inamdar H, Datta A, Manjari KS, et al. Genome sequences of Salmonella enterica serovar typhimurium, Choleraesuis, Dublin, and Gallinarum strains of well- defined virulence in food-producing animals. J Bacteriol. 2011;193:3162–3163. doi: 10.1128/JB.00394-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, et al. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol. 2010;59:307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- 89.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Swofford D. PAUP*: Phylogenetic Analysis Using Parsimony and Other Methods. Sunderland: Sinauer Associates; 2000. [Google Scholar]
- 91.Nei M, Gojobori T. Simple methods for estimating the numbers of synonymous and nonsynonymous nucleotide substitutions. Mol Biol Evol. 1986;3:418–426. doi: 10.1093/oxfordjournals.molbev.a040410. [DOI] [PubMed] [Google Scholar]
- 92.Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- 93.Suzuki Y, Gojobori T. Analysis of coding sequences. In: Salemi M, Vandamme A-M, editors. The Phylogenetic Handbook - a practical approach to DNA and protein phylogeny. 1st edition. Cambridge: Cambridge University Press; 2003. pp. 283–311. [Google Scholar]
- 94.Sokurenko EV, Courtney HS, Maslow J, Siitonen A, Hasty DL. Quantitative differences in adhesiveness of type 1 fimbriated Escherichia coli due to structural differences in fimH genes. J Bacteriol. 1995;177:3680–3686. doi: 10.1128/jb.177.13.3680-3686.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Berggren RE, Wunderlich A, Ziegler E, Schleicher M, Duke RC, et al. HIV gp120-specific cell-mediated immune responses in mice after oral immunization with recombinant Salmonella. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;10:489–495. [PubMed] [Google Scholar]
- 96.Wray C, Sojka WJ. Experimental Salmonella typhimurium infection in calves. Res Vet Sci. 1978;25:139–143. [PubMed] [Google Scholar]
- 97.Boyd EF, Wang FS, Whittam TS, Selander RK. Molecular genetic relationships of the salmonellae. Appl Environ Microbiol. 1996;62:804–808. doi: 10.1128/aem.62.3.804-808.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Swenson DL, Clegg S, Old DC. The frequency of fim genes among Salmonella serovars. Microb Pathog. 1991;10:487–492. doi: 10.1016/0882-4010(91)90115-q. [DOI] [PubMed] [Google Scholar]
- 99.Kelly SM, Bosecker BA, Curtiss R., 3rd Characterization and protective properties of attenuated mutants of Salmonella choleraesuis. Infect Immun. 1992;60:4881–4890. doi: 10.1128/iai.60.11.4881-4890.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Boyd EF, Wang FS, Beltran P, Plock SA, Nelson K, et al. Salmonella reference collection B (SARB): strains of 37 serovars of subspecies I. J Gen Microbiol. 1993;139 Pt 6:1125–1132. doi: 10.1099/00221287-139-6-1125. [DOI] [PubMed] [Google Scholar]
- 101.Hancox LS, Yeh KS, Clegg S. Construction and characterization of type 1 non-fimbriate and non-adhesive mutants of Salmonella typhimurium. FEMS Immunol Med Microbiol. 1997;19:289–296. doi: 10.1111/j.1574-695X.1997.tb01099.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Static adhesion of S. Typhimurium LBH4 expressing different variants of FimH to mannose-containing substrates. Binding of 3H-labled bacteria to Man1 (Man-BSA) and Man5 (RNaseB) was determined as described in ‘Materials and Methods’. Data are the means ± SD of triplicates from one representative experiment of three experiments that were performed.
(TIF)
Static adhesion of representative wild-type and recombinant S. enterica to mannose-containing substrates. Binding of wild-type (A) and recombinant (B) S. enterica strains to Man1 (yeast mannan, red), Man5 (RNaseB, blue) and anti-FimHSE antibody (grey). Attached bacteria were stained with crystal violet and the adhesion was quantified by measuring absorbance at 600 nm. Data are the means ± SD of triplicates from one representative experiment of three performed. The strain tags of systemically non-invasive serovars are in black and the invasive serovars in red.
(TIF)
Man1/Man5 binding ratio calculated for representative wild-type and recombinant strains of S. enterica. Data are the means ± SD of triplicates from one representative experiment of three performed. The strain tags of systemically non-invasive serovars are in black and the invasive serovars in red. ND, not determined.
(TIF)
Bacterial accumulation on Man1-coated surfaces in the parallel plate flow chamber. Bacterial binding to Man-BSA under different shear conditions was recorded for 4 min. Data are the means of two independent experiments.
(TIF)
Effects of point mutations on binding phenotype of S. enterica FimH. Static adhesion of 3H-labeled bacteria to Man1 (Man-BSA, red), Man5 (RNaseB, blue) and anti-FimHSE antibody (grey). Data are the means ± SD of triplicates from one representative experiment of three performed.
(TIF)
List of plasmids.
(RTF)
List of primers.
(RTF)